10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2009; 5(4):304-310. doi:10.7150/ijbs.5.304 This issue Cite
Research Paper
Vesnarinone Represses the Fibrotic Changes in Murine Lung Injury Induced by Bleomycin
1. First Department of Internal Medicine, Yamagata University School of Medicine, Yamagata, JAPAN
2. Otsuka Pharmaceutical Company, Tokushima, JAPAN
3. Department of Internal Medicine, National Cardiovascular Center, Osaka, JAPAN
Received 2008-6-20; Accepted 2009-4-14; Published 2009-4-16
Abstract
We investigated the potential usefulness of vesnarinone, a novel cytokine inhibitor, for the treatment of lung fibrosis using a murine model of bleomycin (BLM)-induced pulmonary fibrosis. Mice were fed a control diet (n=42), or a diet containing low (n=42) or high (n=42) dose of vesnarinone. Dietary intake of vesnarinone minimized the BLM toxicity as reflected by significant decreases in numbers of inflammatory cells, KC, and soluble TNF receptors in the bronchoalveolar lavage fluid. A quantitative evaluation of histology demonstrated significantly mild lung parenchymal lesions in BLM-treated mice fed with diet containing high dose of vesnarinone than in the control diet group. Consistent with the histopathology, hydroxyproline levels in lung tissue from BLM-treated mice fed with diet containing vesnarinone were significantly lower than that from mice fed with control diet. We concluded that vesnarinone inhibits BLM-induced pulmonary fibrosis, at least in part, by the inhibition of acute lung injuries in the early phase.
Keywords: acute lung injury, bleomycin, chemokine, pulmonary fibrosis, vesnarinone
Introduction
Pulmonary fibrosis is an intractable paranchymal lung disease characterized by persistent alveolitis, accumulation of connective tissue and extracellular matrix [1-4]. The pathogenesis of pulmonary fibrosis is not well elucidated. Since the pathological changes of alveolitis and fibrosis are similar to pulmonary fibrosis seen in humans, bleomycin (BLM) models of pulmonary fibrosis have been investigated in various animal species with different routes of administration [4-6]. Although a variety of drugs, compounds, and antibodies have been investigated for their effectiveness, few showed clinical efficacy for preventing the development of pulmonary fibrosis [1-6].
Vesnarinone is a quinolinone derivative, and its pharmacodynamic effects include inhibition of phosphodiesterase III (PDE3) activity, increases in calcium flux and decreases in potassium flux [7-9]. Recently, a novel cytokine-inhibiting activity of vesnarinone has been focused attention, because of the potential mechanisms by which vesnarinone reduces mortality in a murine model of myocarditis [10] or lethal endotoxemia [11]. Indeed, several in vitro studies have shown that vesnarinone suppresses the production of TNF-α and IL-6 in various human cell lines, including peripheral lymphocytes, monocytes, and T-cell lines [12]. Moreover, an in vivo study of endotoxemia in rabbits showed that IV vesnarinone reduced the circulating levels of TNF-α [13]. Cumulative evidence showed that a variety of cytokine are involved in the pathogenesis of pulmonary fibrosis [3]. Thus, we investigated the effects of oral intake of vesnarinone on the development of pulmonary fibrosis in BLM-induced lung injury in mice.
Material and Methods
Animals and Animal Treatments
Male ICR mice, 8 to 10 weeks of age, were purchased from Japan Clea Co. (Tokyo Japan). These animals were divided into the six groups, as shown in table 1. Vesnarinone (Otsuka Pharmaceut. Comp. Tokushima, Japan) were added to the diet. From the daily food intake of mice, we estimated the content of vesnarinone at 100 mg/kg/day (groups L and LB) or 200 mg/kg/day (groups H and HB).
To induce fibrotic changes in the lungs of mice in groups CB, LB, and HB, BLM (150 mg/kg) was intravenously administrated via a tail vein under anesthesia with intraperitoneal injection of thiopental sodium (150mg/kg) on day 0. As a control, physiological salt solution was infused intravenously infused in the groups C, L, and H. All animals were fed the same diet from day -2 to day 28. Thus, the mice were killed at 7 and 28 days after BLM treatment with excess thiopental sodium (450 mg/kg i.p.). Thorax was opened, and lungs were removed for histological studies.
Characteristics of subjects.
| Group Description | Group Name | Vesnarinone (mg/kg) | Bleomycin (mg/kg) | Number of Mouse |
|---|---|---|---|---|
| Control | C | - | - | 18 |
| Low dose | L | 100* | - | 18 |
| High dose | H | 200** | - | 18 |
| Control-bleomycin | CB | - | 150 | 24 |
| Low dose-bleomycin | LB | 100* | 150 | 24 |
| High dose-bleomycin | HB | 200** | 150 | 24 |
*Estimated dose based on 0.083% vesnarinone in the diet.
**Estimated dose based on 0.165% vesnarinone in the diet.
Plasma Concentration of Vesnarinone
Plasma concentrations of vesnarinone were measured on the 7 day of the oral administration. Under anesthesia, whole blood was obtained by direct puncture of right ventricle. Plasma was isolated by centrifugation (3000 rpm) and the concentrations of vesnarinone were determined by high-performance liquid chromatography as previously described [14].
Bronchoalveolar Lavage (BAL)
We performed BAL on 5 to 8 mice in each group on 7 days after the bleomycin infusion. Under anesthesia with intraperitoneal thiopental sodium (150 mg/kg), a 16-gauge canula was wedged into the trachea. 3 ml of phosphate-buffered saline (PBS) was infused into the canula and was recovered, gently to avoid artificial lung injury due to hyperinflation. Cytological preparations were made using centrifugation (Cytospin 2; Shandon, Pittsburgh, PA), and were stained with modified May-Giemsa stain (Diff Quick; American Science Products, McGaw Park, IL). Differential all counts were performed on 200 cells per sample. Cells were also gathered from bronchoalveolar lavage fluids (BALF) by centrifugation at 200 × g for 10 min, and its supernatants were stored at -80ºC until evaluation.
Biochemical Analysis of BALF and Plasma
Total protein levels were evaluated by modified Bradford method [15]. Since tumor necrosis factor (TNF)-α, KC, and macrophage inflammatory protein-2 (MIP-2) are mediators of tissue injury and repair and associated with lung fibrosis, Murine TNF-α (Quantikine; R&D Systems, Minneapolis, MN), murine KC, murine MIP-2, murine soluble TNF-receptor 55 (sTNF-R55), and murine soluble TNF-receptor 75 (sTNF-R75) (all from R&D Systems, Minneapolis, MN) in BALF and plasma were measured by an enzyme-linked immunosorbent assay (ELISA). Hyaluronic acid, which increases in lung injury including fibrotic lung diseases, were quantified using enzyme-linked hyaluronic acid binding protein method (Read Medical Products inc., Westminster, CO). The albumin concentration in BALF was also determined by ELISA (Albuwell; Exocell Inc., Philadelphia, PA).
Histological Analysis
On day 28, both lungs were fixed by an inflation-fixation method in 10% buffered formaldehyde under constant positive pressure (10 cm water pressure) in preparation for histological examination [16]. The fixed lungs were sectioned sagittally, embedded in paraffin, and stained air way with Elastica-Masson or hematoxylin-eosin. For the area analysis of fibrotic changes, a quantitative fibrotic scale (Ashcroft scale) was used [16]. A numerical fibrotic score (Ashcroft scale) was obtained as follows; the severity of the fibrotic changes in each lung section was given as the mean score from the observed microscopic fields. More than 25 fields within each lung section were observed at a magnification of ×100, and each field was assessed individually for severity and allotted a score from 0 (normal) to 8 (total fibrosis). The severity score for each field were averaged and are presented as the average for each lung section. To avoid bias, all histologic specimens were evaluated in a blinded fashion. Each specimen was scored independently by two observers, including a histopathologist; finally, the mean of their individual scores was taken as the fibrotic score.
Hydroxyproline Assay
On day 28, to estimate total lung collagen content, hydroxyproline was measured according to a method previously described with modifications [17]. Briefly, the lung was gently perfused with 3 ml of PBS from the right ventricle. Both lungs were then excised and homogenized (Tissue Tearor; Biospec Products, Inc., Bartlesville, OK) in 2 ml of PBS. 1 ml aliquot was desiccated using a rotary vacuum pump (Savant Instruments, Inc., Farmingdale, NY) and then hydrolyzed in 6 N HCl at 110ºC for 12 hr. Next, 50-μl aliquots were added into 1 ml of 1.4% chloramine T (Sigma Chemical Co., St. Louis, MO), 10% n-propanol, and 0.5 M sodium acetate (pH 6.0). After 20 min of incubation at room temperature, 1 ml of Erlich's solution (1 M p-dimethylaminobenzaldehyde in 70% n-propanol, 20% perchloric acid) was added and allowed to incubate at 65ºC for 15 min. Finally, the level of absorbance was measured at 550 nm using the 100 μl of reaction product and the amount of hydroxyproline was determined against a standard curve generated using known concentrations of hydroxyproline (Sigma Chemical Co.).
Statistical Analysis
Results are reported as mean ± SEM. The data from each subject were first compared using Kruskal-Wallis nonparametric one-way analysis of variance. When significant, the differences between the groups were further compared using nonparametric Mann-Whitney U tests and the Steel-Dwass test with an adjustment of the p values (p<0.05). Correlations were tested by calculating the Spearman correlation coefficient. P <0.05 was taken as the level of statistical significance.
Results
Plasma Concentrations of Vesnarinone
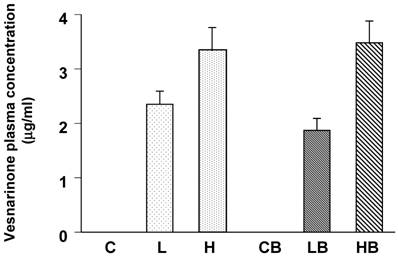
We confirmed the lowest concentration of vesnarinone to be about 2 μg/ml in plasma from the L and LB groups (Fig. 1). Higher concentrations of vesnarinone were also detected in both the H and HB groups (Fig. 1). These levels were half of the plasma concentrations seen clinically when given to patients at a dose of 30 mg/day for the treatment of congestive heart failure [18].
Mouse plasma concentrations of vesnarinone on Day 7 after bleomycin treatment (C; n=5, L; n=5, H; n=5, CB; n=5, LB; n=5, HB; n=5). Data shown are means ± SE.
Analysis of BALF
As shown in Table 2, total cell counts in BALF were significantly high on 7 days after BLM treatment (group CB) when these were compared with those from control group (group C; p<0.01). Significant increases in macrophages, lymphocytes and neutrophils were also noted in BALF from group CB (p<0.01). Importantly, oral intake of vesnarinone at both doses reduced these increases in cell numbers of all cell types in group CB. Oral intake of vesnarinone alone (groups L and H) showed no change in the cellular profiles in BALF of group C.
Cell concentration in BALF 7 days after BLM administration.
| Total cell (×104/ml) | Macrophages (×104/ml) | Neutrophils (×104/ml) | Lymphocytes (×104/ml) | Total Protein (mg/ml) | Albumin (mg/ml) | |
|---|---|---|---|---|---|---|
| C | 10.0 ± 1.7 | 10.0 ± 1.7 | 0.00 ± 0.0 | 0.00 ± 0.0 | 118.0 ± 33.5 | 23.4 ± 4.4 |
| L | 4.67 ± 1.0 | 4.47 ± 0.9 | 0.06 ± 0.0 | 0.14 ± 0.1 | 101.2 ± 22.7 | 23.1 ± 2.5 |
| H | 5.33 ± 0.8 | 5.18 ± 0.8 | 0.09 ± 0.0 | 0.06 ± 0.0 | 91.6 ± 15.1 | 23.1 ± 2.5 |
| CB | 37.7 ± 2.9 | 34.3 ± 2.6 | 1.90 ± 0.4 | 1.50 ± 0.2 | 2251 ± 529 | 168.0 ± 18.5 |
| LB | 22.0 ± 2.6** | 20.6 ± 2.4** | 0.71 ± 0.2** | 0.66 ± 0.1** | 741.3 ± 138** | 90.6 ± 12.0** |
| HB | 19.4 ± 2.2** | 18.6 ± 2.2** | 0.62 ± 0.2** | 0.22 ± 0.1** | 664.4 ± 100** | 108.1 ± 16.0** |
C; n=6, L; n=6, H; n=6, CB; n=8, LB; n=8, HB; n=8. Data are shown as mean ± SE.
**p<0.01 compared with group CB
Total protein and albumin contents in BALF were increased after BLM treatment (group CB) (Table 2). This increase was significantly inhibited by vesnarinone.
The levels of KC, sTNF-R55, sTNF-R75, and hyaluronic acid in groups LB and HB were markedly suppressed (p<0.01) as compared with group CB (Table 3).
Cytokine and chemokine in BALF, 7 days after BLM administration.
| TNF-α (pg/ml) | KC (pg/ml) | MIP-2 (pg/ml) | sTNF-R55 (pg/ml) | sTNF-R75 (pg/ml) | Hyaluronic acid (ng/ml) | |
|---|---|---|---|---|---|---|
| C | 2.2 ± 2.2 | 0.0 ± 0.0 | 0.5 ± 0.3 | 32.6 ± 16.8 | 134.4 ± 16.6 | 2.0 ± 1.3 |
| L | 4.1 ± 2.7 | 0.0 ± 0.0 | 0.7 ± 0.3 | 15.0 ± 7.4 | 145.6 ± 11.8 | 3.3 ± 3.3 |
| H | 0.0 ± 0.0 | 1.2 ± 1.2 | 1.2 ± 0.5 | 0.0 ± 0.0 | 223.3 ± 40.1 | 0.0 ± 0.0 |
| CB | 11.0 ± 3.1 | 31.6 ± 5.5 | 0.6 ± 0.3 | 105.6 ± 14.0 | 3486 ± 476 | 493.1 ± 200 |
| LB | 13.6 ± 2.5 | 7.5 ± 2.6** | 0.6 ± 0.3 | 33.4 ± 10.4** | 1497 ± 357** | 159.2 ± 87.2* |
| HB | 5.9 ± 2.5 | 9.9 ± 2.0** | 0.4 ± 0.2 | 52.8 ± 15.4** | 223 ± 40.1** | 128.0 ± 64.3* |
C; n=5, L; n=5, H; n=5, CB; n=8, LB; n=8, HB; n=8. Data are shown as mean ± SE.
**p<0.01 compared with CB, *p<0.05 compared with group CB
Histologic Finding and Morphometry of Fibrosis
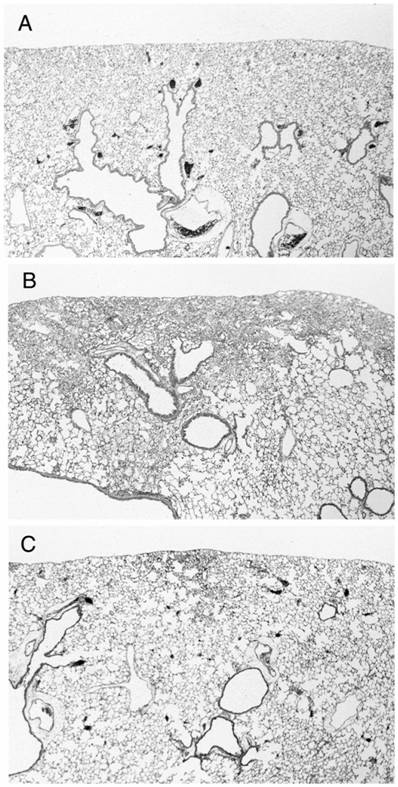
There were no pathological changes in lungs from group C (Fig. 2A). In samples 4 weeks after the treatment revealed focal fibrotic lesions primarily in the subpleural and occasionally in the perivascular areas with consolidation of lung parenchyma, loss of alveolar architecture, and increased cellularity with alveolar macrophages (Fig. 2B).
In contrast to the findings in mice treated with BLM alone, histologic findings at 4 weeks with simultaneous administration of vesnarinone revealed that fibrotic lesions were less focal in the subpleural areas, and fibrotic changes were milder in degree than in mice treated with BLM alone (Fig. 2C).
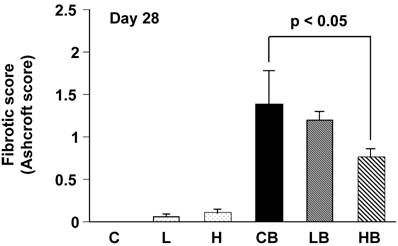
The overall level of the fibrotic change was quantified by the numerical score (Ashcroft score). The scores in groups CB and HB were 1.4 ± 0.4 and 0.7 ± 0.1, respectively. A significant difference in the scores between two groups was demonstrated (Fig. 3).
Representative histological findings of the lungs from control mouse (A: group C, n=4), mouse treated with bleomycin alone (B: group CB, n=6), and bleomycin-treated mouse with simultaneous administration of vesnarinone (C: group HB, n=6).
Quantitative evaluation of fibrotic changes (Ashcroft scale) treated with bleomycin and vesnarinone. Fibrotic score (Ashcroft score) was obtained with a continuous numerical scale for determining the degree of fibrotic changes. Each grade was scored on a scale from 0 to 8, and the average of microscopic field scores was used for comparison (x 100). C; n=4, L; n=4, H; n=4, CB; n=6, LB; n=6, HB; n=6. Data shown are means ± SE.
Collagen Content
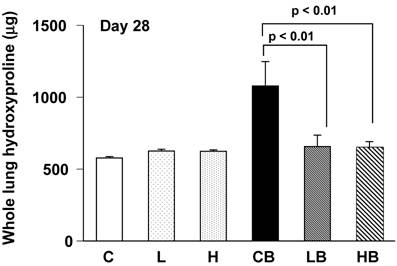
Collagen content was assessed by measuring hydroxyproline in the total lungs 4 weeks after BLM treatment. The hydroxyproline levels in BLM-treated lungs (group CB) were significantly increased from control mice (group C). The hydroxyproline content was lower in lungs treated with BLM and vesnarinone (group LB and HB) than in lungs treated with BLM alone (group CB) (Fig. 4). Mice treated with saline and vesnarinone (group L, H) showed minor increases in pulmonary hydroxyproline content compared with control mice (group C), although no statistical significance was found.
Hydroxyproline content in bleomycin and vesnarinone-treated lungs. To evaluate total lung collagen, hydroxyproline was extracted from lungs of mouse. C; n=5, L; n=5, H; n=5, CB; n=5, LB; n=5, HB; n=5. Data are shown as mean ± SE.
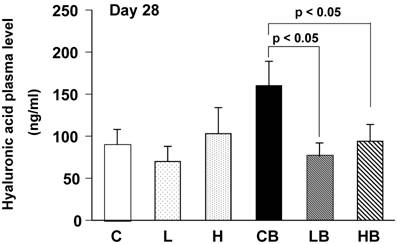
Circulating Hyaluronic Acid Levels
Plasma hyaluronic acid contents were significantly increased in mice treated with BLM alone, reflecting fibrotic changes in the lungs (Fig. 5). Oral administration of vesnarinone ameliorated these levels in BLM-treated mice.
Mouse plasma concentrations of hyaluronic acid on Day 28. Levels of hyaluronic acid in plasma of each mouse were quantified using enzyme-linked hyaluronic acid binding protein method. C; n=12, L; n=12, H; n=12, CB; n=12, LB; n=12, HB; n=12. Data are shown as mean ± SE.
Discussion
In the present study, we investigated whether vesnarinone, a potential cytokine inhibitor, has a protective effect against the fibrotic changes induced by BLM administration in mice. Our study demonstrated that vesnarinone inhibited the acute lung injuries, possibly by blocking the production of KC, and ameliorated the development of pulmonary fibrosis, since it is well known that the degree of the pulmonary fibrosis is dependent on the severity of initial lung injury [19, 20]. Although inhibition of cytokine production by vesnarinone has been shown in various models [10, 12, 21-25], little is known about the use of vesnarinone for the treatment of lung injury or the development of pulmonary fibrosis.
In 7 days after BLM treatment, elevated levels of cell numbers of all kinds of inflammatory cells, and protein and albumin contents in BALF were noted in CB group. Both low and high doses of vesnarinone intake significantly decreased all these levels. In addition, biochemical markers for lung inflammation in BALF including a neutrophilic chemokine, KC, sTNF-Rs, and hyaluronic acids, all which were increased in CB mice, were decreased in LB and HB mice. These data clearly supported the anti-cytokine and chemokine activities of vesnarinone [10, 12, 21-25]. Levels of MIP-2 in BALF were not different among the groups. This data suggested that some restricted chemokines are involved in the early inflammation of BLM-induced lung injury.
Oral administration of vesnarinone did not suppress the levels of TNF-α in BALF (LB and HB mice). The similar result was reported in a study in which acute lung injury caused by lipopolysaccharide and zymosan was suppressed by a type 4 PDE inhibitor, independent of TNF inhibition [26]. Levels of two sTNF-Rs, which are thought to inhibit TNF activities, were significantly decreased in BALF compared with LB and HB mice. Decreased shedding of both type sTNF-Rs may reflect the inhibition of the acute lung injuries induced by BLM, since a variety of inflammatory stimuli—including cytokines, oxidants, and protease—are known to shed sTNF-Rs [27, 28].
In 28 days after BLM administration, our pathological data demonstrated that dietary intake of the high dose, but not the low dose, of vesnarinone significantly reduced the pathological fibrotic changes induced by BLM. In contrast, the levels of both hydroxyproline and hyaluronic acid in lung tissue were significantly decreased in LB and HB mice than in CB mice. These differences in the inhibitory capacity between LB and HB mice may be explained by the fact that lung morphological changes (Ashcroft score) do not always parallel the changes in biochemical markers (hydroxyproline and hyaluronic acid content) in the pulmonary fibrosis [29].
Vesnarinone has a variety of functions including the inhibition of PDE3, increased inward calcium flux, reduced potassium flux, and a novel cytokine inhibiting activity [12, 21-25]. In addition, vesnarinone is reported to inhibit the increase in natural killer cell activity after viral infection [12]. Vesnarinone inhibits E-selectin expression in human umbilical vein endothelial cells [30]. Since nitric oxide is one of the important mediators responsible for the development of lung injury, vesnarinone may ameliorate BLM-induced lung injury by inhibiting the induction of nitric oxide syntheses [22, 23]. Furthermore, a recent study has demonstrated that vesnarinone inhibits the secretion of hyaluronan in myofibroblasts by specifically suppressing hyaluronan synthase activity [31]. All these anti-inflammatory effects of vesnarinone may play roles in the inhibition of BLM-induced lung injury. Although amelioration of experimental acute lung injury has been reported using a nonspecific PDE inhibitor, pentoxyfylline [32], and a PDE4 inhibitor, rolipram [26], vesnarinone may have a specific activity for the suppression of cytokine production [10, 12, 21-25]. Since the degree of the pulmonary fibrosis is closely related to the severity of initial acute lung injury in patients with acute respiratory distress syndrome [19, 20], vesnarinone would ameliorate the development of pulmonary fibrosis due to the suppression of BLM-induced acute lung injury. However, precise mechanisms of amelioration of BLM-induced pulmonary fibrosis by vesnarinone remain to be elucidated.
Unique activities of vesnarinone including inhibition of Fas expression and apoptosis [24] may also ameliorate fibrotic change in mice since ligation of Fas antigen induces apoptosis in lung epithelial cells and develops pulmonary fibrosis [33]. Thus it is possible that vesnarinone ameliorates fibrotic changes by inhibiting apoptosis in lung epithelial cells. Additionally, it has been reported that vesnarinone has antiproliferative effects and apoptosis-inducing activities in various tumor cells. Since these effects are related to known activities of vesnarinone, which induces a negative growth factor, TSC-22 [34], or a cyclin-dependent kinase inhibitor, p21WAF1 [35] and inhibits expression of interleukin-8 [36], the inhibition of fibroblast growth may be mediated by these mechanisms in the development process on BLM-induced pulmonary fibrosis.
In conclusion, vesnarinone is one of the potential therapeutic agents for the treatment of lung fibrosis. Further experiments will be required to understand how this drug reduces the fibrotic response.
Acknowledgements
This study was supported in part by a Grant-in-Aid for Scientific Research (C) from the Ministry of Education, Science, Sports and Culture of Japan (No. 11557044). The authors wish to thank Mr. Arjuna J. Celaya for his English help.
Conflict of Interest
All authors do not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
1. Panos RJ, King TE. Idiopathic pulmonary fibrosis. In: (ed.) Lynch JP, DeRemee RA. Immunologically mediated pulmonary fibrosis. Philadelphia: Lippincott. 1991:1-39
2. Visscher DW, Myers JL. Histologic spectrum of idiopathic interstitial pneumonias. Proc Am Thorac Soc. 2006;3:322-329
3. Wolff G, Crystal RG. Biology of pulmonary fibrosis. In: (ed.) Crystal RG, West JB, Barnes PJ, Weibel ER. The Lung. Philadelphia: Lippincott. 1997:2509-2524
4. Alan F, Goldstein RH. Animal models of pulmonary fibrosis. In: (ed.) Crystal RG, West JB, Barnes PJ, Weibel ER. The Lung. Philadelphia: Lippincott. 1997:2525-2536
5. Moeller A, Ask K, Warburton D, Gauldie J, Kolb M. The bleomycin animal model: a useful tool to investigate treatment options for idiopathic pulmonary fibrosis? Int J Biochem Cell Biol. 2008;40:362-382
6. Nakamura H, Sato S, Takahashi K. Effect of vitamin E deficiency on bleomycin-induced pulmonary fibrosis in the hamster. Lung. 1988;166:161-176
7. Hosokawa T, Mori T, Fujiki H, Kinoshita S, Takemoto K, Imaizumi T, Noda T, Ohura M, Tominaga M, Yabuuchi Y. Cardiovascular actions of OPC-18790: a novel positive inotropic agent with little chronotropic action. Heart Vessels. 1992;7:66-75
8. Itoh H, Kusagawa M, Shimomura A, Suga T, Ito M, Konishi T, Nakano T. Ca2+-dependent and Ca2+-independent vasorelaxation induced by cardiotonic phosphodiesterase inhibitors. Eur J Pharmacol. 1993;240:57-66
9. Toyama G, Kamiya K, Cheng J, Lee JK, Suzuki R, Kodama I. Vesnarinone prolongs action potential duration without reverse frequency dependence in rabbit ventricular muscle by blocking the delayed rectifier K current. Circulation. 1997;18:3696-3703
10. Matsui S, Matsumori A, Matoba Y, Uchida A, Sasayama S. Treatment of virus-induced myocardial injury with a novel immunomodulating agent, vesnarinone. J Clin Invest. 1994;94:1212-1217
11. Matsui S, Matsumori A, Sasayama S. Vesnarinone prolongs survival and reduces lethality in a murine model of lethal endotoxemia. Life Sci. 1994;55:1735-1741
12. Sasayama S, Matsumori A. Vesnarinone: a potential cytokine inhibitor. J Cardiac Fail. 1996;2:251-258
13. Takeuchi K, del Nido PJ, Ibrahim AE, Cao-Danh H, Friehs I, Glynn P, Poutias D, Cowan DB, McGowan FX Jr. Vesnarinone and amrinone reduce the systemic inflammatory response syndrome. Thorac Cardiovasc Surg. 1999;117:375-382
14. Miyamoto G, Sasabe H. Pharmacokinetics of a new positive inotropic agent, 3, 4-Dihydro-6- [4- (3, 4-dimethoxybenzoyl) -1-piperazinyl] S-2 (1H) -quinolinone (OPC-8212), in the rat, rabbit, beagle dog and rhesus monkey. Arzneim -Forsch /Drug Res. 1984;34:394-402
15. Kruger NJ. The Bradford method for protein quantitation. Methods Mol Biol. 1994;32:9-15
16. Ashcroft T, Simpson JM, Timbell V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J Clin Pathol. 1988;41:467-470
17. Woessner JF Jr. The determination of hydroxyproline in tissue and protein samples containing small proportions of this amino acid. Arch Biochem Biophys. 1961;93:440-447
18. Ohnishi A, Ishizaki T. Pharmacokinetic profile of OPC=8212 in human: a new, non-glycoside, inotropic agent. J Clin Pharmacol. 1988;28:719-726
19. Martin C, Papazian L, Payan MJ, Saux P, Gouin F. Pulmonary fibrosis correlates with outcome in adult respiratory distress syndrome. A study in mechanically ventilated patients. Chest. 1995;107:196-200
20. Chida M, Ono S, Hoshikawa Y, Kondo T. Subclinical idiopathic pulmonary fibrosis is also a risk factor of postoperative acute respiratory distress syndrome following thoracic surgery. Eur J Cardiothorac Surg. 2008;34:878-881
21. Matsumori A, Shioi T, Yamada T, Matsui S, Sasayama S. Vesnarinone, a new inotropic agent, inhibits cytokine production by stimulated human blood from patients with heart failure. Circulation. 1994;89:955-958
22. Matsumori A, Okada I, Shioi T, Furukawa Y, Nakamura T, Ono K, Iwasaki A, Sasayama S. Inotropic agents differentially inhibit the induction of nitric oxide synthase by endotoxin in cultured macrophages. Life Sci. 1996;59:121-125
23. Hattori Y, So S, Hattori S, Kasai K, Shimoda S. Vesnarinone inhibits induction of nitric oxide synthase in J774 macrophages and rat cardiac myocytes in culture. Cardiovasc Res. 1995;30:187-192
24. Oyaizu N, McCloskey TW, Than S, Pahwa S. Inhibition of CD4 cross-linking-induced lymphocytes apoptosis by vesnarinone as a novel immunomodulating agent: vesnarinone inhibits Fas expression and apoptosis by blocking cytokine secretion. Blood. 1996;87:2361-2368
25. Manna SK, Aggarwal BB. Vesnarinone suppresses TNF-induced activation of NF-κB, c-Jun kinase, and apoptosis. J Immunol. 2000;164:5815-5825
26. Miotla JM, Teixeira MM, Hellewell PG. Suppression of acute lung injury in mice by an inhibitor of phosphodiesterase type 4. Am J Respir Cell Mol Biol. 1998;18:411-420
27. Nakamura H, Hino T, Kato S, Shibata Y, Takahashi H, Tomoike H. Tumor necrosis factor receptor gene expressions and shedding in human whole lung tissue and pulmonary epithelium. Eur Respir J. 1996;9:1643-1647
28. Hino T, Nakamura H, Abe S, Saito H, Inage M, Terashita K, Kato S, Tomoike H. Hydrogen peroxide enhances shedding of type I soluble tumor necrosis factor receptor from pulmonary epithelial cells. Am J Respir Cell Mol Biol. 1999;20:122-128
29. Saldiva PH, Delmonte VC, de Carvalho CR, Kairalla RA, Auler Junior JO. Histochemical evaluation of lung collagen content in acute and chronic interstitial diseases. Chest. 1989;95:953-957
30. Sato Y, Matsumori A, Sasayama S. Inotropic agent vesnarinone inhibits cytokine production and E-selectin expression in human umbilical vein endothelial cells. J Mol Cell Cardiol. 1995;27:2265-2273
31. Ueki N, Taguchi T, Takahashi M, Adachi M, Ohkawa T, Amuro Y, Hada T, Higashino K. Inhibition of hyaluronan synthesis by vesnarinone in cultured human myofibroblasts. Biochim Biophys Acta. 2000;1495:160-167
32. Marques LJ, Zheng L, Poulakis N, Guzman J, Costabel U. Pentoxyfylline inhibits TNF-α production from human alveolar macrophages. Am J Respir Crit Care Med. 1999;159:508-511
33. Hagimoto N, Kuwano K, Kunitake R, Hara N. Apoptosis and expression of Fas/Fas ligand mRNA in bleomycin-induced pulmonary fibrosis in mice. Am J Respir Cell Mol Biol. 1997;16:91-101
34. Kawamata H, Nakashiro K, Uchida D, Hino S, Omotehara F, Yoshida H, Sato M. Induction of TSC-22 by treatment with a new anti-cancer drug, vesnarinone, in a human salivary gland cancer cell. Br J Cancer. 1998;77:71-78
35. Sato M, Kawamata H, Harada K, Nakashiro K, Ikeda Y, Gohda H, Yoshida H, Nishida T, Ono K, Kinoshita M, Adachi M. Induction of cyclin-dependent kinase inhibitor, p21 WAF1, by treatment with 3,4-dihydro-6-[4-(3,4)-dimethoxybenzoyl]-1-piperazinyl]-2(1H)-quinoline (vesnarinone) in a human salivary cancer cell line with mutant p53 gene. Cancer Lett. 1997;112:181-189
36. Harada K, Supriatno, Yoshida H, Sato M. Vesnarinone inhibits angiogenesis and tumorigenicity of human oral squamous cell carcinoma cells by suppressing the expression of vascular endothelial growth factor and interleukin-8. Int J Oncol. 2005;27:1489-1497
Author contact
![]() Correspondence to: Makoto Sata, M.D., Ph.D., Division of Pulmonology (Cardiology), Department of Internal Medicine, Laboratory of Clinical Chemistry, National Cardiovascular Center, 5-7-1 Fujishiro-dai, Suita, Osaka, 565-8565 JAPAN. TEL: +81-6-6833-5012 (ext.2311); FAX: +81-6-6872-7486; E-mail: msatancvc.go.jp
Correspondence to: Makoto Sata, M.D., Ph.D., Division of Pulmonology (Cardiology), Department of Internal Medicine, Laboratory of Clinical Chemistry, National Cardiovascular Center, 5-7-1 Fujishiro-dai, Suita, Osaka, 565-8565 JAPAN. TEL: +81-6-6833-5012 (ext.2311); FAX: +81-6-6872-7486; E-mail: msatancvc.go.jp

 Global reach, higher impact
Global reach, higher impact