Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2007; 3(1):27-39. doi:10.7150/ijbs.3.27 This issue Cite
Research Paper
Proteomic Identification of 14-3-3ζ as an Adapter for IGF-1 and Akt/GSK-3β Signaling and Survival of Renal Mesangial Cells
1. Department of Anatomy and Cell Biology, Wayne State University School of Medicine, Detroit, MI 48201, USA
2. Department of Ophthalmology, Wayne State University School of Medicine, Detroit, MI 48201, USA
3. Department of Internal Medicine, Wayne State University School of Medicine, Detroit, MI 48201, USA
# Contributed Figure 2A and established stable cells
Received 2006-8-15; Accepted 2006-10-25; Published 2006-10-27
Abstract
Recently we demonstrated that IGF-1 expression is increased in the diabetic kidney and that it may involve in renal hypertrophy and extracellular matrix protein (ECM) accumulation in mesangial cells as seen in diabetic glomerulopathy. The present study investigates the molecular mechanism(s) of IGF-1 and Akt/glycogen synthase kinase-3beta (GSK-3β) signaling pathway in the regulation of fibronectin and cyclin D1 expression and survival of renal mesangial cells. A proteomic approach is also employed to identify protein targets of IGF-1 signaling via GSK-3β inhibition in mesangial cells. We show that IGF-1 (100 ng/ml) significantly increases the protein kinase Akt/PKB activity (1.5-2-fold, p<0.05) within 1-5 minutes, which is completely blocked by the presence of 100 nM Wortmannin (phosphatidyl-inositol 3-kinase inhibitor). Akt activation is coupled with Ser9 phosphorylation and inactivation of its down-stream target GSK-3β. IGF-1 increases the cyclic AMP-responsive element (CRE) binding transcription factor CREB phosphorylation at Ser 133 and CRE-binding activity in mesangial cells, which parallels cyclin D1 and fibronectin expressions. Both proteins are known to have CRE-sequences in their promoter regions upstream of the transcription start site. Suppression of GSK-3β by SB216763 (100 nM) increases CREB phosphorylation, cyclin D1 and fibronectin levels. Two dimensional gel electrophoresis followed by MALDI-TOF mass spectrometric analysis of mesangial proteins reveals that IGF-1 treatment or an inhibition of GSK-3β increases the expression of the phosphorylated Ser/Thr binding signal adapter protein 14-3-3ζ. Immuno-precipitation of 14-3-3ζ followed by Western blotting validates the association of phosphorylated GSK-3β with 14-3-3ζ in renal mesangial cells. Stable expression of a constitutively active GSK-3β(Ser9Ala) induces cell death while overexpression of HA-tagged 14-3-3ζ increases cell viability as measured by MTT assays. These results indicate that the Akt/GSK-3β pathway and the adapter protein 14-3-3ζ may play an important role in IGF-1 signaling and survival of mesangial cells in diabetic nephropathy.
Keywords: Diabetic Glomerulopathy, Insulin-like growth factor 1, Akt/GSK-3β, 14-3-3ζ, mesangial cell survival
1. INTRODUCTION
Diabetic glomerular disease, like all forms of glomerular sclerosis, is characterized by deranged glomerular remodeling consisting of enhanced extracellular matrix (ECM) accumulation and altered cell growth leading to an enlargement of the mesangial region [1, 2]. Recent studies indicate that Insulin-like growth factor –1 (IGF-1) is accumulated in diabetic kidney and involves in the expansion of glomerular mesangium and the development of diabetic glomerular sclerosis. IGF–1 is a multifunctional growth factor produced in a variety of tissues including kidney [3, 4] and is considered to participate in the pathology of diabetic renal disease [5, 6]. Increased expression and accumulation of IGF-1 peptide, mRNA and IGF-1 binding to its receptor are observed in diabetic glomerulopathy [7-9]. In patients with type 1 diabetes, elevated urinary IGF-1 is associated with hypertrophy and progression of kidney disease [10, 11]. The signaling pathways by which IGF-1 promotes cell growth, proliferation and hypertrophy of renal cells remain largely undefined.
Insulin and IGF-1 signaling involves activation of phosphatidyl inositol 3 kinase (PI3K) and the downstream Akt/PKB, which in turn phosphorylates GSK-3α at Ser 21 and GSK-3β at Ser 9, and inhibits its kinase activity [12]. Both isoforms have a similar range of substrate specificities and are regulated in parallel in response to growth factor stimulation. However, disruption of GSK-3β gene in mice results in embryonic lethality, indicating that GSK-3α can't completely substitute for a loss of GSK-3β [13]. GSK-3β has been studied more extensively than the α-form, and we have employed this isoform in our recent studies [14]. In MCF-7S breast cancer cells, an inhibition of GSK-3β by IGF-1 or LiCl, a known inhibitor of GSK-3β, results nuclear accumulation of cyclin D1 and cell cycle progression [15]. Recently, we showed that IGF-1 increases mesangial cell hypertrophy and laminin β1 and γ1 expression in in vitro cell cultures and that the Akt/GSK-3β may participate in these processes [14]. There also exists signal cross-talks between Akt/GSK-3β and calcium-dependent protein phosphatase 2B (PP2B or calcineurin) pathways. Other investigators observed that IGF-I treatment of mesangial cells increases the synthesis of calmodulin and calcineurin activity [16]. Cyclosporin A, an inhibitor of calcineurin, blocks both IGF-1-induced NFATc1 nuclear translocation and up-regulation of fibronectin and collagen IV in mesangial cells [16].
Hyperglycemia-induced ECM gene expression involves activation of several transcription factors including the cyclic AMP responsive element (CRE) binding protein, CREB, and GC binding protein, Sp1. These transcription factors are regulated by high glucose in mesangial cells [17-19]. Most ECM genes bear CREB and Sp1 binding consensus sequences in their promoters [20-23]. The activation of genes bearing CRE is thought to occur by the phosphorylation of CREB at Ser 133 and binding to CRE consensus sequences in the promoter region of genes [24, 25]. We have previously shown that high glucose-induced fibronectin protein synthesis is associated with parallel changes in the level of CREB phosphorylation and CRE binding activity in mesangial cells [26-28]. IGF-1 is a potent activator of CREB in a variety of cells types [29]; however, IGF-1's effects on CREB activity in mesangial cells are not known yet. Here, we demonstrate that IGF-1 increases fibronectin and cyclin D1 expression in mesangial cells and is associated with enhanced phosphorylation and DNA-binding activity of CREB. A proteomic analysis reveals that IGF-1 increases the expression of the signal adapter protein, 14-3-3ζ, in mesangial cells and it is associated with GSK-3β and its down-stream target beta-catenin. 14-3-3 proteins are ubiquitous eukaryotic acidic polypeptides of ~32 kDa that binds to serine or threonine residues of phosphorylated proteins and play a regulatory role in intracellular signal transduction, cell-cycle progression, differentiation and apoptosis. Therefore, Akt/GSK-3β and the scaffold protein 14-3-3ζ may play a role in IGF-1 signaling to regulate cell cycle progression, ECM expression and survival of renal mesangial cells.
2. MATERIALS AND METHODS
Materials
Insulin-like growth factor-1, PI3-K inhibitor, Wortmannin, and antibodies for cyclin D1, and α-actin were purchased from Upstate Biotechnology, Lake Placid, NY. Antibodies for phosphorylated and non-phosphorylated GSK-3β, and 14-3-3ζ antibodies and its competitive peptides were obtained from Santa Cruz, CA. Anti-cyclin D1 and phosphorylated Akt substrate motif specific antibodies were purchased form Cell Signaling Technology, Danvers, MA. MTT [3-(4,5-dimethylthiazol-2-yl)-2-,5- diphenyltratrazolium bromide], Hoechst Dye 33342, anti-fibronectin and fluorescence-labeled secondary antibodies were purchased from Molecular Probes, Eugene, OR. SH-5 (Akt inhibitor) was from Alexis (San Diego, CA) while GSK-3β inhibitor, SB216763, was obtained from Calbiochem, San Diego. The enhanced chemiluminescence (ECL) system was obtained from Amersham (Arlington Heights, IL). DMEM and F-12 nutrient mixture (Ham's) were from GIBCO (Grand Island, NY). The gel-shift assay kit was purchased from PROMEGA, Wisconsin.
Cell Culture
An immortalized rat mesangial cell line was cultured in medium containing DMEM and Ham's F-12 (3:1 ratio) supplemented with 2.25% fetal calf serum (FCS) and 0.5 mg/ml gentamicin at 37°C in a humidified chamber with a 5% CO2-95% air mixture [14]. Monolayers at 60-70% confluence were serum-starved overnight and varying amounts of IGF-1 (100 ng/ml) was added for different time intervals. For this time course, IGF-1 with 24 h treatment was added first, then on the second day IGF-1 was added at 6, 2 or 0 h to the respective cultures before harvesting the cells. Thus, all cells including the control maintain a similar condition and length of serum-starvation during the entire experimental procedure [14]. MES cells were harvested and proteins extracted in RIPA buffer. The protein concentrations were determined using a Coomassie-based reagent from Pierce with BSA as the standard. When the inhibitors are included in the culture, they were added 30 minute before adding IGF-1, and were present throughout the period of incubation.
SDS-PAGE, Western Blotting and Immunoprecipitation
Sodium dodecyl sulfate polyacrylamide gel electrophoresis and Western blot analysis of proteins were performed as described previously [14]. Immunoprecipitation of mesangial proteins associated with 14-3-3ζ was performed using Protein G/A-agarose-conjugated 14-3-3ζ antibodies and the bound proteins were specifically dissociated with a 10x higher concentration of a competitive peptide derived from 14-3-3ζ (Santa Cruz). Five hundred microgram of protein extracts were incubated with 50 μl of pre-absorbed Protein G/A-agarose-anti-14-3-3ζ antibodies overnight. The bound proteins were eluted with ten-fold excess of the 14-3-3ζ specific peptide. The resulting proteins were separated on SDS-PAGE and subjected to Western blotting.
Akt/PKB activity assay
The kinase activity of Akt/PKB in mesangial cell extracts was determined using an antibody-based immunoprecipitation assay kit from Upstate Biotechnology, Lake Placid, NY, as described before [30].
Fluorescence Immunohistochemistry
Mesangial cells were grown either in four-chambered tissue culture glass slides (NUNC, Naperville, IL) and exposed to IGF-1 (100 ng/ml) for different time periods (0-24 h). Cells were fixed with freshly prepared paraformaldehyde (4%) for 2 h in ice or 4 oC overnight, washed 10 min each with PBS (3 times) and blocked with 5% horse serum in PBS for 1 h at room temperature. Following a 30 minutes wash with PBS, cells were incubated with primary antibodies for 2 h at 37 oC in a humidified chamber. Primary antibody dilution for chicken anti-fibronectin antibodies was 1:200 (v/v) and mouse anti-α-tubulin was 1:500 (v/v). After washing with PBS, cells were further incubated with corresponding secondary antibodies conjugated with Alexa Fluor 488 or RITC at 1:500 dilution for 1 h at 37 oC in a darkened humidified chamber. After washing with PBS again, mesangial cells were mounted with an aqueous mounting medium with anti-fade agent and sealed with nail polish. The cell-associated fluorescence was observed in an Olympus BX51 Fluorescence microscope fitted with a DP70 CCD camera and images were analyzed by image analysis software of the microscope and combined by Microsoft Adobe Photosoft.
Gel-shift assay
The gel-shift, or electrophoretic mobility-shift, assay provides a simple and rapid method for detecting DNA-binding activity of proteins. We investigated the CREB activity of mesangial nuclear proteins by using a commercially available gel-shift assay kit from Promega as described before [21]. An 18-mer oligonucleotide containing CRE consensus sequence 5'-TGACGTCA-3' was 5'-labeled with T4 polynucleotide kinase and (γ-32P)ATP, and the radiolabeled CRE were separated on a G-25 Sephadex Spin column (Boehringer Mannheim). After incubation at 30°C for 10 min with 32P-CRE, nuclear extracts (7.5 µg protein) were subjected to 6% nondenaturing polyacrylamide gels prepared with TBE gel formulation (supplied by the manufacturer). The gels were run at 100 V for 1 h until the Bromophenol blue dye front is three quarters down the gel, using 0.5x TBE running buffer. The gel was covered with Saran Wrap and exposed to X-ray films. For competitive and noncompetitive assays, 10xcold CRE or GC sequence (a sequence specific for Sp1 transcription factor), were respectively used.
Two-Dimensional Gel Electrophoresis and MALTI-TOF Mass Spectrometry
The proteomic analysis to identify proteins in mesangial cells were performed at the Michigan Proteome Consortium, Core facility at the University of Michigan, Ann Arbor [31]. Protein samples were prepared in 7M Urea, 2M Thiourea, 0.002% Bromophenol Blue, 2 mM tributyl phosphine and 0.5% IPG Buffer. Due to the presence of some interfering materials in the cellular extracts, proteins were precipitated with TCA and re-suspended in the above buffer again. First dimensional isoelectric focusing was performed in a BioRad Protean IEF cell (pH 4-7 and 18 cm strips) and the second dimension in a Protean Plus Dodeca Cell [Bio Rad]. Gels in triplicates from each condition, e.g., control, IGF-1 treated (100 ng/ml) or SB216763 (100 nM GSK3β inhibitor) for 24 h, were stained with Colloidal Commassie blue to detect protein spots. Bio-rad PDQuest 2D software was used to analysis and quantitate the spot intensities. Automated spot picking of proteins of interest was performed by the robotic system Ettan Spot Picker (Amersham Pharmacia Biotech), and the in-gel tryptic digestion was achieved using a MassPrep Robotic Workstation (MicroMass, UK). Tryptic digests were analyzed using matrix-assisted laser desorption/ionization (MALDI) time of flight (MALDI-TOF) ms or ms/ms mass spectrometry using α-cyano 4-hydroxy cinnamic acid as the carrier matrix (Applied Biosystems). Peptide masses were submitted to SwissProt and NCBInr databases for peptide mass fingerprinting (PMF) and protein identification using MS-Fit for ms data search [http://prospector.ucsf.edu/] or MASCOT MS/MS Ion Search for ms/ms data analysis [http://www.matrixscience.com]. Peptides were assumed to be carbamidomethylated at cysteine residues, and allowed up to 1 missed tryptic cleavage and 50-ppm mass tolerance.
Real-time quantitative PCR
Fibronectin mRNA expression was analyzed by real-time quantitative PCR using the ABI Prism 7900HT sequence detection system and SYBR Green PCR Master Mix from Applied Biosystems (Foster City, CA). Primers were designed using Primer Express v 2.0 [Applied Biosystems] and synthesized by Invitrogen (Carlsbad, CA).
Primer sequences of genes for the real-time Q-PCR are:
1. Fibronectin (Fn) [Accession no. X93167]
forward (region 209-229) 5'-CTGGGGTCA CGTACCTCTTCA-3'
reverse (region 389-369) 5'-AGTCGGTAGCCTGCTATACGG-3';
2. S18: [NM_138946]
forward (region 24-44) 5'-GCACAGTGTTTGTAGAGCCTG-3'
reverse (region 170-149) 5'-GCCCTGGAACTTATTGATCGGG-3'.
The real time PCR reaction mixture contained 1X SYBR Green PCR Master Mix, 400 nM forward and reverse primers, and 10 ng total cDNA in a final volume of 25 μl. The PCR cycling was programmed as 95 oC for 15s and 60 oC for 20s for 40 cycles followed by the construction of a melting curve through increasing the temperature from 60 oC to 95 oC at a ramp rate of 2% for 20 min. The real time PCR samples were evaluated using a single predominant peak as a quality control. Ct values were used to calculate the relative expression level of fibronectin mRNAs that were normalized to endogenous cellular 18S. The numbers of real time PCR cycles at which fluorescent signals reached a detection threshold was set within the exponential phase of the PCR.
Generation of stably transfected GSK-3β(S9A) and HA-tagged 14-3-3ζ expressing mesangial cells
The constitutively active Ser 9 to Ala mutated GSK-3β(S9A) and haemagglutinin-(HA)-tagged 14-3-3ζ in pcDNA3.1 plasmids were used to generate stably overexpressing mesangial cells and a corresponding empty pcDNA3.1 control cell line. The expression vector for GSK-3β(S9A) was kindly provided by Dr. Woodgett from the University of Toronto Health Sciences, Toronto, and the HA-tagged 14-3-3ζ plasmid was provided by Drs. Kosie Bialkowska and Joan Fox of Cleveland Clinic Foundation, Cleveland, OH. Rat mesangial cells were plated in a six-well plate and grown overnight to ~50% confluence and serum-starved. Cells were transfected with 1.0 μg DNA per well in 100 μl serum-free DMEM containing 20 μl of Lipofectamine Plus [Invitrogen] in duplicate wells. Three hours later, the media was replaced with serum containing DMEM:F-12 (4:1 ratio) medium containing G418 (400 μg/ml). When the cells reach 90-95% confluence they were trysinized and transferred to 10 cm plates and maintained in media containing the same amount of G418 (400 μg/ml).
Cell Viability Assay
MTT assay for cell proliferation and viability was performed in 24 well plates and with 0.5 mg/ml MTT in each well as recently described in our laboratory [14, 31]. Mesangial cells (1x104 cells) were grown to 70% confluence and serum-starved overnight. IGF-1 100 ng/ml was added for 4-24 h and MTT was added for 3 h. The media was removed and cells were kept in 100 μl of DMSO for 10 minutes. The resulting color was diluted with 500 μl of distilled H2O and detected at 570 nm using a Gemini Microplate reader.
Statistical Analysis
Results are expressed as means +/-SE of indicated number of experiments. Student's t test was used to compare differences between cultures. A value of P<0.05 was consider statistically significant.
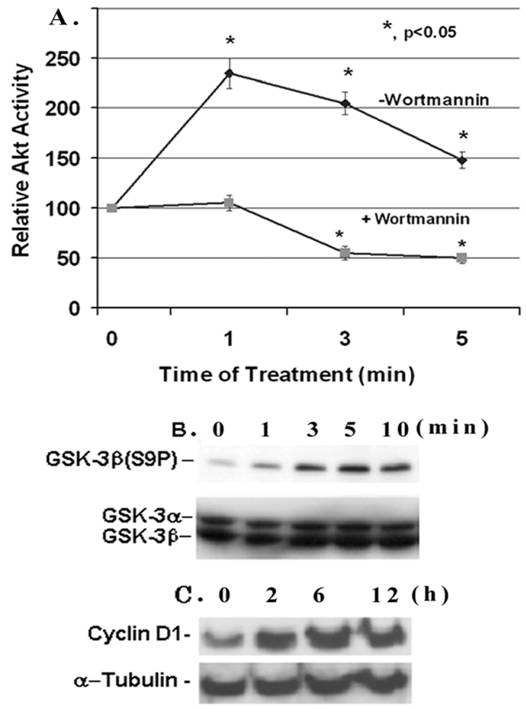
Effect of IGF-1 on AKT/GSK-3β activity and cyclin D1 expression in mesangial cells. (A) Mesangial cells were grown to 60-70% confluence, serum-starved overnight and treated with IGF-1 (100 ng/ml) for 1-5 min with or without 100 nM Wortmannin. Cell extracts (500 μg protein) were immunoprecipitated with anti-Akt antibodies and kinase activity was determined by using an Akt/PKB assay kit from Upstate Biotechnology. The basal Akt activity was ~4.8 pmol 32Pi incorporated into Akt peptides/0.5 mg protein/10 min (n=5). (B) Mesangial cells were serum-starved overnight and incubated with IGF-1 (100 ng/ml) for different time periods: 0, 1, 3, 5, and 10 min, respectively, and 30 μg proteins were subjected to Western Blotting with anti-phospho-GSK-3β antibodies (n=3). The membrane was stripped and reprobed with anti-GSK-3α/β antibodies. (C) Mesangial cells were treated with IGF-1 (100 ng/ml) for 0, 2, 6 and 12 h and were subjected to Western blotting for cyclin D1 and reprobed for α-tubulin (a representative of n=3).
3. RESULTS
IGF-1 regulates the Akt/GSK-3β pathway and cyclin D1 expression in mesangial cells
IGF-1 is a cell growth factor and the signaling pathway involves the activation of PI3-K and Akt kinase. Here, we examine the effect of IGF-1 on Akt activity and Ser 9 phosphorylation of its target GSK-3β in mesangial cells. The Akt activity in mesangial cells increases within 1 minute of IGF-1 (100 ng/ml) addition and reaches a maximal stimulation of ~2.4- fold versus the control at time 0 (p, <0.05, n=5). Afterwards it falls down but maintains a higher activity than the control (Figure 1A). The Akt activity is completely suppressed by the presence of 100 nM Wortmannin, an inhibitor of up-stream PI3-kinase. IGF-1 activation of Akt is associated with an increase in the phosphorylation of GSK-3β at Ser9 as detected by the anti-GSK-3β(S9P) antibodies on Western blots (Figure 1B). The GSK-3β phosphorylation is increased within 1 min of IGF-1 addition, and is highest at 5 min. However, total GSK-3β is not affected by IGF-1. Furthermore, IGF-1 treatment of mesangial cells leads to an increase in the expression of the cell cycle regulator cyclin D1 (Figure 1C). The expression is enhanced gradually from 0-12 h upon IGF-1 treatment. Under these experimental conditions, IGF-1 does not alter α-tubulin expression.
IGF-1 increases fibronectin mRNA and protein expression in mesangial cells
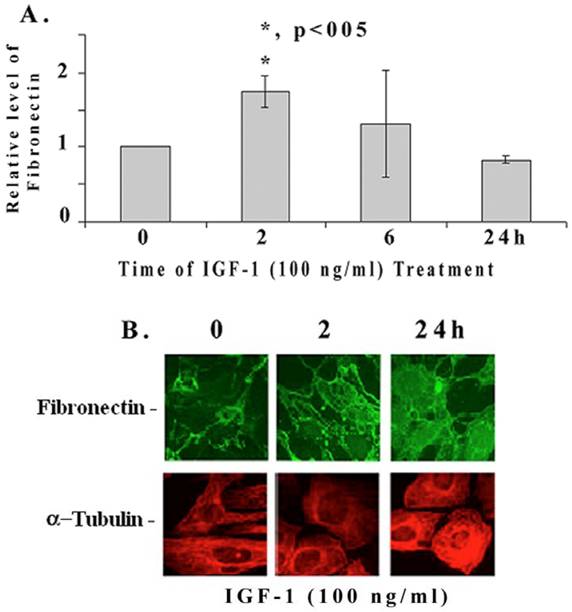
We further examined if IGF-1 regulates the expression of fibronectin mRNA and protein in mesangial cells by quantitative RT-PCR and immunohistology. IGF-1 increases fibronectin mRNA levels significantly at 2 h (1.76+/-0.3 folds, p<0.05, n=4), then decreases at 6 and 24 h (Figure 2A). On the other hand, fibronectin protein levels are increased at 2 (as well as at 6h) and 24 h as revealed by the immunofluorescence microscopy (Figure 2B). In addition, IGF-1 also increases fibronectin protein levels in mouse and human mesangial cells (data not shown). α-tubulin expression appears to be slightly elevated at 24 h of IGF-1 treatment as well.
Quantitative RT-PCR and fluorescence immunohistochemical staining of fibronectin. (A) Mesangial cells were incubated with IGF-1 (100 ng/ml) for different time intervals (0-24 h). Total RNA was isolated using Trizol reagent and performed the real-time quantitative PCR as described in Methods. The expression level of fibronectin were normalized to S-18 and the relative fibronectin mRNA expression is presented (n=4). The symbol (*) represents statistically significant change as compared to controls at 0 time. [B] Mesangial cells were grown in four-chambered glass slides (NUNC, Naperville, IL) and exposed to IGF-1 (100 ng/ml) for different time periods (0, 2, and 24 h). Cells were fixed with freshly prepared paraformaldehyde, permeabilized using 1% Nonidet P-40 detergent, and stained for immunofluorescence with appropriate primary and fluorescent-labeled secondary antibodies as described under Methods. The pictures are representatives of 3 different experiments for each protein.
IGF-1 increases phosphorylation of CREB at Ser133 and its DNA-binding activity in mesangial cells
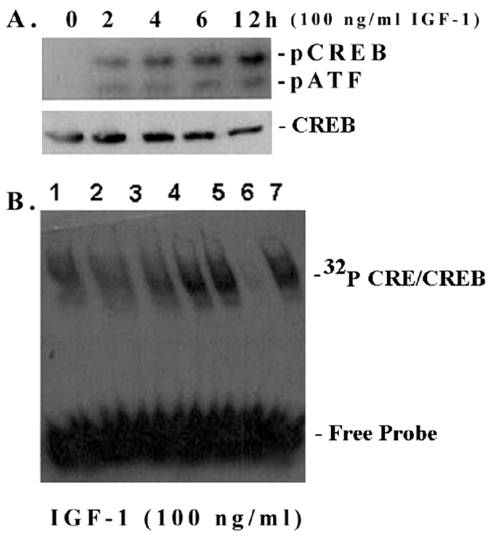
Most ECM genes, including fibronectin, and cyclin D1 contain in their promoters cis-acting elements for cAMP-responsive element binding transcription factor CREB [21-23]. Therefore, we investigated whether IGF-1 has an influence on CREB phosphorylation and the CRE DNA-binding activity in mesangial cells. As shown in Figure 3A, IGF-1 increases the phosphorylation of CREB at Ser133 in a time-dependent manner (0-12 h) as detected by an antibody directed against the phosphorylated CREB at Ser 133 [upper band]. The antibody also detects another band below, the phospho-ATF, which has a similar motif. However, the total CREB content is not changed. In addition, IGF-1 also increases the CRE-binding activity of mesangial nuclear extracts (Figure 3B). The binding is specifically inhibited by a 10-fold excess of cold CRE, a competitive sequence; but not by the GC consensus sequence of Sp1 transcription factor binding site (non-competitive sequence).
Inhibition of GSK-3β increases CREB phosphorylation and cyclin D1 expression
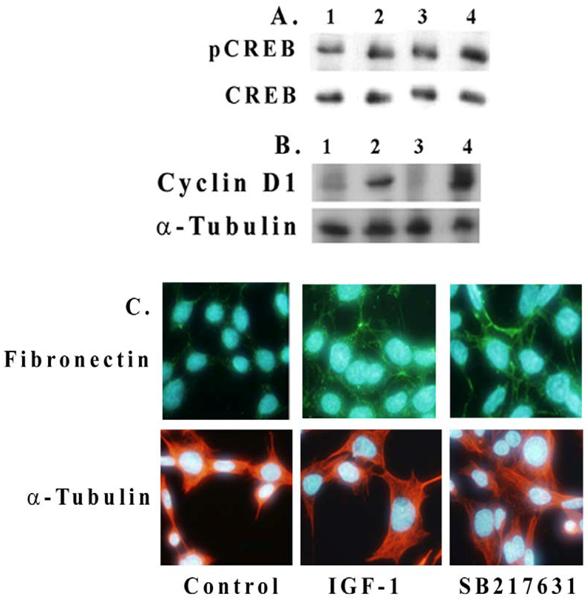
We examined here whether an inhibition of Akt or GSK-3β has an effect on IGF-1-mediated CREB phosphorylation and cyclin D1 expression in mesangial cells. As shown in Figure 4A, IGF-1 increases CREB phosphorylation in 2 h (lanes 1 and 2) and an inhibition of GSK-3β by 100 nM SB216763 further enhances the level of CREB phosphorylation (lanes 2 and 4). Similar results are also seen for cyclin D1 expression in Figure 4B (lanes 2 and 4). An inhibition of Akt by SH-5 (2 μM) reduces both CREB phosphorylation and cyclin D1 expression (lane 3 in Figures 4A and 4B). Fibronectin expression is also increased by IGF-1 or upon the incubation of mesangial cells with GSK-3β inhibitor, SB216763 (100 nM) alone for 24 h (Figure 4C). In general, IGF-1 or GSK-3β inhibition increases the cell and nuclear size as compared with controls.
CREB phosphorylation and CRE-binding in mesangial cells. (A) CREB phosphorylation and total CREB contents were detected in nuclear extracts of mesangial cells on Western blots as described in Methods. A representative of n=3 is shown. (B) EMSA: Nuclear extracts (6 μg protein) were incubated with 32P-CRE (~100,000 cpm) for 10 min and applied to 6% non-denaturing minigels at 100 V for 45 min until dye reaches the bottom of the gel. Lane 1. Time 0 (control); lane 2. 2 h; lane 3. 4 h; lane 4. 6 h; lane 5. 12 h; land 6. 12 h + 10-fold excess of cold CRE (competitive inhibition) and lane 7. 12 h + 10 fold excess a Sp1 DNA fragment (non-competitive control). A representative of n=3 is shown.
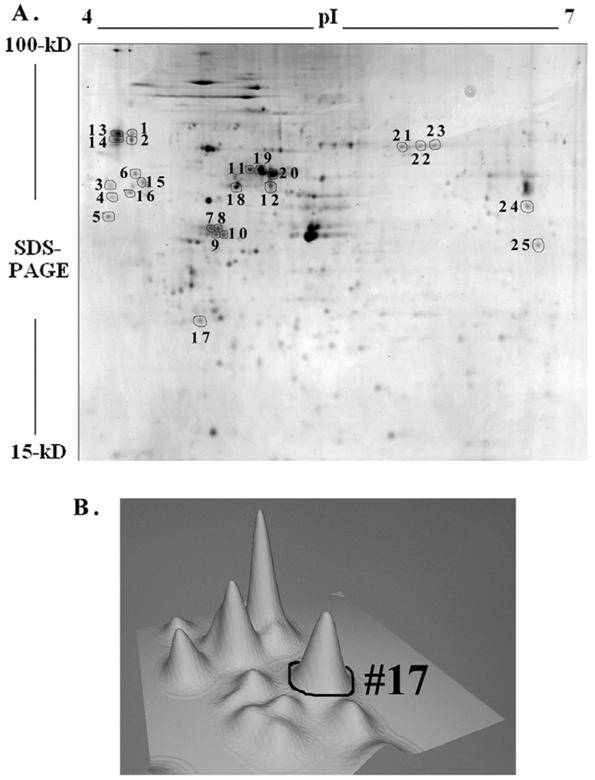
Two-dimensional gel electrophoresis and MALFI-TOF mass spectrometry of mesangial proteins
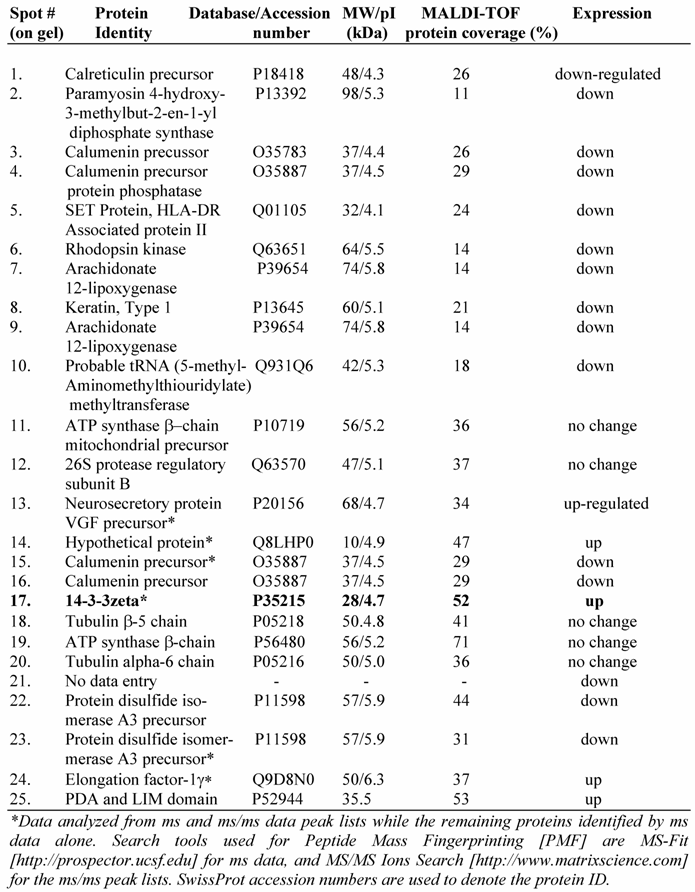
To further understand the IGF-1 signaling and protein expression involving GSK-3β, we compared proteome profiles of mesangial cells after treatment with IGF-1 (100 ng/ml) or SB216763 (100 nM, GSK-3β inhibitor) for 24 h against the control. Cell lysates were resolved by 2-D gel electrophoresis and stained with Colloidal Coomassie blue and digitized. The images from the control (without treatment) and IGF-1 or SB216763 treated cells were compared by 2D gel software PDQuest for changes in the protein expression. Three hundred and ninety six distinctive protein spots were commonly detected in all 9 gels (triplicates for each treatment condition) and they were subjected to statistical analysis using 95% confidential intervals. The differential expression of protein spots beyond 0.5-2-fold was considered down or up regulated by IGF-1 and GSK-3β treatment. Fifty two spots were found commonly changed by both treatments. Twenty five spots containing some of the commonly regulated spots (as shown in Figure 5A) were subjected to automatic gel cutting, in-gel tryptic digestion and MALDI-TOF ms or ms/ms analysis for protein identification. The 3D view of proteins around the spot #17 is shown in Figure 5B to demonstrate that the proteins chosen for analysis were not overlapped with other spots. Similar analysis was also performed for the other spots selected for identification. Peptide mass fingerprinting (PMF) was performed using both NCBInr and SwissProt protein sequence database search engines.
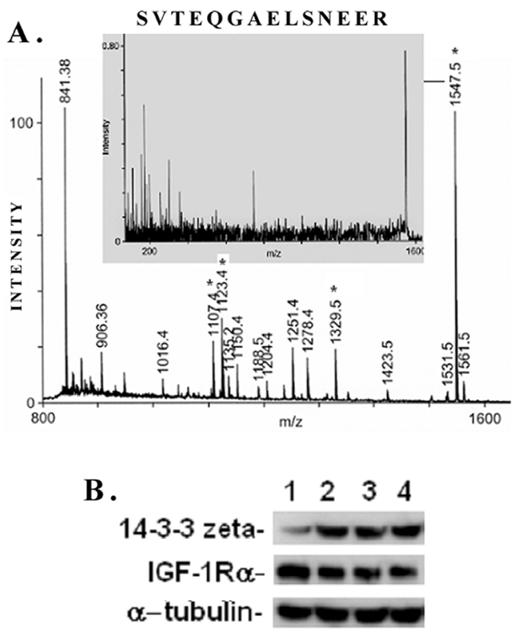
A typical ms/ms spectra for the proteins, e.g., that of the spot #17 which is identified as the phospho-protein docking protein 14-3-3ζ, is shown in Figure 6A. A list of proteins identified by the 2D-gel/mass spectrometric analysis is shown in Table 1. As seen from this list, the fact that IGF-1 treatment or an inhibition of GSK-3β promotes protein synthesis elongation factor γ1 (EF-γ1) goes parallel to cell growth activity of IGF-1. At the same time, both IGF-1 and GSK-3β also down regulate endoplasmic reticulum-associated proteins, such as calreticulin, calumenin and protein disulfide isomerase A3, suggesting ER stress of these cells. Among the proteins identified, the expression of the phospho-Ser/Thr binding adapter protein 14-3-3ζ (shown in bold letters in Table 1) was further investigated as this protein is considered to be involved in cell cycle control and survival involving various protein kinase/phosphatase signaling pathways. The 2D/mass spectrometric and PMF data is further validated as IGF-1 increases 14-3-3ζ expression on Western blots (Figure 6B, upper panel). On the other hand, IGF-1 receptor α level is marginally decreased probably due to the ligand-receptor complex internalization and recycling (Figure 6B, middle panel). α-Tubulin expression is not changed under these conditions.
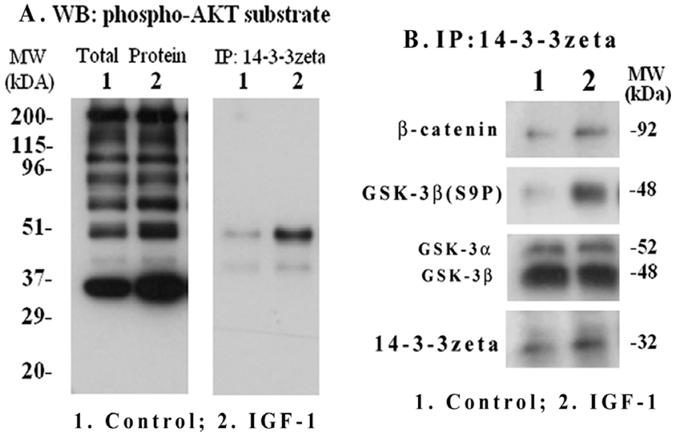
IGF-1 increases Akt target phosphorylation and phospho-GSK-3β association with 14-3-3ζ
We investigated if down-stream phosphoprotein targets of Akt including GSK-3β are associated with the phospho-protein binding adapter 14-3-3ζ in mesangial cells and whether IGF-1 has an influence on their interaction. Μesangial cells were treated with IGF-1 (100 ng/ml) for 2 h and total cellular extracts were subjected to Western blotting with primary antibodies targeted against a phosphorylated Akt peptide motif (R-X-R-X-X-S*/T*); therefore, it recognizes most phosphoproteins that are targeted by Akt. As shown in Figure 7A (left panel), Akt targeted phosphoproteins of different molecular sizes were increased by IGF-1. To further examine which of these Akt targets will bind to 14-3-3ζ, the 14-3-3ζ immunoprecipitates were subjected to Western blotting with the same anti-phospho-Akt motif antibodies. The results reveal that a major band at 48-kDa and another weak band at 40-kDa are bound to 14-3-3ζ (Figure 7A, right). GSK-3β is a 48-kDa protein and is a target of Akt. Therefore, the 14-3-3ζ immune complexes were further blotted with antibodies against phosphorylated GSK-3β(S9P) and β-catenin (a target of GSK-3β). As shown in Figure 7B, the presence of GSK-3β(S9P) and β-catenin are detected in the 14-3-3ζ complex. In addition, the binding of 14-3-3ζ itself is increased while total GSK-3α/β level is not changed. However, the presence of cyclin D1 and CREB in the 14-3-3ζ immunoprecipitates was not detected under the experimental conditions.
Identification of a few highly expressed mesangial cell proteins by MALDI-TOF mass spectrometry and peptide mass fingerprinting
Effect of Akt and GSK-3β inhibition on CREB phosphorylation and cyclin D1 and fibronectin expression. Mesangial cells were grown to 70% confluence and serum-starved overnight. Inhibitors for Akt/PKB (2 μM, SH-5) and GSK-β (100 nM, SB216763) were pre-treated for 30 min before adding IGF-1 (100 ng/ml) for 2 h. Cell extracts (30 μg protein) were subjected to Western blotting for (A) phosphorylated and total CREB and (B) for cyclin D1. Lane 1. Control; lane 2. IGF-1, 2h; lane 3. IGF-1, 2h+SH-5 (2 μM); and lane 4. IGF-1, 2h+SB216763 (100 nM). Representative blots from three different experiments are shown. (C). Effect of IGF-1 (100 ng/ml) or SB216763 (100 nM) on fibronectin expression upon 24 h exposure was determined by immunofluorescence microscopy. Nuclear staining was performed with Hoechst Dye 33342 and fibronectin with Alexis 488 (n=3).
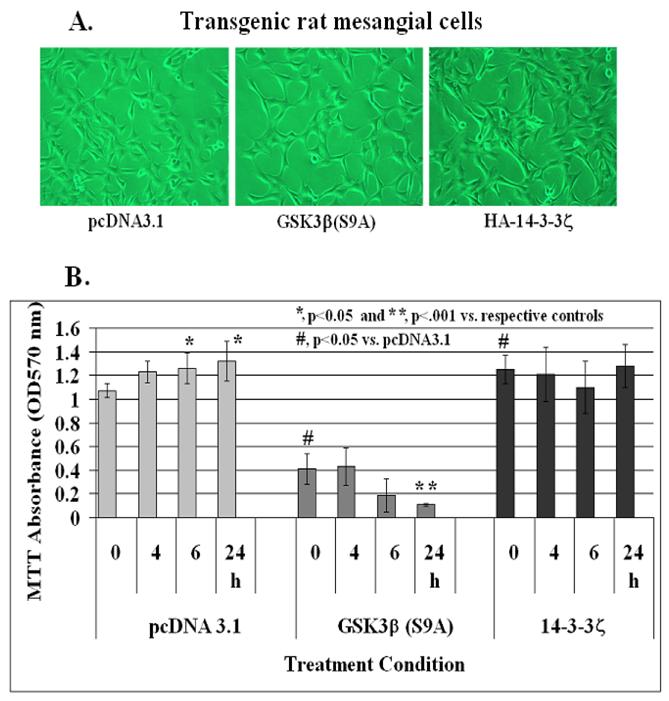
Effect of stable expression of constitutively active GSK-3β[S9A] and HA-14-3-3ζ on IGF-1-mediated mesangial cell survival
In order to elucidate further a role for GSK-3β and 14-3-3ζ in IGF-1 signaling and mesangial cell survival, a phosphorylation insensitive Ser 9 to Ala mutant GSK-3β(S9A), which is a constitutively active kinase, and HA-tagged 14-3-3ζ were transfected in rat mesangial cells. After 3 passages with G418, the cells were examined for GSK-3β and HA-14-3-3ζ expression on Western blots. There was ~1.5-fold increases in GSK-3β levels in GSK-3β(S9A) stables cells and ~2-fold increased level of 14-3-3ζ in HA-14-3-3ζ expressing mesangial cells. No morphological differences among these three cell line is observed under the phase contrast microscope (Figure 8A). Control pcDNA3.1, GSK-3β(S9A) or HA-tagged 14-3-3ζ stable mesangial cells were grown to ~70-80% confluence in 24 well plates. IGF-1 at 100 ng/ml was added to each well for 0, 4, 6 or 24 h and cell viability was assayed by MTT. As shown in Figure 8B, IGF-1 increases mesangial cell viability at 6 and 24 h significantly (p<0.05 versus control at time 0 in pcDNA3.1 cells, left panel). However, cell viability and IGF-1 effects are reduced in GSK-3β(S9A) overexpressors significantly (p<0.05 between pcDNA3.1 and GSK-3β(S9A) at 0 time, and p<0.001 between GSK-3β(S9A) at 0 and 24 h, middle panel). On the other hand, MTT absorbance is increased significantly (p<0.05 versus pcDNA3.1 control at 0 time) in HA-14-3-3ζ expressing cells and IGF-1 treatment does not have an additional effect on mesangial cell viability (right panel).
4. DISCUSSION
IGF-1 is one of the growth factors that are implicated in renal growth, hypertrophy and the progression of diabetic glomerulopathy because of its persistent accumulation in the diabetic kidney. Recently, we demonstrated that higher expression of IGF-1 in the diabetic kidney is associated with increases in ECM protein levels and cell cycle control factors such as cyclin D1 and p21Cip1 [14]. In addition, the phosphorylation of the transcription factor CREB at Ser 133 is also increased in the diabetic kidney without affecting the total CREB content (unpublished data). In this study, using a proteomic approach, we demonstrate that IGF-1 regulates Akt/GSK-3β signaling pathway, fibronectin expression and survival of mesangial cells and that the phospho-protein docking adaptor 14-3-3ζ interacts with GSK-3β to regulate cell survival. It is also noteworthy that IGF-1 induces the expression of fibronectin mRNA at 2 h maximally and then returns to the level of the control while the elevation of protein content continues up to 24 h. These results suggest that mRNA and protein levels do not always correlate and that post-transcriptional mechanism(s) of gene expression regulation may be equally important in determining the overall accumulation of ECMs in the diabetic glomerulopathy. In this regard, we have recently shown that TGF-β1 increases fibronectin and laminin N-linked glycosylation in renal mesangial cells, which retards the rate of ECM degradation [32]. It is still unknown whether IGF-1 is involved in ECM glycosylation and degradation; however, an inactivation of matrix metalloproteinase (MMP-2) activity by IGF-1 is observed in mesangial cells isolated from non-obesed diabetic mice, which correlates with a decrease in the degradation of collagen type IV [33]. Recently, in a microarray study, we observed low level of ECM mRNA expression in mouse mesangial cells whereas the protein levels are consistently increased in mesangial cells and in the diabetic kidney [34]. Furthermore, we found that treatment of a mouse mesangial cell line in culture with IGF-1 increases mRNA stability for fibronectin at 24 h when compared with non-treated control cells (data not shown). Hence, a small change in the transcriptional rates and/or mRNA stability may exert a profound effect at the level of cell function in chronic diseases such as diabetes and its vascular complications.
Two-dimensional gel electrophoresis of mesangial proteins. Mesangial cells were cultured for 24 h with or without 100 ng/ml IGF-1 or GSK-3β inhibitor, SB 216763 (100 nM) in triplicates. Cellular proteins were subjected to 2D gel electrophoresis and stained with Colloidal Coomassie blue. A representative 2-D gel image of the Control (triplicates were run for each condition) and twenty-five spots were subjected to MALDI-TOF ms and/or ms/ms analysis is shown in Figure A. Three-dimensional view of the spots around #17 is depicted in (B) as a representative to ensure that spots selected are not overlapped.
IGF-1 signaling leads to activation of the Akt activity in mesangial cells and phosphorylation of its down-stream substrate GSK-3β at Ser 9 [14]. This IGF-1 signaling pathway parallels an increase in the expression of the G0/G1 cell cycle regulation factor cyclin D1, which is further stimulated by the inhibition of GSK-3β and completely blocked by Akt inhibitors (Figure 4B). Many ECM genes including fibronectin bear several cis-elements in their promoters such as cyclic AMP regulatory element (CRE), which is recognized by the CREB family of transcription factors [21-23]. We have previously shown that high glucose, glucosamine and TGF-β1 increases CREB phosphorylation and CRE-binding activity in mesangial cells and that these effects are mediated in part by protein kinases A and C, and they are involved in fibronectin and laminin expression [20, 24]. The transcriptional activation of the cyclin D1 gene expression is also mediated by cis-elements containing CREB sites [35]. Our observation that IGF-1 is a stimulator of CREB phosphorylation at Ser 133 and an activator of CRE consensus DNA-binding activity goes parallel to fibronectin and cyclin D1 expression. An inhibition of GSK-3β by SB216763 stimulates both CREB phosphorylation and cyclin D1 expression suggesting a role for the Akt/GSK-3β pathway and transcription factor CREB in cell cycle regulation and ECM gene expression. GSK-3β does not target CREB at S133 but can be phosphorylated at Ser 129, a motif created by phosphorylated S133 [36]. Thus, the increase in the phosphorylation of CREB at Ser133 by IGF-1 or upon suppression of GSK-3β activity, observed in this study, appears to be an indirect effect either due to the inhibition of protein phosphatase(s) or activation of protein kinase(s) that target CREB at Ser 133. Further studies will be needed to answer these questions.
MALDI TOF Mass spectrometry. (A) Peptide mass fingerprint spectra of Spot #17 and identification of protein 14-3-3ζ generated by Applied Biosystems (ABI) Proteomics Analyzer (MALDI-TOF/TOF) is shown here. Four peptides (*) were subjected to automatic ms/ms analysis, and protein identification was performed by both automatic and manual searches using Mascot MS/MS Ion Search or MS-Tag search tools. Both NCBI and SwissProt were used to search the protein database. (B) Proteomic data on IGF-1-induced 14-3-3ζ expression in mesangial cells is validated on Western blots. MES cells were treated with 100 ng/ml IGF-1 for different time intervals (0, 2, 4, and 6h, respectively), and subjected to Western blots for 14-3-3ζ, IGF-1 receptor α and α-tubulin. IGF-1 stimulates 14-3-3ζ expression while IGF-1Rα expression is decreased marginally probably due to ligand-receptor internalization and remodeling. α-tubulin is not affected. The experiments were performed in triplicates.
IGF-1 activation of Akt substrate phosphorylation and interaction of phospho-GSK-3β with 14-3-3ζ. Mesangial cells were grown to 70-80% confluence and serum-starved overnight. IGF-1 (100 ng/ml) was added for 2 h and cells were harvested as described in Methods. Total extracts were subjected to SDS-PAGE and Western blotting with phospho-Akt motif antibodies (Figure 7A, left panel). 14-3-3ζ immunoprecipitates were probed with the phospho-Akt motif antibodies (Figure 7A, right panel). In addition, the 14-3-3 ζ immunoprecipitates were subjected to Western blotting for 14-3-3 ζ, GSK-3α/β, phosphorylated GSK-3β(S9P) and β-catenin (Figure 7B). The blots are representatives of three repeats.
Much interest has arisen to develop inhibitors of GSK-3β for several therapeutic uses, including the treatment of neurodegenerative diseases, type 2 diabetes, bipolar disorders, and chronic inflammatory disease. However, a direct inhibition of GSK-3β expression or blocking its activity might be expected to cause clinically deleterious effects inducing uncontrolled cell proliferation and tumor growth. Therefore, a comparative proteome analysis and the identification of IGF-1 target components of the Akt/GSK-3β pathway will help us to understand the IGF-1 signaling mechanism and glomerular mesangial cell function. Towards this goal, we began to undertake a 2D-gel/MALDI-TOF mass spectrometry-based proteomic approach of identifying abundant protein expression in mesangial cells after treatment of the cells with IGF-1 or with an inhibitor of GSK-3β, SB216763. Twenty-five of the expressed proteins were initially identified by mass spectrometry and peptide mass fingerprinting as shown in Table 1. One of the proteins identified by this technique is the phospho-Ser/Thr-binding signal adapter protein 14-3-3ζ (spot #17 in 2D-gel), whose expression is increased by IGF-1 and is found to be associated with GSK-3β by co-immunoprecipitation (Figures 5 and 6). We draw attention to this particular protein because 14-3-3 proteins are known to involve in the interaction with intracellular phospho-proteins and they are implicated in IGF-1 signaling [37], signal integration [38], cell cycle regulation and survival [39, 40]. Insulin and IGF-1 receptors are known to interact with similar cytoplasmic proteins and they share a common signal-transduction pathway. However, functionally they affect different cellular processes. For example, IGF-1 is more effective than insulin in promoting cell mitogenesis whereas insulin regulates cellular metabolic activities. 14-3-3 proteins selectively bind to IGF-1 receptor, but not to insulin receptor, and may contribute to the differences in IGF-1 and insulin receptor signal transduction and their down-stream cellular function [37].
Effect of stable expression of constitutively active GSK-3β(S9A) or 14-3-3ζ on mesangial cell viability. Mesangial cells stably overexpressing pcDNA3.1 (control), Ser9 to Ala mutated GSK-3β(S9A) or HA-tagged 14-3-3ζ were treated with 100 ng/ml IGF-1 for 0, 4, 6, and 24 h. Cell viability was determined by MTT as described in Methods. The symbol (*) represents statistically significant change compared with corresponding controls at 0 time for each cell line. The symbol (#) represents significant change when compared with the pcDNA3.1 control. The experiments were performed in triplicates and were repeated twice.
The association of GSK-3β with 14-3-3ζ has been demonstrated for the regulation of tau phosphorylation in neuronal cells [41]. Our findings that 14-3-3ζ is associated with GSK-3β and its down-stream signaling target β-catenin may provide a molecular and biochemical mechanism for GSK-3β regulation of β-catenin nuclear translocation and cell cycle progression in mesangial cells [14]. Recently, GSK-3β and 14-3-3 proteins have been shown to induce myocardial apoptosis, hypertrophy and fibrosis in streptozotocin-induced diabetic mice [42]. In addition to the identification of 14-3-3ζ in the present study, the proteomic analysis also reveals that IGF-1 down-regulates several calcium-binding endoplasmic reticular proteins including calreticulin and calumenin (Table 1) indicating cell stress and calcium release from the ER storage. The regulation of ER calcium-binding proteins may lead to activation of calcium signaling and gene expression [43]. In fact, calcium/calmodulin dependent protein kinases and calcineurin play an important role in mesangial cell growth and ECM expression [12], and may influence 14-3-3ζ interaction with phospho-protein targets to alter signaling pathways. Our findings that stable expression of 14-3-3ζ increases mesangial cell viability while expression of the non-phosphorylated active form of GSK-3β(S9A) reduces cell survival indicates that GSK-3β and 14-3-3ζ contribute to IGF-1 signaling and mesangial cell survival (Figure 8). GSK-3β is a negative regulator of IGF-1 signaling and cell survival; therefore, stable overexpression of this constitutively active kinase leads to blockade of IGF-1 effects on mesangial cell survival. On the other hand, 14-3-3ζ expression increases cell viability suggesting that it may trap or counteract the action of proapoptotic factors such as BAD and Bax [40]. Further attempts are being made to identify the Akt and GSK-3β-targeted phosphoproteins that interact with 14-3-3ζ using mesangial cells that express the HA-tagged 14-3-3ζ stably, pull-down protein capture methods and 2D-gel/MALDI-TOF mass spectrometry.
In summary, we have demonstrated that IGF-1 stimulates Akt/GSK-3β pathway leading to CREB phsophorylation and CRE-binding activity in glomerular mesangial cells. IGF-1 increases fibronectin mRNA and protein levels in mesangial cells; however, mRNA and protein expression patterns do not go parallel. A proteomic analysis of IGF-1 signaling via the GSK-3β pathway identifies the scaffolding protein 14-3-3ζ to be up-regulated and associated with phosphorylated GSK-3β in mesangial cells. Stable expression of a phosphorylation insensitive and constitutively active form of GSK-3β(S9A) or HA-tagged 14-3-3ζ in mesangial cells alters IGF-1-mediated cell survival. The results imply that Akt/GSK-3β and 14-3-3ζ may mediate IGF-1 signaling and contribute to cell growth, matrix gene expression and survival of renal mesangial cells in diabetic nephropathy leading to the development of glomerular sclerosis and end-stage renal disease.
Acknowledgements
This study is supported by the American Diabetes Association Career Development Award (7-03-CD-14) and the Robert W. Schrier, MD, Young Investigator Grant of the National Kidney Foundation to Dr. Singh. The support from Research to Prevent Blindness to the Department of Ophthalmology is also acknowledged. We thank Ms. T. S. Devi, BS, LLB, for helping us in tissue culture and Western blotting experiments.
Conflict of interests
The authors have declared that no conflict of interest exists.
References
1. Mauer SM, Steffes MW, Brown DM. The kidney in diabetes. Am J Med. 1981 ;70:603-612
2. Osterby R, Gundersen HG, Horlyck A, Nyberg G, Westberg G. Diabetic glomerulopathy. Structural characteristics of the early and advanced stages. Diabetes. 1983 ;32:79-82
3. Hammerman MR, Miller SB. The growth hormone insulin-like growth factor axis in the kidney revisited. Am J Physiol. 1993 ;265:F1-F14
4. Flyvbjerg A, Kessler U, Dorka B, Fund B, Orskov H, Kiess W. Transient increase in renal insulin-like growth factor binding proteins during initial kidney hypertrophy in experimental diabetes in rats. Diabetologia. 1993 ;35:589-593
5. Heilig CW, Liu Y, England RL, Freytag SO, Gilbert JD, Heilig KO, Zhu M, Concepcion LA, Brosius FC 3rd. D-glucose stimulated cell GLUT1 expression and basal and IGF-1-sensitive glucose uptake in rat mesangial cell: implications for diabetic nephropathy. Diabetes. 1997 ;46:030-1039
6. Horney MJ, Shirley DW, Kurtz DT, Rosenzweig SA. Elevated glucose increases mesangial cell sensitivity to insulin-like growth factor I. Am J Physiol. 1998 ;274:F1045-F1053
7. Bach LA, Stevanson JL, Allen TJ, Jerums G, Herington AC. Kidney insulin-like growth factor-I mRNA levels are increased in post pubertal diabetic rats. J Endocrinol. 1991 ;129:5-10
8. Marshall SM, Flyvbjerg A, Frystyk J, Korsgaard L, Orskov H. Renal insulin-like growth factor I and growth hormone receptor binding in experimental diabetes and after unilateral nephrectomy in the rat. Diabetologia. 1991 ;34:632-639
9. Verrotti A, Cieri F, Petitti MT, Morgese G, Chiarelli F. Growth hormone and IGF-I in diabetic children with and without microalbuminuria. Diab Nutr Metab. 1999 ;12:271-276
10. Cummings EA, Sochett EB, Dekker MG, Lawson ML, Daneman D. Contribution of growth hormone and IGF-I to early diabetic nephropathy in type 1 diabetes. Diabetes. 1998 ;47:1341-1346
11. Flyvbjerg A. Role of growth hormone, insulin-like growth factors [IGFs] and IGF-binding proteins in the renal complications of diabetes. Kidney Int. 1997 ;60(Suppl):S12-S19
12. Gooch JL, Tang Y, Ricono JM, Abboud HE. Insulin-like growth factor-I induces renal cell hypertrophy via a calcineurin-dependent mechanism. J Biol Chem. 2001 ;276:42492-42500
13. Woodgett JR. Molecular cloning and expression of glycogen synthase kinase-3/Factor A. EMBO J. 1990 ;9:2431-2438
14. Jiang Y, Cheng DW, Levi E, Singh LP. IGF-1 increases laminin, cyclin D1 and P21Cip1 expression in glomerular mesangial cells: An investigation of the intracellular signaling pathway and cell cycle progression. J Cell Biochem. 2006 ;98:208-220
15. Hoeflich KP, Luo J, Rubie EA, Tsao MS, Jin O, Woodgett JR. Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature. 2000 ;406:86-90
16. Hamelers IH, van Schaik R, Sipkema J, Sussenback JS, Steenbergh PH. Insulin-like growth factor triggers nuclear accumulation of cyclin D1 in MCR-7S breast cancer cells. J Biol Chem. 2002 ;277:47645-47652
17. Kreisberg JI, Radnik RA, Kreisberg SH. Phosphorylation of cAMP responsive element binding protein after treatment of mesangial cells with high glucose plus TGF beta or PMA. Kidney Int. 1996 ;50:805-810
18. Verrecchia F, Rossert J, Mauviel A. Blocking sp1 transcription factor broadly inhibits extracellular matrix gene expression in vitro and in vivo: implications for the treatment of tissue fibrosis. J Invest Dermatol. 2001 ;116:755-763
19. Poncelet AC, Schnaper HW. Sp1 and Smad proteins cooperate to mediate transforming growth factor-beta 1-induced alpha 2[I] collagen expression in human glomerular mesangial cells. J Biol Chem. 2001 ;276:6983-6992
20. Zdunek M, Silbiger S, Lei J, Neugarten J. Protein kinase CK2 mediates TGF-beta1-stimulated type IV collagen gene transcription and its reversal by estradiol. Kidney Int. 2001 ;60:2097-2108
21. Singh LP, Andy JC, Anyamale V, Greene K, Alexander M, Crook ED. The hexosamine biosynthesis pathway-mediated fibronectin synthesis in mesangial cells is associated with increases in CREB phosphorylation and nuclear CRE binding. The Involvement of protein kinases A and C. Diabetes. 2001 ;50:2355-2362
22. O'Neill BC, Suzuki H, Loomis WP, Denisenko O, Bomsztyk K. Cloning of rat laminin gamma 1-chain gene promoter reveals motifs for recognition of multiple transcription factors. Am J Physiol. 1997 ;273:F411-F420
23. Patel RS, Odermatt E, Schwarzbauer JE, Hynes RO. Organization of the fibronectin gene provides evidence for exon shuffling during evolution. EMBO J. 1987 ;6:2565-2572
24. Walton KM, Rehfuss RP, Chrivia JC, Lochner JE, Goodman RH. A dominant repressor of cyclic adenosine 3'5'-monophosphate [cAMP]-regulated enhancer-binding protein activity inhibits the cAMP-mediated induction of somatostatin promoter in vivo. Mol Endocrinol. 1992 ;6:647-655
25. Pugazhenthi S, Boras T, O'Connor D, Meintzer MK, Heidenreich KA, Reusch JE. Insulin-like growth factor I-mediated activation of the transcription factor cAMP response element-binding protein in PC12 cells. Involvement of p38 mitogen-activated protein kinase-mediated pathway. J Biol Chem. 1999 ;274:2829-2837
26. Singh LP, Greene K, Alexander M, Crook ED. Hexosamines and Transforming Growth Factor beta utilize similar signaling pathways to mediated matrix protein synthesis in mesangial cells. Am J Physiol-renal Physiol. 2004 ;286:F409-F416
27. Singh LP, Alexander M, Greene K, Crook ED. Overexpression of the complementary DNA for human glutamine:fructose-6-phosphate amidotransferase in mesangial cells enhances glucose-induced fibronectin and transcription factor cyclic adenosine monophosphate-responsive element binding protein phosphorylation. J Invest Med. 2003 ;51(1):32-41
28. Singh LP, Crook ED. Hexosamine stimulation of laminin protein synthesis in cultured mesangial cells: Role of Protein Kinase C and Protein Kinase A. Am J Physiol - Renal Physiol. 2000 ;279:F646-F654
29. Leinninger GM, Backus C, Uhler MD, Lentz SI, Feldman EL. Phosphatidylinositol 3-kinase and Akt effectors mediate insulin-like growth factor-I neuroprotection in dorsal root ganglia neurons. FASEB J. 2004 ;18:544-1546
30. Singh LP, Generette D, Crook ED. Glucose-induced insulin resistance of phosphatidylinosito 3-OH kinase and AKT/PKB is mediated by the hexosamine biosynthesis pathway. J Diabs Compl. 2001 ;15:88-96
31. Molloy MP, Phadke ND, Chen H, Tyldesley H, Garfin DE, MaddocK JR, Andrews PC. Profiling the alkaline membrane proteome of Caulobacter crescentus with two-dimensional electrophoresis and mass spectrometry. Proteomics. 2002 ;2:899-910
32. Jiang Y, Cheng DW, Crook ED, Singh LP. Transforming growth factor-β1 regulation of laminin γ1 and fibronectin expression and survival of mouse mesangial cells. Mol Cell Biochem. 2005 ;278:165-175
33. Lupia E, Elliot SJ, Lenz O, Zheng F, Hattori M, Striker GE, Striker LJ. IGF-1 decreases collagen degradation in diabetic NOD mesangial cells: implication of diabetic nephropathy. Diabetes. 1999 ;48:1638-1644
34. Cheng DW, Jiang Y, Shalev A, Kowluru A, Crook ED, Singh LP. An Analysis of high glucose and glucosamine-induced gene expression and oxidative stress in renal mesangial cells. Arch Physiol Biochem. accepted for publication
35. Nagata D, Suzuki E, Nishimatsu H, Satonaka H, Goto A, Omata M, Hirata Y. Transcriptional activation of the cyclin D1 gene is mediated by multiple cis-elements including Sp1 and a cAMP-responsive element in vascular endothelial cells. J Biol Chem. 2001 ;276:662-669
36. Salas TR, Reddy SA, Clifford JL, Davis RJ, Kikuchi A, Lippman SM, Menter DG. Alleviating the suppression of glycogen synthase kinase-3beta by Akt leads to the phosphorylation of cAMP-response element-binding protein and its transactivation in intact cell nuclei. J Biol Chem. 2003 ;278:41338-41346
37. Furlanetto RW, Dey BR, Lopaczynski W, Nissley SP. 14-3-3 proteins interact with the insulin-like growth factor receptor but not the insulin receptor. Biochem J. 1997 ;327:765-771
38. Chow C-W, Dvais RJ. Intergration of calcium and cyclic AMP signaling pathways by 14-3-3. Mol Cell Biol. 2000 ;20:702-712
39. Peng CY, Graves PR, Thoma RS, Wu Z, Shaw SA, Piwnica-Worms H. Mitotic and G2 checkpoint control: regulation of 14-3-3 protein binding by phosphorylation of Cdc25C on Ser-216. Science. 1997 ;277:1501-1505
40. Zha J, Harada H, Yang E, Jockel J, Korsmeyer SJ. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X(L). Cell. 1996 ;87:619-828
41. Agarwal-Mawal A, Qureshi HY Cafferty PW, Yuan Z Han D, Lin T Paudel HK. 14-3-3 connects glycogen synthase kinase-3βto tau within a brain microtubule-assocaited tau phosphorylation complex. J Biol Chem. 2003 ;278:12722-12728
42. Gurusamy N, Watanabe K, Ma M, Prakash P, Hirabayashi K, Zhang S, Muslin AJ, Kodama M, Aizawa Y. Glycogen synthase kinase 3βtogether with 14-3-3 protein regulates diabetic cardiomyopathy: effect of losartan and tempol. FEBS Lett. 2006 ;580:1932-1940
43. Humez S, Legrand G, Vanden-Abeele F, Monet M, Marchetti P, Lepage G, Crepin A, Dewailly E, Wuytack F, Prevarskaya N. Role of endoplasmic reticulum calcium content in prostate cancer cell growth regulation by IGF and TNFalpha. J Cell Physiol. 2004 ;201:201-213
Author contact
![]() Correspondence to: Lalit P. Singh, Ph.D., Department of Anatomy and Cell Biology, Wayne State University School of Medicine, 540 East Canfield Avenue, #8332, Detroit, MI 48201. Phone: 313-576-5032 Fax: 313-577-3125 Email: plsinghwayne.edu
Correspondence to: Lalit P. Singh, Ph.D., Department of Anatomy and Cell Biology, Wayne State University School of Medicine, 540 East Canfield Avenue, #8332, Detroit, MI 48201. Phone: 313-576-5032 Fax: 313-577-3125 Email: plsinghwayne.edu

 Global reach, higher impact
Global reach, higher impact