Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2009; 5(3):249-255. doi:10.7150/ijbs.5.249 This issue Cite
Research Paper
In vivo evidence of hepato- and reno-protective effect of garlic oil against sodium nitrite-induced oxidative stress
1. Department of Zoology, Faculty of Science, Mansoura University, Mansoura, Egypt;
2. Department of Pathology and Department of Genetics, Stanley S. Scott Cancer Center, Louisiana State University Health Science Center, New Orleans, Louisiana, USA;
3. Department of Ob Gyn and Biochemistry, Stanley S. Scott Cancer Center, Louisiana State University Health Science Center, New Orleans, Louisiana, USA.
Received 2008-12-21; Accepted 2009-2-26; Published 2009-3-10
Abstract
Sodium nitrite (NaNO2), a food color fixative and preservative, contributes to carcinogenesis. We investigated the protective role of garlic oil against NaNO2-induced abnormalities in metabolic biochemical parameters and oxidative status in male albino rats. NaNO2 treatment for a period of three months induced a significant increase in serum levels of glucose, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), bilirubin, urea and creatinine as well as hepatic AST and ALT. However, significant decrease was recorded in liver ALP activity, glycogen content, and renal urea and creatinine levels. In parallel, a significant increase in lipid peroxidation, and a decrease in glutathione content and catalase activity were observed in the liver and the kidney. However, garlic oil supplementation showed a remarkable amelioration of these abnormalities. Our data indicate that garlic is a phytoantioxidant with powerful chemopreventive properties against chemically-induced oxidative stress.
Keywords: Food additives, Sodium nitrite, Oxidative stress, Garlic oil, Liver, glycogen, Alanine aminotransfrase, Aspartate aminotransfrease, Alkaline phoasphatase
Introduction
Natural and synthetic food additives approved by the U.S. Food and Drug Administration are commonly used to maintain or improve safety, the nutrient value, and the taste and texture of food [1]. Although many of the 3,000 these additives enhance our food supply, others are the subject of fierce controversy. The discovery that children at the age of nursery consume food containing great amounts of additives prompted the scientific community to oversee this issue. Sodium nitrite (NaNO2) is present in vegetables and is routinely used as a color fixative and preservative for meats and fish [2]. The hazardous effect of NaNO2 derives from the reaction of nitrites with amines to produce nitrosamines, and with amides to produce nitrosamides. The toxic effects of nitrates and nitrites are well documented in mammalians, including impairment of reproductive function [3], hepatotoxicity and methaemogobenemia [4], dysregulation of inflammatory responses and tissue injury [5], growth retardation [6], and endocrine disturbance [7]. The wide use of nitrates as preservatives in food technology elevates the importance of studying their effects.
Although NaNO2 is generally accepted as a weak carcinogen [8, 9], Wistar rats exposed to 0.3% NaNO2 in their drinking water for at least one year developed squamous papillomas of the forestomach [10]. Using F344 rat in a multiorgan carcinogenesis model, 0.3% NaNO2 given in the drinking water for 28 weeks increased the incidence of forestomach neoplasm in the post-initiation period [11]. Thus, it cannot be precluded that NaNO2 has very weak carcinogenic potential, particularly in the squamous epithelium of the forestomach. Moreover, in combination with other chemicals, NaNO2 has also been shown to form carcinogens or to enhance carcinogenesis. For instance, highly carcinogenic N-nitroso-compounds are produced when nitrite reacts with secondary amines and N-alkyl amides under acidic conditions in vitro [8, 12] and in vivo [13]. Other studies have demonstrated that treatment with NaNO2 in combination with phenolic compounds [11, 14] or ascorbic acid [15] strongly enhanced forestomach carcinogenesis in a rat two-stage carcinogenesis model.
Recent trends in controlling and treating diseases tend to favor natural antioxidant compounds rather than synthetic ones [16]. The human diet, which contains large number of natural compounds, is essential in protecting the body against the development of diseases, and the garlic Allium sativum is one of the well known plant with remarkable anti-carcinogenic properties is [16, 17]. Garlic, is a commonly worldwide used food, and its medical properties have been well recognized since the ancient times. Garlic is known for its antibacterial [18], anticarcinogenic [19], hypolipidemic [20], hypoglycemic [21], antifungal [22], and anti-atherosclerotic [23] properties, and an antioxidant against free radicals [24, 25].
Here, we investigated the role of garlic oil in preventing NaNO2-induced abnormalities in the biochemical parameters associated with the oxidative stress in male albino rats.
Materials and methods
Chemicals
NaNO2 (Sigma Aldrich, St Louis, MO) was applied as a freshly prepared solution and given by gavages at a dose of 80 mg/kg body weight as previously described [26]. Garlic oil was purchased from El-Captain Company (Cairo, Egypt). Garlic oil was given by gavages at a dose of 5ml/kg as described [27].
Animals
Male Albino Rats (Rattus rattus) weighing about 100-120 g were used in this study. The animals were kept under good ventilation and received a balanced diet and water ad libitum throughout the experimental period. Rats were divided into four main groups (n= 6) as follow: 1) Control group received, standard diet without any treatment; 2) Garlic oil-treated group, received standard diet, which was supplemented orally with garlic oil at a dose of 5 ml/kg body weight for a period of 3 months; 3) NaNO2-treated group, received standard diet supplemented orally with sodium nitrite at dose of 80 mg/kg body weight for a period of 3 months; and 4) NaNO2+garlic oil-treated group, received standard diet and were supplemented orally with similar doses of NaNO2 and garlic oil as group 3 for similar period of 3 months.
At the end of the experimental period, overnight fasted animals were sacrificed by cervical dislocation, and blood samples were collected in centrifuge tubes. Serum was separated from coagulant blood by centrifugation at 860g for 20 min, and then quickly frozen at -20°C for biochemical analysis. Small pieces of liver and kidney tissues were separated, weighed, homogenized in ice cold water and stored at -20°C for subsequent measurements.
Biochemical analysis
Serum glucose level was determined using the Biomerieux reagent kits [28]. Liver glycogen content was determined according to the method described by Van-Handle [29]. Serum alanine aminotransfrase (ALT) and aspartate aminotransfrease (AST) activities were determined according to the method described by Reitman and Frankel [30], whereas alkaline phoasphatase (ALP) activity was estimated by Belfield method [31]. Total protein, bilirubin, urea and creatinine levels were determined using Diamond Diagnostic Kit as previously reported [32-33]. The product of lipid peroxidation, thiobarbituric acid reactive substances (TBARS), was determined as previously described [34]. Glutathione (GSH) content was estimated by the method of Prins and Loose [35], and the activity of catalase was determined by the method of Aebi [36].
Statistical analysis
The data was analyzed using the Statistical Package for Social Science program (S.P.S.S. 11). For comparison between different experimental rat groups, one way analysis of variance (ANOVA) was used followed by Tukey's test. The results were expressed as means + SE and % of change, and P < 0.05 was considered to be statistically significant.
Results
A number of biochemical parameters were examined in the serum collected from each group and the results are summarized in table 1. While rats fed on standard diet supplemented with garlic oil did not show any significant changes in the majority of the parameters examined, a significant increase in serum levels of glucose, bilirubin, urea and creatinine as well as the activity of the AST, ALT and ALP enzymes were observed in rats treated with NaNO2 for a period of three months. The total protein content significantly decreased in serum (Table 1). However, supplementation of NaNO2- intoxicated rats with garlic oil ameliorated the nitrite adverse effects as evidenced by a significant increase of serum total protein content, and a decrease of serum glucose, bilirubin, urea and creatinine levels, as well as the activity of AST, ALT and ALP enzymes (Table 1). The same parameters were examined in liver and kidney tissues, and the data are shown in table 2. In the NaNO2-treated rats, a statistically significant inhibition of hepatic glycogen and total protein contents, and the activity of the enzymes ALT and AST as well as the levels of renal urea, creatinine and total protein content (Table 2). The activity of hepatic ALP was significantly increased in the NaNO2-treated rats (Table 2). However, administration of garlic oil to the NaNO2-intoxicated rats significantly restored these parameters in the liver and kidney organs (Table 2).
Further, we assessed oxidative stress parameters and antioxidant activity in the liver and the kidney and the results are summarized in table 3. The data indicate that TBARS concentration increased significantly, while GSH content, as well as catalase activity were decreased in both organs of NaNO2-intoxicated rats (Table 3). However, combination of garlic oil with NaNO2 reduced TBARS concentration and restored the levels of GSH as well as the activity of catalase (Table 3).
Serum biochemical parameters in different rat groups. Results are presented as means ± SE (n=5) and % of change. AST: aspartate aminotransfrease ; ALT: alanine aminotransfrase; ALP: alkaline phoasphatase
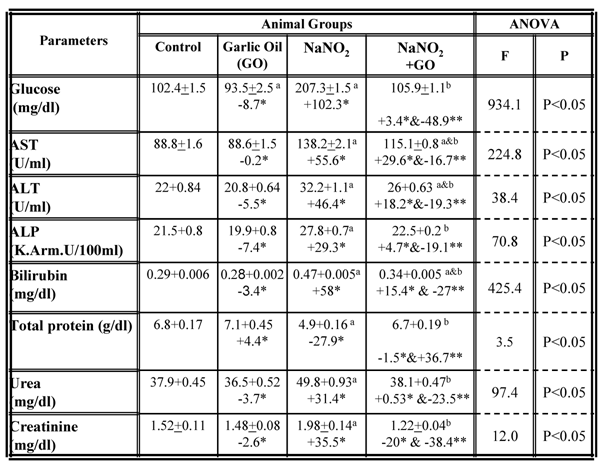
a: Compared to control group; b: Compared to NaNO2 treated rats group
* % of change compared with control group.
** % of change compared with NaNO2 treated rats group.
Hepatic and renal biochemical parameters in different rat groups. Results are presented as means ± SE (n=5) and % of change. AST: aspartate aminotransfrease ; ALT: alanine aminotransfrase; ALP: alkaline phoasphatase
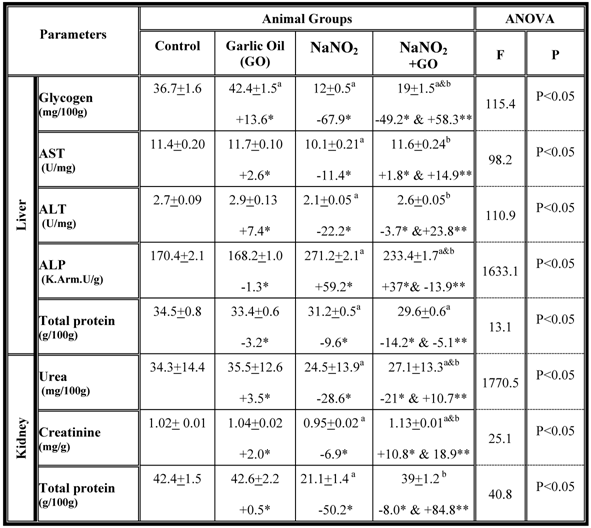
a: Compared to control group; b: Compared to NaNO2 treated rats group
* % of change compared with control group.
** % of change compared with NaNO2 treated rats group.
Hepatic and renal oxidative stress and antioxidant parameters in different rat groups. Results are presented as means ± SE (n=5) and % of change. TBARS: Thiobarbituric acid reactive substance; GSH: Glutathione; CAT: Catalase; MDA: malondialdehyde
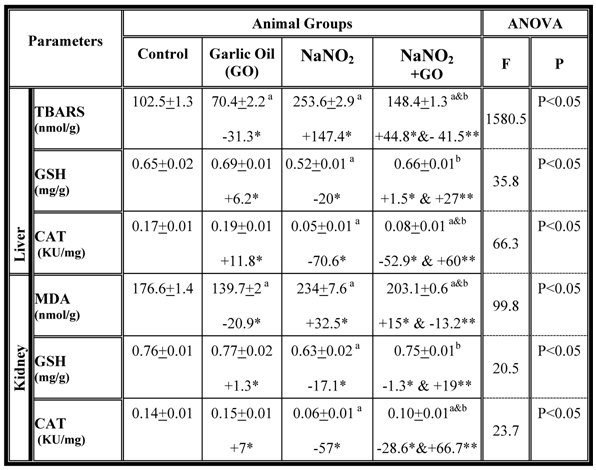
a: Compared to control group; b: Compared to NaNO2 treated rats group
* % of change compared with control group.
** % of change compared with NaNO2 treated rats group.
Discussion
The NaNO2 and other additives may react with amines of the foods in the stomach and produce nitrosamines and free radicals. Such products may increase lipid peroxidation, which can be harmful to different organs including liver and kidney [37]. On the other hand, these free radicals, known to cause oxidative stress, can be prevented or reduced by dietary natural antioxidants through their capacity to scavenge these products [38]. The present study was undertaken to determine whether garlic oil can prevent and/or reduce NaNO2-induced oxidative stress by examining different biochemical parameters of oxidative damage in the serum, the liver and the kidney of male rats.
Our results clearly showed that there was a significant increase in serum glucose concentration and a decrease in liver glycogen content of NaNO2-treated rats. The findings suggest nitrite-stimulation of gluconeogenesis [39], and glucose shift from tissue to blood or an impairment of glucose mobilization. Furthermore, nitroso-compounds can alter the antioxidant system causing disturbance in the metabolic processes leading to hyperglycemia [40]. However, serum glucose and liver glycogen levels were ameliorated upon garlic oil supplementation. The hypoglycemic effect of garlic oil and its organo-sulfur compounds might be due to their ability to enhance insulin secretion [41, 42].
Our results also indicate an inhibitory effect NaNO2 on the biosynthesis of protein, which was restored by garlic oil supplementation. These data suggest a stimulation of the thyroid and the adrenal glands by NaNO2 which can lead to a blockade in protein synthesis, fast breakdown, increased rate of free amino acids, and decreased protein turnover [43]. In addition, nitrite interactions results into nitric oxide release, which can inhibit total protein synthesis [44]. However, the increase in bilirubin concentration as well as the activity of AST, ALT and ALP enzymes in the serum of NaNO2-treated rats could be attributed to the toxic effect of nitroso-compounds, formed in the acidic environment of the stomach, in causing severe hepatic necrosis [45]. These abnormalities were prevented by supplementation of garlic oil, perhaps due to its role in stabilizing the cell membrane and protect the liver from free radical-mediated liver cell toxicity [46].
In response to NaNO2 treatment, urea and creatinine were increased in the serum but decreased in the kidney, suggesting an impairment of kidney functions. These effects could also be attributed to the changes in the threshold of tubular re-absorption, renal blood flow and glomerular filtration rate [47]. Garlic oil showed a clear improvement in kidney functions, perhaps due to the antioxidant properties of garlic in scavenging free radicals leading to reduced levels of nitric oxide and lipid peroxidation. Moreover, NaNO2-inhibited glutathione content and catalase enzyme activity in the liver and the kidney may be attributed to the observed induction of lipid peroxidation [48]. However, garlic improved the antioxidant mechanism due to the ability of Diallyl disulfide and Diallyl trisulfide present in garlic oil in modulating the oxidative stress and detoxifying enzyme system [49, 50, 51].
In conclusion, from the results achieved it can be concluded that the administration of garlic has an extremely beneficial role in overcoming the occurred adverse effects of chronic ingestion of sodium nitrite, which is probably through its excellent antioxidant properties and highly nutritional values.
Acknowledgements
Dr. Ouhtit is supported by the Louisiana Cancer Research Consortium in New Orleans.
Conflict of Interest
The authors have declared that no conflict of interest exists.
References
1. National Toxicology Program. NTP toxicology and carcinogenesis studies of sodium nitrite (CAS No. 7632-00-0) drinking water studies in F344/N rats and B6C3F1 mice. Natl Toxicol Program Tech Rep Ser. 2001;495:1-274
2. Kilgore WW, Li MY. Food Additives and Contaminations. In: (ed.) Doull J, Khassen CD, Amdur MD. Cassarett and Doulls, Toxicology: The Basic Science of Poisons, 2nd ed. New York: Macmillian. 1980:593-607
3. Sleight SD, Sinha DP, Uzoukwu M. Effect of sodium nitrate on reproductive performance of pregnant sows. J Am Vet Med Assoc. 1972;161:819-823
4. Swann PF. The toxicology of nitrate, nitrite and N-nitroso compounds. J Sci Food Agrec. 1975;26:1761-1770
5. Blanquat DG, Fritsch F, Cazotles C. Effect of dietary nitrate and nitrite on experimentally induced inflammation in the rat. Intern. J Tiss React. 1983;27:173-180
6. Prasad J. Effect of high nitrate diet on thyroid glands in goats. Ind J Animal Sci. 1983;53:791-794
7. Jahries G, Hesse VI, Schone LH, Mehnert E. Influence of nitrates and plant goitrgens on thyroid hormone, somated in status and growth of swine. Mj Vet Med. 1986;41:528-530
8. Maekawa A, Ogiu T, Onodera H, Furuta K, Matsuoka C, Ohno Y, Odashima S. Carcinogenicity studies of sodium nitrite and sodium nitrate in F-344 rats. Food Chem Toxicol. 1982;20:25-33
9. Inai K, Aoki Y, Tokuoka S. Chronic toxicity of sodium nitrite in mice, with reference to its tumorigenicity. Gann. 1979;70:203-8
10. Mirvish SS, Bulay O, Runge RG, Patil K. Study of the carcinogenicity of large doses of dimethylnitramine. N-nitroso-L-proline, and sodium nitrite administered in drinking water to rats. J Natl Cancer Inst. 1980;64:1435-42
11. Hirose M, Tanaka H, Takahashi S, Futakuchi M, Fukushima S, Ito N. Effects of sodium nitrite and catechol, 3-methoxycatechol, or butylated hydroxyanisole in combination in a rat multiorgan carcinogenesis model. Cancer Res. 1993;53:32-7
12. Aoyagi M, Matsukura N, Uchida E, Kawachi T, Sugimura T, Takayama S, Matsui M. Induction of liver tumors in Wistar rats by sodium nitrite given in pellet diets. J Natl Cancer Inst. 1980;65:411-4
13. Hecht SS. Approaches to cancer prevention based on an understanding of N-nitrosamine carcinogenesis. Proc Soc Exp Biol Med. 1997;216:181-91
14. Kawabe M, Takaba K, Yoshida Y, Hirose M. Effects of combined treatment with phenolic compounds and sodium nitrite on two-stage carcinogenesis and cell proliferation in the rat stomach. Jpn J Cancer Res. 1994;85:17-25
15. Yoshida Y, Hirose M, Takaba K, Kimura J, Ito N. Induction and promotion of forestomach tumors by sodium nitrite in combination with ascorbic acid or sodium ascorbate in rats with or without N-methyl-N-nitro-N-nitrosoguanidine pre-treatment. Int J Cancer. 1994;56:124-8
16. Craig W, Beck L. Phytochemicals: Health Protective Effects. Can J Diet Pract Res. 1999;60:78-84
17. Agarwal MK, Iqbal M, Athar M. Garlic oil ameliorates ferric nitrilotriacetate (Fe-NTA)-induced damage and tumor promotion: implications for cancer prevention. Food Chem Toxicol. 2007;45:1634-40
18. Johnson MG, Vaughn RH. Death of Salmonella typhimurium and Escherichia coli in the presence of freshly reconstituted dehydrated garlic and onion. Appl Microbiol. 1969;17:903-905
19. Hussain SP, Jannu LN, Roa AR. Chemopreventive action of garlic on methylcholanthrene induced carcinogenesis in the uterine cervix of mice. Cancer Lett. 1990;49:175-180
20. Bordia A, Bansal HC, Arora SK, Singh SV. Effect of essential oils of garlic and onion on alimentary hyperlipidemia. Atherosclerosis. 1975;21:15-19
21. Jain RC, Vjas CR. Garlic in alloxan induced diabetic rabbits. Am J Clin Nutr. 1975;28:684-685
22. Amer M, Taha M, Tosson Z. The effect of aqueous garlic extract on the growth of dermatophytas. Inter Dermatol. 1980;19:285-287
23. Bordia A, Verma SK. Effect of garlic feeding on regression of experimental atherosclerosis in rabbits. Artery. 1980;7:428-437
24. Morihara N, Sumioka I, Ide N, Moriguchi T, Uda N, Kyo E. Aged garlic extract maintains cardiovascular homeostasis in mice and rats. J Nutr. 2006;136:777S-781S
25. Banerjee SK, Mukherjee PK, Maulik SK. Garlic as an antioxidant: the good, the bad and the ugly. Phytother Res. 2003;17:97-106
26. Kohn MC, Melnick RL, Frank YE, Portier CJ. Pharmacokinetics of sodium nitrite-induced methemoglobinemia in the rat. DMD. 2002;30:676-683
27. Chen HW, Tsai CW, Yang JJ, Liu CT, Kuo WW, Lii CK. The combined effects of garlic oil and fish oil on the hepatic antioxidant and drug-metabolizing enzymes of rats. British J Nutr. 2003;89:189-200
28. Trinder P. A colorimetric method for the determination of glucose. Ann Clin Biochem. 1969;6:24-26
29. Van-Handle E. Estimation of glycogen in small amounts of tissue. Anal Biochem. 1965;11:256-262
30. Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxaloacetic and glutamic pyruvic transaminase. Am J Clin. 1957;28:56-63
31. Belfield A, Goldberg DM. Revised assay for serum phenyl phosphatase activity using 4-amino-antipyrine. Enzyme. 1971;12:561-73
32. Palton CJ, Crouch SR. Enzymatic determination of serum urea by modified Berthelot reaction. Anal Chem. 1977;49:464-469
33. Henry RJ. Principles and Techniques. Clinical Chemistry, 2nd Ed. Harper and Row. 1974:525.
34. Esteribauer H, Cheeseman K. Determination of aldehdic lipid peroxidation products: Malonaldehyde and 4- hydroxyonenal. Enzymol. 1990;186:407-421
35. Prins HK, Loose JA. Glutathione “Chapter 4”. In: (ed.) Yunis JJ. Biochemical Methods in Red Cell Genetics. London: Academic Press. 1969:126-129
36. Aebi HE. Catalase ed HV. In: (ed.) Bergmeyer HV. Method of enzymatic analysis vol 3. Berlin: Verlag Chemie. 1983:273-286
37. Choi SY, Chung MJ, Sung NJ. Volatile N-nitrosamine inhibition after intake of Korean green tea and Maesil (Prunus mume SIEB. et ZACC.) extracts with an amine-rich diet in subjects ingesting nitrate. Food Chem Toxicol. 2002;40:949-957
38. Aruoma OI. Free radicals, oxidative stress and antioxidants in human health and disease. J Am Oil Chem Soc. 1998;75:199-212
39. Wiechetek M, Garwacki S, Karlik W, Lewicki J, Souffrant W. Effect of nitrite on ureagenesis and carbohydrate metabolism in isolated rat hepatocytes. Arch of Environ Contamin Toxicol. 1992;24:375-380
40. Anil KB, Manju B, Giridhar S, Deepak B. Protective role of Vitamin E pre-treatment on N-nitrosodiethylamine induced oxidative stress in rat liver. Chem Biol Interact. 2005;20:101-102
41. Liu CT, Hse H, Lii CK, Chen PS, Sheen LY. Effects of garlic oil and diallyl trisulfide on glycemic control in diabetic rats. Eur J Pharmacol. 2005;516:165-173
42. Eidi A, Eidi M, Esmaeili E. Antidiabetic effect of garlic (Allium sativum L.) in normal and streptozotocin induced diabetic rats. Phytomedicine. 2006;13:624-629
43. Eremin YN, Yocharina MG. Effect of nitrites on the state of thyroid gland in iodine deficiency and different diets. Vopr Pitan. 1981;5:60-62
44. Kolpakov V, Gordon D, Kulik TJ. Nitric oxide-generating compounds inhibit total protein and collagen synthesis in cultured vascular smooth muscle cells. Circ Res. 1995;76:305-309
45. Kalantari H, Salehi M. The protective effect of garlic oil on hepatotoxicity induced by acetaminophen in mice and comparison with N-acetylcysteine. Saudi Med J. 2001;22:1080-4
46. Hikino H, Tohkin M, Kiso Y, Namiki T, Nishimura S, Takeyama K. Antihepatotoxic Actions of Allium sativum Bulbs1. Planta Med. 1986;52:163-168
47. Zurovsky Y, Haber C. Antioxidants attenuate endotoxin-generation induced acute renal failure in rats. Scand J Urol Nephrol. 1995;29:147-154
48. Shahjahan M, Vani G, Shyamaladevi CS. Effect of Solanum trilobatum on the antioxidant status during diethyl nitrosamine induced and phenobarbital promoted hepatocarcinogenesis in rat. Chem Biological interact. 2005;156:113-123
49. Popova MA, Popove CS. Effect of chemical agents on some enzyme activities and on the stability of membrane structures. Bulg J Vet Med. 2005;8:163-171
50. Saravanan G, Prakash J. Effect of garlic (Allium sativum) on lipid peroxidation in experimental myocardial infarction in rats. J Ethnopharmacol. 2004;94:155-158
51. Pedraza-Chaverrí J, Maldonado PD, Barrera D, Cerón A, Medina-Campos ON, Hernández-Pando R. Protective effect of diallyl sulfide on oxidative stress and nephrotoxicity induced by gentamicin in rats. Mol Cell Biochem. 2003;254:125-130
Author biography
Allal Ouhtit is a principal investigator and a member of the Stanley S Scott Cancer Center at the Louisiana State University (LSU) Health Sciences Center. He received his MPh in Neuroscience and his PhD in Biochemistry from the University Claude Bernard Lyon-I, France. He completed his postdoctoral work at the International Agency for Research on Cancer (IARC). In 2001, he became an Assistant Professor at the Queens University of Belfast, UK. He joined the LSU Health Sciences Center in 2005. His group focuses on two areas of research: 1) understanding the signaling mechanisms by which the cell adhesion receptors CD44 and CD146 regulate breast cancer metastasis; and 2) identification of powerful combinations of herbal antioxidants for chemoprevention of cancer and other diseases. Dr Ouhtit is an internationally respected scientist with awards for outstanding and innovative research.
![]() Correspondence to: Allal Ouhtit, Ph D., M Ph., Stanley S. Scott Cancer Center, Louisiana State University Health Science Center, CSRB Building, Room# 748C, 533 Bolivar Street, New Orleans, LA 70112. Ph: 1-504-568-2896 (Office); Fax: 1-504-568-2932; aouhtiedu
Correspondence to: Allal Ouhtit, Ph D., M Ph., Stanley S. Scott Cancer Center, Louisiana State University Health Science Center, CSRB Building, Room# 748C, 533 Bolivar Street, New Orleans, LA 70112. Ph: 1-504-568-2896 (Office); Fax: 1-504-568-2932; aouhtiedu

 Global reach, higher impact
Global reach, higher impact