Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2009; 5(3):226-243. doi:10.7150/ijbs.5.226 This issue Cite
Review
A Curriculum Vitae of Teeth: Evolution, Generation, Regeneration
University of Athens, Faculty of Biology, Department of Cell Biology and Biophysics, Athens, Greece
Received 2008-11-19; Accepted 2009-2-21; Published 2009-2-24
Abstract
The ancestor of recent vertebrate teeth was a tooth-like structure on the outer body surface of jawless fishes. Over the course of 500,000,000 years of evolution, many of those structures migrated into the mouth cavity. In addition, the total number of teeth per dentition generally decreased and teeth morphological complexity increased. Teeth form mainly on the jaws within the mouth cavity through mutual, delicate interactions between dental epithelium and oral ectomesenchyme. These interactions involve spatially restricted expression of several, teeth-related genes and the secretion of various transcription and signaling factors. Congenital disturbances in tooth formation, acquired dental diseases and odontogenic tumors affect millions of people and rank human oral pathology as the second most frequent clinical problem. On the basis of substantial experimental evidence and advances in bioengineering, many scientists strongly believe that a deep knowledge of the evolutionary relationships and the cellular and molecular mechanisms regulating the morphogenesis of a given tooth in its natural position, in vivo, will be useful in the near future to prevent and treat teeth pathologies and malformations and for in vitro and in vivo teeth tissue regeneration.
Keywords: epithelial-mesenchymal interactions, teeth evolution, development and regeneration
1. Introduction
A huge amount of literature is devoted to the origin, evolution, organogenesis, pathology and therapy of teeth. There have been tremendous advances in recent years towards a better understanding of the regulation of teeth development [1, 2]. The immense interest in this subject is quite justified since, apart from the intrinsic scientific merit, teeth congenital abnormalities account for 20% of all inherited disorders, whereas, oral pathology occupies a leading position in the list of human diseases [3, 4].
Teeth are highly mineralized appendages found in the entrance of the alimentary canal of both invertebrates and vertebrates. They are associated mainly with prehension and processing of food, but they also frequently serve other functions, such as defense, display of dominance and phonetic articulation in humans. Generally, when speaking of teeth we usually refer to the dentition of vertebrates. Teeth with the basic microscopic anatomy similar to that of recent vertebrates first appeared at Ordovicium, approx. 460 million years ago. Some jawless fish developed superficial, dermal structures known as odontodes [5, 6] (Fig. 1). Those small tooth-like structures were located outside the mouth and served various functions, including protection, sensation and hydrodynamic advantage. The encroachment of odontodes into the oropharyngeal cavity created the buccal teeth, which covered the entire surface and later were localized to the jaw margins. Dietary habits and ecological adaptations have driven the teeth of vertebrates to acquire numerous anatomical forms and shapes, as represented by incisors, canines, premolars and molars [7].
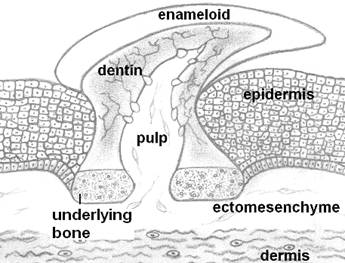
The main body of a tooth consists of a calcified tissue called the dentine, which is secreted by odontoblasts, cells of cranial neural crest (cnc) origin [8]. Dentine is composed of collagen, dentine sialophosphoprotein, dentine matrix protein and hydroxylapatite. Dentine surrounds the pulp, which is rich in fibroblast-like cells, blood vessels and nerves. The upper part of the dentine is usually covered by a layer of enamel, which is secreted by ameloblasts, oral epithelial cells. Enamel, the hardest tissue of the human body, is collagen-free. Its main proteins are amelogenin (90%), ameloblastin, enamelin and tuftelin. The root firmly supports the tooth within an alveolar socket by means of the periodontium. The visible part of a tooth in the oral cavity is referred to as the clinical crown [7]. Teeth are generated through highly orchestrated mutual inductive interactions between two major cell types: stomodeal ectoderm and cranial, neural crest-derived ectomesenchyme cells. In some animals the endodermal epithelium directly participates in teeth formation [9]. Morphological differences between individual teeth of a dentition arise mainly from differences in the spatiotemporal expression of several, odontogenic genes. These genes encode transcription factors that regulate the synthesis of various signaling factors [10]. These signaling factors mediate inductive interactions between the odontogenic tissue layers and affect cell multiplication, cell death and cytodifferentiation [11]. How these inductive interactions were modified during evolution to generate the numerous anatomical features of teeth is a major interest in evolutionary biology. Interestingly, genes and signaling factors playing leading roles in teeth morphogenesis are also involved in the development of many other organs in various animals [10, 12].
Odontodes, the ancestors of teeth, looked like placoid scales of recent sharks. Odontodes consisted of a dentine cone with a pulp cavity and covered by a hypermineralized tissue like enamel or enameloid. They were attached to the integument by a bony base.
The plethora of molecules involved [e.g., fibroblast growth factors (FGFs), bone morphogenetic proteins (BMPs), sonic hedgehog (SHH), wingless integrated (WNTs)] and the complexity of interactions (e.g., activation, inhibition, regulatory loops) inevitably lead with some frequency to homeostatic disorganization, which results in congenital abnormalities, such as tooth agenesis, which is the most commonly inherited disorder [3, 4]. Most human congenital teeth malformations are caused by mutations in developmentally regulated genes [6]. The fact that, an embryonic tooth bud can develop in vitro [13] indicates that the expression of teeth-related genes is not restricted only in vivo. Mutations that alter teeth act at many levels of control, i.e., the development of the embryonic bud, the morphogenesis of the bell stage, the production of enamel and dentin and the formation of the roots [1, 2]. The mechanisms of this genetic control are surely encoded at the molecular and submolecular levels. These mechanisms are beginning to be studied. The favored animal model for such studies is the common laboratory mouse, since teeth development in mice is similar to that of man. Additionally, the same set of genes functions in mouse as in man during teeth development; there are only minor differences in the expression patterns of these genes, and mutations in counterpart genes cause similar defective phenotypes (e.g., mouse Tabby and human EDA) [14].
Tooth damage and loss is quite frequent (>7%) and adversely affects mastication, articulation, facial esthetics and psychological health. Historically, surgeons have used several procedures to replace lost and repair damaged teeth, including tooth allotransplantation, autotransplantation, dental implants (metal or ceramics) and artificial dentures. Since these procedures sometimes have questionable therapeutic efficacy for various reasons (e.g., lack of biocompatibility between implants and human tissues, lack of osteointegration, damage to surrounding tissues, etc.), regenerative medicine promises to overcome these difficulties with biological procedures [15, 16]. New strategies have been designed in an attempt to achieve biological replacement of tooth tissues and whole teeth both in vivo and in vitro [13, 15, 16, 17].
Several excellent reviews on teeth have been recently published e.g., [1, 2, 6, 18, 19]; unfortunately, space limitations do not allow us to discuss and/or cite a significant proportion of them. In this review, we briefly present the current status of the entire field of odontology. We will review the most important aspects of teeth evolution, morphogenesis and biological restoration, from the pioneering studies to the most recent developments. We aimed to provide sufficient information for even the casual reader that may not be quite familiar with the subject.
2. Origin and Evolution of Teeth
2.1 The ancestors of teeth were dermal appendages
In a few organisms there is substantial evidence to suggest that teeth may have derived from both ectoderm and endoderm [20, 21]. In most cases, teeth evolved from scale-like epidermal structures, the odontodes, which “migrated” into the mouth after enough mutations. This process is visible in modern sharks, which have placoid scales on the skin that grade into the teeth on the jaws. In certain cases, however, dermal denticles did not transform into teeth and underwent independent evolution [22]. Natural selection has favored toothed organisms, which have a major advantage in their ability to capture and process food. Teeth can be classified into three types, based on where they are formed: jaw, mouth and pharyngeal. The close relationship between past and present teeth can be demonstrated by a phylogenetic analysis. Using this type of analysis, amelogenin appears to have been duplicated from SPARC (SPARC, secreted protein, acidic, rich in cysteine), 630,000,000 years ago, i.e., long before the Cambrian explosion [23, 24].
2.2. During evolution the number of teeth per dentition decreased
Variations in tooth number may represent an important factor for mammalian diversification. The evolutionary pathway from fish to reptiles to mammals is characterized by a reduction in the number of teeth (from polyodonty to oligodonty) and of their generations (from polyphyodonty to di- and/or monophyodonty) as well as an increase in morphological complexity of the teeth (from homodonty to heterodonty) [7, 25]. Some organisms (e.g., killer whales, rats, elephants) develop their dentition only once in their life; others (e.g., turtles, birds, toothless whales, anteaters) have lost their dentition and are characterized by adontia. Adontia in many organisms is considered to be secondary, since the embryo possesses tooth germs that undergo apoptosis before birth [26, 27]. Region-specific tooth loss has been a common trend in vertebrate evolution. Some organisms retained a high number of teeth, however: Opossum (50 teeth), sirenoids (possess 44 molars) and some dolphins (bearing more than 200 relatively similar teeth, having thus lost heterodonty and returned to homodonty). Interestingly, some teeth that were lost during evolution reappeared in an atavistic sense [28], thus violating the “law” of irreversibility in evolution. If we could understand the mechanism of spontaneous re-acquisition of lost properties, we might be able to apply this knowledge to the clinical, biological restoration of lost teeth. Along those lines, understanding the rules of polyphyodonty will surely support tooth regenerative efforts. How and why evolutionary tooth loss occurs is not known, but several interesting hypotheses have been proposed. For example, there could be a loss of a tooth-type-specific initiation message, attenuation of the inductive and/or inhibitory signal or a reduction in the concentration of required proteins. In support of this last idea, the lack of canines and premolars in the mouse upper diastema has been attributed to the weak expression of the PAX9 gene [29, 30].
Changes in the number and morphology of teeth may reflect a significant factor in the generation of new species in mammals. The most common feature is the loss of various teeth, perhaps as a result of a mutation in tooth-related genes. For example, rodents lack lateral incisors, canines and premolars. Sheep have lost their upper incisors and the canines. An analysis of mutant mice phenotypes has clearly indicated that specific mutations (e.g., GLI2-/-,GLΙ3+/-) cause phenotypes that resemble several ungulates that lack all upper incisors [3, 29]. It is worth noting that in placental mammals teeth tend to disappear over the course of evolution in an order that is opposite the order of their appearance during eruption [7, 9]. A reaction/diffusion model of morphogenesis has been used to explain this phenomenon. According to this model, repeated structures (e.g., vertebrae, phalanges, feathers, color patterns, teeth) arise as a result of the coordination of two molecules, an activator and an inhibitor. Two well known examples of such interacting molecules are FGF8/BMP4 [31] and ectodin/BMP4 [32]. Teeth located at a distance from the center of the morphogenetic field tend to disappear due to field attenuation [33].
2.3. Evolution favored an increase in teeth complexity
Diet and mastication are regarded as central factors in teeth evolution. There is a strong correlation between teeth form (e.g., cardiform, villiform, incisor, canine, molariform) and feeding habits. During evolution, mammals, which originated from reptile-like ancestors, (Diapsida), developed in each side of their skull two openings (temporal fenestrae) behind the orbit that are still present in a modified form in modern mammals. This opening has been used as a rigid place for the attachment of powerful masticatory muscles. This evolutionary event allowed a much more efficient exploitation of the food caloric energy needed to support high levels of activity. Cynodonts (more advanced, mammal-like reptiles) changed their dentition from one designed for catching and holding prey before swallowing it whole to one designed for better mastication of food, with specialized, molar-like teeth endowed with randomly placed enameloid pustules [34]. The most important anatomic and functional feature of the masticatory surface of an erupted tooth is the cusps [35]. Cusp number, morphology, topology and orientation are species-specific; these features also differ between teeth of the same mammal. Those disparities are due to differential, spatiotemporal cell multiplication and programmed cell death of the inner enamel epithelium cells during embryonic and post-embryonic development [11].
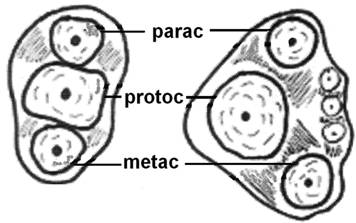
The evolution of the mammalian jaw and teeth created occlusal surfaces that are adequate for a great variety of foods. For example, Triconodont organisms were endowed with teeth bearing three major cusps in a (more or less) straight line (Fig. 2) and other smaller cusps on an external, rounded cingulum. This arrangement increases the ability of the teeth to crush and grind food, thus giving rise to mastication. In Symmetrodont organisms (extinct mammals), the central cusp was separated from the other two outer cusps so that a triangle was formed on the occlusal surface of the upper molars; later, comparable, geometrically complementary structures were formed on the occlusal surface of the lower molars too, resulting in a dramatic increase in the masticatory efficiency of the molars [34, 35].
Evolution of the tribosphenic teeth. Diagram explaining the evolution of the in-straight-line cusps of upper molars (left), to shaping the triangle (right) aiming to better grinding of the food. (parac=paracone, protoc=protocone and metac=metacone).
2.4. Hypothetical models seek to unravel the evolution of the dentitions
Evolutionary biology has not yet provided an explanation of the evolution of teeth and cusps. Two controversial hypothetical models, the field model [36] and the clone model [37], have been proposed. The field model postulates that heterodonty is due to graded values of hypothetical morphogens [36, 38]. In this model, each dental quadrant is divided into three subfields: incisors (key tooth, 1st, mesial incisor), canines, and molariforms (key tooth, 1st, mesial molar). Each tooth develops according to its position in the field. Teeth belonging to the same field have graded similarities according to the distance from the field origin: the third human molars, which generally develop later but disappear earlier than the other molars, are the most variable since they are at a position in which the morphogenetic field is weak. This model further suggests that multicuspid teeth of mammals derive their evolutionary origin from the union of many single reptilian tooth germs [39]. It is speculated that the signals that were used in our ancestors to develop each tooth separately were combined in modern species to create a single tooth with a more complicated morphology. Each tooth cusp evolved independently under specific genetic control, and the same sets of genes function for all cusps; thus, a reaction-diffusion mechanism may underlie the activation of genes at specific locations and times to create the crown patterns [5, 39].
The clone model states that each tooth is derived from specific ectomesenchymal cells that are instructed to form a tooth of a given shape [37]. The clone model argues that even the most complicated mammalian molars arise from the differentiation of only one tooth blastema of one conical reptilian tooth and that each cusp was formed from a clone of a cnc cell, perhaps from a single committed cell [40]. After producing a “stem precursor,” the clone grows forward or backward, gradually losing its shape potential. Indeed, the patterns of mouse molars are simplified from first to third molar. The linear arrangement of teeth at the jaw margins may result from the narrowness of the tooth-forming area. One hypothesis states that the widespread periodicity in the pattern results from each tooth blastema acting either as a source of an inhibitor that diffuses and inhibits the development of adjacent teeth or as a sink that consumes all available substances in the vicinity [1, 5]. It is thought that the inhibitory fields are short-lived where teeth develop in close proximity or even unite [22].
In support of the aforementioned two models, the initiation of each different tooth class (e.g., incisors, molars) in different parts of the oral cavity (distal, proximal) has been attributed to local cells responding to specific groups of homeobox genes [41]. Specifically, the proximal dental epithelium secretes FGF8, which induces the expression of the PAX9, BARX1, DLX1 and DLX2 homeobox genes in the proximal mesenchyme; these genes direct the formation of molariform teeth. BMP4 is secreted by the distal epithelium and induces in the distal mesenchyme the expression of genes (MSX1, MSX2 and ALX4) that direct incisor formation. The orderly expression and combination of these gene products direct the extent and the location of the various morphogenetic fields within the developing dentition and thus the tooth type. The size of the field is influenced by the expression of a signaling molecule, ectodysplasin (encoded by EDA, a member of the tumor necrosis factor -TNF family of ligands), and/or its receptors. High EDA activity means supernumerary teeth, surprisingly only premolar-like, appearing distal to the first molar; low EDA activity leads to missing molars, as presented by the Tabby and Downless mouse phenotypes [42]. Those three models (field, clone, homeobox code) have been elegantly unified into a single model to explain dental patterning [43]; this model encompasses the clones of the migrating cnc cells, the homeobox genes of the mesenchymal cells and the signaling molecules secreted by the dental epithelium. These models are based on strong theoretical and clinical data [44] and have been recently analyzed and supported, indicating that clearly there is a multifactorial etiology in the development of the dentition [2, 45].
3. Teeth Development, Genetics and Diseases
3.1. Dental epithelium interacts with cranial neural crest cells to form teeth
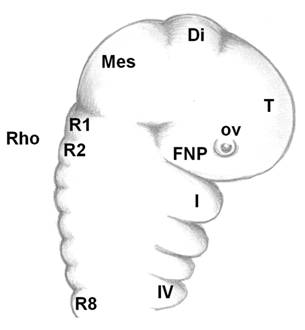
As the neural tube forms, the dorsal ectoderm synthesizes the signaling protein WNT6; whereas, in the neural plate, members of the BMPs family are produced. Where these two embryonic tissues intersect, active cell multiplication occurs in both the ectoderm and neuroderm. These multiplying cells express the FOXD3 gene, which instructs these cells to form two dorsal, longitudinal rows of ectomesenchyme on both sides of the neural tube to create a transient population of highly nomadic cells, the neural crest cells. The embryonic brain is subdivided into the forebrain, midbrain and hindbrain (rhombencephalon), which is further subdivided into eight rhombomeres (R1-R8, Fig. 3). The archenteron continues to develop in a posterior to anterior direction and participates in pharyngeal arch formation. The pharyngeal arches contain a central blood vessel, the aortic arch, surrounded by paraxial mesoderm. This core is enveloped by a sheet of cnc cells; this cells, in their turn, are covered by continuous sheets of epidermal ectoderm and internal endoderm. The first pharyngeal arch forms the upper and the lower jaws. Massive layers of the oropharyngeal epithelium (stomodeum) migrate over and overlap the pharyngeal arches; odontogenic cells from the neural crest have already migrated and populated the region by this time. Although oral teeth are thought to arise exclusively from the ectoderm, pharyngeal teeth may also be derived from the endoderm epithelium [9, 20, 21]. Cranial neural crest (cnc) cells, although of ectodermal origin, undergo “mesenchymalization,” a process justifying their designation as ectomesenchymal cells [46]. Interestingly, before the onset of their migration, the cnc cells express Hox genes; after arrival at their destination places (first pharyngeal arch), they do not express Hox genes. This fact suggests that the acquired identity is maintained [46]. Some of the cnc cells from the forebrain region migrate ventrally between the surface ectoderm and local mesoderm and establish the frontonasal prominence, where upper incisors form. Cranial neural crest cells from the midbrain and the three first rhombomeres populate the first pharyngeal arch, where all other teeth develop on the rest of the maxilla and the whole mandible. The homeobox genes LHX6 and LHX7 appear to have critical roles in directing the cnc cells to their correct destinations [47]. There, the cnc cells multiply actively to produce the main body of the pharyngeal arch. Upon arrival and arrangement of the cnc cells, teeth develop by multiple, reciprocal, inductive molecular interactions between the dental epithelium (perhaps with cranial paraxial mesoderm and endoderm too) and the underlying ectomesenchyme in the maxilla and mandible (Fig. 4a) [8].
Tissue recombination experiments performed between ED 8.0-11.5 have shown that the earliest odontogenic potential resides in the dental epithelium rather than the cnc cells and that the patterning information for tooth initiation and type is present in the oral ectoderm prior to epithelial thickening [48]. Later (ED 12), this potential is lost from the epithelium and acquired by the ectomesenchymal cells, which in turn regulate differentiation of the epithelial cells. This acquisition by ectomesenchymal cells was demonstrated when mouse embryonic molar mesenchyme was combined with chick embryonic epithelium and found to result in the formation of tooth germs [48]. The mutual, accurate, spatiotemporal orchestration of the epithelial/mesenchymal communication is significant, as the loss of these interactions results in teeth abnormalities, as in the absence of teeth in birds and in the lower jaw of anurans.
The cnc cells give rise (a little later) to various tooth cell types (odontoblasts, which produce dentine; cementoblast, which secrete cementum to cover the root dentine; osteoblasts, which participate in the formation of dental alveoli; and fibroblasts, which synthesize collagen for the periodontic ligament). Cells from the oral epithelium remain and differentiate locally into enamel-producing ameloblasts [49].
Linear, simplified sagittal view of the cephalic part of a vertebrate embryo. Telencephalon (T)+diencephalon (Di)=forebrain. Mesencephalon (Mes=midbrain). Οcular vesicle (ο.v.). FNP=frontonasal prominence. Rhombencephalon (Rho=hindbrain). Rhombomeres (R1-R8). The four first (Ι-IV) branchial arches. Branchial arch I separates into upper and lower jaws (maxilla and mandible, respectively).
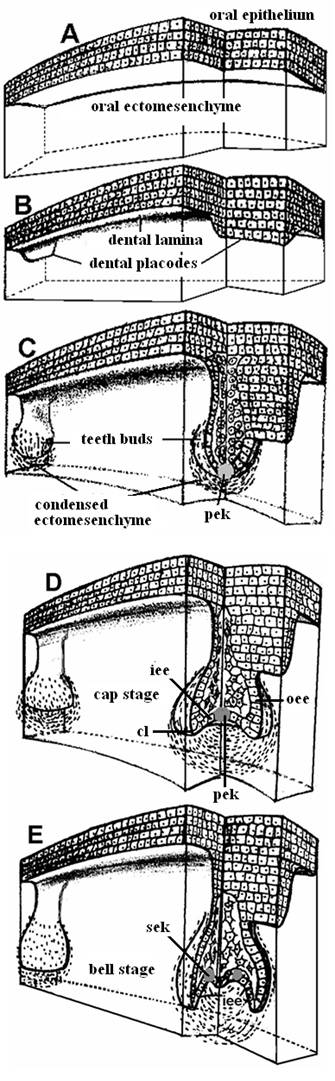
Stages in teeth development: (A) Pre-patterned oral ectoderm is in close contact with cranial, neural crest ectomesenchyme. At this stage (ED 10) the odontogenic potential resides in the epithelium. (B) The epithelial cells secrete specific signals in different areas, proliferate and form a band of epithelial tissue, the dental lamina and the dental placodes. (C) At the sites of the dental placodes the epithelial cells proliferate and intrude within the mesenchyme forming the tooth buds. At this developmental stage the odontogenic potential is lost form the epithelium and granted to the ectomesenchyme. (D) The bud folds in and acquires initially the form of an inverted cap and later the form of a bell (E). [cl = cervical loop, iee = inner enamel epithelium, oee = outer enamel epithelium, pek = primary enamel knot, sek = secondary enamel knots].
3.2. FGF8 and BMP4 are master molecules of odontogenesis
The complicated, sequential, reciprocal interactions between the dental epithelium and dental ectomesenchyme that are required for tooth formation are mediated by the spatiotemporal expression of tooth-related genes (approx. 300) and the secretion of growth and transcription factors (approx. 100) that are reiteratively used in regulatory loops [18]. Epithelial cells secrete specific sets of growth factors [e.g., FGFs (FGF3, FGF4, FGF8, FGF10), BMPs (BMP2, BMP4)] and signaling molecules [SHH and WNTs (WNT3, WNT7, WNT10)] some of which regionalize the oral ectoderm (FGF8: molar=proximal=posterior domain, BMP4: incisor=distal=anterior=mesial domain) before the arrival of the cranial neural crest cells. The stimulus dividing the oral ectoderm into proximal and distal domains is of endoderm origin. The new qualities of ectodermal domains greatly influence the fate determination of the cnc cells that migrate and populate the first branchial arch. The distinction between proximal and distal domains is achieved by cells responding according to their proximity to the source of the signal. BMPs and FGFs are expressed in both the ectoderm and ectomesenchyme, whereas SHH and WNTs are expressed only in the ectoderm. Proteins that are secreted in one germ layer may diffuse to other layers, however. An important regulatory function of BMP4 is to inhibit FGF8 secretion. It has been suggested that BMP4 acts antagonistically with FGF8 to produce localized sites of ectomesenchyme that express PAX9 and specify where teeth will develop [31, 50]. Particularly BMPs have been suggested to play a role in the formation of periodic patterning by inhibiting spreading of FGF signaling. A lack of BMP4 signal results in the down regulation of MSX1 expression and distal extension of BARX1 into the incisor region [31]. Insufficiency in BMP signaling (e.g., through loss of BMP receptors or overexpression of BMP inhibitors) results in various defects in different cusps and teeth, suggesting differential requirements for the level of BMP signaling [51]. SHH is secreted early in the whole presumptive dental epithelium and specifies the sites of oral ectoderm proliferation, invagination into the ectomesenchyme and tooth initiation [52, 53]. The expected type of tooth (e.g., molar) can be changed to another type (e.g., incisor) by both up regulating the expression of incisor determinants (e.g., MSX1) and down regulating molar determinants (e.g., BARX1) [31, 51]. In general, the experimental modulation of various molecules can alter homeobox gene expression in competent tissues, resulting in altered teeth number, size and shape. For example, the addition of noggin (a neutralizer of BMPs) to early (ED 9-10) mouse mandibular arches results in the transformation of incisors to molars [54].
Regardless of its location and its type on the jaw (anterior: incisor, canine; posterior: premolar, molar) the development of each tooth passes through the same, four morphological stages: initiation, bud or blastema, cap and bell, corresponding to determination of tooth type/size/number, followed by morphogenesis, differentiation and mineralization, respectively (Fig. 4).
3.3. The initiation stage of tooth development is characterized by the formation of the dental lamina
The initiation of tooth development begins at the end of the fifth week of human gestation [10th embryonic day (ED 10) of mouse development]. During the “initiation stage,” we understand the molecular and cellular processes that determine the exact type, position and orientation of each tooth on the developing jaws. The cooperation of the WNT7b and SHH genes delimitates oral (non-odontogenic) from dental (odontogenic) epithelium and thereby restricts SHH expression to the dental epithelium [55]. Then, (ED 10-11) cells of the dental epithelium start proliferating as a narrow, horseshoe-like ribbon, the dental lamina, whose shape reflects the future (ED 11) dental arches [5] (Fig. 4b). The first morphological indication of tooth-family development on the dental lamina is the formation of ectodermal placodes (ED 11.5), i.e., embryonic epithelial thickenings preceding the local appearance of an ectodermal organ. Research has identified FGFs and WNTs as activators and BMPs as inhibitors of placode formation [56]. The signals providing the relevant positional information are not yet known in detail; however, at ED 10, the expression of PAX9 is induced in the mouse ectomesenchyme by epithelial FGF8 [31]. The simultaneous presence of BMP2 and BMP4 in the vicinity inhibits PAX9 expression, and teeth do not form [50]. However, PAX9 double mutant mice progress beyond initiation to the bud stage, suggesting that there may be other initiation-specific genes, such as PITX2 and SHH [50]. Other tooth-related genes that are expressed at the initiation stage include MSX1, MSX2, DLX1, DLX2 and LEF1, but tooth development is arrested at the initiation stage only when both MSX1 and MSX2 or both DLX1 and DLX2 genes are inactivated [3, 29]. It is worth noting that knockout mutations of most teeth-related genes do not cause developmental arrest at a very early stage and cannot prevent formation of the dental lamina. Therefore, a safeguard mechanism must exist, probably gene redundancy. An example of this phenomenon is the persistence of the dental lamina in birds although their teeth were lost over 60 million years ago [57].
3.4. The dental epithelium proliferates, invaginates into the ectomesenchyme and forms the tooth bud
Dental placodes secrete molecules from all four growth and transcription factor families (BMPs, FGFs, SHH and WNTs), which induce the expression of many genes (e.g., PAX9, MSX1/2, RUNX2, BMPs, FGFs, Activin, LEF1, DLX1, BARX1, LHX6/7, GLI1/2/3) in the mesenchyme (Figure 4). Specifically, epithelial BMP4 (via MSX1) induces the production of mesenchymal BMP4, whereas epithelial FGF8 induces mesenchymal activin βA. BMPs and FGFs activate MSX1, whereas FGFs induce the expression of PAX9 and RUNX2 [57, 58, 59]. At 20 positions of the human embryo dental lamina [7th - 9th week of human gestation and at corresponding positions of the mouse embryo (ED 11-11.5)], the epithelial cells, under the influence of BMP4 and activin βΑ, start proliferating (early bud stage, ED 12.5) and intrude within the mesenchyme in a cylinder-like structure with a bulb-like bud at the end (ED 13.5). The bud stage is characterized by the appearance of a tooth blastema without a clear arrangement of cells (Fig. 4c). Then, ectomesenchymal cells proliferate and accumulate around each epithelial bud. The expression of PAX9 is necessary for mesenchymal condensation. Interestingly, in PAX9 mutants MSX1 expression is normal before ED 12, whereas, PAX9 knockouts at ED 13 do not express MSX1. A little later, the innermost cells of the epithelial, gland-like bud acquire a star-like shape and start synthesizing glycosaminoglycanes. Water is also drawn in between the cells to stretch them apart. This internal part of the tooth bud contains the stellate reticulum and the intermediate layer, which affect the folding of the inner enamel epithelium. Some cells within the stellate reticulum of continuously growing teeth (e.g., mice incisors) have been identified as putative stem cells [17]. During the bud stage of tooth development, the odontogenic potential is lost from the epithelium (around ED 11.5-12) and gained by the ectomesenchyme [48]. This interplay involves a complicated spatiotemporal expression and inhibition of several genes. Unlike in the initiation stage, in the early blastema stage, several mutations in key genes (e.g., LEF1, MSX1, PAX9) have been reported to affect odontogenesis in a syndromic fashion [3, 4, 29, 55, 56].
3.5. The tooth bud transforms into a cap by differential proliferation and infolding of the epithelium
Mesenchymal cells secrete various extracellular molecules, such as tenascin and syndecan. These extracellular molecules bind and therefore increase the local concentration of many growth factors. A differential concentration induces differential multiplication in the epithelial layer and transformation of the semi-spherical tooth bud into a semi-pyramidal structure that is continuous with the dental lamina at the tip of the cone. This event initiates tooth morphogenesis, as the epithelial base of the cone marks the future site of the tooth crown. BMP4 is a good candidate for a mesenchymal signal that induces the transition from the bud to the cap stage. If BMP4 is absent from the mesenchyme, the dental-specific genes LEF1, MSX1 and PAX9 are not expressed, and tooth development is arrested at the bud stage [4, 27, 60]. At ED 12, WNT and BMP4 induce a histologically distinct epithelial mass, the enamel knot, at the center of the base of this pyramid [61]. A specific feature of the enamel knot cells is the absence of cell division. Protein p21, a well known inhibitor of cyclin-dependent G1 kinases, does not allow enamel knot cells to enter S phase and facilitates their elimination by apoptosis [62]. The prevailing notion is that the enamel knot is a transient organizer that conveys morphogenetic information to adjacent cells and disappears by apoptosis after performing its function. The cells of the enamel knot produce mitogenic factors (mainly FGFs) that diffuse and induce spatiotemporal-specific epithelial cell proliferation. Knot cells express p21 and FGF4 under the influence of BMP4 and WNT, respectively, along with many (approx. 50) other signals [63]. BMP4 is a key molecule in the induction of apoptosis in many systems, such as rhombomers, digits and apical ectodermal ridge [64]. The apoptotic removal of the primary enamel knot cells starts at the end of the cap stage (ED 15); the knot is lost by the bell stage (ED 16). FGF4 protects neighboring epithelial and mesenchymal cells from apoptosis. In vitro, however, the inhibition of apoptosis does not influence teeth and cusp morphogenesis [65]. The expanding epithelium folds inside the core of the bud in an anterior to posterior direction. The whole structure acquires the form of an overturned cap (Fig. 4d). The rim of this structure constitutes the cervical loop, which grows rapidly downwards [66]. The epithelium inside the cap is characterized as the inner enamel epithelium (iee). The outer part of the cap is covered by the outer enamel epithelium (oee). Between these two epithelial sheets are the vacuolized cells of the stellate reticulum and an intermediate cell layer. The structure, including the iee, oee, stellate reticulum and the intermediate layer, is known as the enamel or dental organ. The mesenchyme that is condensed “under” the iee and between the cervical loop is the dental papilla (future dental pulp), whereas the mesenchyme that is condensed around the dental papilla and dental organ is called the dental follicle and gives rise to cementoblasts, osteoblasts and fibroblasts [7, 49].
3.6. Bell formation
Since cusp position and height are tooth- and species-specific, their correct spacing and size must be regulated by an accurate control mechanism. In multicuspid mouse teeth, the primary enamel knot induces (at various time-points, e.g., ED 15, ED 15.5, ED 16) the formation of secondary enamel knots in genetically specified parts of the inner enamel epithelium [67]. The key signaling molecule for the formation of a knot is BMP4. The formation of the first secondary knot at ED 15 marks the beginning of the bell stage of tooth development. Later, the second, third and fourth secondary knots form. The iee continues infolding according to the organizing signals that emanate from the primary and secondary knots, displaces the stellate reticulum and acquires the form of a bell (ED 16.5) (Fig. 4e). In contrast to epithelium no distinct pattern in cell proliferation in the dental mesenchyme has been observed. Knots, being at the tips of the epithelial infolding, coordinate the formation and determine the position and the height of the corresponding cusps on the crown [68]. The enamel organ is clearly separated from the dental papilla, the cusps start to form and the crown increases. The height of each cusp seems to depend on the timing of the appearance of the corresponding secondary enamel knot. The development of a tooth always starts from the highest secondary cusp, regardless of the phylogenetic age of the cusp. For example, the phylogenetically youngest but fairly large metacone appears before the evolutionarily oldest but smaller paracone [67, 68]. Crown morphogenesis and cytodifferentiation occur during the bell stage [69, 70]. During this stage, the cells differentiate in situ, and the crown takes its final shape. The mesenchymal cells at the border of the dental pulp attach to the basement membrane of the iee, take a cylindrical form and transform into odontoblasts that secret predentine. Immediately after predentine deposition, the cells of the iee take a columnar shape and differentiate into ameloblasts that start synthesizing and depositing pre-enamel prisms. These two substances, secreted by two different cell populations and mineralized by different mechanisms, produce the actual tooth matrix by apposition of hydroxylapatite crystals [71]. The cells of the intermediate layer considerably help the process of enamel formation and, after tooth eruption, transform into the basal layer of the epithelial attachment by regaining the ability to undergo mitosis. At this stage, the dental lamina disintegrates, leaving the tooth “free” from the epithelium. Finally, the pulp and enamel organ are “enclosed” in a form of condensed mesenchyme, which constitutes the dental follicle. Cells of this layer differentiate into cementoblasts, osteoblasts and fibroblasts [7].
To date, more than 50 genes have been identified as being actively transcribed in the enamel knots. Many of those genes produce growth and signaling molecules (BMPs, FGFs, SHH, WNTs, etc.). These genes are expressed as nested patterns around the enamel knots, and these patterns demarcate the future pattern of the cusps [38]. The spreading of differentiation from the knots downwards is correlated with a gradual spreading of the expression of many knot genes as well. The orderly appearance of various knots in the same epithelium suggests that there is an accurate mechanism that guarantees the exact position, spacing and timing of the appearance of secondary knots. This mechanism might include molecules emanating from each forming knot and inhibiting the appearance of an inappropriately positioned knot [18]. BMPs, SHH, FGFs, as well as ectodin, follistatin and noggin (widely distributed BMP inhibitors) play primary roles in this inhibitory reaction, regulating (particularly ectodin) the distance between the forming cusps [51]. The same set of molecules seems to play comparable roles in other organs as well [56]. It must be stressed that in multicuspid teeth the primary enamel knot is not associated with a cusp; only secondary knots are. Like primary knots, secondary knots express FGF4, do not proliferate and are removed apoptotically.
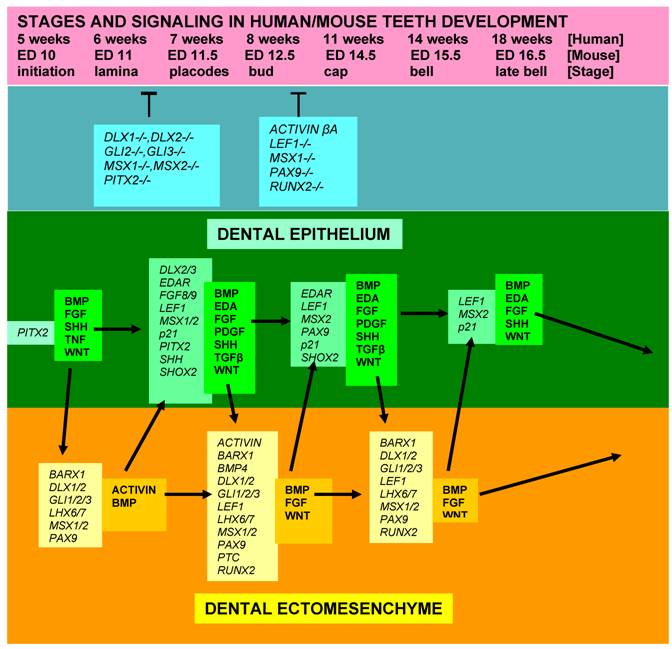
In conclusion, most of the signaling molecules that regulate epithelial-ectomesenchymal interactions during tooth development are members of the TGF (transforming growth factors), FGF, BMP, Hedgehog, EDA, Notch and WNT families. The ectomesenchymal expression of MSX1 and PAX9 remains strong from the initiation stage through the bell stage. The closely overlapping expression patterns of PAX9 and Msx1 genes are consistent with a role in epithelial-mesenchymal interactions. Their activity is down regulated at ED16 (Fig. 5).
Diagram showing stages of teeth development, a few genetic factors affecting phenotypes, and some signalling molecules and growth factors expressed in the epithelial and mesenchymal components of developing teeth. [While most teeth-related genes exhibit, in general, similar expression patterns in the developing teeth in both humans and mice, several genes, including MSX1, FGF8, PAX9, and SHOX2, show some slightly different expression profiles]. [Arrows = activation; (T) = inhibition at the indicated stages; italic fond = genes; regular, bold fond = growth factors].
3.7. Teeth acquire their final form and shape early in development
Odontoblasts produce and excrete collagen I, which is a major component of predentine. Predentine is calcified by hydroxylapatite to dentine in the presence of high concentrations of tenascin and alkaline phosphatase. After excretion and apposition of dentine, ameloblasts start producing pre-enamel. As predentine and pre-enamel are deposited, they are mineralized by hydroxylapatite to dentine and enamel, respectively [72]. Enamel constitutes the outermost covering of the adult amphibian, reptilian and mammalian teeth. Although the layer of ameloblasts is continuous, in many animals (e.g., mouse, horse) enamel-free areas are present on the cusp tips at the time of eruption [70]. Apposition of the mineralized tissues starts at the tip of each cusp and continues in successive layers, so that the crown of the teeth is completely formed. After performing their function, ameloblasts undergo apoptosis before tooth eruption. Human enamel cannot be made anew, and injuries to it are permanent. In contrast, dentine continues to be made throughout life.
Pulp cells that contact the HERS (Hertwig's epithelial root sheath) are instructed to differentiate into odontoblasts, which form the root dentine. Then, the HERS disintegrates and dental follicle cells differentiate into cementoblasts. Apposition of root dentin and cementum continues after the eruption of the teeth. Root formation in humans is usually completed 2 - 3 years after the tooth erupts [73]. The periodontal ligament suspends each tooth in its alveolar socket to allow limited movements of the teeth and prevent squeezing of apical blood vessels and nerve fibers during chewing. Unlike many other human organs that grow as long as the body grows, teeth cannot continue to grow once formed. Mammalian teeth change their shape only as a result of wear and tear, since the form and the shape of each tooth is completed before its eruption. Consequently, the variety in the number, dimensions, distances of cusps, lophs, basins, crests, etc. in molars is due only to differences that arise during tooth ontogenesis. Several vertebrates are exceptions to this rule and able to change the shape of their replacement teeth according to feeding conditions during their lifetime [74]. In many vertebrates, tooth number increases throughout life. In others, tooth number is stable and characteristic of a particular species [7].
3.8. Numerous genes and mutations affect teeth and dentition phenotypes
Teeth morphology exhibits high heritability and is regulated by many genes. Analyses of gene expression patterns in dental epithelium and mesenchyme have revealed association of numerous genes with teeth development. Whether a normal dentition appears in the mouth at all or with a defective phenotype depends largely on the expression of an array of teeth-related genes [2, 3, 4, 10, 29]. Despite our extensive knowledge of the spatiotemporal expression of specific genes during teeth formation, no single gene has yet been directly connected experimentally or naturally with ontogenesis or the lack of a specific tooth [3, 4, 29]. Developmental defects usually occur in teeth tissues as a result of mutations in genes encoding signaling molecules and transcription factors. These developmental defects may appear alone (isolated, as represented by hypodontia in PAX9 mutants) or in combination with defects in other tissues (syndromic, as displayed by hypodontia due to mutations in many genes, such as MSX1, AXIN2, EDA, PITX2 and SHH) [14, 58, 75]. The heredity of teeth features obeys universal genetic rules; however, very few features are inherited in a Mendelian manner; most dental polymorphies are controlled by more than 300 genes [29]. Some of them, such as EDA (ectodysplasin), HED (ectodermal dysplasia), MSX1 and PAX9, play key roles in determining dentition phenotype [76].
Mutations in many of those genes are known to cause dental defects in various mammals, including humans. MSX1 and PAX9 are among the best studied mesenchymal transcription factors. They are considered to be of primary importance in teeth development, given their expression pattern in teeth tissues during development, the phenotype of mice with knockout mutations in these genes and by their association with tooth agenesis [77, 78, 79]. Mutations in the PAX9 gene result in partial or total adontia, although PAX9 mutant mice have tooth germs in the correct positions. MSX1 mutations predominantly affect the second and third molars. Mutants in RUNX2 cause supernumerary teeth, whereas AMEL, DLX3 and ENAM mutations are associated with the well known amelogenesis imperfecta [14]. There is evidence [2, 29, 50] that specific mutations in mice generally affect all teeth of the same family (e.g., molars); this fact is not true in humans. Teeth-related genes of each family seem to act synergistically, meaning that teeth development is arrested at the initiation stage only if both gene members are mutated (e.g., MSX1+MSX2 or DLX1+DLX2). The extent to which genes influence teeth patterning is clearly demonstrated by the high proportion (20%) of people affected by the hereditary condition of tooth agenesis. In addition, whereas non-syndromic tooth agenesis is typically inherited in an autosomal dominant manner, similar phenotypes have also been reported to be inherited in an autosomal recessive or X-linked manner [4]. Studies of MSX1 and PAX9 mutations in human families revealed extensive variation in tooth number among affected individuals, reflecting co-expression of various, cryptic genetic differences that lead to phenotypic variation in dental patterning. These findings support models that consider teeth initiation and morphogenesis to be influenced by numerous genetic, epigenetic and environmental factors [78, 79].
The ostensible symmetry and identity displayed by corresponding upper and lower jaw teeth might suggest that the same fundamental genetic circuits regulate the generation of those teeth. Detailed morphological analysis of homologous teeth in the two jaws has revealed significant differences in form and shape, however. It is not yet known how and why the ectomesenchymal cells located in the developing mandible respond differently to ectodermal signaling than those located in the developing maxilla. Mice that have a doubly mutated activin βΑ gene have maxillary molars that continue to grow normally even though all other teeth stop developing at the tooth bud stage. Conversely, double DLX1/2 mice mutants possess all other teeth except maxillary molars. The presence of mandibular molars in these mutants is attributed to the expression of DLX5/6 only in the mandibular molar region. Differential expression of noggin in the dental epithelium displays different effect on maxillary and mandibular third molars [51]. Therefore, different genetic pathways may exist in upper and lower jaw molar specification [63]. Additionally, human upper molars possess three roots, whereas lower molars bear only two. Evolutionary studies have also indicated that teeth at corresponding positions of the upper and lower jaws may have evolved independently [80]. Lastly, in the lower jaw of the mouse there is no trace of tooth initiation in the diastema, but in the upper jaw diastemal tooth rudiments progress to the bud stage before arresting in development [26, 27].
3.9. Teeth pathology and clinical treatment
The major acquired pathological conditions that affect teeth concern the erosion of non-living elements (enamel and dentin), damage to living tissues (pulp and periodontium) and the loss of whole teeth. Teeth loss is a problem of great magnitude since approx. 35% of the world population is edentulous by age 65. Although the human crown is protected externally by the considerably hard and durable enamel, and the human root is impacted into the alveolar bone, teeth undergo extensive wear and tear. The mouth is the major gate of entry into the human body and takes in a lot of foreign, harmful substances. Many of them (e.g., alcohol, acids, aromatic carbohydrates, bacteria, etc.) cause significant damages inside the buccal cavity; so much so that stomatopathology is classified fairly systematically as the second frequent human pathology, following general viral infections. Even saliva, depending on its pH and ionic strength, can contribute to the deterioration of enamel integrity. Carious lesions top the list of dental pathologies; injury and fracture are not infrequent. In many cases, bacteria can colonize the space between the alveolar bone and teeth and destroy periodontal tissues, resulting in teeth loss if left untreated [81].
Human ameloblasts die before tooth eruption; therefore, there is no possibility for secondary physiological enamel production and restoration/regeneration of the worn out enamel layer. As with other crystalline structures, worn out enamel sites undergo spontaneous mild remineralization by incorporating calcium and phosphate ions that are present in the mouth. This process occurs at a very slow rate, however, and is not able to compensate for the enamel loss due to bacterial activity and acids. Fluorides augment “natural” enamel remineralization. In contrast to ameloblasts, odontoblasts remain active throughout life. After complete formation of the root, they very slowly lay down secondary dentine; consequently, as the animal ages, the pulp cavity of the teeth continually grows smaller. Of high clinical interest is the fact that another type, the tertiary dentine, is produced in response to certain stimuli, such as tooth decay, attrition and incidental or intentional trauma. There are two types of tertiary dentine; reactionary, laid down by the living odontoblasts, and restorative, produced by progenitor pulp cells [82, 83, 84]. The list of teeth diseases is considerably supplemented by the frequent congenital abnormalities, such as adontia, amelogenesis imperfecta, supernumerary teeth, etc., [3, 4, 75].
The classical therapeutic approaches include extrinsic dental interventions, such as tooth filling, tooth extraction and implantation of an inert, artificial (metal, ceramic) substitute. Those interventions are not always free of unpleasant and/or adverse side-effects [13, 85, 86]. In addition, those approaches fail quite frequently and have considerable costs. Leading causes of the long-term therapeutic inefficacy of the usual dental interventions include the lack of mechanical and immunological properties of the periodontium and the crevicular sulcus, respectively, the incompatibility between dental tissues and non-biological substitutes and the insufficiency of host bone in the jaw to accommodate the implant. Therefore, the need for a better, biologically-oriented therapeutic approach is urgent. In this respect, regenerative medicine, which seeks ways to imitate natural physiological mechanisms of organ initiation and morphogenesis, could be of help [87, 88, 89].
4. Is Human Teeth Regeneration a Prospective Clinical Reality or a Fantasy?
4.1 Physiological repair is a widespread property of many tissues and organs
The most common teeth pathologies involve lesions on the crown that are caused by caries and/or injuries, pulp inflammation, diseases of the periodontium and teeth loss. Dental surgeons clinically treat these pathologies by substituting the lost physiological tissue/organ with a nonbiological, artificial material. Although usually the outcome is fairly good, pathological repeats are common [85, 86]. Therefore, an ambitious dream of numerous dentists is to be able to substitute the artificial material with a biological, cell-based one that is able to form a genuine replica of the damaged tooth part or the entire lost tooth. Tissue and organ regeneration has become an extensive, multidisciplinary research field with clear purposes and hopeful clinical prospects for a pleiad of human tissues and organs, such as bones, muscles, liver, heart and kidney, among others [87, 88, 89]. There are hopeful clinical prospects because representatives from all animal phyla are endowed with considerable, although varying, regenerative capabilities [90]. The enormous regenerative differences that are observed, even between closely related species, could be due to an inability to secondarily express some components, for reasons that are not yet clearly understood. Nearly every organ harbors in particular niches specific cells that are known today as somatic (or adult) stem cells [13, 17, 19, 89, 91]. The term “stem cells” includes pluripotent cells that have an unlimited capacity to divide and are specifically adapted for permanent survival. A pluripotent cell is endowed with the capacity to differentiate into cells of all three germ layers (ecto-, endo- and mesoderm). Stem cells are usually classified into two major categories: embryonic cells, which are of blastocyst inner cell mass origin and are pluripotent and adult cells, which are located in various tissues and are usually multipotent (meaning that they can give rise only to cells from two germ layers). An adult stem cell may divide into two daughter cells; one of them remains in situ as an adult stem cell; the other differentiates to compensate for cell loss if needed for homeostatic purposes. Physiologically, an adult stem cell becomes aware of the loss of its normal neighbors, multiplies and produces daughter cells, some of which differentiate so as to replace the lost ones.
Many scientists believe that in the near future, scientists will be able to reproduce ex vivo and in vitro the in vivo development of a genuine replica of an organ. There have been various experimental results reported that indicate that the regeneration of various complicated organs, such as the eye [92] and teeth [93], is quite feasible. For example, there is a plethora of tissue-specific stem cell types that can be manipulated in the lab to induce tissue-specific differentiation [13, 17, 19, 87, 89, 91]. Appropriate culture methods are available as well as biocompatible, biodegradable, three-dimensional support materials [94]. The induction signals are also fairly well known. What is not known yet is how to obtain an easily accessible source of human somatic stem cells, what are the quantities of cells and growth factors to be combined, what should their spatial arrangement be and how is the teeth size, shape and purposeful development controlled [17, 19, 94].
4.2. Biological tooth repair and regeneration
Considering the aforementioned challenges and gaps in knowledge, it is reasonable to ask whether the scientific evidence for the capacity for teeth regeneration is actually substantial and reliable or whether the whole idea is unrealistic and fed by unjustified enthusiasm. According to many experts, guarded optimism might be justified, since teeth tissue restoration/regeneration occurs naturally and can be reproduced in vitro [13, 15, 17, 19, 83, 84, 94, 95]. Various animal species (e.g., mice, voles) can replace physically worn teeth parts of varying teeth types (mouse incisors, vole molars) with stem cells [17, 19]. In addition, numerous animals (most nonmammalian species) are endowed with the ability to continually replace lost teeth throughout life via de novo formation of tooth germs (polyphyodonty) [96]. Normally, humans do not have such abilities; however, it is possible that the regenerative potential in humans is underestimated and that some of components might be able to be reactivated under certain circumstances [97, 98]. For example, stem cells have been isolated from mesodermal human dental tissues, such as the periodontal ligament [99], dental follicle [100], dental pulp [101] and bone marrow [102, 103]. When cultivated under appropriate conditions, they may be able to differentiate into tooth-related cells that can produce cementum, periodontal ligament, alveolar bone and dentine. The search for a source of epithelial, tooth-related stem cells, i.e., able to express enamel proteins, is still underway [104]; at present the only human source is the tooth germ of young children. Recent advances in our knowledge and technology have allowed us to harbor great expectations of the possibility of reviving latent, intrinsic biological powers to totally or partially restore and repair tooth erosions [83, 84, 101, 102]. Such advances include studies of the biological regeneration of tooth tissues in vivo and the generation complete teeth in vitro [105, 106].
Practically, to achieve this goal one only needs cells that are able to multiply, cooperate and reform the missing part. To avoid immunological rejection or immunosuppressive interventions, a dentist must find and easily isolate cells from each individual patient. This kind of protocol poses numerous problems and could postpone clinical applications. Currently, there are two main branches of research that approach the idea of teeth tissue regeneration. The first and more accessible branch deals with the restoration/repair of partial teeth damages, such as the deep lesions caused by bacterial activity. This line of research seeks to reactivate existing but latent reparative capabilities and/or use teeth-related stem cells to repair the damaged part of the tooth by cell multiplication and production of the missing material. (b) The second and more long-term branch of research involves using stem cells and applying conventional tissue engineering techniques to create a replica of the desired missing tooth.
(a) Partial tooth repair
Gradual enamel and dentin loss and pulp exposure caused by carries or injury induces living odontoblasts to restart the production of what is called reactionary dentin. If the lesion is of high magnitude, causing the death of odontoblasts, then growth factors (mainly members of the TGFβ family) are liberated from the destructed extracellular matrix. These growth factors recruit pulp perivascular stem cells, which migrate to the injured region and produce reparative dentine [107]. This natural process demonstrates that cells in various pulp locations (mainly around vessels) have the ability to differentiate into odontoblast-like cells following dental injury [83, 84] and that there is a lifelong potential for the synthesis and organized apposition of small quantities of dentin. If this activity could be harnessed and imitated, scientists may be able to achieve satisfactory results at the clinical level [95]. In addition, it is thought that the identification of various enamel proteins (mainly amelogenin) in odontoblasts [108] could lead to the ability to synthesize and deposit dentin and enamel at regions of extensive damage in a purpose-directed and biased manner [109]. Another cell-based approach that is achievable in the near future is the acquisition of adult stem cells and the cultivation/multiplication of these cells in a nonbiological, biodegradable scaffold of shape complementary to the lost tooth part. Conditions should be chosen to guide odontoblast and ameloblast differentiation and the orderly production of materials needed to correctly replace the missing ones.
Thus far, there have been numerous in vitro and in vivo studies performed on partial tooth repair that have generated exciting and strongly promising results [83, 84, 101, 102, 110]. These studies have been mainly performed in animal models, however, so it remains to be seen whether these achievements can be directly applied to humans.
(b) Replacement of a whole tooth
The replacement of an entire lost human tooth by a replica seems to be a realistic target; however, we are still seeking this goal. According to many experts the achievement of that goal may not occur for many decades. Several experimental approaches to whole tooth replacement have been designed and performed, but they can all be considered to belong in a single class of “teeth bioengineering”. This procedure involves the in vitro production of a bioengineered tooth germ by recapitulating/imitating the embryonic epithelial-mesenchymal interactions, ectopically transplanting (into renal capsule, omentum, chorioallantoic membrane, anterior chamber of eye) the tooth germ, subsequently implanting this germ in place of the missing tooth and then growing the fairly normal tooth according to its place in the jaw. To successfully achieve this goal, the scientist needs:
(b.i) Tooth-related cells from each patient, preferably isolated stem cells from the corresponding teeth tissues that are endowed with odontogenic potential. Such cells, particularly those of mesenchymal origin, are already identified in human tissues [99, 100, 101, 102]. At present, the only readily available source of odontogenic epithelial stem cells is the apical niche of the mouse incisor [17]. Odontogenic epithelial cells of human origin may also be obtained from teeth of young children (impacted third molar) and from other mammalian sources (e.g., pig) [111]. In addition, stem cell technology has succeeded in differentiating human bone marrow stem cells into enamel-producing cells [112]. The experimental recombination of either dissociated epithelial and mesenchymal cells or intact dental epithelium and mesenchyme creates tooth-like structures that have an organized apposition of the main tooth constituents [104, 113, 114, 115].
(b.ii) Culture techniques that permit a fast expansion that would yield the needed cell quantities to condition the microenvironment and allow for cell-cell interactions that would lead to purposeful cell differentiation. This requirement might prove to be the rate limiting step of the whole procedure, particularly for epithelial cells, which grow extremely slow in culture. Nevertheless, cultured mammalian cells from dissociated tooth buds manage to sort out, cooperate and form tooth-like structures [104, 116].
(b.iii) An adequate microenvironment supporting 3D tooth-like growth, leading to the production of a genuine tooth-like bud or even a tooth replica. The usual approach to achieving a bio-tooth with a natural three-dimensional shape involves use of an artificial, tooth-shaped biodegradable scaffold. Various scaffold materials have already been employed for this purpose and have yielded promising results. Seeding such scaffolds with cultured dental cells results in the production of ordered teeth components (e.g., enamel, dentin, root) though with miniature dimensions [104, 111].
(b.iv) The ability to implant the bioengineered bio-tooth germ into the prepared empty alveolar socket under conditions that permit the development of the root, periodontal ligament and osteointegration. This goal has already been achieved, proving that the jaw of an adult animal can accept a bioengineered tooth bud, nurture it and cooperate with it in a way that allows for tooth growth, morphogenesis and eruption [19, 113, 117, 118].
All of these above mentioned requirements have been already met experimentally, though they have not been applied clinically. Nonetheless, the obstacles to achieving the target seem unsurpassable at present. For example, we still lack an easily accessible source of odontogenic cells from an adult (mostly aged person). In those cases where cells are available, there is no way of controlling the size and shape of the needed bio-tooth, and the time needed to generate the tooth is long. Theoretically, an ideal option is physiological regeneration, where the replica of the missing tooth is produced in situ: local stem or tissue cells that are resident in situ after the removal of a tooth can be instructed to differentiate or transdifferentiate, respectively, to repeat primary odontogenesis. Practically, this scenario is not realistic for the foreseeable future, since human oral tissues that are left behind after the removal of a tooth do not possess (as far as we know) cell sources or structures that are comparable to those of nonmammalian species that retain the ability to replace missing teeth. In humans, permanent teeth form as a localized proliferation of the dental lamina of the pre-existing deciduous tooth [7]. This process happens only once and regenerative capabilities disappear along with the dental lamina. In contrast, other vertebrates constantly replace lost teeth. In particular, zebrafish [119] replace teeth in a strikingly similar way as humans replace decidual teeth. Therefore, the analysis of the molecular loops that regulate the process of successional tooth formation might reveal a way to induce a third dentition in the patients.
5. Conclusions
This review provides insight into how teeth are made in nature and how we might make them using our accumulating knowledge and technology. There are many teeth types, such as cutting incisors, tearing canines, grinding premolars and molars. Continuously growing teeth are common in many animals (e.g., mouse incisors, vole molars), and some organisms (e.g., sharks) can replace lost teeth throughout life. There are also species variations in the extent of enamel coverage on teeth (e.g., man, mouse, horse, elephant). Despite such differences, most recent teeth, including those of humans, originate from a common precursor and develop under similar molecular instruction. Tooth defects or missing dentition compromises human health physically and psychiatrically. Evolutionary and developmental biologists, as well as tissue engineers, are working together to investigate and compare the tissue origin, patterning and growth of various teeth parts in an effort to restore healthy and/or repair defective tissue. Our understanding of these biological processes may serve as a foundation for the future design and fabrication of regenerated teeth. Research continues with the goal of being able exploit natural processes to generate new therapies. To that end, we have extended our knowledge of the cellular and molecular biology and the genetic circuits involved in the epithelial-ectomesenchymal interactions. We have acquired numerous somatic stem cell lines with higher plasticity than what was previously thought possible, and we have learned that missing components of a morphogenetic field can be replaced by similar components from other tissues. Future work will continue to explore the possibility of tooth tissue restoration in vivo and the regeneration of whole teeth, both in vivo and in vitro. It seems now that the recent convergence of the human genome project and other projects in different scientific and technological fields has significantly enriched our arsenal of tools that can be applied towards our goal, i.e., teeth tissue regeneration.
There is no doubt that it will take a long time before even partial restoration of dental tissues, let alone complete tooth regeneration, will be achieved both in vivo and in vitro and applied in clinical practice. Nonetheless, there are reasons to be optimistic. It is hoped that by continuing to improve our understanding in these areas, we will be able to improve the way we diagnose and treat pathologies affecting teeth, whether they arise from genetic or environmental factors, injury or disease.
Acknowledgements
This work was funded by the Special Account for Research Grants of the National and Kapodistrian University of Athens, and by the State Scholarships Foundation of Greece.
Conflict of Interest
The authors have declared that no conflict of interest exists.
References
1. Thesleff I. The genetic basis of tooth development and dental defects. Am J Med Genet Part A. 2006;140A:2530-2535
2. Townsend G, Harris EF, Lesot H, Clauss F, Brook A. Morphogenetic fields within the human dentition: A new, clinically relevant synthesis of an old concept. Arch Oral Biol. 2008 [Epub ahead of print]
3. Line SRP. Variation of tooth number in mammalian dentition: connecting genetics, development, and evolution. Evol Devel. 2003;5(3):295-304
4. Kapadia H, Mues G, D'Souza R. Genes affecting tooth morphogenesis. Orthod Craniofac Res. 2007;10(4):237-244
5. Butler PM. Ontogenic aspects of dental evolution. Int J Dev Biol. 1995;39(1):25-34
6. Smith MM, Coates MI. Evolutionary origins of the vertebrate dentition: phylogenetic patterns and developmental evolution. Eur J Oral Sci. 1998;106(Suppl 1):S482-S500
7. Peyer B. Comparative Odontology. US: University of Chicago Press. 1968
8. Lumsden AG. Spatial organization of the epithelium and the role of neural crest cells in the initiation of the mammalian tooth germ. Development. 1988;103(Supp l):S155-S169
9. Imai H, Osumi N, Eto K. Contribution of foregut endoderm to tooth initiation of mandibular incisor in rat embryos. Eur J Oral Sci. 1998;106(Suppl 1):S19-S23
10. Thesleff I, Vaahtokari A, Partanen AM. Regulation of organogenesis. Common molecular mechanisms regulating the development of teeth and other organs. Int J Dev Biol. 1995;39(1):35-50
11. Matalova E, Tucker AS, Sharpe PT. Death in the life of a tooth. J Dent Res. 2004;83(1):11-16
12. Koussoulakos S. Vertebrate limb development: from Harrison's limb disk transplantations to targeted disruption of Hox genes. Anat Embryol (Berl). 2004;209(2):93-105
13. Thesleff I, Tummers M. Stem cells and tissue engineering: prospects for regenerating tissues in dental practice. Med Princ Pract. 2003;12(Suppl 1):S43-S50
14. Fleischmannova J, Matalova E, Tucker AS, Sharpe PT. Mouse models of tooth abnormalities. Eur J Oral Sci. 2008;116(1):1-10
15. Chai Y, Slavkin HC. Prospects for tooth regeneration in the 21st century: a perspective. Microsc Res Tech. 2003;60(5):469-479
16. Ferreira CF, Magini RS, Sharpe PT. Biological tooth replacement and repair. J Oral Rehabil. 2007;34(12):933-939
17. Bluteau G, Luder HU, De Bari C, Mitsiadis TA. Stem cells for tooth engineering. Eur Cell Mater. 2008;16:1-9
18. Jernvall J, Thesleff I. Reiterative signaling and patterning during mammalian tooth morphogenesis. Mech Dev. 2000;92(1):19-29
19. Yen AH, Sharpe PT. Stem cells and tooth tissue engineering. Cell Tissue Res. 2008;331(1):359-372
20. Zerina J, Smith MM. Origin and evolution of gnathostome dentitions: a question of teeth and pharyngeal denticles in placoderms. Biol Rev Camb Philos Soc. 2005;80(2):303-345
21. Soukup V, Epperlein HH, Horácek I, Cerny R. Dual epithelial origin of vertebrate oral teeth. Nature. 2008;455(7214):795-798
22. Reif W. Evolution of dermal skeleton and dentition in vertebrates: the odontode-regulation theory. Evol Biol. 1982;15:287-368
23. Sire J-Y, Delgado S, Fromentin D, Girondot M. Amelogenin: lessons from evolution. Arch Oral Biol. 2005;50(2):205-212
24. Kawasaki K, Buchanan AV, Weiss KM. Gene duplication and the evolution of vertebrate skeletal mineralization. Cells Tissues Organs. 2007;186(1):7-24
25. Salazar-Ciudad I, Jernvall J. How different types of pattern formation mechanisms affect the evolution of form and development. Evol Dev. 2004;6(1):6-16
26. Witter K, Lesot H, Peterka M, Vonesch JL, Mísek I, Peterková R. Origin and developmental fate of vestigial tooth primordia in the upper diastema of the field vole (Microtus agrestis, Rodentia). Arch Oral Biol. 2005;50(4):401-409
27. Keränen SV, Kettunen P, Åberg T, Thesleff I, Jernvall J. Gene expression patterns associated with suppression of odontogenesis in mouse and vole diastema regions. Dev Genes Evol. 1999;209(8):495-506
28. Kurtén B. Return of a lost structure in the evolution of the felid dentition. Soc Scient Fenn Comm Biol. 1963;26:1-12
29. Stock DW. The genetic basis of modularity in the development and evolution of the vertebrate dentition. Philos Trans R Soc Lond B Biol Sci. 2001;356(1414):1633-1653
30. Turecková J, Sahlberg C, Åberg T, Ruch JV, Thesleff I, Peterkovà R. Comparison of expression of the msx-1, msx-2, BMP-2 and BMP-4 genes in the mouse upper diastemal and molar tooth primordia. Int J Dev Biol. 1995;39(3):459-468
31. Neubüser A, Peters H, Balling R, Martin GR. Antagonistic interactions between FGF and BMP signalling pathways: a mechanism for positioning the sites of tooth formation. Cell. 1997;90(2):247-255
32. Laurikkala J, Kassai Y, Pakkasjärvi L, Thesleff I, Itoh N. Identification of a secreted BMP antagonist, ectodin, integrating BMP, FGF, and SHH signals from the tooth enamel knot. Dev Biol. 2003;264(1):91-105
33. Weiss KM, Stock DW, Zhao Z. Dynamic interactions and the evolutionary genetics of dental patterning. Crit Rev Oral Biol Med. 1998;9(4):369-398
34. Crompton AW, Parker P. Evolution of the mammalian masticatory apparatus. Amer Sci. 1978;66:192-201
35. Polly PD. Development and evolution occlude: evolution of development in mammalian teeth. Proc Natl Acad Sci USA. 2000;97(26):14019-14021
36. Butler PM. Dental merism and tooth development. J Dent Res. 1967;46(5):845-850
37. Osborn JW. The evolution of dentitions. The study of evolution suggests how the development of mammalian dentitions may be controlled. Am Sci. 1973;61(5):548-559
38. Jernvall J, Keränen SV, Thesleff I. Evolutionary modification of development in mammalian teeth: Quantifying gene expression patterns and topography. Proc Natl Acad Sci USA. 2000;97:14444-14448
39. Ruch JV. Patterned distribution of differentiating dental cells: facts and hypotheses. J Biol Buccale. 1990;18(2):91-98
40. Osborn JW. On the control of tooth replacement in reptiles and its relationship to growth. J Theor Biol. 1974;46(2):509-527
41. Sharpe PT. Homeobox genes and orofacial development. Connect Tissue Res. 1995;32(1-4):17-25
42. Tucker AS, Headon DJ, Courtney JM, Overbeek P, Sharpe PT. The activation level of the TNF family receptor, Edar, determines cusp number and tooth number during tooth development. Dev Biol. 2004;268(1):185-194
43. Mitsiadis TA, Smith MM. How do genes make teeth to order through development? J Exp Zoolog B Mol Dev Evol. 2006;306(3):177-182
44. Brook AH. A unifying aetiological explanation for anomalies of human tooth number and size. Arch Oral Biol. 1984;29(5):373-378
45. Brook AH, Griffin RC, Smith RN, Townsend GC, Kaur G, Davis GR, Fearne J. Tooth size patterns in patients with hypodontia and supernumerary teeth. Arch Oral Biol. 2008 [Epub ahead of print]
46. Wada H, Makabe K. Genome duplications of early vertebrates as a possible chronicle of the evolutionary history of the neural crest. Int J Biol Sci. 2006;2(3):133-141
47. Grigoriou M, Tucker AS, Sharpe PT, Pachnis V. Expression and regulation of Lhx6 and Lhx7, a novel subfamily of LIM homeodomain encoding genes, suggests a role in mammalian head development. Development. 1998;125(11):2063-2074
48. Mina M, Kollar EJ. The induction of odontogenesis in non-odontogenic mesenchyme combined with early murine mandibular arch epithelium. Arch Oral Biol. 1987;32:123-127
49. Slavkin HC. Embryonic tooth formation. A tool for developmental biology. Oral Sci Rev. 1974;4(0):7-136
50. Peters H, Neubüser A, Balling R. Pax genes and organogenesis: Pax9 meets tooth development. Eur J Oral Sci. 1998;106(Suppl 1):S38-S43
51. Plikus MV, Zeichner-David M, Mayer JA, Reyna J, Bringas P, Thewissen JG, Snead ML, Chai Y, Chuong CM. Morphoregulation of teeth: modulating the number, size, shape and differentiation by tunning Bmp activity. Evol Dev. 2005;7(5):440-457
52. Hardcastle Z, Mo R, Hui CC, Sharpe PT. The Shh signalling pathway in tooth development: defects in Gli2 and Gli3 mutants. Development. 1998;125(15):2803-2811
53. Dassule HR, Lewis P, Bei M, Maas R, McMahon AP. Sonic hedgehog regulates growth and morphogenesis of the tooth. Development. 2000;127(22):4775-4785
54. Tucker AS, Matthews KL, Sharpe PT. Transformation of tooth type induced by inhibition of BMP signaling. Science. 1998;282(5391):1136-1138
55. Sarkar L, Cobourne M, Naylor S, Smalley M, Dale T, Sharpe PT. Wnt/Shh interactions regulate ectodermal boundary formation during mammalian tooth development. Proc Natl Acad Sci USA. 2000;97(9):4520-4524
56. Jung HS, Francis-West PH, Widelitz RB, Jiang TX, Ting-Berreth S, Tickle C, Wolpert L, Chuong CM. Local inhibitory action of BMPs and their relationships with activators in feather formation: implications for periodic patterning. Dev Biol. 1998;196(1):11-23
57. Chen Y, Zhang Y, Jiang TX, Barlow AJ, St Amand TR, Hu Y, Heaney S, Francis-West P, Chuong CM, Maas R. Conservation of early odontogenic signaling pathways in Aves. Proc Natl Acad Sci USA. 2000;97(18):10044-10049
58. Mostowska A, Kobielak A, Trzeciak WH. Molecular basis of non-syndromic tooth agenesis: mutations of MSX1 and PAX9 reflect their role in patterning human dentition. Eur J Oral Sci. 2003;111(5):365-370
59. Åberg T, Wang XP, Kim JH, Yamashiro T, Bei M, Rice R, Ryoo HM, Thesleff I. Runx2 mediates FGF signaling from epithelium to mesenchyme during tooth morphogenesis. Dev Biol. 2004;270(1):76-93
60. Maas R, Bei M. The genetic control of early tooth development. Crit Rev Oral Biol Med. 1997;8(1):4-39
61. Vaahtokari A, Åberg T, Jernvall J, Keränen S, Thesleff I. The enamel knot as a signaling centre in the developing mouse tooth. Mech Dev. 1996;54(1):39-43
62. Jernvall J, Åberg T, Kettunen P, Thesleff I. The life history of an embryonic signaling center: BMP4 induces p21 and is associated with apoptosis in the mouse tooth enamel knot. Development. 1998;125(2):161-169
63. Ferguson C, Tucker AS, Christensen L, Lau AL, Matzuk MM, Sharpe PT. Activin is an essential early mesenchymal signal in tooth development that is required for patterning of the murine dentition. Genes Development. 1998;12:2636-2649
64. Pizette S, Niswander L. BMPs negatively regulate structure and function of the limb apical ectodermal ridge. Development. 1999;126(5):883-894
65. Coin R, Kieffer S, Lesot H, Vonesch JV, Ruch JV. Inhibition of apoptosis in the primary enamel knot does not affect specific tooth crown morphogenesis in the mouse. Int J Dev Biol. 2000;44(4):389-396
66. Luan X, Ito Y, Diekwisch TG. Evolution and development of Hertwig's epithelial root sheath. Dev Dyn. 2006;235(5):1167-1180
67. Salazar-Ciudad I, Jernvall J. A gene network model accounting for development and evolution of mammalian teeth. Proc Natl Acad Sci USA. 2002;99(12):8116-8120
68. Coin R, Lesot H, Vonesch JL, Haikel Y, Ruch JV. Aspects of cell proliferation kinetics of the inner dental epithelium during mouse molar and incisor morphogenesis: a reappraisal of the role of the enamel knot area. Int J Dev Biol. 1999;43(3):261-267
69. Slavkin HC. Molecular determinants during dental morphogenesis and cytodifferentiation: a review. J Craniofac Genet Dev Biol. 1991;11(4):338-349
70. Lyngstadaas SP, Møinichen CB, Risnes S. Crown morphology, enamel distribution, and enamel structure in mouse molars. Anat Rec. 1998;250(3):268-280
71. Yokozeki M, Afanador E, Nishi M, Kaneko K, Shimokawa H, Yokote K, Deng C, Tsuchida K, Sugino H, Moriyama K. Smad3 is required for enamel biomineralization. Biochem Biophys Res Commun. 2003;305(3):684-690
72. Goldberg M, Septier D, Lécolle S, Chardin H, Quitana MA, Acevedo AC, Gafni G, Dillouya D, Vermelin L, Thoneman B, Schmalz G, Bissila-Mapahou P, Carreau JP. Dental mineralization. Int J Dev Biol. 1995;39(1):93-110
73. Marks SCJr, Schroeder HE. Tooth eruption: theories and facts. Anat Rec. 1996;245(2):374-393
74. Streelman JT, Webb JF, Albertson RC, Kocher TD. The cusp of evolution and development: a model of cichlid tooth shape diversity. Evol Dev. 2003;5(6):600-608
75. Bailleul-Forestier I, Berdal A, Vinckier F, de Ravel T, Fryns JP, Verloes A. The genetic basis of inherited anomalies of the teeth. Part 2: syndromes with significant dental involvement. Eur J Med Genet. 2008;51(5):383-408
76. Courtney JM, Blackburn J, Sharpe PT. The Ectodysplasin and NFkappaB signalling pathways in odontogenesis. Arch Oral Biol. 2005;50(2):159-163
77. Peters H, Neubüser A, Kratochwil K, Balling R. Pax9-deficient mice lack pharyngeal pouch derivatives and teeth and exhibit craniofacial and limb abnormalities. Genes Dev. 1998;12(17):2735-2747
78. Brook AH, Lath DL, Brook BJ, Smith RN. Further consideration of the aetiology of developmental anomalies of the dentition. Zadzinska E, ed. Current Trends in Dental Morphology Research, Lodz: University of Lodz. 2005:467-474
79. Townsend G, Hughes T, Luciano M, Bockmann M, Brook A. Genetic and environmental influences on human dental variation: A critical evaluation of studies involving twins. Arch Oral Biol. 2008 [Epub ahead of print]
80. Luckett PW. An ontogenetic assessment of dental homologies in therian mammals. In: (ed.) Szalay FS, Novacek MJ, McKenna MG. Mammal Phylogeny: Mesozoic differentiation, multituberculates, monotremes, early therians and marsupians. New York: Springer Verlag 1993:182-204
81. Pickles MJ. Tooth wear. Monogr Oral Sci. 2006;19:86-104
82. Sloan AJ, Waddington RJ. Dental pulp stem cells: what, where, how? Int J Paediatr Dent. 2009;19(1):61-70
83. Goldberg M, Lacerta-Pinheiro S, Jegat N, Six N, Septier D, Priam F, Bonnefoix M, Tompkins K, Chardin H, Denbesten P, Veis A, Poliard A. The impact of bioactive molecules to stimulate tooth repair and regeneration as part of restorative dentistry. Dent Clin North Am. 2006;50(2):277-298
84. Tziafas D. The future role of a molecular approach to pulp-dentinal regeneration. Caries Res. 2004;38(3):314-320
85. Esposito M, Hirsch JM, Lekholm U, Thomsen P. Biological factors contributing to failures of osseointegrated oral implants. (II). Etiopathogenesis. Eur J Oral Sci. 1998;106(3):721-764
86. Christensen GJ. How to kill a tooth. J Am Dent Assoc. 2005;136(12):1711-1713
87. Weaver CV, Garry DG. Regenerative biology: a historical perspective and modern applications. Regen Med. 2008;3(1):63-82
88. Carlson BM. Some principles of regeneration in mammalian systems. Anat Rec B New Anat. 2005;287(1):4-13
89. Stocum DL, Zupanc GK. Stretching the limits: stem cells in regeneration science. Dev Dyn. 2008;237(12):3648-3671
90. Koussoulakos S, Kiortsis V. Regeneration: Birth of experimental biology, physiology, recent research trends. Trends Compar Biochem Physiol. 1993;1:201-218
91. Weissman IL. Stem cells: units of development, units of regeneration, and units in evolution. Cell. 2000;100(1):157-168
92. Chow RL, Altmann CR, Lang RA, Hemmati-Brivanlou A. Pax6 induces ectopic eyes in a vertebrate. Development. 1999;126(19):4213-4222
93. Lemus D, Coloma L, Fuenzalida M, Illanes J, Paz de la Vega Y, Ondarza A, Blanquez MZ. Odontogenesis and amelogenesis in interacting lizard-quail tissue combinations. J Morphol. 1986;189(2):121-129
94. Yu J, Shi J, Jin Y. Current approaches and challenges in making a bio-tooth. Tissue Eng Part B. 2008;14(3):307-319
95. Shi S, Bartold PM, Miura M, Seo BM, Robey PG, Gronthos S. The efficacy of mesenchymal stem cells to regenerate and repair dental structures. Orthod Craniofac Res. 2005;8(3):191-199
96. Sire JY, Davit-Beal T, Delgado S, Van Der Heyden C, Huysseune A. First-generation teeth in nonmammalian lineages: evidence for a conserved ancestral character? Microsc Res Tech. 2002;59(5):408-434
97. Saulnier N, Di Campli C, Zocco MA, Di Gioacchino G, Novi M, Gasbarrini A. From stem cell to solid organ. Bone marrow, peripheral blood or umbilical cord blood as favorable source? Eur Rev Med Pharmacol Sci. 2005;9(6):315-324
98. Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453(7193):314-321
99. Seo BM, Miura M, Sonoyama W, Coppe C, Stanyon R, Shi S. Recovery of stem cells from cryopreserved periodontal ligament. J Dent Res. 2005;84(10):907-912
100. Handa K, Saito M, Tsunoda A, Yamauchi M, Hattori S, Sato S, Toyoda M, Teranaka T, Narayanan AS. Progenitor cells from dental follicle are able to form cementum matrix in vivo. Connect Tissue Res. 2002;43(2-3):406-408
101. Liu H, Gronthos S, Shi S. Dental pulp stem cells. Methods Enzymol. 2006;419:99-113
102. Kawaguchi H, Hirachi A, Hasegawa N, Iwata T, Hamaguchi H, Shiba H, Takata T, Kato Y, Kurihara H. Enhancement of periodontal tissue regeneration by transplantation of bone marrow mesenchymal stem cells. J Periodontol. 2004;75(9):1281-1287
103. Kawazoe Y, Katoh S, Onodera Y, Kohgo T, Shindoh M, Shiba T. Activation of the FGF signaling pathway and subsequent induction of mesenchymal stem cell differentiation by inorganic polyphosphate. Int J Biol Sci. 2008;4(1):37-47
104. Honda MJ, Tsuchiya S, Sumita Y, Sagara H, Ueda M. The sequential seeding of epithelial and mesenchymal cells for tissue-engineered tooth regeneration. Biomaterials. 2007;28(4):680-689
105. Nakahara T, Ide Y. Tooth regeneration: implications for the use of bioengineered organs in first-wave organ replacement. Hum Cell. 2007;20(3):63-70
106. Ikeda E, Tsuji T. Growing bioengineered teeth from single cells: potential for dental regenerative medicine. Expert Opin Biol Ther. 2008;8(6):735-744
107. Smith AJ, Lesot H. Induction and regulation of crown dentinogenesis: embryonic events as a template for dental tissue repair? Crit Rev Oral Biol Med. 2001;12(5):425-437
108. Nagano T, Oida S, Ando H, Gomi K, Arai T, Fukae M. Relative levels of mRNA encoding enamel proteins in enamel organ epithelia and odontoblasts. J Dent Res. 2003;82(12):982-986
109. Nakashima M, Reddi AH. The application of bone morphogenetic proteins to dental tissue engineering. Nat Biotechnol. 2003;21(9):1025-1032
110. Nakashima M, Akamine A. The application of tissue engineering to regeneration of pulp and dentin in endodontics. J Endod. 2005;31(10):711-718
111. Sonoyama W, Liu Y, Fang D, Yamaza T, Seo BM, Zhang C, Liu H, Gronthos S, Wang CY, Shi S, Wang S. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS ONE. 2006;1:e79
112. Hu B, Nadiri A, Kuchler-Bopp S, Perrin-Schmitt F, Peters H, Lesot H. Tissue engineering of tooth crown, root, and periodontium. Tissue Eng. 2006;12(8):2069-2075
113. Ohazama A, Modino SA, Miletich I, Sharpe PT. Stem-cell-based tissue engineering of murine teeth. J Dent Res. 2004;83(7):518-522
114. Duailibi MT, Duailibi SE, Young CS, Bartlett JD, Vacanti JP, Yelick PC. Bioengineered teeth from cultured rat tooth bud cells. J Dent Res. 2004;83(7):523-528
115. Modino SA, Sharpe PT. Tissue engineering of teeth using adult stem cells. Arch Oral Biol. 2005;50(2):255-258
116. Duailibi SE, Duailibi MT, Vacanti JP, Yelick PC. Prospects for tooth regeneration. Periodontol 2000. 2006;41:177-187
117. Nakao K, Komine A, Suenaga M, Nakao K, Tsuji T, Tomooka Y. Tooth regeneration from newly established cell lines from a molar tooth germ epithelium. Biochem Biophys Res Commun. 2007;355(3):758-763
118. Nait Lechguer A, Kuchler-Bopp S, Lesot H. Crown formation during tooth development and tissue engineering. J Exp Zoolog B Mol Dev Evol. 2009 [Epub ahead of print]
119. Stock DW. Zebrafish dentition in comparative context. J Exp Zoolog B Mol Dev Evol. 2007;308(5):523-549
Author contact
![]() Correspondence to: Stauros Koussoulakos, University of Athens, Faculty of Biology, Department of Cell Biology and Biophysics, Panepistimiopolis 15784, Athens - Greece. e-mail: skoussouuoa.gr
Correspondence to: Stauros Koussoulakos, University of Athens, Faculty of Biology, Department of Cell Biology and Biophysics, Panepistimiopolis 15784, Athens - Greece. e-mail: skoussouuoa.gr

 Global reach, higher impact
Global reach, higher impact