10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2007; 3(7):455-462. doi:10.7150/ijbs.3.455 This issue Cite
Research Paper
Adjuvant effect of anti-4-1BB mAb administration in adoptive T cell therapy of cancer
1. Division of Surgical Oncology, University of Michigan, Ann Arbor, MI 48109-5932, USA.
2. Shiga University of Medical Science, Shiga, Japan.
3. Bristol-Myers Squibb Company, Princeton, NJ, USA.
* These two authors contributed equally to the work.
Received 2007-11-13; Accepted 2007-11-20; Published 2007-11-20
Abstract
Administration of anti-4-1BB mAb has been found to be a potent adjuvant when combined with other therapeutic approaches, e.g. chemotherapy, cytokine therapies, anti-OX40 therapy, and peptide or DC vaccines. However, the adjuvant effect of anti-4-1BB mAb administration in adoptive T cell therapy of cancer has not been fully evaluated. In this report, effector T cells were generated in vitro by anti-CD3/anti-CD28 activation of tumor-draining lymph node (TDLN) cells and used in an adoptive immunotherapy model. While T cells or anti-4-1BB alone showed no therapeutic efficacy in mice bearing macroscopic 10-day pulmonary metastases, T cells plus anti-4-1BB mediated significant tumor regression in an anti-4-1BB dose dependent manner. Mice bearing microscopic 3-day lung metastases treated with T cells alone demonstrated tumor regression which was significantly enhanced by anti-4-1BB administration. NK cell depletion abrogated the augmented therapeutic efficacy rendered by anti-4-1BB. Cell transfer between congenic hosts demonstrated that anti-4-1BB administration increased the survival of adoptively transferred TDLN cells. Using STAT4-/- mice, we found that modulated IFNγ secretion in wt TDLN cells after anti-CD3/CD28/4-1BB activation in vitro was lost in similarly stimulated STAT4-/- TDLN cells. Additionally, anti-4-1BB administration failed to augment the therapeutic efficacy of T cell therapy in STAT4-/- mice. Together, these results indicate that administered anti-4-1BB mAb can serve as an effective adjuvant to augment the antitumor reactivity of adoptively transferred T cells by recruiting the host NK cells; increasing the persistence of infused effector T cells, and modulating the STAT4 molecular signaling pathway.
Keywords: Anti-4-1BB, T cells, Cancer, Adoptive immunotherapy, NK cells, STAT4
1. Introduction
Adoptive transfer of tumor-reactive T cells represents an effective immunotherapeutic strategy for cancer treatment. To enhance the efficacy of T cell therapy, various strategies have been employed as an adjunct to cell transfer. These combined therapies include cell transfer in concert with the exogenous administration of cytokines (i.e., IL-2) [1,2]; local tumor therapies (i.e., irradiation) [3] or genetic modulation [4]; active immunization (i.e. DC vaccines) [5]; or blockade of immunosuppressive mechanisms (i.e. B7-H1 blockade) [6].
4-1BB (CD137) is an important co-stimulating receptor that is expressed by activated NK cells, T cells and DCs [7,8]. 4-1BB ligation can be accomplished with agonistic mAb to 4-1BB[9,10]. Engagement of 4-1BB on T cells using anti-4-1BB mAb provides a third signal to lymphoid cells in conjunction with the stimulus via the TCR-CD3 complex and CD28. We reported that 4-1BB ligation during CD3/CD28 co-activation of tumor-draining lymph node (TDLN) cells in vitro shifted T cell responsiveness toward a type 1 cytokine pattern with markedly elevated IFNγ and GM-CSF secretion [11]. Importantly, these TDLN cells were more effective in mediating antitumor reactivity in vivo than those activated via CD3 and CD28. TDLN cells activated through CD3/CD28/4-1BB ligation showed significantly decreased T cell apoptosis and necrosis compared with T cells activated via CD3 alone or CD3/CD28 together. This latter observation suggested that 4-1BB ligation inhibited activation-induced cell death in TDLN cells [11]. 4-1BB/4-1BBL interaction has been described in the in vitro generation of human CD19 specific CD8+ T cells for adoptive immunotherapy clinical trials [12]. More recently, van Rijn et al. compared the activation of human T cells via CD3 alone or CD28 and/or human 4-1BB and reported superior activation via co-stimulation compared to CD3 activation alone [13].
Anti-4-1BB mAb has also been administrated to mediate therapeutic effects in vivo. Chen and co-workers were the first to report that anti-4-1BB mAb can be administered in vivo to mediate antitumor responses against established weakly immunogenic tumors in animal models [14]. In addition to mono-therapy, most of the recent studies have involved the administration of anti-4-1BB mAb as an adjuvant with other therapeutic approaches. For example, anti-4-1BB was administrated as a component in combined treatment of cancer with chemotherapy [15], or with IL-12 therapy [16]. Anti-4-1BB was also used in conjunction with different monoclonal antibodies against other co-stimulatory molecules [17,18]. For example, concomitant therapeutic administration of agonist anti-4-1BB and anti-OX40 mAbs mediated rejection of established tumors and elicited powerful CD8 T cell responses in animal models [17]. In addition, it was reported that regression of poorly or non-immunogenic tumors required active immunization with a peptide vaccine plus anti-4-1BB injection [19]. In weakly and poorly immunogenic tumor models, we examined the effects of stimulating 4-1BB by administering anti-4-1BB mAb after tumor lysate-pulsed dendritic cell (TP-DC) vaccination. In established pulmonary and subcutaneous tumor models, anti-4-1BB synergistically enhanced tumor regression after TP-DC vaccination, and improved the survival of treated animals [20].
It is apparent from these reports that anti-4-1BB administration can provide potent in vivo co-stimulation of cellular immune responses and may therefore serve as a useful reagent in cancer immunotherapy. However, the adjuvant effect of anti-4-1BB mAb administration in adoptive T cell therapy of cancer has not been fully examined to date. In this study, we documented that anti-4-1BB administration has a significant adjuvant effect in the context of adoptive T cell therapy and have examined several mechanisms involved with this effect.
2. Materials and Methods
Mice
Female C57BL/6 (B6) mice with a CD45.2 phenotype were purchased from the Harlan Laboratories (Indianapolis , IN). Female CD45.1 mice on C57BL/6 background were purchased from The Jackson Laboratory (Bar Harbor, ME). The STAT4-/- knockout (KO) mice on B6 background were provided by Dr. Mark Kaplan from Indiana University. All the mice were maintained in specific pathogen-free conditions and were used for experiments at 8 weeks of age or older. Recognized principles of laboratory animals care (NIH publication No. 85-23, revised 1985) were followed, and the University of Michigan Laboratory of Animal Medicine approved all animal protocols.
Murine tumor cells
MCA 205 murine tumors are 3-methylcholanthrene-induced weakly immunogenic fibro- sarcomas that are syngeneic to B6 mice. Tumors were maintained in vivo by serial subcutaneous (s.c.) transplantation in B6 mice and were used within the eighth transplantation generation. Tumor cell suspensions were prepared from solid tumors by enzymatic digestion in 50 ml of Hank's balanced salt solution (HBSS) (GIBCO, Grand Island, NY) containing 40 mg of collagenase, 4 mg of deoxyribonuclease I and 100 units of hyaluronidase (Sigma Chemical Co., St. Louis, MO) for 3 hours at room temperature. Tumor cells were washed in HBSS 3 times before s.c. injection in mice to induce TDLN.
TDLN preparation
To induce TDLN, B6 mice or Stat 4-/- knockout mice were inoculated with 1 x 106 tumor cells in 0.1 ml of phosphate buffered saline (PBS) s.c. in the lower flank. Nine days later, inguinal TDLN were removed aseptically. Multiple TDLN were pooled from groups of mice. Lymphoid cell suspensions were prepared by mechanical dissociation with 25-gauge needles and pressed with the blunt end of a 10-mL plastic syringe in HBSS. The resultant cell suspension was filtered through nylon mesh and washed in HBSS.
Activation of TDLN cells
TDLN cells were activated with 1.0 μg/ml anti-CD3 mAb plus 1.0 μg/ml anti-CD28 mAb (BD Pharmingen, San Diego, CA) immobilized in 24-well plates (4 x 106 cells/2ml/well) or 6-well plates (20 x 106 cells/10ml/well) at 37oC with 5% CO2 for two days. For antibody immobilization, each well of a 24-well cell culture plate (Costar, Cambridge, MA) was coated with 1 ml of anti-CD3 plus anti-CD28 at 4°C overnight or at room temperature for 5 to 6 hours. After antibody activation, the cells were harvested and counted. The cells were then expanded in complete media (CM) [11] containing human recombinant IL-2 (Chiron Therapeutics, Emeryville, CA) starting at a concentration of 3 x 105 cells per ml in 6-well culture plate (Costar) for three days. The concentration of IL-2 was 80 international units (IU)/ml. At the end of the cell expansion, cells were harvested and counted to determine the fold of expansion and were used for adoptive transfer.
In some experiments, wild type (wt) or Stat4-/- TDLN cells were activated with anti-CD3 and anti-CD28 (1.0 μg/ml each) plus soluble anti-4-1BB mAb (rat IgG1, BD Pharmingen) at 2.5 μg/ml. Secondary cross-linking antibody (anti-rat IgG1) was used (10 μg/ml) together with anti-4-1BB mAb. Supernatant was then collected at the end of cell activation for cytokine analyses.
Treatment of established pulmonary metastases
Pulmonary metastases were established via tail vein injection of viable MCA 205 cells (2 x 105 ) in B6 or Stat4-/- mice. Three or ten day tumor-bearing mice were infused intravenously (i.v.) with TDLN cells activated with anti-CD3/anti-CD28 and expanded in IL-2. Commencing on the day of the cell transfer (day 0), intraperitoneal (i.p.) injections of IL-2 (40,000IU) were administered in 0.5 ml of PBS and continued twice daily for eight doses. To some groups, anti-4-1BB mAb (25 or 100 μg/ mouse) was administrated i.p. in 0.5 ml of PBS on day 0 and day 3. Agonistic anti-4-1BB mAb (1D8) was kindly provided by Bristol-Myers Squibb Company, Pharmaceutical Research Institute (Princeton, NJ). At least five mice were used in each experimental group. Approximately 17 days (for 3-day lung metastases) or 24 days (for 10-day lung metastases) after tumor injection, all mice were sacrificed, and lungs were harvested for enumeration of pulmonary metastatic nodules. The metastases appeared as discrete white nodules on the black surface of lungs insufflated with a 15% solution of India ink.
In vivo NK cell depletion
Anti-NK1.1 hybridoma cells (HB191; American Type Culture Collection, Manassas, VA) were used to produce the PK136 mAb for NK cell depletion. MAb was purified from ascites fluid by ImmunoPure Immobilized Protein A/G gel (Pierce, Biotechnology, Rockford, IL). The optimal procedure for in vivo NK depletion was 400 μg PK136 mAb /mouse i.p. one day before T cell transfer followed by a second injection 5 days later. This was determined in preliminary functional assays of NK activity and flow cytometry (data not shown).
Preparation of splenocytes
Spleens were harvested from control (PBS) or anti-4-1BB mAb treated mice. The weight was scaled and spleens were dissociated into cell suspension individually. The cell suspension was treated with lysis buffer (0.83% NH4Cl, 0.1% KHCO3, and 0.004% EDTA) for 1 minute to deplete erythrocytes. The resultant cells were washed twice, resuspended, and the total number of splenocytes was counted. 3x10^6 cells of each sample were used for the FACS analysis.
Preparation of blood samples
Blood samples were collected into EDTA-coated tube. 70 μl of blood samples was treated with blood cell lysis buffer (0.8% NH4Cl, 0.08% NaHCO3, and 0.004% EDTA) for 1 minute to deplete erythrocytes. The resultant cells were then washed three times, counted and used for FACS staining.
Fluorescence activated cell sorter (FACS) analysis
To evaluate the effect of anti-4-1BB on the in vivo persistence of adoptively transferred TDLN cells, congeneic mice were used as donors for TDLN cells. TDLN cells were induced in CD45.1 B6 mice, activated with anti-CD3/anti-CD28 and expanded in IL-2. These cells were then infused intravenously into naive CD45.2 B6 mice on day 0. Anti-4-1BB (100 μg) was administrated i.p. on day 0 and day 3. Each group (PBS or anti-4-1BB treated) consisted of 15 mice that received 5×106 – 7.5×106 cells per mouse. Blood samples were collected on days 1, 4, and 9 after adoptive transfer. Spleens were also harvested on these days. Five mice from each group were used at each time point. After lysis of RBCs, Fc receptors were blocked with anti-CD16/CD32 mAb, and cells were stained using R-PE–conjugated anti-CD45.1 (A20) and PE-Cy5 conjugated anti-CD3 mAbs (both from BD Pharmingen). Isotype-matched control mAbs were used as recommended by the manufacturer. Cells were fixed with 2% paraformaldehyde, and analyzed within 24 hours in a FACScan flow cytometer (Becton Dickson & Co., Mountain View, CA). Immediately before analysis, a known quantity of 50 µl of polystyrene microbeads (Bangs Laboratories, Fishers, IN) was added to each sample. For each sample, 1.2×106 events were acquired and analyzed. FL2 versus FL3 dot plots were gated on forward and side scatter properties of the adoptively transferred cells, and the number of CD45.1/CD3 double positive cells were recorded. The absolute number of cells of interest in each blood sample was calculated using the following formula: (number of cells of interest analyzed in sample) × (number of beads added to sample) / (number of beads analyzed in sample). Data acquired from samples stained with isotype control Abs were subtracted from the data obtained from corresponding samples stained with the Abs of interest. The number of CD45.1/CD3 positive cells in spleens was calculated as (number of cells of interest in sample) x (total number of splenocytes)/ (3x10^6).
Assessment of cytokine release
TDLN cells were activated with anti-CD3/anti-CD28 in the presence or absence of soluble anti-4-1BB mAb plus secondary cross-linking antibody as described above. The supernatant was then collected and analyzed for IFNγ secretion using commercially available enzyme linked immunofluorescence assay (ELISA) kits (BD Pharmingen). Briefly, 96 well plates (Costar) were coated with capture anti-mouse IFNγ (at 1:250 dilution) at 4ºC overnight. Plates were then washed three times with PBS-Tween 20 (0.05%)(PBST) before being blocked with PBS-FBS (10% v/w) for 1 h at 37°C. Plates were washed again with PBST and incubated with serially diluted standard (recombinant mouse IFNγ, starting at 2000 pg/ml) and supernatant samples for 2 h at room temperature. Plates were washed five times before IFNγ levels in the samples were determined by incubation with biotin anti-mouse IFNγ (at 1:250 dilution) plus Streptavidin-HRP (horseradish peroxidase) for 1h at room temperature. Plates were washed seven more times before the substrate was added. After terminating the enzymatic reaction with 2N H2SO4, ODs were measured on a microplate reader at 490nm.
Statistical analysis
In adoptive transfer model, the significance of differences in numbers of metastatic nodules between experimental groups was determined using one-way analysis of variance followed by a Newman-Keuls post hoc test. Two-sided p values of <0.05 were considered statistically significant between two groups. Student t-test was used to analyze cytokine release data.
3. Results
1. Administered anti-4-1BB mAb can serve as an effective adjuvant to augment the antitumor reactivity of adoptively transferred TDLN cells
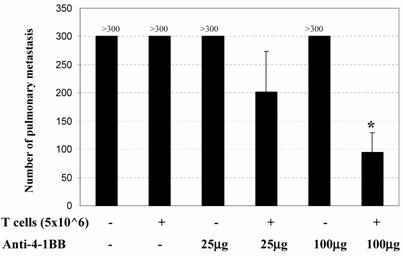
The in vivo adjuvant effect of anti-4-1BB monoclonal antibody was examined in an adoptive immunotherapy model. We evaluated the antitumor activity of adoptively transferred TDLN cells and/or anti-4-1BB administration in animals with advanced (10-day) MCA 205 pulmonary metastases. Effector T cells were TDLN cells activated with anti-CD3/anti-CD28 followed by expansion in IL-2 as described in the Materials and Methods. Mice with 10-day established MCA 205 pulmonary metastases were treated by the adoptive transfer of TDLN cells with or without anti-4-1BB mAb administration. Approximately two weeks after treatment, all mice were euthanized for enumeration of pulmonary metastatic nodules. As shown in Figure 1, mice treated with T cells alone or anti-4-1BB alone showed no therapeutic efficacy. However, the combination of T cell transfer plus anti-4-1BB administration resulted in the reduction of metastatic tumor in an anti-4-1BB dose-dependent fashion. At a higher dose (100μg) of anti-4-1BB used in these experiments, T cell therapy combined with anti-4-1BB administration resulted in significant tumor regression compared with all other groups (p<0.05). There experiments have therefore demonstrated a synergism between T cell adoptive transfer and anti-4-1BB injection in generating an immune response within the host to reject advanced tumor burdens.
Anti-4-1BB mAb administration augments the efficacy of adoptive T cell therapy in the treatment of 10-day established MCA 205 pulmonary metastases. B6 mice were inoculated i.v. with 2 x 105 MCA 205 tumor cells to induce pulmonary metastases. Mice with 10-day established pulmonary metastases were treated either by the adoptive transfer of anti-CD3/anti-CD28-activated MCA 205 TDLN cells alone, or with anti-4-1BB alone at 25 μg or 100 μg i.p. on days 0 and 3 following T cell adoptive transfer on day 0, or with T cells plus anti-4-1BB administration. Commencing on the day of the cell transfer (day 0), intraperitoneal (i.p.) injections of IL-2 (40,000IU) were administered in 0.5 ml of PBS and continued twice daily for eight doses. *p<0.05 compared with any other groups. Data are representative of two independent experiments.
2. Anti-4-1BB administration-enhanced therapeutic efficacy is partially mediated by host NK cells
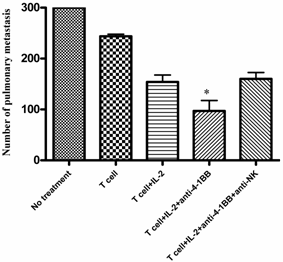
We identified NK cells as well as CD4+ and CD8+ T cells as the effectors involved in the tumor rejection response associated with tumor lysate-pulsed dendritic cell vaccination plus anti-4-1BB therapy in one of our previous reports [20]. To examine the role played by the host NK cells during adoptive T cell transfer plus anti-4-1BB-mediated antitumor reactivity, we depleted NK cells during the course of the combined therapy. In these antibody depletion experiments, mice were inoculated with MCA 205 tumor cells i.v. on day -3 and received the first depleting anti-NK1.1 mAb (400 μg, i.p.) on day -1. On day 0, anti-CD3/anti-CD28-activated MCA 205 TDLN cells were transferred accompanied by IL-2 administration as described in the Materials and Methods. Anti-4-1BB (100μg, i.p) was administrated on day 0 and day 3. A second dose of NK depleting mAb was administered on day 5. The adequacy of depletion was documented by flow cytometry of splenocytes retrieved from the treated mice (data not shown). Lungs were harvested on day 15 and pulmonary metastases were enumerated. As depicted in Figure 2, the antitumor efficacy of T cell therapy with IL-2 injection was significantly enhanced by the administration of anti-4-1BB mAb. However, this enhancement was significantly abrogated by the depletion of NK cells. These data suggested that NK cells are essential in the antitumor activity of T cell transfer plus anti-4-1BB administration.
Anti-4-1BB mAb-enhanced therapeutic efficacy is partially mediated by host NK cells. Treatment of 3-day established MCA 205 pulmonary metastases was performed as in Figure 1, but neutralizing anti-NK1.1 mAb was used to deplete NK cells. MCA 205 tumor cells were injection on day -3 to establish pulmonary metastasis. Anti-NK1.1 (400 μg/mouse) was injected i.p. to deplete host NK cell on day -1 and day 5. Activated and expanded TDLN cells (2x10^6) were transferred on day 0 with IL-2 administration. Anti-4-1BB mAb (100 μg) was administrated i.p. on day 0 and day 3. Lungs were harvested two weeks later for enumeration of pulmonary metastatic nodules. *p < 0.05 versus all other groups. Data are representative of two independently performed experiments.
3. Anit-4-1BB administration increases the survival of adoptively transferred TDLN cells
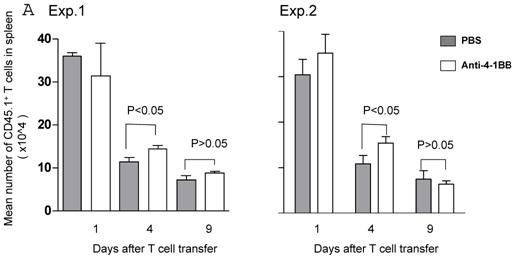
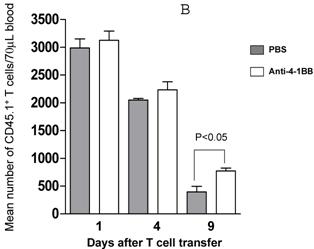
We proceeded to evaluate the survival of adoptively transferred TDLN cells by using the CD45.1/B6 congeneic mouse as a donor strain. TDLN cells were induced in these mice and activated with anti-CD3 plus anti-CD28 followed by IL-2 expansion as in previously experiments. Activated and expanded TDLN cells were then transferred (i.v) into CD45.2/B6 mice. Anti-4-1BB (100 μg) was administrated (i.p.) on the same day as cell transfer and 3 days later. Serial splenocyte and blood samples were obtained at various time points after cell infusion, and the absolute numbers of CD45.1+/CD3+ cells were assessed in the spleen and in the blood respectively. The persistence of the transferred TDLN cells in the spleen (mean number of 5 mice each group) is illustrated in Figure 3A. In two of two experiments performed, at an early time point (day 1), there was minimal difference regarding the persistence of the infused CD45.1+/CD3+ cells between the groups with or without anti-4-1BB administration. However, 4 days later, we found significantly (p<0.05) more donor cells in the group of mice which had received anti-4-1BB. This difference disappeared in the spleen at a later time point (day 9). In blood (Figure 3B), the infused CD45.1+/CD3+ donor cells persisted in higher numbers in the hosts administrated with anti-4-1BB than in those without anti-4-1BB administration. These differences reached statistical significance (P<0.05) at the late time point (day 9) evaluated in this study after adoptive transfer. These experiments indicate that anti-4-1BB administration increased the survival of adoptively transferred TDLN cells.
Anti-4-1BB administration enhances the survival of adoptively transferred TDLN cells. CD45.2 mice received intravenous adoptive transfer of activated and expanded CD45.1 TDLN cells on day 0. Anti-4-1BB (100μg/mouse) was administrated i.p on day 0 and day 3. Spleens were harvested and blood samples were collected on days 1, 4, and 9 after cell infusion. The absolute number of donor CD45.1+CD3+ cells was determined using fluorochrome-conjugated mAb staining and flow cytometry analysis as described in Materials and Methods. A, Data are reported as the mean number of CD45.1+CD3+ cells of 5 mice/group in the spleen. Two independently performed experiments are shown. B, Mean number of CD45.1+CD3+ cells detected per 70 microliter of blood per mouse. There were 5 mice each group.
4. Anti-4-1BB-elicited therapeutic efficacy involves STAT4 molecular signaling pathway
We previously examined the effects of anti-4-1BB on anti-CD3/anti-CD28 activated TDLN cells in vitro [11]. In that study, addition of anti-4-1BB polarized anti-CD3/anti-CD28 activated TDLN cells towards a type 1 profile and augmented their therapeutic efficacy in the adoptive immunotherapy of established tumor. We subsequently reported that anti-4-1BB mAb administration augmented the antitumor efficacy of DC-based vaccines, and the enhanced antitumor efficacy was once again due to the polarization effect of administered anti-4-1BB in shifting tumor-reactive T cells toward the type 1 phenotype [20]. STAT4 is well-known to be an important transcription factor for the development of Th1 cells [21]. To identify the molecular mechanisms which may be involved in the adjuvant effect of anti-4-1BB, we evaluated the role of STAT4 signaling pathway in the following experiments.
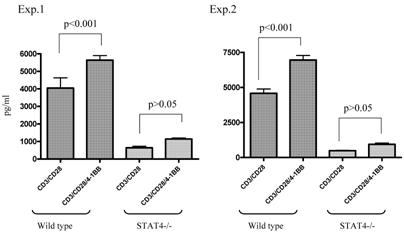
We used STAT4-/- knockout mice to compare the immune response of anti-4-1BB-cultured STAT4-/- TDLN cells with that of similarly treated wt TDLN cells. As shown in Figure 4, in two of the two independently performed experiments, ligation of 4-1BB during anti-CD3/anti-CD28 activation significantly enhanced the IFNγ production by anti-CD3/anti-CD28-activated wt TDLN cells (p<0.001). However, STAT4-/- TDLN cells activated by anti-CD3/anti-CD28 secreted comparable (p>0.05) amounts of IFNγ either with or without the use of anti-4-1BB. These in vitro data suggested that the STAT4 signaling pathway may be involved in 4-1BB-induced immune function of TDLN cells.
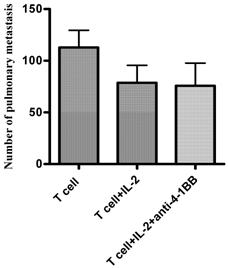
As a corollary, we examined involvement of the STAT4 signaling pathway when anti-4-1BB was used in vivo. Anti-CD3/anti-CD28-activated and IL-2-cultured STAT4-/- TDLN cells were adoptively transferred into tumor-bearing STAT4-/- mice, followed by IL-2 administration. A separate group of mice also received anti-4-1BB administration (Figure 5). While T cells with IL-2 administration mediated tumor regression to certain extent compared with T cells alone, anti-4-1BB administration failed to enhance the therapeutic effect mediated by T cells plus IL-2 in the STAT4-/- hosts.
These in vitro and in vivo results support the conclusion that the STAT4 signaling pathway is involved in anti-4-1BB-evoked antitumor immunity during adoptive T cell therapy of cancer.
Lack of modulated IFNγ secretion in STAT4-/- TDLN cells post CD3/CD28/4-1BB activation in vitro. TDLN cells from wt or STAT4-/- mice were activated with anti-CD3/anti-CD28 with or without anti-4-1BB followed by culture in IL-2. Supernatants at the end of cell culture were collected to examine the IFNγ secretion using ELISA. Two independently performed experiments are shown.
Anti-4-1BB administration fails to augment the therapeutic efficacy of T cell therapy in STAT4-/- mice. MCA 205 TDLN cells were induced in STAT4-/- mice and were activated with anti-CD3/anti-CD28 followed by IL-2 expansion to generate effector T cells for adoptive therapy. Three-day MCA205 pulmonary metastases were established in the STAT4-/- mice as in Figure 3. Activated and expanded STAT4-/- TDLN cells (2x10^6) were adoptively transferred into tumor bearing STAT4-/- mice followed by IL-2 injection (4x10^4 IU twice daily for 4 days) with or without anti-4-1BB administration (100μg on day 0 and day 3 relevant to T cell transfer on day 0). Fifteen to 17 days after tumor injection, all mice were euthanized for enumeration of pulmonary metastasis nodules. Data are representative of two independently performed experiments.
4. Discussion
In this study, we evaluated the adjuvant effect of administrated anti-4-1BB monoclonal antibody in adoptive T cell therapy of cancer. Anti-4-1BB administration augmented the therapeutic efficacy of adoptively transferred TDLN cells in mediating the regression of established metastatic tumor in a dose dependent fashion and prolonged the survival of the treated mice. Mechanistically, the salutary effects of anti-4-1BB when administered in conjunction with T cell transfer are mediated via several pathways. These include the modulation of host NK cells, increased survival/persistence of the infused T cells, and the involvement of STAT4 signaling pathway.
Several reports examining anti-4-1BB alone as an antitumor therapy have found CD8+ cells to be critical [14,22]. The major role of CD8+ cells as effectors may relate to the relatively greater expression of 4-1BB on CD8+ cells compared to CD4+ cells after T cell receptor engagement [23]. Another host component that may also be playing a significant role in cancer therapy are NK cells [24,25]. Indeed, we previously found that the in vivo antitumor reactivity of anti-4-1BB mAb plus DC vaccination was mediated by CD8+ cells, CD4+ cells, and NK cells [20]. Wilcox et al. also reported that 4-1BB-stimulated NK cells promote the expansion of CD8+ cytolytic T cells in vitro, thus providing another form of help [26]. In our current study, depletion of NK cells in the receipt mice significantly abrogated anti-4-1BB augmented therapeutic efficacy of adoptively transferred TDLN cells, hence demonstrating an important role played by host NK cells in the combined therapy with T cell transfer plus anti-4-1BB. Yang and colleagues reported that wild type cells from the K1735 melanoma (K1735-WT) were rejected following vaccination with cells K1735-1D8 transfected to express scFv from the anti-4-1BB monoclonal antibody 1D8, and that NK cells were needed for this rejection. Furthermore, tumors harvested after mice had been transplanted with K1735-1D8 cells or a mixture of K1735-1D8 and K1735-WT cells contained more NK cells. They also found that the percentage of NK cells was higher in B16-1D8 melanomas expressing anti-4-1BB scFv than in the B16-WT tumors [27]. These studies revealed a clear association of tumor infiltrating NK cells and tumor rejection. While NK cells were found to interact with the adaptive immune system in the type 1 polarization process in our previously described anti-4-1BB administration/DC vaccine setting [20], the mechanisms involved in the present anti-4-1BB administration/T cell transfer study remain to be further elucidated.
Utilization of congenic animals allowed us to track adoptively transferred TDLN cells using a mAb to CD45.1. We observed that administration of anti-4-1BB mAb following T cell transfer resulted in significantly increased in vivo persistence of the infused donor cells. Several groups have previously reported that anti-4-1BB can promote survival of CD8+ T cells [28-30]. Takahashi et al [18] reported that the combined in vivo administration of anti-4-1BB mAb with staphylococcal enterotoxin A in mice resulted in an increased persistence of staphylococcal enterotoxin A-reactive CD8+ T cells in the treated hosts compared to a deletion of these antigen-reactive T cells if anti-4-1BB was not given. Kwon and coworkers have previously reported that both CD4+ and CD8+ T cells activated with anti-CD3 mAb increased survival in vitro when 4-1BB was ligated [31,32]. Our results extend these observations by demonstrating that the administrated anti-4-1BB mAb was able to increase the persistence of adoptively transferred TDLN cells in spleen and in peripheral blood. The lymphodepleted host environment appears to promote the persistence of transferred effector T cells resulting in improved therapeutic effects [33]. These results provide evidence to support the development of novel strategies to extend the survival and persistence of adoptively transferred T cells in the tumor-bearing host in order to increase the therapeutic effectiveness. In one of our recent reports, we found that ligation of 4-1BB during anti-CD3/anti-CD28 activation of TDLN cells in vitro significantly increased the cell yield by preventing activation-induced cell death via a mechanism involving the up-regulation of Bcl-2 and Bcl-xL molecules [34]. Further studies to evaluate if anti-4-1BB administration enhances the survival of effector cells in vivo by modulating anti-apoptotic Bcl gene family molecules, as well as the studies to demonstrate anti-4-1BB administration-induced cell proliferation and trafficking in vivo would provide further evidence for anti-4-1BB-induced antitumor activity in adoptive immunotherapy of cancer. In addition, the survival of tumor Ag specific T cells could be investigated by using transgenic animal models, such as the OT-1/OVA model, as we have successfully used in one of our previous studies [20].
We described in this study that co-stimulation of 4-1BB with CD3 and CD28 significantly augmented IFNγ production by the activated wt TDLN cells. We have previously reported that T cells that manifest type 1 cytokine release in response to tumor antigen are requisite for mediating tumor regression in an adoptive transfer model, and that type 2 responsive T cells are immunosuppressive [35]. We have also reported that the type 1 (IFN-γ): type 2 (IL-10) cytokine ratio of adoptively transferred VPLN cells correlated with their ability to mediate tumor regression in patients with advanced renal cell cancers [1]. The Th1 response is mediated through STAT4 [21,36], whereas Th2 differentiation is STAT6-dependent [37,38]. In this study, the role of STAT4 in this phenomenon was revealed by using the STAT4-/- mice. In vitro, the modulated IFNγ secretion by CD3/CD28/4-1BB-activated wt TDLN cells was lost in STAT4-/- TDLN cells. In adoptive transfer experiments, anti-4-1BB administration failed to augment the therapeutic efficacy of T cell therapy in STAT4-/- mice as they did in wt hosts. Our in vitro and in vivo experiments have thus provided direct evidence and support the conclusion that the STAT4 signaling pathway is involved in anti-4-1BB elicited antitumor immunity in adoptive T cell therapy of cancer. Nevertheless, in our adoptive transfers conducted in this regards, both the donor and the host were STAT4-deficient. Since anti-4-1BB was administered systemically right after T cells were infused, this antibody could impact both on the infused T cells and on the host T cells. To further understand which cells require STAT4 in order for anti-4-1BB to mediate its effects, effector T cells can be generated from wt donors and then transferred into tumor-bearing STAT4-/- hosts, and visa versa, plus anti-4-1BB administration. These criss-cross adoptive transfers should allow us to understand if the administrated anti-4-1BB has a direct effect on the transferred cells or on the host.
Acknowledgements
The authors would like to thank Ms. Eleanore Kotowski for her excellent assistance in the preparation of this manuscript. This work was supported in part by NIH grant CA82529 and the Gillson Longenbaugh Foundation.
Conflict of Interests
The authors have declared that no conflict of interest exists.
References
1. Chang AE, Li Q, Jiang G. et al. Phase II trial of autologous tumor vaccination, Anti-CD3-activated vaccine-primed lymphocytes, and Interleukin-2 in stage IV renal cell cancer. J Clin Oncol. 2003;21:884-890
2. Topalian SL, Solomon D, Avis FP. et al. Immunotherapy of patients with advanced cancer using tumor-infiltrating lymphocytes and recombinant interleukin-2: a pilot study. J Clin Oncol. 1988;6:839-853
3. Ganss R, Ryschich E, Klar E. et al. Combination of T-cell therapy and trigger of inflammation induces remodeling of the vasculature and tumor eradication. Cancer Res. 2002;62:1462-1470
4. Huang H, Li F, Gordon JR. et al. Synergistic enhancement of antitumor immunity with adoptively transferred tumor-specific CD4+ and CD8+ T cells and intratumoral lymphotactin transgene expression. Cancer Res. 2002;62:2043-2051
5. Lou Y, Wang G, Lizée G. et al. Dendritic cells strongly boost the antitumor activity of adoptively transferred T cells in vivo. Cancer Res. 2004;64:6783-6790
6. Strome SE, Dong H, Tamura H. et al. B7-H1 blockade augments adoptive T-cell immunotherapy for squamous cell carcinoma. Cancer Res. 2003;63:6501-6505
7. Kwon BS, Tan KB, Ni J. et al. A newly identified member of the tumor necrosis factor receptor superfamily with a wide tissue distribution and involvement in lymphocyte activation. J Biol Chem. 1997;272:14272-14276
8. Wilcox RA, Chapoval AI, Gorski KS. et al. Expression of functional CD137 receptor by dendritic cells. J Immunol. 2002;168:4262-4267
9. Takahashi C, Mittler RS, Vella AT. Differential clonal expansion of CD4 and CD8 T cells in response to 4-1BB ligation: contribution of 4-1BB during inflammatory responses. Immunol Letters. 2001;76:183-191
10. Lee HW, Park SJ, Choi BK. et al. 4-1BB promotes the survival of CD8+ T lymphocytes by increasing expression of Bcl-xL and Bfl-1. J Immunol. 2002;169:4882-4888
11. Li Q, Carr A, Ito F. et al. Polarization effects of 4-1BB during CD28 costimulation in generating tumor-reactive T cells for cancer immunotherapy. Cancer Res. 2003;63:2546-2552
12. Numbenjapon T, Serrano LM, Chang WC. et al. Antigen-independent and antigen-dependent methods to numerically expand CD19-specific CD8+ T cells. Exp Hematol. 2007;35:1083-1090
13. van Rijn RS, Simonetti ER, Hagenbeek A. et al. Quantitative assessment of human T lymphocytes in RAG2(-/-)gammac(-/-) mice: the impact of ex vivo manipulation on in vivo functionality. Exp Hematol. 2007;35:117-127
14. Melero I, Shuford WW, Newby SA. et al. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nature Med. 1997;3:682
15. McMillin DW, Hewes B, Gangadharan B. et al. Complete regression of large solid tumors using engineered drug-resistant hematopoietic cells and anti-CD137 immunotherapy. Hum Gene Ther. 2006;17:798-806
16. Li Q, Pan PY, Gu P. et al. Role of immature myeloid Gr-1+ cells in the development of antitumor immunity. Cancer Res. 2004;64:1130-1139
17. Lee SJ, Rossi RJ, Lee SK. et al. CD134 Costimulation Couples the CD137 Pathway to Induce Production of Supereffector CD8 T Cells That Become IL-7 Dependent. J Immunol. 2007;179:2203-2214
18. Takahashi C, Mittler RS, Vella AT. 4-1BB is a bona fide CD8T cell survival signal. J Immunol. 1999;162:5037-5040
19. Wilcox R, Flies D Zhu G. et al. Provision of antigen and CD137 signaling breaks immunological ignorance, promoting regression of poorly immunogenic tumors. Clin Invest. 2002;109:651-659
20. Ito F, Li Q, Shreiner AB. et al. Anti-CD137 Monoclonal Antibody Administration Augments the Antitumor Efficacy of Dendritic Cell-Based Vaccines. Cancer Res. 2004;64:8411-8419
21. Zhang S, Kaplan MH. The p38 mitogen-activated protein kinase is required for IL-12-induced IFN-gamma expression. J Immunol. 2000;165:1374-1380
22. Miller RE, Jones J, Le T. et al. 4-1BB-specific monoclonal antibody promotes the generation of tumor-specific immune responses by direct activation of CD8 T cells in a CD40-dependent manner. J Immunol. 2002;169:1792-1800
23. Taraban VY, Rowley TF, O'Brien L. et al. Expression and costimulatory effects of the TNF receptor superfamily members CD134 (OX40) and CD137 (4-1BB), and their role in the generation of anti-tumor immune responses. Eur J Immunol. 2002;32:3617-3627
24. Wang G, Tschoi M, Spolski R. et al. In vivo antitumor activity of interleukin 21 mediated by natural killer cells. Cancer Res. 2003;63:9016-9022
25. Brady J, Hayakawa Y, Smyth MJ. et al. IL-21 induces the functional maturation of murine NK cells. J Immunol. 2004;172:2048-2058
26. Wilcox R, Tamada K, Strome S. et al. Signaling through NK cell-associated CD137 promotes both helper function for CD8+ cytolytic T cells and responsiveness to IL-2 but not cytolytic activity. J Immunol. 2002;169:4230-4236
27. Yang Y, Yang S, Ye Z. et al. Tumor cells expressing anti-CD137 scFv induce a tumor-destructive environment. Cancer Res. 2007;67:2339-2344
28. Halstead ES, Mueller YM, Altman JD. et al. In vivo stimulation of CD137 broadens primary antiviral CD8+ T cell responses. Nat Immunol. 2002;3:536-541
29. Cooper D, Bansal-Pakala P, Croft M. 4-1BB (CD137) controls the clonal expansion and survival of CD8 T cells in vivo but does not contribute to the development of cytotoxicity. Eur J Immunol. 2002;32:521-529
30. Laderach D, Movassagh M, Johnson A. et al. 4-1BB co-stimulation enhances human CD8(+) T cell priming by augmenting the proliferation and survival of effector CD8(+) T cells. Internatl Immunol. 2002;14:1155-1167
31. Lee H-W, Nam K-O, Seo SK. et al. 4-1BB cross-linking enhances the survival and cell cycle progression of CD4T lymphocytes. Cell Immunol. 2003;223:143-150
32. Lee H-W, Park S-J, Choi BK. et al. 4-1BB promotes the survival of CD8+ T lymphocytes by increasing expression of Bcl-xl and Bfl-1. J Immunol. 2002;169:4882-4888
33. Dudley ME, Wunderlich JR, Yang JC. et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncology. 2005;23:2346-2357
34. Kroon H, Li Q, Teitz -Tennenbaum S. et al. 4-1BB co-stimulation of effectror T cells for adoptive immunotherapy of cancer: Involvement of Bcl gene family members. J Immunotherapy. 2007;30:406-416
35. Aruga A, Aruga E, Tanigawa K. et al. Type 1 versus type 2 cytokine release by Vbeta T cell subpopulations determines in vivo antitumor reactivity: IL-10 mediates a suppressive role. J Immunol. 1997;159:664-673
36. Stamm LM, Satoskar AA, Ghosh SK. et al. STAT-4 mediated IL-12 signaling pathway is critical for the development of protective immunity in cutaneous leishmaniasis. Eur J Immunol. 1999;29:2524-2529
37. Chen Z, Lund R, Aittokallio T. et al. Identification of novel IL-4/Stat6 regulated genes in T lyphocytes. J Immunol. 2003;171:3627-3635
38. Stamm LM, Taisanen-Sokolowski A, Okano M. et al. Mice with STAT6-targeted gene disruption develop a Th1 response and control cutaneous leishmaniasis. J Immunol. 1998;161:6180-6188
Author contact
![]() Correspondence to: Dr. Qiao Li or Dr. Alfred E. Chang, Division of Surgical Oncology, University of Michigan Comprehensive Cancer Center, 1500 E. Medical Center Drive, 3303 CC, Ann Arbor, MI 48109-5932. Phone (734) 936-4392; Fax (734) 647-9647; E-mail: qiaoliedu or aechangedu.
Correspondence to: Dr. Qiao Li or Dr. Alfred E. Chang, Division of Surgical Oncology, University of Michigan Comprehensive Cancer Center, 1500 E. Medical Center Drive, 3303 CC, Ann Arbor, MI 48109-5932. Phone (734) 936-4392; Fax (734) 647-9647; E-mail: qiaoliedu or aechangedu.

 Global reach, higher impact
Global reach, higher impact