10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2005; 1(4):146-151. doi:10.7150/ijbs.1.146 This issue Cite
Research Paper
Co-expression of zinc binding motif and GFP as a cellular indicator of metal ions mobility
1. Department of Clinical Microbiology, Faculty of Medical Technology, Mahidol University, Bangkok, Thailand.
2. Department of Parasitology, Faculty of Medical Technology, Mahidol University, Bangkok, Thailand.
Received 2005-10-5; Accepted 2005-11-21; Published 2005-12-1
Abstract
A significant role of zinc-binding motifs on metal mobility in Escherichia coli was explored using a chimeric metal-binding green fluorescent protein (GFP) as an intracellular zinc indicator. Investigation was initiated by co-transformation and co-expression of two chimeric genes encoding the chimeric GFP carrying hexahistidine (His6GFP) and the zinc-binding motif fused to outer membrane protein A (OmpA) in E. coli strain TG1. The presence of these two genes was confirmed by restriction endonucleases analysis. Co-expression of the two recombinant proteins exhibited cellular fluorescence activity and enhanced metal-binding capability of the engineered cells. Incorporation of the zinc-binding motif onto the membrane resulted in 60-fold more binding capability to zinc ions than those of the control cells. The high affinity to metal ions of the bacterial surface influenced influx of metal ions to the cells. This may affect the essential ions for triggering important cell metabolism. A declining of fluorescent intensity of GFP has been detected on the cell expressed of zinc binding motif. Meanwhile, balancing of metal homeostasis due to the presence of cytoplasmic chimeric His6GFP enhanced the fluorescent emission. These findings provide the first evidence of real-time monitoring of intracellular mobility of zinc by autofluorescent proteins.
Keywords: chimeric green fluorescent protein, zinc ions, co-transformation, co-expression, metal mobility
1. Introduction
Zinc is an essential trace element required for the normal growth and viability of organisms [1]. This metal ion plays critical roles in many cellular processes including cofactor of enzymes, nuclear factor and hormone. A large number of enzymes and proteins contain zinc as a structural or catalytic cofactor such as alkaline phosphatase, RNA polymerase, superoxide dismutase and zinc finger proteins. In some eukaryotes, level of intracellular zinc triggers various important cell events. Depleting of zinc ions from the cell leads to cell death and apoptosis meanwhile deficiency of zinc ions in human can cause anemia, loss of appetite, defective of immune function, loss of epithelium integrity and growth retardation [2, 3]. Although zinc is not redox active under physiological conditions, excess of zinc ions can cause toxic to cells. Zinc toxicity may mediate via binding to other cation binding sites which subsequently alter the appropriate function of proteins and cofactors. The intracellular level of zinc ions is needed to be regulated to maintain viability and cell function. Precise regulatory systems have been evolved in many organisms to control the uptake, distribution, storage, and detoxification of zinc ions (for recent review see [4]). Several families of integral membrane proteins play role to transport zinc ions across the membranes into and out of the cells. In Escherichia coli, zinc homeostasis is accomplished largely through the transcriptional control of zinc uptake and efflux transporters [5, 6]. In mammalian cells, the transmembrane transporters (e.g. ZIP and ZnT families) are involved in the regulatory processes. However, little is known on the molecular mechanism and location of intracellular zinc storage. Recent studies revealed that no persistent cytoplasmic pool (zinc quota) of free zinc exists in E. coli under steady-state growth conditions [7]. In eukaryotic cells, labile zinc level has been estimated at the low-nanomolar range [8]. Monitoring of intracellular zinc level usually requires the fluorescent zinc-specific probes e.g. zinquin, zinpyr-1 and TSQ [9, 10, 11]. Increasing of the intracellular labile zinc upon up taking from the extracellular medium can be visualized by the fluorescent intensity of cells. Depletion of intracellular pools by metal chelators e.g. TPEN (membrane-permeable) and CaEDTA (membrane-impermeable) reveals a rapid decrease of fluorescence. However, limitation has been on the high background of fluorescence, photobleaching and cell toxicity.
Herein, we explore the feasibility of using metal-binding green fluorescent proteins together with the expression of the zinc-binding motif as tools to study the metal mobility of the cells. The co-expression of the chimeric genes encoding the zinc-binding motif fused to outer membrane protein (OmpA) [12] and the chimeric GFPs [13] were performed in E. coli strain TG1. With an advantage of autofluorescence of the GFP, depletion of zinc ions and metal homeostasis were monitored in real time.
2. Materials and Methods
Bacterial strain and plasmids
E. coli strain TG1 [(lac-pro), Sup E, thi1, hsd D5/F' tra D36, pro A+ B+, lacI, lacZ, M15; (ung+, dut+)] was used as host. Plasmids pGFPuv (Clontech Laboratories, USA), pHis6GFPuv [13] and pEVZn [12] were used for co-transformation and co-expression.
Enzymes and chemicals
Restriction endonucleases and molecular weight marker (λ/HindIII) were obtained from New England Biolabs. Chelating Sepharose Fast Flow gel was purchased from Pharmacia Biotech, Sweden. All other chemicals were of analytical grade and commercially available.
Co-transformation of chimeric genes
Transformation of chimeric genes into E. coli was performed according to the standard procedure. A plasmid pEVZn, which has previously been proven to express polyhistidine on the cell surface, was firstly transformed into E. coli strain TG1. These cells were grown and treated with 50 mM CaCl2 to become the competent cells. The plasmid of either pGFPuv or pHis6GFPuv was then co-transformed into the cells. Cells were subsequently spread onto LB agar containing 100 mg/l ampicillin. Transformants those possessed greenish fluorescence (observed under UV transiluminator) were picked and subcultured onto the LB media for isolation. Plasmids were extracted from the cells by Miniprep standard protocol as described by Maniatis. Successfulness of co-transformation was verified via restriction endonucleases analysis.
Verification of co-expression of two recombinant proteins
Cells carrying these two plasmids were grown in liquid LB medium containing 100 mg/l ampicillin at 35ºC with shaking for overnight. Cultures were pelleted by centrifugation then washed twice and resuspended in phosphate buffered saline (PBS; 50 mM Na2HPO4, 0.3 M NaCl, pH 7.4). Cell suspension was loaded onto the Immobilized Metal Affinity Chromatography (IMAC) charged with zinc ions. Unbound cells were removed by washing with phosphate buffer. Binding of cells to the gel was detected by determination of fluorescence emission under UV translumination and fluorescence microscope. Bound cells were eluted with 500 μl phosphate buffer containing 20 mM EDTA. Eluate was collected and subjected to microplate fluorescence reader for fluorescence measurement (excitation at 395 nm and emission at 508 nm).
Effect of zinc ions on fluorescence emission of engineered cells
Cells carrying pEVZn co-transformed with either pGFPuv or pHis6GFPuv were cultivated to allow co-expression between zinc-binding motif on the cell surface and native GFP or His6GFP in the cytoplasm. Cultures were harvested, washed, and resuspended in 5 mM HEPES buffer, 0.85 % (w/v) NaCl, pH 7.1. Cell suspensions were adjusted to 0.5 OD measured at 600 nm. Aliquots of 50 μl cell suspension were further incubated with various concentrations (50 nM-50 mM) of ZnSO4 in 96-well microtiter plates. Effect of zinc ions on fluorescent emission of engineered cells was investigated. The fluorescence intensity was measured at 5, 15, 30, 45, 60, 120, 180, and 240 min using the microplate fluorescence reader.
3. Results and discussion
On this, we report the first evidence of using the chimeric GFP as an intracellular zinc indicator in E. coli. Expression of the zinc-binding motif on the surface membrane clearly indicates that limitation of zinc uptake into the cell decreases the fluorescent intensity of the GFP. Meanwhile, the presence of cytoplasmic metal-binding region (His6GFP) plays an active role for metal homeostasis.
Co-expression of chimeric GFP and zinc binding motif
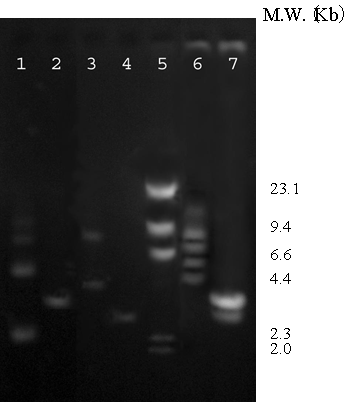
Co-transformation of the two chimeric genes encoding zinc-binding motif fused to outer membrane protein A (OmpA) [12] and chimeric green fluorescent protein carrying hexapolyhistidine (His6GFP) [13] in E. coli strain TG1 was performed. The presence of these two chimeric genes was verified via restriction endonucleases analysis. With respect to the presence of a unique EcoRI site on each plasmid (pEVZn and pHis6GFPuv), the extracted plasmid was cleaved by EcoRI and subsequently analyzed on an agarose gel electrophoresis. As shown in Figure 1, two corresponding bands (3.9 and 3.3 Kb) were observed as compared to the pEVZn and the pHis6GFPuv, respectively (lanes 2, 4 and 7).
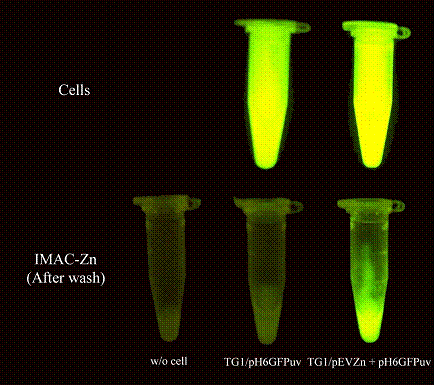
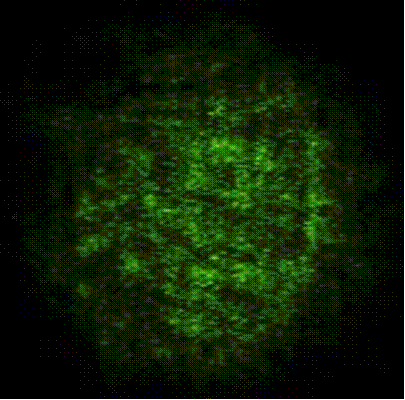
To further confirm the co-expression of these two recombinant proteins, cells those exhibited green fluorescence were grown and expression of the zinc-binding motif on the cell surface was investigated via binding to the immobilized metal affinity chromatography (IMAC) charged with zinc ions. Binding of engineered cells (TG1/pEVZn + pHis6GFPuv) on the IMAC gel was determined by using UV transiluminator as compared to cells expressing His6GFP intracellularly. As shown in Figure 2, the engineered cells exhibited not only a strong fluorescence as comparable to the control cell (upper panel) but these cells also provided the metal-binding capability (lower panel). Immobilized cells were further visualized via a fluorescence microscope as represented in Figure 3. All these findings inferred not only the success of co-expression but also exploring a feasibility of applying this approach as a tool for verification of metal-binding avidity of the engineered cells. Similar investigations have also been reported by using phase contrast microscopy, electron transmission and fluorescence micrograph or confocal laser scanning microscope [14, 15, 16].
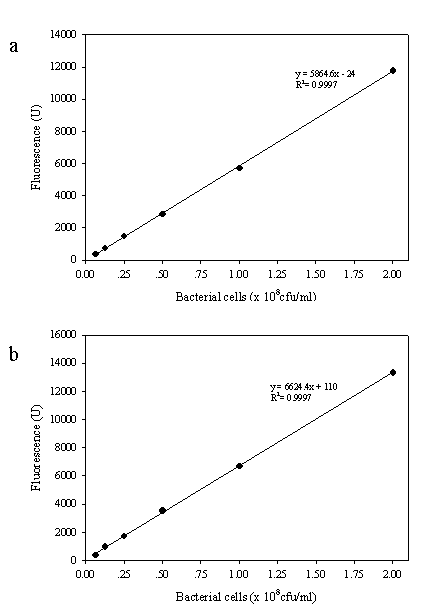
To further calculate the metal-binding capability of the engineered cells, a linear correlation between fluorescence intensity and amount of by-standing cells on the IMAC gel was determined. Figure 4 showed such the correlation of cells expressing His6GFP intracellularly (Figure 4a) or co-expression of His6GFP and zinc-binding motif (Figure 4b). Both cases demonstrated the linear correspondence with a correlation coefficient very close to 1. In addition, the engineered cells exhibited a bit higher fluorescence emission per se than the cell expressing His6GFP. From these data, calculation could easily be taken by dividing of the fluorescence intensity of cells eluted from the gel by the amount of gel. The amounts of engineered cells and cells expressing intracellular His6GFP immobilized onto the gel were 0.98 × 108 cfu/ml and 0.0173 × 108 cfu/ml, respectively. This finding suggested that the metal-binding capability of engineered cell was approximately 60-fold higher than those of the control cells. The strong binding affinity is mainly attributable to the presence of zinc-binding motif consisting of several histidine residues throughout the bacterial surface membrane. These basidic amino acids provide more affinity to zinc ions than the polar head group of the membranous material or the peptidoglycan layer of control cells [17].
Location of metal-binding regions on fluorescence responses
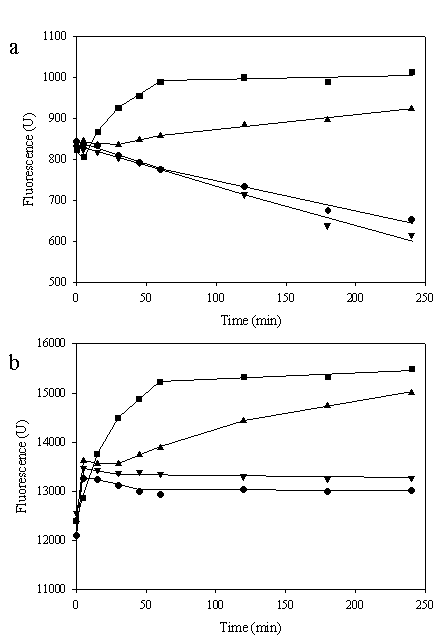
To investigate whether location of zinc-binding motif affected intracellular metal mobility, the alteration in fluorescent intensity of engineered cells was monitored. For this purpose, co-transformation between pEVZn and pGFPuv was performed in a similar manner and applied for comparison. All the cells were incubated with various concentrations (50 nM-50 mM) of ZnSO4 and the fluorescence responses were measured. As shown in Figure 5a, a gradually reduction of fluorescent emission of cells expressing zinc-binding motif on the cell surface and native GFP (TG1/pEVZn + pGFPuv) according to time was observed in the absence of zinc ions. Low concentration of zinc ions (50 nM) had no significant effect on the fluorescent intensity while contrarily metal at higher concentrations (50 μM – 50 mM) enhanced the fluorescence of the cells. In case of cells expressing zinc-binding motif on the cell surface together with intracellular His6GFP, the declining of fluorescent emission in the absence of metal ions was not observed (Figure 5b). More importantly, when various concentrations of zinc ions (50 nM – 50 mM) were applied the enhancement effect on the fluorescent intensity was revealed as concentration dependent. A plausible explanation to the discrepancy observation was the competitive binding to metal ions between the zinc-binding motif on the cell surface and intracellular metal-binding region. In case of the native GFP, availability of zinc ions passing through the cellular membrane is limited by binding of the zinc-binding motif on the cell surface. We have previously reported that zinc ions enhanced the fluorescent emission of GFP in vivo [18]. Therefore, depletion of zinc resulted in a reduction of the fluorescent intensity of native GFP. Supportive evidence is that fluorescence emission of native GFP expressed in the TG1 host without expression of the zinc-binding motif on the cell surface is up to 8-10 fold higher than those of the cells carrying pEVZn and pGFPuv (data not shown). Furthermore, an alteration on colony morphology of the engineered cells is detected upon metal deprivation. This is in a good agreement with the reduction of cell viability in the presence of zinc chelator (TPEN) [11].
It is worth to state that there was no significant difference in fluorescent emission between cells expressing His6GFP and cells co-expressing zinc-binding motif in the absence of metal ions (Figure 4). The enhancement effect of zinc ions on the fluorescence emission has readily been observed even at very low amount of metal applied to the cells (Figure 5b). Explanation is that the presence of intracellular polyhistidine may act as a high affinity-binding domain capable of efficiently capturing metals from the environment, which may increase the uptake and in turn modulate the function of metal ions for the cells. Supportive evidences can be taken into accounts i) E. coli strain TG1 expressing His6GFP intracellularly provided more metal accumulation than the others [19], ii) the hexahistidine provided a high affinity toward divalent cations as has been demonstrated by the association to chelating membrane lipid [20, 21] and IMAC-Zn2+ binding [13], iii) the relative mobility of zinc ions across the cell membrane in a similar fashion was observed upon addition of a membrane-impermeable chelator e.g. CaEDTA [10] and iv) the histidine residue plays a major role in many zinc-transport systems [22, 23].
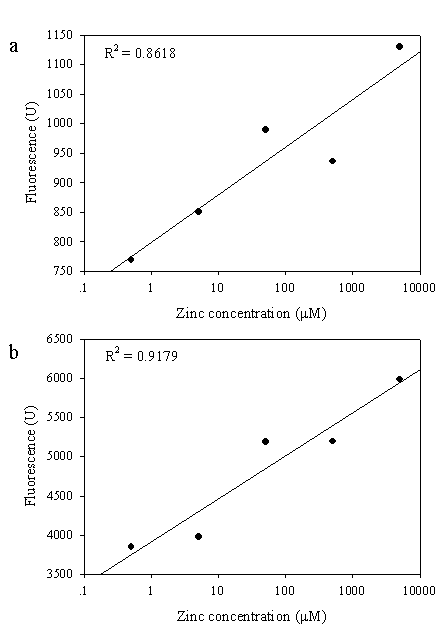
All these findings suggest not only the significant role of zinc-binding motif on the metal regulation at the cellular level but also supporting a high feasibility to apply the chimeric GFPs as an intracellular metal indicator in the future [24]. For circumstances, linear response between zinc concentrations and fluorescent intensity of the engineered cells was investigated (Figure 6). Cells co-expressing the zinc-binding motif and His6GFP provided a linear response ranging from 0.5 μM - 5 mM with the correlation coefficient greater than 0.9 (Figure 6b). However, little is known on the enhancement of the fluorescent emission upon addition of zinc or cadmium ions to the cells expressing chimeric GFPs. Further work is required to understand whether the hexapolyhistidine exert their action by mediating direct transfer of the metal ions into the chromophore region as in case of the intra or intermolecular mechanism of copper transfer from the N-terminal histidine rich region to the active site of Cu/Zn SOD [25] or by increasing the local concentration of the metal around the chromophore of the GFP.
Acknowledgements
This project was supported by a grant from the annual budget (B.E. 2546-2550) of Mahidol University. S.B. was supported by the Royal Golden Jubilee Ph.D. Scholarship from the Thailand Research Fund under the supervision of V.P. and Y.S. was supported by the collaborative Ph.D. fellowship from Royal Thai government.
Conflict of interest
The authors have declared that no conflict of interest exists.
References
1. Vallee B, Falchuk K. The biochemical basis of zinc physiology. Physiol Rev. 1993 ;73:79-118
2. Prasad A. Recognition of zinc-deficiency syndrome. Nutrition. 2001 ;17:67-69
3. Rudolf E, Cervinka M. Depletion of endogenous zinc stores induces oxidative stress and cell death in human melanoma cells. Acta Medica (Hradec Kralove). 2004 ;47:91-96
4. Liuzzy J, Counsins R. Mammalian zinc transporters. Annu Rev Nutr. 2004 ;24:151-172
5. Lee JL, Barrett JA, Poole RK. Genome-wide transcriptional response of chemostat-cultured Escherichia coli to zinc. J Bacteriol. 2005 ;187:1124-1134
6. Yamamoto K, Ishihama A. Transcriptional response of Escherichia coli to external zinc. J Bacteriol. 2005 ;187:6333-6340
7. Outen C, O'Halloran T. Femtomolar Sensitivity of Metalloregulatory Proteins Controlling Zinc Homeostasis. Science. 2001 ;292:2488-2492
8. Sensi S, Canzoniero L, Yu S, Ying H, Koh J, Kerchner G, Choi D. Measurement of intracellular free zinc in living cortical neurons: routes of entry. J Neurosci. 1997 ;17:9554-9564
9. Duffy J, Miller C, Rutschilling G, Ridder G, Clegg M, Keen C, Daston G. A decrease in intracellular zinc level precedes the detection of early indicators of apoptosis in HL-60 cells. Apoptosis. 2001 ;6:161-172
10. Frederickson CJ, Suh SW, Koh JY, Cha YK, Thompson RB, LaBuda CJ, Balaji RV, Cuajungco MP. Depletion of intracellular zinc from neurons by use of and extracellular chelator in vivo and in vitro. J Histochem Cytochem. 2002 ;50:1659-1662
11. Devergnas S, Chimienti F, Naud N, Pennequin A, Coquerel Y, Chantegrel J, Favier A, Seve M. Differential regulation of zinc efflux transporters ZnT-1, ZnT-5 and ZnT-7 gene expression by zinc levels: a real-time RT-PCR study. Biochem Pharmacol. 2004 ;68:699-709
12. Mejare M, Ljung S, Bulow L. Selection of cadmium specific hexapeptides and their expression as OmpA fusion proteins in Escherichia coli. Protein Eng. 1998 ;11:489-494
13. Prachayasittikul V, Isarankura Na Ayudhya C, Mejare M, Bulow L. Construction of a chimeric histidine6-green fluorescent protein: role of metal on fluorescent characteristic. Thammasat Int J Sc Tech. 2000 ;5:61-68
14. Sousa C, Cebolla A, de Lorenzo V. Enhanced metalloadsorption of bacterial cells displaying poly-His peptides. Nature Biotechnol. 1996 ;14:1017-1020
15. Xu Z, Lee S. Display of polyhistidine peptides on the Escherichia coli cell surface by using outer membrane protein C as an anchoring motif. Appl Environ Microbiol. 1999 ;65:5142-5147
16. Kjaergaard K, Schembri M, Klemm P. Novel Zn2+-chelating peptides selected from a fimbria-displayed random peptide library. Appl Environ Microbiol. 2001 ;67:5467-5473
17. Beveridge T, Koval S. Binding of metals to cell envelopes of Escherichia coli K-12. Appl Environ Microbiol. 1981 ;42:325-335
18. Prachayasittikul V, Isarankura Na Ayudhya C, Bulow L. Lighting E. coli cells as biological sensors for Cd2+. Biotechnol Lett. 2001 ;23:1285-1291
19. Isarankura Na Ayudhya C. Engineering of chimeric protein for binding to metal ions (PhD Thesis). Bangkok, Thailand: Mahidol University. 2000
20. Prachayasittikul V, Isarankura Na Ayudhya C, Tantimongcolwat T, Galla HJ. Nanoscale orientation and lateral organization of chimeric metal-binding green fluorescent protein on lipid membrane determined by epifluorescence and atomic force microscopy. Biochem Biophys Res Commun. 2005 ;326:298-306
21. Prachayasittikul V, Isarankura Na Ayudhya C, Hilterhaus L, Hinz A, Tantimongcolwat T, Galla HJ. Interaction analysis of chimeric metal-binding green fluorescent protein and artificial solid-supported lipid membrane by quartz crystal microbalance and atomic force microscopy. Biochem Biophys Res Commun. 2005 ;327:174-182
22. Lee S, Grass G, Haney C, Fan B, Rosen B, Anton A, Nies D, Rensing C. Functional analysis of the Escherichia coli zinc transporter ZitB. FEMS Microbiol Lett. 2002 ;215:273-278
23. Kirschle C, Huang L. ZnT7, a novel mamalian zinc transporter, accumulates zinc in the Golgi apparatus. J Biol Chem. 2003 ;278:4096-4102
24. Baubet V, Mouellic HL, Campbell AK, Lucas-Meunier E, Fossier P, Brulet P. Chimeric green fluorescent protein-aequorin as bioluminescent Ca2+ reporters at the single-cell level. Proc Natl Acad Sci USA. 2000 ;97:7260-7265
25. Battistoni A, Pacello F, Mazzetti A, Capo C, Kroll J, Langford P, Sansone A, Donnarumma G, Valenti P, Potilio G. A histidine-rich metal binding domain at the N terminus of Cu, Zn-superoxide dismutases from pathogenic bacteria: a novel strategy for metal chaperoning. J Biol Chem. 2001 ;276:30315-30325
Figures
Restriction endonucleases analysis. Lane 1 pEVZn uncut, lane 2 pEVZn/EcoRI, lane 3 pHis6GFPuv uncut, lane 4 pHis6GFPuv/EcoRI, lane 5 λ/HindIII, lane 6 Co-transformation (pEVZn + pHis6GFPuv) uncut, lane7 Co-transformation (pEVZn + pHis6GFPuv)/EcoRI
Determination of metal-binding capability of engineered cells via fluorescent analysis. Upper panel suspension of cells expressing His6GFP intracellularly (TG1/pHis6GFPuv) and cells co-expressing zinc-binding motif on the cell surface and His6GFP in the cytoplasm (TG1/pEVZn + pHis6GFPuv). Lower panel metal-binding capability of engineered cells immobilized onto the IMAC gel charged with zinc ions
Metal-binding capability of engineered cells co-expressing zinc-binding motif on the cell surface and His6GFP intracellularly to the immobilized zinc ions on the IMAC gel determined by fluorescence microscope (400 X magnification)
Linear correlation between fluorescent intensity and amount of cells expressing His6GFP intracellularly (TG1/pHis6GFPuv) (a) and engineered cells co-expressing zinc-binding motif on the cell surface and His6GFP in the cytoplasm (TG1/pEVZn + pHis6GFPuv) (b)
Effect of various concentrations of zinc ions on fluorescence level of E. coli co-expressing zinc-binding motif on the cell surface and native GFP (a) or His6GFP (b) in the cytoplasm. *Values represented means (n ≥ 2). Symbols: ● (no metal); ▼ (50 nM); ▲ (50 μM); ■(50 mM)
Linear correlation between zinc concentration and fluorescence level of E. coli co-expressing zinc-binding motif on the cell surface and native GFP (a) or His6GFP (b) in the cytoplasm
Author contact
![]() Corresponding address:
Corresponding address:
Assoc. Prof. Dr. Virapong Prachayasittikul, Department of Clinical Microbiology, Faculty of Medical Technology, Mahidol University, 2 Prannok Rd., Bangkok-Noi, Bangkok, Thailand 10700, Telephone: 662-849-6318 Telefax: 662-849-6330. E-mail: mtvprmahidol.ac.th

 Global reach, higher impact
Global reach, higher impact