Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2009; 5(2):128-134. doi:10.7150/ijbs.5.128 This issue Cite
Research Paper
Assay Validation for KIM-1: human urinary renal dysfunction biomarker
Department of Biomarker Services, Covance Central Laboratory Services Inc., 8211 SciCor Drive, Indianapolis, Indiana 46214-2985, USA
Received 2008-10-17; Accepted 2009-1-17; Published 2009-1-19
Abstract
Urinary kidney injury molecule (KIM-1) is a sensitive quantitative biomarker for early detection of kidney tubular injury. The objective of the present work was to analytically validate this urinary renal injury biomarker. Duo-set reagents from R&D were used to develop the ELISA and validate the assay's linearity, intra-run precision, inter-run precision, lower limit of quantification, recovery, dilutional verification, reference range, stability, and length of run. The reference range data suggests that the healthy population falls within the assay range (59 - 2146 pg/mL) and upper limit of quantitation for this assay is 17168 pg/mL for the patient population. This is a robust assay to detect urinary levels of KIM-1, which serves as a non-invasive sensitive, reproducible, and potentially high-throughput method to detect early kidney injury in drug development studies.
Keywords: KIM-1, ELISA, urinary biomarker, validation.
Introduction
Acute kidney injury (AKI) has been defined as a rapid decline in glomerular filtration rate that occurs over hours or days [1, 2]. AKI is common, and absolute incidence of AKI has increased [3], diagnosis of which is frequently based on measurements of blood urea nitrogen (BUN) [4]. BUN and serum creatinine are not very specific or sensitive for the diagnosis of AKI because they are affected by many renal and non-renal (age, sex, race, muscle mass, nutritional status, infection, and volume of distribution, certain medications) [5-7] factors that are independent of kidney injury or kidney function.
Clearly, there is a need to identify a more specific AKI biomarker that is produced at the site of injury, can be measured easily in blood or urine and should be reasonably stable in body fluids to substantially improve the diagnosis. Several biomarkers have been studied in animal models and human of AKI [4], and kidney injury molecule-1 (KIM-1) is a recently discovered tubular protein that is markedly induced after ischemic reperfusion injury and in response of a number of other proximal tubular toxins, such as cisplatin and folic acid [8, 9]. KIM-1 is a type I transmembrane glycoprotein, with an ectodomain containing Ig-like domain and a mucin domain [8]. It is undetectable in healthy kidney tissue, but expressed at very high levels in dedifferentiated proximal tubule epithelial cells in human and rodent kidneys after ischemic or toxic injury [8, 10].
Hence, KIM-1, the new biomarker of kidney injury and glomerular filtration, holds the promise of substantially improving the diagnostic approach to acute kidney injury [9, 11]. Previously, an ELISA has been designed and validated for quantitating KIM-1 in rat urine as an animal model, but this first generation ELISA has not been tested on human urine [12]. Subsequently, an ELISA has been developed previously to measure KIM-1 in human urine [13]. These KIM-1 reagents are not commercially available, therefore there is a need to develop, evaluate, and validate a high-throughput commercially available method for quantifying KIM-1 in human urine. Analytically validating KIM-1 would provide clinical studies with the means to quantify the KIM-1 biomarker so that it can be evaluated for clinical practice. Therefore, the goal of the study was to analytically validate KIM-1 in human urine and demonstrate appropriate clinical performance using the ELISA from a R&D duo-set [14].
This assay is a Sandwich ELISA based on: 1) uniformly coating goat anti-human KIM-1 in the micro-plate well, followed by washing and then blocking plates non-specifically, 2) binding of human KIM-1 in the sample to the immobilized KIM-1 antibody, 3) the binding of a biotinylated goat anti-human KIM-1 detection antibody, 4) wash away of unbound materials, followed by conjugation of streptavidin-horseradish peroxidase to the immobilized biotinylated antibodies, 5) wash away of free enzyme, and 6) quantification of immobilized antibody-enzyme conjugates by monitoring horseradish peroxidase activities in the presence of the substrate 3,3',5,5'-tetra-methylbenzidine. The enzyme activity is measured spectrophotometrically by increased absorbance at 450 nm, corrected from the absorbance at 540 nm, after acidification of formed products. Since the increase in absorbance is directly proportional to the amount of captured KIM-1 in the unknown sample, the concentration of KIM-1 can be derived by interpolation from a reference curve generated in the same assay with reference standards of known concentrations of human KIM-1 [14]. The standard curve concentrations used for all the KIM-1 ELISAs were 2000, 1000, 500, 250, 125, 62.5, and 31.25 pg/mL and the absorbances read approximately 2.5, 1.5, 0.9, 0.5, 0.25, 0.12, and 0.05 respectively. The curve fit for the analysis of the standard curve was four parametric logistic (4PL).
Materials and Methods
Sample collection from Healthy population: For the determination of the reference interval, blood and urine samples were collected from healthy healthcare professionals (10 males and 10 females). Individuals were informed of the testing to be performed and only the specified testing was performed on the collected reference specimens. Analyses were conducted with the full respect for the individual's right to confidentiality. Information regarding their age, gender and race was also collected with the healthcare professional's consent to calculate the glomerular filtration rate (GFR). Urine creatinine and serum creatinine was assayed for each of these samples. Freshly collected urine samples were allowed to sit at room temperature for 30 minutes to sediment, and the supernatant was aliquoted and stored at -70 oC until analysis.
Construction of KIM-1 sandwich ELISA (R&D Cat# DY1750, Minneapolis, MN): The wells of Nunc-Maxisorp EIA plates were coated by diluting the capture antibody (72 μg/mL) to a working concentration of 0.4 μg/mL in PBS with 100 μL in each well. The plate was sealed and incubated overnight at room temperature. Each well was aspirated and washed using an automated microplate washer (Bio-Tek) with 400 μL of Wash Buffer (0.05% Tween-20 in PBS), repeating the process two times for a total of three washes. The plates were blocked by adding 300 μL of reagent diluent (1% BSA in PBS, 0.2 μm filtered) to each well and incubated at room temperature for 2 hours. After washing as in the previous step, 100 µL of standard recombinant human KIM-1 (0-2000 pg/mL), control, and urine sample was pipetted to the designated well, covered with an adhesive strip, and placed on the orbital shaker at 400 rpm at room temperature for 2 hours. The plate was washed using the same wash protocol as before, and 100 μL of the biotinylated goat anti-human KIM-1 detection antibody diluted in reagent diluent to a working concentration of 400 ng/mL was added to each well. The plate was covered with a new adhesive strip, incubated at room temperature for 2 hours with continued shaking at 400 rpm. Washing step was repeated and 100 μL of streptavidin-HRP diluted to a working dilution was added to each well. The plate was protected from light, covered with a new adhesive strip, shaken at 400 rpm, and incubated at room temperature for 20 minutes. After washing, 100 µL substrate solution was added to all wells, protected from light, covered with an adhesive strip, shaken at 400 rpm, and incubated at room temperature for 7 minutes. The reaction was stopped by adding 50 µL of stop solution to all wells. The absorbance was measured using a plate reader (BioTek Elx800) at 450 nm with an absorbance correction at 540 nm. The urinary KIM-1 concentration was calculated based on the standard curve and expressed in absolute terms (pg/mL).
Evaluation of the KIM-1 ELISA: The validation of the KIM-1 ELISA was evaluated by measuring linearity, intra-run precision, inter-run precision, analytical sensitivity, recovery, dilution verification, reference range, stability and length of run. Details of the criteria for each are described in the result section.
Sample collection and analysis: Urine samples were collected asceptically directly in urine cups and stored at -70 oC within 4 hours. All statistical analysis was carried out using the program EP evaluator [15] (version 8.0, DG Rhoads).
Analytical Testing: Urinary creatinine was measured by the Jaffe rate-blanked creatinine assay using a Roche/ Hitachi 911 system (Roche Diagnostics, Indianapolis , IN, USA). Serum enzymatic creatinine was measured using the Roche/Hitachi P800 analyzer. Glomerular filteration rate (GFR) was calculated using the MDRD (modification of diet in renal disease) formula 186 X (SerumCreatinine)-1.154 X (Age)-0.203 X (0.742 if female and 1.000 if male) X (1.210 if African American and 1.000 if others) (mL/min/1.73m2) [16].
Results
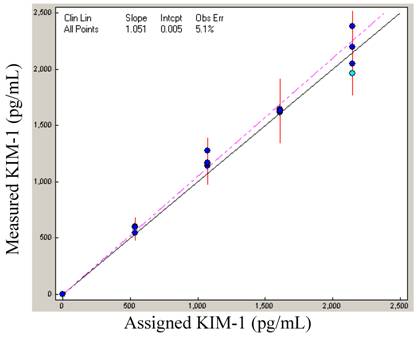
Linearity: In order to know the range of the assay, it is important to define the linear range of the assay. To define the range, a high standard was serially diluted with reagent diluent to give a series of specimens (100 + 0, 75 + 25, 50 + 50, 25 + 75, and 0 + 100). Each sample was assayed for KIM-1 in duplicates (N = 4), and slope calculated. The plotted linearity shows a slope of 1.051, and the data demonstrates that the assay is acceptably linear to 2146 pg/mL (Figure 1).
Linearity: KIM-1 linearity was demonstrated with the high standard diluted proportionately with buffer.
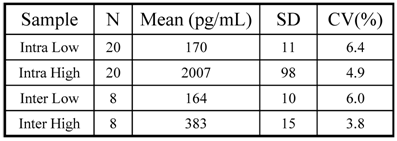
Precision: Two sets (low and high) of pooled urine samples were created and each set was run on two separate plates for N=20, % CV calculated for each to give intra-low and intra-high precision. The intra-low sample gave an average reading of 170 ± 11 pg/mL (CV = 6.4 %, N = 20), while the intra-high sample averaged 2007 ± 98 pg/mL (CV = 4.9 %, N = 20). Low and high pools were also run on 8 separate plates, and % CV calculated to give an inter-low and inter-high precision (Table 1). The mean low value for inter-low precision was 164 ± 10 pg/mL (CV = 6.0 %, N = 8), and that of inter-high precision was 383 ± 15 pg/mL (CV = 3.8 %, N = 8).
KIM-1 assay precision
Sensitivity: The assay's lower limit of quantification (LLOQ) is defined as the lowest concentration at which an across run %CV can be generated that is less than 20%. A series of samples in the concentration range of 57 to 384 pg/mL were generated to provide multiple low samples that were aliquoted and frozen. Samples were thawed and analyzed on 10 separate plates over several days. The %CV of each sample was determined from the 10 analytical runs and the lowest sample that demonstrated inter-assay precision (%CV) of less than 20% was the LLOQ of the assay. The LLOQ for the assay as read from the curve is defined as 59 pg/mL (%CV = 19.8).
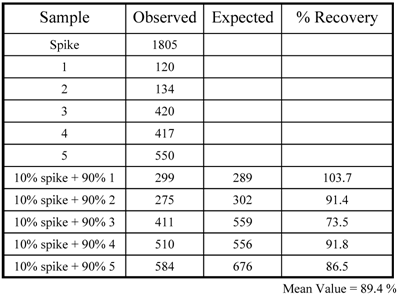
Recovery: In order to establish the % recovery, 5 urine samples were identified that read between 120 - 550 pg/mL and one high sample that read 1805 pg/mL. A second set of 5 samples were generated by mixing 10 % of the high sample with 90 % of the low samples. These samples were then assayed for KIM-1 activity. Based on the observed concentration of KIM-1 for the original 5 samples, the expected values were calculated. The average recovery of the samples was found to be 89.4 % (Table 2).
KIM-1 recovery; where 1:10 represents a mixture of 90% of sample and 10% of spike. The expected value was compared with the observed value and recovery calculated.
Dilution Verification: The dynamic range for the measurement of KIM-1 has been defined as 59 - 2146 pg/mL. This range poses difficulty for samples that are higher than the defined range. The problem is typically resolved by diluting the sample so that the reading falls within the defined range. The reading is then multiplied with the dilution factor to generate the true value. This is valid only if the dilution process is linear. A high sample (694.40 pg/mL) was diluted 1:2, 1:4, 1:8, 1:16, 1:32, and 1:64 in reagent diluent and each diluted reading was assayed for % recovery. The high sample diluted appropriately to 1:8, with 87.0 % recovery, demonstrating that the high sample can be diluted at least 8 fold, indicating the upper limit of quantification (upper limit of quantification = upper limit of linearity X highest dilution allowed) to be 17168 pg/mL (2146 X 8) (Figure 2).
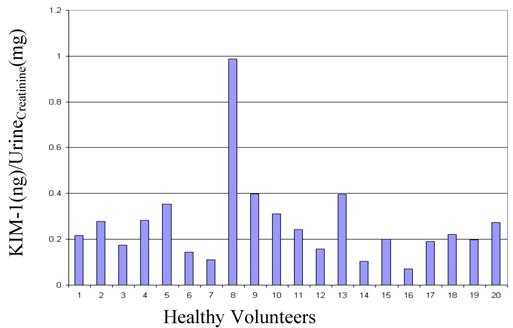
Reference Range: Urine and serum was collected from 20 healthy donors (10 males and 10 females), assayed for urinary KIM-1, urinary creatinine and serum creatinine. KIM-1 ranged from 60 - 837 pg/mL, and when normalized to urinary creatinine, the results spanned the range of 0.070 - 0.399 KIM-1(ng)/Urinecreatinine(mg), with one outlier at 0.988 (Figure 3). The MDRD calculated GFR for all the volunteers was > 65.00 (Table 3).
Dilution Verification: A urine specimen with a high endogenous KIM-1 concentration was serially diluted with reagent diluent, assayed and % recovery calculated. Most diluted samples recovered between 80 - 120 % of expected concentration.

Summary of baseline characteristics of healthy population.
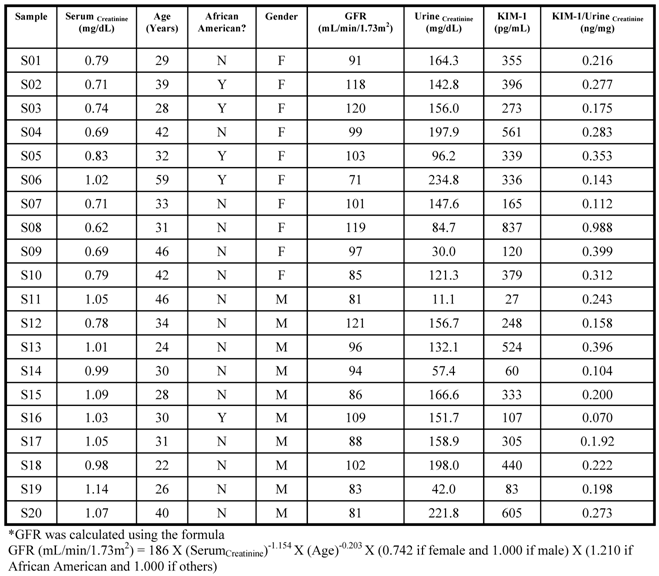
Reference Interval: Urine specimens collected from 20 healthy subjects was assayed to determine the reference range of KIM-1. Values for healthy volunteers ranged from 60 - 837 pg/mL, and the ratios KIM-1(ng/mL)/UrineCreatinine(mg/mL) ranged from 0.070 - 0.399, with one outlier at 0.988.
Length of run: In order to make sure that there was no plate effect, and the samples read the same on any well in the plate, we placed QC samples at the end of lanes 6, 9 and 12. The SD calculated during the intra-run precision was 11 pg/mL (CV = 6.4%) for intra-low, and 98 pg/mL (CV = 4.9%) for intra-high. The 3 SD range was established and applied to the QC samples on the plate. The range was calculated to be 142 - 210 pg/mL for the low and 350 - 471 pg/mL for the high QC samples. QC samples in lanes 6, 9, and 12 fell within range confirming the length of run experiment.
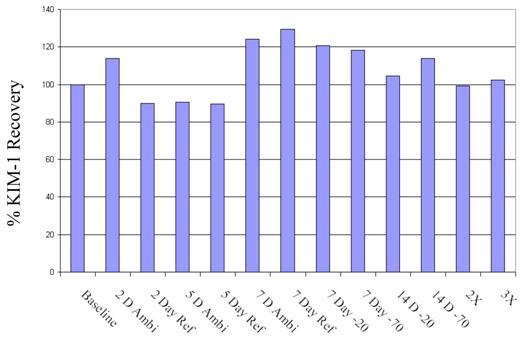
Stability: To test the stability of the samples over time, freeze-thaw cycles, and storage temperatures (room temperature, 4, -20, -70 oC), aliquoted samples (N=6) in 21 different tubes were subjected to a host of conditions. The % recovery was compared to the baseline value for all 6 samples. KIM-1 was found to be stable up to 7 days refrigerated and ambient conditions. Samples recovered within 99-114 % when subjected to 3 freeze-thaw cycles, and also at -20 oC, and -70 oC at 14 days (Figure 4). Stability studies up to 1 year are under way.
Stability: Recovery of KIM-1 at ambient, refrigerated, -20, and -70 oC over 14 days; and 2 and 3 times freeze-thaw was compared to the baseline samples (N = 6). The plotted data is the mean value for the 6 samples. The mean value for sample 1-6 across different conditions is 56.44 ± 33.22, 253.43 ± 37.43, 668.62 ± 199.19, 315.29 ± 35.15, 396.76 ± 77.61, 401.66 ± 51.63 pg/mL. Sample 1 reading 56.44 is below the LLOQ, therefore a high standard deviation is not unusual.
Discussion
The US FDA and European Medicines Agency (EMEA) have completed a major milestone under their Critical Path and Roadmap to 2010 through an extensive process of data generation and analysis and “qualified” seven new nephrotoxicity biomarkers for use in regulatory decision-making. This consortium of public-private partnership led by the Critical Path Institute, generated and submitted the data for seven urinary proteins- KIM-1, albumin, total protein, β2-microglobulin, cystatin C, clusterin, and trefoil factor-3. Efforts are underway to qualify these markers for human studies and clinical trials [17, 18]. The evaluated product is the only commercially available KIM-1 assay to support broad use of this newly recognized regulatory biomarker.
There is a growing body of evidence that supports KIM-1 as a specific marker for ischemic renal injury, and therefore a sensitive, validated assay to measure urinary levels of KIM-1 is required for clinical research. A rigorous validation of the KIM-1 assay to optimize the performance for high-throughput routine clinical research was performed. The commercially available KIM-1 assay described in this paper is suitable to assay KIM-1 in human urine.
This paper reports development of a commercially available quantitative assay for measuring KIM-1 in human urine. This assay could have direct implications for evaluation of nephrotoxicity in humans where reliance on serum creatinine is questioned. A significant advantage of this KIM-1 assay is the ease of use, analytical sensitivity, and rapid throughput, making the assay reliable for diagnostic and prognostic measurements. All the solutions are prepared easily using commercially available buffers and the recombinant KIM-1 protein was used as the assay standard. The KIM-1 assay has dynamic range of 0-2146 pg/mL and a lower limit of quantification of 59.0 pg/mL with inter-assay variability of 19.8 %. The performance characteristics of the ELISA are consistent over time, making it a robust assay for research and clinical research use. This study, for the first time, gives us data on the short-term stability of the KIM-1. The long-term stability data is being generated as well. The experiment on dilution verification show appropriate recovery up to a 1:8 dilution, indicating no interference in the urine matrix from any other factors. This is further confirmed by the spike recovery results giving approximately 90 % recovery.
The only other human KIM-1 data has been published by Han et. al [10]. Their normalized KIM-1 levels in normal healthy individuals were less than those of other acute renal failure patients at 0.48 ± 0.19 (ng)/Urinecreatinine(mg). The exact normal values were not published. The determined reference range (mean ± 2SD) in our lab with 19 donors (one outlier removed) was 0.228 ± 0.188 (ng)/Urinecreatinine(mg), and including the outlier was 0.266 ± 0.386 (ng)/Urinecreatinine(mg). The value of 0.988 (ng)/Urinecreatinine(mg) was well above the 3SD range, which is why it was considered an outlier.
Investigators in the past have relied on multiple classical laboratory tests to detect AKI, but the biggest disadvantage these tests have is time delay between injury and detection making them marginally effective biomarkers. Some of the disadvantages current biomarkers face are: lack of stability, lack of stability due to pH of urine which means the analyte has to be neutralized soon after collection, need to add stabilizers soon after collection, inhibition due to metals, lack of specificity, and onset of elevation and sustainability. KIM-1 has excellent frozen stability and the literature indicates excellent sensitivity to AKI. The KIM-1 ELISA assay also provides the throughput to handle large number of samples over extended period of time.
In conclusion, analytical validation of the KIM-1 assay demonstrates this assay can be used reliably and routinely in a standard clinical research setting. The analytical performance of this assay permits meaningful comparison of data between trials. KIM-1 has a great potential as a biomarker of renal injury, and the R&D duo set assay provides the ability for any researcher to generate KIM-1 data.
Acknowledgements
We would like to thank the staff of Covance Central Laboratories, Indianapolis for providing the healthy urine, and serum specimens in this study to verify the reference range.
Conflict of Interest
The authors have declared that no conflict of interest exists.
References
1. Lassnigg A, Schmidlin D, Mouhieddine M, Bachmann LM, Druml W, Bauer P, Hiesmayr M. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. J Am Soc Nephrol. 2004;15(6):1597-605
2. VanBiesen W, Vanholder R, Lameire N. Defining acute renal failure: RIFLE and beyond. Clin J Am Soc Nephrol. 2006;1(6):1314-19
3. Waikar SS, Curhan GC, Wald R, McCarthy EP, Chertow GM. Declining mortality in patients with acute renal failure, 1988 to 2002. J Am Soc Nephrol. 2006;17(4):1143-50
4. Edelstein CL. Biomarkers of acute kidney injury. Adv Chronic Kidney Dis. 2008;15(3):222-34
5. Stevens LA, Lafayette RA, Perrone RD. et al. Laboratory evaluation of kidney function. In: (ed.) Schrier RW. Diseases of the Kidney and Urinary Tract 8th ed. Philadelphia, PA: Lippincott, Williams and Wilkins. 2007
6. Star RA. Treatment of acute renal failure. Kidney Int. 1998;54(6):1817-31
7. Vaidya VS, Ferguson MA, Bonventre JV. Biomarkers of acute kidney injury. Annu Rev Pharmacol Toxicol. 2008;48:463-93
8. Ichimura T, Bonventre JV, Bailly V, Wei H, Hession CA, Cate RL, Sanicola M. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem. 1998;273(7):4135-42
9. Ichimura T, Hung CC, Yang SA, Stevens JL, Bonventre JV. Kidney injury molecule-1: a tissue and urinary biomarker for nephrotoxicant-induced renal injury. Am J Physiol Renal Physiol. 2004;286(3):F552-63
10. Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62(1):237-44
11. Bonventre JV. Kidney Injury Molecule-1 (KIM-1): a specific and sensitive biomarker of kidney injury. Scand J Clin Lab Invest Suppl. 2008;241:78-83
12. Vaidya VS, Ramirez V, Ichimura T, Bobadilla NA, Bonventre JV. Urinary kidney injury molecule-1: a sensitive quantitative biomarker for early detection of kidney tubular injury. Am J Physiol Renal Physiol. 2006;290(2):F517-29
13. Vaidya VS, Ramirez V, Ichimura T, Bobadilla NA, Bonventre JV. Urinary kidney injury molecule-1: a sensitive quantitative biomarker for early detection of kidney tubular injury. Am J Physiol Renal Physiol. 2006;290(2):F517-29
14. R&D Systems Inc. Minneapolis, MN, USA. KIM-1 assay kit instructions for use (ed 751761; 5/08). R&D Systems Inc: Minneapolis, MN, USA. 2008
15. Rhoads DG. http://www.dgrhoads.com
16. NKDEP. http://www.nkdep.nih.gov/professionals/gfr_calculators/orig_con.htm
17. CPATH. http://www.c-path.org/pdf/PSTC_nephro_VXDS_summary_final.pdf
18. FDA. http://www.fda.gov/bbs/topics/NEWS/2008/NEW01850.html
Author contact
![]() Correspondence to: Gordon F. Kapke, Ph.D., Tel.: 317-273-7901; Fax: 317-273-7990; E-mail: gordon.kapkecom
Correspondence to: Gordon F. Kapke, Ph.D., Tel.: 317-273-7901; Fax: 317-273-7990; E-mail: gordon.kapkecom

 Global reach, higher impact
Global reach, higher impact