Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2009; 5(2):182-191. doi:10.7150/ijbs.5.182 This issue Cite
Research Paper
Association between Apoptotis and CD4+/CD8+ T-Lymphocyte Ratio in Aseptic Loosening after Total Hip Replacement
1. Department of Orthopaedics, University of Duisburg-Essen, Pattbergstrasse 1-3, 45239 Essen, Germany
2. Department of Forensic Pathology, Institute of Legal Medicine, Charité Universitätsmedizin Berlin, Turmstrasse 21, 10559 Berlin, Germany
3. Institute of Pathology and Neuropathpology, University Hospital Essen, University of Duisburg-Essen, Hufelandstrasse 55, 45122 Essen, Germany
Received 2008-11-25; Accepted 2009-2-1; Published 2009-2-3
Abstract
Particle-induced osteolysis is a major cause of aseptic loosening after total joint replacement. While the osteolytic cascade initiated by cytokine release from macrophages has been studied extensively, the involvement of T-lymphocytes in this context is controversial and has been addressed by only a few authors. In a former study we detected that the quantity of T-lymphocytes may be influenced by apoptosis in patients with aseptic loosening. In this study we intended to find out more details about the apoptosis-induced shifting of the T-cell number. We focused our interest on the CD4+ and CD8+ T-cells and their relative ratio. Caspase-3 cleaved was evaluated immunohistochemically to detect apoptotic T-cells in capsules and interface membranes from patients with aseptic hip implant loosening and a varying degree of caspase-3 cleaved expression in CD4+ and CD8+ T-lymphocytes was detected. Moreover, a relationship between the intensity of the apoptotic reactions and the radiological extent of osteolysis was observed. The number of CD4+ cells was decreased in the presence of strong apoptotic reactions, respectively extensive osteolysis, while CD8+ cells were affected to a much lower degree. Thus, the CD4+/CD8+ ratio changed from 1.0 in cases with only small areas of periprosthetic osteolysis and minimally intense apoptosis to 0.33 in cases with large areas of osteolysis. This may suggest a causal relationship between the apoptosis-induced shift in the CD4+/CD8+ ratio and the osteolysis respectively aseptic loosening. It is possible that these findings may lead to a new understanding of particle-induced osteolysis.
Keywords: Wear Debris, Osteolysis, Apoptosis, T-Lymphocytes, Immunohistochemistry
1. Introduction
Particle-induced osteolysis is a major cause of aseptic loosening after hip joint replacement. Periprosthetic osteolysis is initiated by an aseptic inflammatory response to phagocytosis of implant wear particles resulting in increased proliferation and differentiation of osteoclast precursors into mature osteoclasts [1]. Progressive osteolysis can result in implant instability and failure, eventually requiring revision surgery. The complexity of this topic is reflected by the large number of published studies on particle-induced bio-reactivity. While the osteolytic cascade initiated by cytokine release from macrophages has been studied extensively, the involvement of T-lymphocytes in this context is controversial and has been addressed by only a few authors [2]. Most of them focused their interest on the affects of lymphocyte-mediated sensitivity on implant perfomance [3]. In previous studies, besides increased apoptosis of macrophages, giant cells and fibroblasts, we also observed apoptosis of T-lymphocytes in the capsules and interface membranes of patients with aseptic hip implant loosening, but not in the capsules of patients without joint replacements [4, 5]. In addition to other immunohistochemical markers, apoptotic reactions were identified by caspase-3 cleaved. Caspases cleave a number of different substrates in the cytoplasm or nucleus leading to many of the morphological features of apoptotic cell death [6]. In the apoptotic effector pathway, caspase-3 is probably best correlated with apoptosis in mammalian cells [7]. On closer inspection of the T-lymphocytes in the tissues we saw varying intensities of apoptosis, related to the extent of osteolysis [5]: In the early stages of osteolysis we found no caspase-3 cleaved reaction in T-cells. As the extent of osteolysis increased, we observed a corresponding increase in the intensity of the apoptotic reactions. But the capsules and interface membranes of the patients with the most advanced osteolysis, showed no apoptotic reactions in the T-lymphocytes. Depending on the extent of osteolysis and degree of apoptotic reactions, the number of T-cells also differed and we observed a greater number of these cells in the earlier stages than in the advanced stages. In that study we did not differentiate between the different subsets of T-lymphocytes. A differentiation could be made by the “clusters of differentiation” (CD). These are co-receptors, that most T-lymphocytes express if released from the thymus to peripheral tissues. They play a major role in determining the lymphocytes' function [8, 9]. The most common receptors are CD4 and CD8. T-cells expressing CD4 are also known as CD4+ T-cells. CD4 is predominantly expressed on the surface of helper T-cells, CD8 on cytotoxic killer cells. CD4+ T-cells are specific for antigens presented by major histocompatibility complex II (MHC II) molecules on the surface of antigen-presenting cells (APC) such as macrophages, dendritic cells and B-lymphocytes. CD8+ T-cells are specific for the class I MHC molecules on non-body-own proteins [10].
In the current study we intended to find out if wear particle-induced apoptosis in T-lymphocytes influences the balance between the different subsets of T-lymphocytes, as is seen in the pathogenesis of impaired wound healing, myasthenia gravis, liver inflammation after sepsis and other diseases [11-13]. As these studies focused their interest on the CD4+ and CD8+ T-lymphocytes and the value of the CD4+/CD8+ ratio, we also concentrated on this aspect.
2. Materials and Methods
2.1. Patients
We investigated specimens from patients with total hip arthoplasty (Group 1), patients with primary total hip replacement for osteoarthritis (Group 2) and autopsies (Group 3). Groups 1 and 2 consisted of consecutive cases operated at the Department of Orthopaedics of the University of Duisburg-Essen, Group 3 consisted of cases autopsied at the Institute of Legal Medicine, University of Hamburg. This collective of cases has already been used in a previous study by our group [5]. The study was approved by the local Ethics Committee.
Group 1: The group consisted of twenty-one patients, thirteen female and eight male. The mean age was 70.3, ranging from 45 to 85 (Standard Deviation (SD): 11.4). The mean survival time (time in situ) of the implant was 12.2 years, ranging from 3 to 20 years (SD: 5.3). Three patients had already had one previous exchange, in one case it was the second and in another case it was the third exchange. In each case the articulations of the loosened prostheses were a metal femoral head and a polyethylene cup. All patients had undergone primary total hip replacement for primary osteoarthritis. Patients with osteonecrosis, rheumatoid arthritis, and relevant comorbidities were not included. In addition, cases with loosening due to surgical technique or initial implant malpositioning were excluded. Eleven patients received a cemented polyethyelene acetabular component and cemented stem manufactured from a stainless-steel alloy. Uncemented implants were used in eight cases manufactured from a titanium alloy. Hybrid-fixation was performed in two cases with an uncemented titanium acetabular component and a cemented stainless-steel stem. For further clinical data of the patients included in Group 1 see Table 1. The reason for revision surgery was aseptic loosening of a hip implant. The presence of infection was excluded by analysis of inflammatory markers in the blood (C-reactive protein, leucocytosis), by culture of joint fluid obtained by aspiration from the hip joint for twenty-one days and by histology of hip joint capsules and interface membrane. Loosening was diagnosed preoperatively using modified radiographic criteria described previously: Loosening of cemented acetabular components was defined as migration of the acetabular component, a new fracture in the cement mantle, or a circumferential radiolucent line between the bone and the cement mantle that was not visible on the immediately postoperative radiographs [14]. Loosening of cemented femoral components was defined as subsidence of the femoral component, fracture of the cement or stem, or a circumferential radiolucent line at the bone-cement interface [15]. Debonding of the femoral component with a radiolucent line of smaller than two millimetres between the shoulder of the prosthesis and the cement was not considered to represent loosening. Uncemented acetabular and femoral components were evaluated according to the system described by McAuley et al. [16]. The femoral and acetabular bone defects seen in the radiographs were classified according to the classification of Paprosky [17].
Clinical data Group 1 (patients with aseptic loosening of hip implant)
| Case No. | Age | Sex | Years after Implantation | Number of revisions | Component loosening / Components exchanged | Cemented fixation | Paprosky score | ||
|---|---|---|---|---|---|---|---|---|---|
| Acetabular | Stem | Acetabular | Stem | ||||||
| 1 | 78 | M | 16 | 1 | X | Hybrid | 1 | X | |
| 2 | 55 | F | 3 | 1 | X | Yes | X | 1 | |
| 3 | 72 | M | 10 | 1 | X | Yes | 2a | X | |
| 4 | 69 | F | 8 | 4 | X | No | 2a | X | |
| 5 | 82 | M | 7 | 1 | X | Yes | 2a | X | |
| 6 | 50 | M | 2 | 1 | X | X | Hybrid | 2b | 1 |
| 7 | 61 | F | 12 | 1 | X | X | No | 2b | 1 |
| 8 | 45 | M | 11 | 1 | X | No | 2c | 1 | |
| 9 | 63 | F | 6 | 2 | X | Yes | X | 2 | |
| 10 | 70 | F | 9 | 1 | X | Yes | X | 2 | |
| 11 | 81 | F | 19 | 1 | X | Yes | 2b | X | |
| 12 | 73 | M | 5 | 1 | X | Yes | 2b | X | |
| 13 | 83 | F | 16 | 1 | X | Yes | 2b | X | |
| 14 | 71 | M | 17 | 1 | X | X | Yes | 2b | 2 |
| 15 | 82 | F | 16 | 1 | X | X | No | 2c | 3 |
| 16 | 54 | F | 20 | 2 | X | X | No | 3b | 3 |
| 17 | 79 | F | 20 | 1 | X | Yes | 3b | X | |
| 18 | 85 | F | 10 | 1 | X | Yes | X | 3b | |
| 19 | 77 | M | 8 | 1 | X | No | X | 3 | |
| 20 | 79 | F | 19 | 1 | X | No | 3c | X | |
| 21 | 67 | F | 3 | 3 | X | No | X | 4 | |
| Mean | 70,3 | 12,2 | |||||||
| SD | 11,4 | 5,3 | |||||||
aF = female; M = male
bAcet. = acetabular
Group 2: Group 2 consisted of sixteen patients who underwent primary total hip replacement for osteoarthritis. Patients with osteonecrosis, rheumatoid arthritis and serious secondary diseases were not included. Ten patients were female and six were male. The mean age was 64.8, ranging from 44 to 75 (SD: 9.8).
Group 3: Group 3 consisted of six patients who had been autopsied at the Department of Forensic Pathology at the Institute of Legal Medicine, University of Hamburg and who had no historical or radiological evidence of osteoarthritis of the hip at the time of death. Three patients were female and three were male. The mean age was 57.7, ranging from 47 to 80 (SD: 12.2).
2.2. Specimens
Group 1: Tissues were harvested from the newly formed hip joint capsules as well as from the interface membranes of the acetabular and femoral periprosthetic regions after removal of prostheses from patients undergoing exchange hip arthroplasty.
Group 2: In Group 2 hip capsules were retrieved during primary total hip arthroplasty.
Group 3: In Group 3 hip capsules were retrieved during autopsy.
2.3. Tissue processing
Formalin-fixed and paraffin-embedded tissues from patients were retrieved from the files of the Department of Pathology and Neuropathology, University Hospital of Essen, University of Duisburg-Essen and the Institute of Legal Medicine, University of Hamburg.
2.4. Hematoxylin Eosin (HE) staining
Sections were cut to a thickness of 4 µm and mounted on glass slides. The staining was performed using Mayers Hämalaun (Merck 109249) and Erythrosin B (Merck 115936) solutions.
2.5. Immunohistochemistry
Semi-thin serial sections were cut and mounted on protein-coated glass slides. After dewaxing in xylene and rehydration in a series of alcohols, endogenous peroxidase activity in the tissue was blocked with 5% hydrogen peroxidase for five minutes. This was followed by incubation with the primary antibodies to caspase-3 cleaved (polyclonal antibody, dilution of 1:200; Zytomed Systems, Berlin, Germany), CD4 (monoclonal antibody, dilution of 1:40; Zytomed Systems, Berlin, Germany) and CD8 (monoclonal antibody, dilution of 1:150; Dako, Glostrup, Denmark) for thirty minutes at room temperature using an automated autostainer (Autostainer, Dako-Cytomation, Glostrup, Denmark). Afterwards, a second incubation with Zytomed POLHRP-100 (brown) or POLAP-100 (red) as well as visualisation according to the manufacturer's instructions (Zytomed Systems, Berlin, Germany) was performed. The specificity of the immunoreactions was checked by omission of the primary antibody. Tonsil tissue served as positive control for caspase-3 cleaved, respectively for T-cell markers CD4 and CD8.
For double immunohistochenistry, the same caspase-3 cleaved antibody and CD4 respectively CD8 antibody were used. Antigen retrieval was carried out at 98°C for 10 min in a water bath (Target retrieval buffer, DAKO). The caspase-3 cleaved/CD4 and caspase-3 cleaved/CD8 double labelings were done in two steps. First, caspase-3 cleaved labeling with an immunhistochemical staining technique based on a horseradish peroxidase (HRP)-labeled polymer that is conjugated to secondary antibodies (ZytoChemPlus HRP Polymer Kit, Zytomed Systems) was done. Staining was completed by an incubation with 3,3´-diaminobenzidine + substrate-chromogen (Zytomed Systems), which results in a brown-colored precipitate at the antigen site. Second, CD4 or CD8 labeling was done with an alkaline phosphatase -labeled polymer (ZytoChemPlus AP Polymer Kit, Zytomed Systems) and developed with a Permanent Red chromogenic substrate system (Zytomed Systems). At the end of the procedure, nuclei were counterstained with hematoxylin for 5 min.
2.6. Light microscopy
Before analysis, the sections were coded and blinded. All areas of each biopsy section were examined independently by two observers, a consultant pathologist (M.T.) and an orthopaedic resident (S.L.) specially trained in histology for this project. The results were equalized by a consensus read out. The sections were scored for the presence of caspase-3 cleaved in T-lymphocytes: The percentage of caspase-3 cleaved positive T-lymphocytes per total lymphocytes was evaluated and a semi-quantitatively proportion score was attributed on a 0-5 scale, as follows: 0 = no staining, 1 = rare positive cells (1-5%), 2 = scattered clusters of positive cells (6-20%), 3 = positive staining in quite a large number of cells (21-50%), 4 = strong staining in most cells (51-80%), 5 = strong staining in nearly all cells (81-100%).
Furthermore, the sections were investigated for the presence of CD4+ and CD8+ T-lymphocytes. For each CD4 stained section, three random fields of a minimum of 100 cells each were counted at a magnification of 200x. The same areas were selected in the CD8 stained sections for counting. All data are reported as means of the respective section.
Finally, the sections were scored semi-quantitatively for the presence of microscopic-sized polyethylene particles and metal particles (for example titanium) on a 0-5 score: 0 = no particles, 1 = rare particles, 2 = few scattered clusters of particles, 3 = considerable number of particles in several regions, 4 = many particles in most regions, 5 = particles diffusely distributed in the whole tissue.
2.7. Statistical Analysis
We calculated the CD4+/CD8+ ratio by division of the assigned semi-quantitative scores of both T-lymphocyte subsets.
The Mann-Whitney-U test was applied for comparison of caspase-3 cleaved immunoreactivity in Groups 1 and 2. This test was based on the exact permutation distribution, since the sample sizes are small. Comparisons with p-values < 0.05 were considered to be significant. No adjustment for multiple testing was applied since the statistical analysis was performed in an exploratory manner. The software SPSS 12.0 (SPSS Inc. Headquarters, Chicago, Illinois, USA) was used to carry out the statistical computations.
3. Results
3.1. Histology
Group 1: HE staining of the interface tissue revealed typical features of membranes that form in response to arthroplasty-derived particles, i.e. numerous fibrous cells in a bed of connective tissue. In the capsule tissue HE staining showed the fibrous membrane with collagen fibres, and the synovial membrane with loose connective tissue, fibrous and synovial cells.
Varying quantities of wear debris, including metal and polyethylene particles, were found in the capsule and interface tissues, accompanied by a cellular infiltrate of T-lymphocytes, macrophages, giant cells, fibroblasts and synovial cells. Scores for the presence of polyethylene and metal particles in capsules and interfaces are presented in Table 2. All kind of cells, including T-lymphocytes were situated predominantly on the bone-near side of the capsules and interface membranes.
Immunohistochemical results Caspase-3 cleaved, CD4 and CD8 in Group 1 (patients with aseptic loosening of hip implant)
| Capsule | Acetabular-interface | Stem-interface | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No | PEa | MEa | Ca-3b | CD4c | CD8c | Ratiod | PEa | MEa | Ca-3b | CD4c | CD8c | Ratiod | PEa | MEa | Ca-3b | CD4c | CD8c | Ratiod |
| 1 | 2 | 1 | 0 | 27 | 37 | 0,73 | 5 | 1 | 0 | 39 | 52 | 0,75 | − | − | − | − | − | − |
| 2 | 1 | 5 | 0 | 39 | 39 | 1,00 | − | − | − | − | − | − | 2 | 5 | 0 | 52 | 52 | 1,00 |
| 3 | 3 | 1 | 2 | 30 | 40 | 0,75 | 3 | 1 | 2 | 26 | 36 | 0,72 | − | − | − | − | − | − |
| 4 | 2 | 2 | 2 | 25 | 38 | 0,66 | 5 | 4 | 2 | 42 | 55 | 0,76 | − | − | − | − | − | − |
| 5 | 2 | 1 | 1 | 24 | 39 | 0,62 | 2 | 4 | 2 | 52 | 65 | 0,80 | − | − | − | − | − | − |
| 6 | 1 | 1 | 0 | 55 | 55 | 1,00 | 1 | 5 | 0 | 38 | 38 | 1,00 | 1 | 5 | 0 | 29 | 40 | 0,73 |
| 7 | 3 | 2 | 1 | 30 | 47 | 0,64 | 4 | 1 | 2 | 33 | 49 | 0,67 | 4 | 1 | 0 | 54 | 54 | 1,00 |
| 8 | 2 | 2 | 2 | 29 | 46 | 0,63 | 3 | 1 | 3 | 29 | 62 | 0,47 | 2 | 1 | 0 | 45 | 53 | 0,85 |
| 9 | 4 | 4 | 2 | 39 | 53 | 0,74 | − | − | − | − | − | − | 1 | 1 | 2 | 36 | 53 | 0,68 |
| 10 | 1 | 4 | 2 | 40 | 55 | 0,73 | − | − | − | − | − | − | 2 | 1 | 1 | 34 | 50 | 0,68 |
| 11 | 2 | 1 | 1 | 27 | 50 | 0,54 | 4 | 1 | 2 | 30 | 60 | 0,50 | − | − | − | − | − | − |
| 12 | 2 | 1 | 2 | 39 | 52 | 0,75 | 2 | 2 | 1 | 36 | 59 | 0,61 | − | − | − | − | − | − |
| 13 | 2 | 2 | 2 | 32 | 39 | 0,82 | 3 | 2 | 1 | 19 | 33 | 0,58 | − | − | − | − | − | − |
| 14 | 1 | 2 | 2 | 18 | 30 | 0,60 | 4 | 4 | 2 | 39 | 56 | 0,70 | 4 | 5 | 2 | 37 | 62 | 0,60 |
| 15 | 3 | 3 | 3 | 28 | 41 | 0,68 | 3 | 3 | 2 | 35 | 52 | 0,67 | 5 | 5 | 2 | 26 | 52 | 0,50 |
| 16 | 3 | 3 | 3 | 17 | 38 | 0,45 | 3 | 3 | 3 | 36 | 60 | 0,60 | 3 | 3 | 3 | 28 | 53 | 0,53 |
| 17 | 2 | 2 | 2 | 16 | 40 | 0,40 | 4 | 5 | 3 | 19 | 38 | 0,50 | − | − | − | − | − | − |
| 18 | 1 | 1 | 0 | 26 | 42 | 0,62 | − | − | − | − | − | − | 1 | 4 | 0 | 26 | 53 | 0,49 |
| 19 | 2 | 2 | 3 | 15 | 39 | 0,38 | − | − | − | − | − | − | 1 | 1 | 3 | 15 | 35 | 0,43 |
| 20 | 3 | 5 | 0 | 14 | 41 | 0,34 | 4 | 4 | 0 | 13 | 39 | 0,33 | − | − | − | − | − | − |
| 21 | 1 | 1 | 0 | 12 | 33 | 0,36 | − | − | − | − | − | − | 1 | 4 | 0 | 19 | 51 | 0,37 |
| Ø | 2,0 | 2,2 | 1,4 | 27,7 | 42,6 | 0,6 | 3,3 | 2,7 | 1,7 | 32,4 | 50,3 | 0,6 | 2,3 | 3,0 | 1,1 | 33,4 | 50,7 | 0,7 |
| SE | 0,8 | 1,3 | 1,0 | 10,4 | 6,85 | 0,2 | 1,1 | 1,5 | 1,0 | 9,7 | 10,4 | 0,2 | 1,4 | 1,8 | 1,2 | 11,7 | 6,6 | 0,2 |
a PE = polyethylene; ME = metal. The sections were scored semi-quantitatively for the presence of microscopic-sized polyethylene and metal particles (for example titanium) on a 0-5 score: 0 = no particles, 1 = small number of particles in only a few regions, 2 = several particles in some regions, 3 = quite a large number of particles in a large number of regions, 4 = many particles in most regions, 5 = many particles in the whole tissue.
b Ca-3 = The sections were scored separately for the presence of caspase-3 cleaved T-lymphocytes. The scores were assigned semi-quantitatively on a 0-5 scale, as follows: 0 = no staining, 1 = rare positive staining or trace staining (1-5%), 2 = scattered clusters of positive cells (6-20%), 3 = positive staining in quite a large number of cells (21-50%), 4 = strong staining in most cells (51-80%), 5 = strong staining in nearly all cells (81-100%). − = No change of acetabulum respectively stem.
c The sections were investigated for the presence of CD4+ and CD8+ T-lymphocytes. For each CD4 stained section, three random fields of a minimum of 100 cells each were counted at a magnification of 200x. The same areas were selected in the CD8 stained sections for counting. All data are reported as means of the respective section. − = No change of acetabulum respectively stem.
d Ratio = CD4+/CD8+ ratio
Group 2: In the capsule samples from patients with osteoarthritis HE staining showed a thickening of the synovial membrane accompanied by a focal proliferation of synovial cells. In advanced cases hyperaemia as well as lymphocytic infiltration of the fibrous tissue was observed. Fibroblasts and macrophages were seen in lower numbers than in Group 1. Giant cells were consistently not observed.
Group 3: HE staining showed physiological capsule tissue: A fibrous membrane with collagen fibres and a synovial membrane with loose connective tissue, fibrous and synovial cells.
3.2. Immunohistochemistry
3.2.1. Caspase-3 cleaved
Group 1: The results for Group 1 are presented in Table 2. Both the interface membranes and capsules from patients with aseptic loosening of their hip prosthesis frequently showed a positive reaction in the T-lymphocytes. Caspase-3 cleaved immunoreactivity was also observed in macrophages, giant cells, fibroblasts and synovial cells. Results for these types of cells have already been published [5].
The caspase-3 cleaved/CD4 and caspase-3 cleaved/CD8 double immunohistochemistry showed caspase-3 cleaved reactions almost always in CD4 cells (Figure 2).
Group 2 and 3: No caspase-3 cleaved immunoreactivity was detected in T-lymphocytes or other cells.
3.2.2. CD4 and CD8
Group 1: The results for Group 1 are presented in Table 2. The tissue samples showed variable numbers of lymphocytes. In most samples the number of lymphocytes was obviously increased in comparison to control group 3 with a mean T-cell number per random field of 70.3 in the capsules, 82.7 in the acetabular interfaces and 84.1 in the stem interfaces. In all cases we found more CD8+ than CD4+ cells.
Group 2: We found an increase in the number of T-cells compared to tissues of patients without osteoarthrosis, especially in the number of CD4+ cells. The mean T-cell number per random field was 77.8. In all cases we found more CD4+ than CD8+ cells.
Group 3: As is characteristic for normal tissue only a few T-cells (mean 11.2) were detected and CD4+ T-cells were dominating.
3.3. Statistical analysis
In the Mann-Whitney-U test the comparison of caspase-3 cleaved expression in capsules between Group 1 (patients with aseptic loosening) and Group 2 (patients with osteoarthritis) showed significant differences for T-lymphocytes (p<0.001), macrophages (p<0.001) and fibroblasts (p<0.001).
The CD4+/CD8+ ratio ranged between 0.33 and 1.0 in Group 1 with a mean score of 0.6 (SD 0.2) in the capsules, 0.6 (SD 0.2) in the acetabular interfaces and 0.7 (SD 0.2) in stem interfaces. In Group 2 the CD4+/CD8+ ratio ranged between 1.1 and 1.6 with a mean score of 1.3 (SD 0.1). In Group 3 the CD4+/CD8+ ratio ranged between 1.2 and 1.4 with a mean score of 1.2 (SD 0.1).
4. Discussion
Most studies attempting to explain the aetiology of aseptic loosening of joint prostheses have concentrated on macrophages which are thought to play the central role [2, 18]. The role of T-lymphocytes in aseptic loosening is largely unknown. While some studies of the cellularity of periprosthetic tissues from patients with aseptic loosening suggest the presence of a substantial quantity of activated T-cells, other reports are controversial, reporting only unactivated or low numbers of lymphocytes [19-22]. The quantity of T-lymphocytes may be influenced by apoptosis, which we described in a previous study [4, 5]. In this study we intended to find out more details about the apoptosis-induced shifting of the T-cell number in patients with aseptic loosening. The collective of cases and specimens stained by caspase-3 cleaved in a former study [5], was reinvestigated for CD4+ and CD8+ T-lymphocytes, because we wanted to find out if these two T-lymphocyte subsets are influenced by wear particle-induced apoptosis in the same manner.
While in previous studies [4, 5] detection of T-lymphocytes was performed semi-quantitatively by morphological means, in the actual study we evaluated their presence by immunohistochemistry and number of respective cell types was counted by using random fields. Thus, we could confirm our previous observation that in Groups 1 (total hip arthoplasty) and 2 (primary total hip replacement for osteoarthritis) there is an increased lymphocytic infiltration such as would occur in the presence of chronic inflammation. This can be attributed to wear debris respectively osteoarthritis. In Group 3 (without osteoarthrosis) a low number of lymphocytes appropriate to physiological capsule tissue was found.
In terms of apoptotic reactions in T-lymphocytes we observed a varying degree of caspase-3 cleaved staining in the capsules and interface membranes of patients with aseptic loosening (Group 1), while the tissues of patients without arthoplasty showed no apoptotic reactions (Groups 2 and 3). The intensity of the apoptotic reactions tends to follow the radiological changes classified by Paprosky et al. [17]: In the early stages of osteolysis with a Paprosky score of acetabular 1 respectively femoral 1 we found no caspase-3 cleaved reaction in T-cells. As the extent of osteolysis increased, we observed a corresponding increase in the intensity of the apoptotic reactions. But the capsules and interface membranes of the patients with the most advanced osteolysis and a Paprosky score of 3c acetabular respectively 4 femoral showed no apoptotic reactions in the T-lymphocytes. This might be caused by the fact that the morphological aspect of the tissues was similar to scar tissue. Moreover, we also found many necrotic areas.
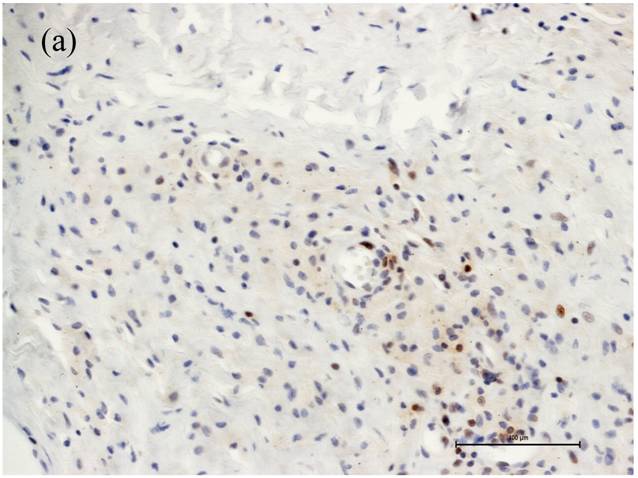
Associated with the increasing apoptosis in T-lymphocytes the number of CD4+ cells was decreased in cases with large areas of osteolysis (Fig. 1a), while the number of CD8+ cells was affected to a much lower extent (Fig. 1b). Moreover the caspase-3 cleaved/CD4 and caspase-3 cleaved/CD8 double immunohistochemistry showed caspase-3 cleaved reactions almost always in CD4 cells (Figure 2). Thus, the CD4+/CD8+ ratio changed from 1.0 in cases with small areas of periprosthetic osteolysis and minimally intense apoptosis to 0.33 in cases with large areas of osteolysis, especially observed in the interface membranes. We therefore speculate that the apoptotic reactions in CD4+ T-lymphocytes are one reason for the inversed CD4+/CD8+ ratio in patients with aseptic loosening. Our observation that the number of CD4+ cells is related to the extent of osteolysis could be the reason why Hercus et al. observed a predominance of CD4+ in the interface membranes of most patients with aseptic loosening [23]. In a former study we observed no proliferation of T-lymphocytes neither in the capsules nor in the interface membranes, so the decrease of CD4+ seems not be influenced by a lower proliferation rate but by a higher apoptotic rate.
The level of apoptotic reactions and the number of CD4+ and CD8+ T-lymphocytes was not influenced by the type of endoprosthesis or the sex and age of patients.
The control groups in our study always showed positive CD4+/CD8+ ratios (>1) with an average score of 1.3 in Group 2, and 1.2 in Group 3. Scores between 1 and 1.5 can be regarded as normal in the tissues of human beings. We speculate that the diminished CD4+ expression, caused by apoptosis, and the relatively increased number of CD8+ T cells are involved in the pathophysiological process of aseptic loosening. As mentioned before, it is known that in various pathophysiological processes, for example wound healing, the balance of the different T-cell subsets influences the outcome of healing [11, 24]: An impaired function or complete lack of CD4+ T-cells leads to diminished wound repair. In contrast, isolated depletion of CD8+ lymphocytes promotes healing by enhancing mechanical strength and collagen formation [11, 25]. It is conceivable that the abundance of CD8+ T-cells leads to a down-regulation of the mechanical strength in the interface membranes and capsules, which finally results in the necrotic tissues observed in patients with pronounced osteolysis. This consideration is supported by the fact that helper T-cells (CD4+) possess the ability to control the immune response while cytotoxic T-cells (CD8+) can cause tissue damage and to a certain extent influence the local response.
Moreover, more recent in vitro studies have demonstrated that CD4+ and CD8+ sub-populations are able to influence osteoclastogenesis: Especially regulatory T cells, a subset of CD4+ T-lymphocytes, inhibit osteoclast differentiation from Peripheral Blood Mononuclear Cells (PBMCs) [26, 27]. We therefore conclude that apoptotic reactions in CD4+ T cells are harmful because we speculate that the slowdown of CD4+ cells is jointly responsible for an activation of osteoclasts.
At this point it is not clear which factors are responsible for induction of apoptosis in T-lymphocytes, especially in the CD4+ T cells. It was demonstrated that in other inflammatory diseases an increased production of nitric oxide (NO) causes activated T-lymphocytes to undergo apoptosis [28, 29]. Dalton et al. observed impaired apoptosis of CD4+ T-cells in iNOS (inducible nitric oxide synthase) knockout mice, suggesting that iNOS is jointly responsible for apoptosis in CD4+ T-cells [29]. Increased expression of iNOS by macrophages has also been noticed in connection with loose total hip replacements. We therefore assume that iNOS is one reason for the described apoptotic reactions in T-lymphocytes.
In parallel to the decrease of the CD4+/CD8+ ratio we observed an influx of macrophages in the tissues from patients with advanced loosening of the hip implant who had a Paprosky score of 2c to 3b in the acetabular tissue, respectively 3 in the femoral tissue. It may be speculated if the increased expression of iNOS is responsible for the decrease of the CD4+/CD8+ ratio. Furthermore, as described in a former study of our group apoptotic reactions in the macrophages does not compensate the increasing number of these cells [5]. Further studies are necessary to clarify the exact mechanism and to determine the pathogenetic role of the shift in the CD4+/CD8+ ratio. These studies might also clarify if the change in CD4+/CD8+ ratio is really, as supposed by us, the cause or the effect of aseptic osteolysis. Understanding of these mechanisms could lead to a concept for therapeutic action to treat or prevent aseptic loosening.
Number of CD4+ and CD8+ T-cells in case of increased apoptotic reactions respectively large extent of osteolysis. Magnifications are at original x 200. (a) The number of CD4+ cells was decreased in cases with large areas of osteolysis. (b) The number of CD8+ cells were affected to a much lower extent.
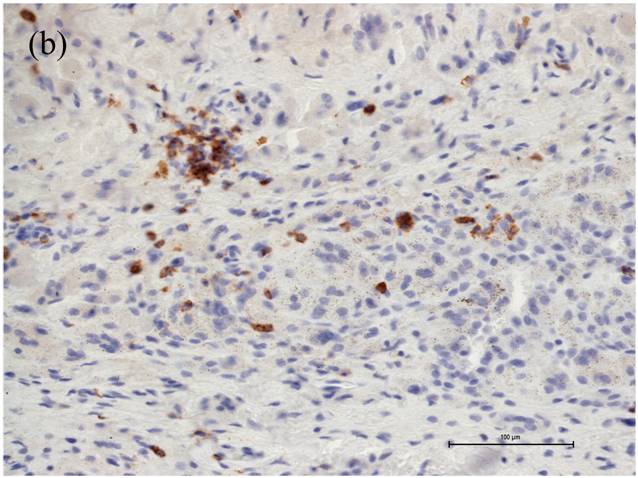
The caspase-3/CD4 double immunohistochemistry showed caspase-3 cleaved reactions (brown stained) almost always in CD4 cells (red stained). Magnifications are at original x 600. Scale bar: 100µm.
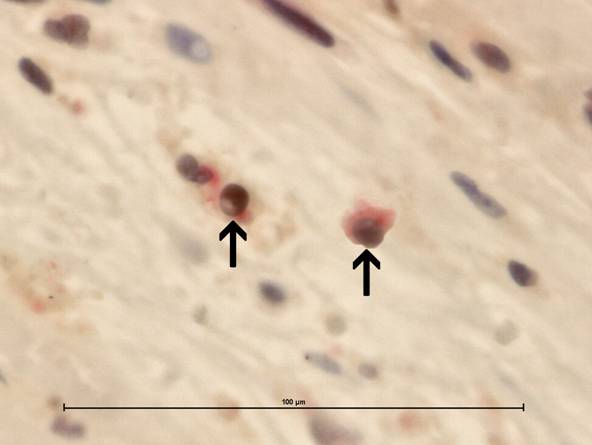
5. Conclusions
In this study, apoptosis of T-lymphocytes was detected in vivo in the capsules and interface membranes of patients with aseptic hip implant loosening. The apoptotic reactions led to a CD4+/CD8+ ratio in which CD8+ predominated. This may suggest a causal relationship with osteolysis and aseptic loosening. This is substantiated by the fact that the apoptotic reactions in the lymphocytes and the CD4+/CD8+ ratio correlate with the stage of osteolysis in aseptic loosening. It is possible that these findings may lead to a new understanding of particle-induced osteolysis.
Acknowledgements
This study was supported by a grant from the Verein der Freunde und Förderer des Evangelischen Krankenhauses Essen-Werden e.V.
We thank Mrs. N. Cramer for technical assistance.
Conflict of Interest
The authors have declared that no conflict of interest exists.
References
1. Silva MJ, Sandell LJ. What's new in orthopaedic research. J Bone Joint Surg Am. 2002;84A:1490-6
2. Purdue PE, Koulouvaris P, Potter HG. et al. The cellular and molecular biology of periprosthetic osteolysis. Clin Orthop Relat Res. 2007;454:251-61
3. Hallab NJ, Anderson S, Stafford T. et al. Lymphocyte responses in patients with total hip arthroplasty. J Orthop Res. 2005;23:384-91
4. Landgraeber S, Toetsch M, Wedemeyer C. et al. Over-expression of p53/BAK in aseptic loosening after total hip replacement. Biomaterials. 2006;27:3010-20
5. Landgraeber S, von Knoch M, Löer F. et al. Extrinsic and intrinsic pathways of apoptosis in aseptic loosening after total hip replacement. Biomaterials. 2008;29:3444-3450
6. Degterev A, Boyce M, Yuan J. A decade of caspases. Oncogene. 2003;22:8543-67
7. Martin SJ, Green DR. Protease activation during apoptosis: death by a thousand cuts? Cell. 1995;82:349-52
8. Janeway CAJr. The T cell receptor as a multicomponent signalling machine: CD4/CD8 coreceptors and CD45 in T cell activation. Annu Rev Immunol. 1992;10:645-74
9. Weiss A, Littman DR. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263-74
10. Gao G, Jakobsen B. Molecular interactions of coreceptor CD8 and MHC class I: the molecular basis for functional coordination with the T-cell receptor. Immunol Today. 2000;21:630-6
11. Schäffer M, Barbul A. Lymphocyte function in wound healing and following injury. Br J Surg. 1998;85:444-60
12. Li X, Xiao BG, Xi JY. et al. Decrease of CD4(+)CD25(high)Foxp3(+) regulatory T cells and elevation of CD19(+)BAFF-R(+) B cells and soluble ICAM-1 in myasthenia gravis. Clin Immunol. 2008;126:180-8
13. Wesche-Soldato DE, Chung CS, Gregory SH. et al. CD8+ T cells promote inflammation and apoptosis in the liver after sepsis: role of Fas-FasL. Am J Pathol. 2007;171:87-96
14. Hodgkinson JP, Shelley P, Wroblewski BM. et al. The correlation between the roentgenographic appearance and operative findings at the bone-cement junction of the socket in Charnley low friction arthroplasties. Clin Orthop. 1988:105-9
15. Harris WH, McGann WA. Loosening of the femoral component after use of the medullary-plug cementing technique. Follow-up note with a minimum five-year follow-up. J Bone Joint Surg Am. 1986;68:1064-6
16. McAuley JP, Moore KD, Culpepper WJ 2nd. et al. Total hip arthroplasty with porous-coated prostheses fixed without cement in patients who are sixty-five years of age or older. J Bone Joint Surg Am. 1998;80:1648-55
17. Paprosky WG, Bradford MS, Younger TI. Classification of bone defects in failed prostheses. Chir Organi Mov. 1994;79:285-91
18. Sargeant A, Goswami T. Pathophysiological aspects of hip implants. J Surg Orthop Adv. 2006;15:111-2
19. Arora A, Song Y, Chun L. et al. The role of the TH1 and TH2 immune responses in loosening and osteolysis of cemented total hip replacements. J Biomed Mater Res A. 2003;64:693-7
20. Farber A, Chin R, Song Y. et al. Chronic antigen-specific immune-system activation may potentially be involved in the loosening of cemented acetabular components. J Biomed Mater Res. 2001;55:433-41
21. Baldwin L, Flanagan BF, McLaughlin PJ. et al. A study of tissue interface membranes from revision accord knee arthroplasty: the role of T lymphocytes. Biomaterials. 2002;23:3007-14
22. Li TF, Santavirta S, Waris V. et al. No lymphokines in T-cells around loosened hip prostheses. Acta Orthop Scand. 2001;72:241-7
23. Hercus B, Saeed S, Revell PA. Expression profile of T cell associated molecules in the interfacial tissue of aseptically loosened prosthetic joints. J Mater Sci Mater Med. 2002;13:1153-6
24. Schäffer M, Bongartz M, Hoffmann W. et al. MHC-class-II-deficiency impairs wound healing. J Surg Res. 2007;138:100-5
25. Barbul A, Breslin RJ, Woodyard JP. et al. The effect of in vivo T helper and T suppressor lymphocyte depletion on wound healing. Ann Surg. 1989;209:479-83
26. Grcević D, Lukić IK, Kovacić N. et al. Activated T lymphocytes suppress osteoclastogenesis by diverting early monocyte/macrophage progenitor lineage commitment towards dendritic cell differentiation through down-regulation of receptor activator of nuclear factor-kappaB and c-Fos. Clin Exp Immunol. 2006;146:146-58
27. Kim YG, Lee CK, Nah SS. et al. Human CD4+CD25+ regulatory T cells inhibit the differentiation of osteoclasts from peripheral blood mononuclear cells. Biochem Biophys Res Commun. 2007;357:1046-52
28. Kusmartsev S, Gabrilovich DI. STAT1 signaling regulates tumor-associated macrophage-mediated T cell deletion. J Immunol. 2005;174:4880-91
29. Dalton DK, Wittmer S. Nitric-oxide-dependent and independent mechanisms of protection from CNS inflammation during Th1-mediated autoimmunity: evidence from EAE in iNOS KO mice. J Neuroimmunol. 2005;160:110-21
Author contact
![]() Correspondence to: Stefan Landgraeber, M.D., Department of Orthopaedics, University of Duisburg-Essen, Pattbergstrasse 1-3, 45239 Essen, Germany. Phone +49-201-4089-2146; Fax +49-201-4089-2722; Email stlandyde
Correspondence to: Stefan Landgraeber, M.D., Department of Orthopaedics, University of Duisburg-Essen, Pattbergstrasse 1-3, 45239 Essen, Germany. Phone +49-201-4089-2146; Fax +49-201-4089-2722; Email stlandyde

 Global reach, higher impact
Global reach, higher impact