Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2009; 5(4):377-387. doi:10.7150/ijbs.5.377 This issue Cite
Research Paper
Chemoprevention of rat liver toxicity and carcinogenesis by Spirulina
1. Department of Pathology and Department of Genetics, Stanley S. Scott Cancer Center, Louisiana State University Health Science Center, New Orleans, Louisiana, USA;
2. Department of Ob Gyn and Biochemistry, Stanley S. Scott Cancer Center, Louisiana State University Health Science Center, New Orleans, Louisiana, USA;
3. Department of Zoology, Faculty of Science, Mansoura University, Mansoura, Egypt.
Received 2009-4-15; Accepted 2009-5-21; Published 2009-6-2
Abstract
Spirulina platensis (SP) is a filamentous cyanobacterium microalgae with potent dietary phyto-antioxidant, anti-inflammatory and anti-cancerous properties. The present study aimed to investigate the chemopreventive effect of SP against rat liver toxicity and carcinogenesis induced by dibutyl nitrosamine (DBN) precursors, and further characterized its underlying mechanisms of action in HepG2 cell line. Investigation by light and electron microscopy showed that DBN treatment induced severe liver injury and histopathological abnormalities, which were prevented by SP supplementation. The incidence of liver tumors was significantly reduced from 80 to 20% by SP. Immunohistochemical results indicated that both PCNA and p53 were highly expressed in the liver of DBN-treated rats, but were significantly reduced by SP supplementation. Molecular analysis indicated that SP treatment inhibited cell proliferation, which was accompanied by increased p21 and decreased Rb expression levels at 48hrs post-treatment. In addition, SP increased Bax and decreased Bcl-2 expression, indicating induction of apoptosis by 48hrs. This is the first report of the in vivo chemopreventive effect of SP against DBN-induced rat liver cytotoxicity and carcinogenesis, suggesting its potential use in chemoprevention of cancer.
Keywords: Spirulina platensis, Phyto-antioxidant, Liver toxicity, Hepatocellular carcinoma, Electron microscopy, p53
Introduction
Spirulina platensis (SP) is a cyanobacterium being used in many countries as nutritional supplement for human and animal consumption. SP has been labeled as a powerful food, rich in proteins, carbohydrates, polyunsaturated fatty acids, sterols and some more vital elements like calcium, iron, zinc, magnesium, manganese and selenium. It is a natural source of vitamin B12, vitamin E, ascorbic acid, tocopherols and whole spectrum of natural mixed carotene and xanthophyll phytopigments (1-3).
SP is well known for its anti-inflammatory and anti-cancerous properties. A hot water extract of SP has been orally administered to patients as an anti-cancer and anti-viral agent. SP is best known as an immune booster by stimulating natural killer (NK) cells and co-operative action of IL-12 and IL-18 for NK-mediated IFN gamma production (4). These SP-stimulated NK cells can fight illnesses other than cancer. SP hinders the growth of oral cancer. SP extract has been shown to inhibit tumor initiation in Syrian hamster cheek pouch mucosa painted with 7,12-dimethylbenz[a]anthracene (5). Such an inhibitory effect may be attributed to the repair of carcinogen-damaged DNA, and SP has been suggested as an efficient radical scavenger (6-9). Other studies have reported that the unique polysaccharides of SP enhance cell nucleus enzyme activity and potentiate the process of DNA repair (10, 11)
Since SP has many therapeutic roles including anticancer properties, the present study is the first of the in vivo effects of SP on DBN-induced hepatotoxicity and carcinogenesis in the rat liver and its possible underlying molecular mechanisms on cell proliferation and apoptosis using HepG2 human liver cancer cell line as a model.
Materials and Methods
Cells, compounds and chemicals. HepG2 hepatocellular carcinoma cells kindly provided by Dr. Hollenbach (LSU Health Sciences Center, New Orleans) were cultured in DMEM with 4500 mg/l glucose and supplemented with 10% fetal bovine serum and 1% penicillin and streptomycin (0.1 ml/well). Mesenchymal stem cells (MSCs) kindly provided by Dr. David Welsh (LSU Health Sciences Center, New Orleans) were cultured in MEM-Alpha (Cat # 12561, GIBCO, CA, USA) supplemented with 16.5% of Bovine Serum Albumin (Cat# S-11550, Atlanta Biologicals, GA, USA) and 1% L-Glutamine (GIBCO, Cat# 25030, GIBCO, CA, USA). SP algae used in this work was cultured in our laboratory of algal technology at Mansoura University, under optimal conditions on Zarrouk medium as previously described (12). Algal mass was harvested every 3 weeks by continuous centrifuge, air dried and ground to powder form. Chemicals were supplied by Sigma Chemical Co. (St Louis, MO) unless otherwise stated. The dibutylnitrosoamine solution (DBN) was prepared by mixing 2000 ppm of sodium nitrite (NaNO2) with 1000 ppm of dibutylamine (DBA) in drinking water.
Alamar Blue Cell proliferation assay. HepG2 cells were seeded in 96-well plates in appropriate medium and incubated at 37 ºC and 5% CO2. SP was applied at different concentrations, in triplicates, to the wells to achieve final concentrations ranging from 0 to 800 µg/ml. The suppression of cell growth was determined using the Alamar Blue assay (Alamar Biosciences, Sacramento, CA) as previously reported (13). Briefly, the concentration of alamar blue added to the cells was in an amount equal to 1% of the total medium volume. The oxidized form of this dye, which is non-toxic, is converted to the reduced form by mitochondrial enzyme activity of the viable cells. The shift in fluorescence was measured at 600 nm with excitation wavelength at 570 nm in a Fluorometer (LabSystems Fluoreskan-II), 4 hours after addition of the dye and the results expressed as percent of controls. This method provides an accurate measure of the number of cells metabolizing the dye to a reduced state and is a sensitive and accurate means of monitoring cell proliferation. In order to ascertain the specificity of observed effects the MSCs were used as an example of non-cancerous cell type. MSCs were treated with the highest dose of SP (800 µg/ml), and growth of cells was evaluated using the alamar blue assay as described above.
Western blot analysis of molecular pathways affected by SP treatment. HepG2 cells were treated with increasing concentrations of SP ranging from 0 to 800μg/ml. Western blot analysis was performed as previously described (14). Proteins were extracted with a lysis buffer and precipitated using RIPA buffer (Santa Cruz Biotechnology, CA, USA). Protein samples (60μg) were boiled for 5 min in an equal volume of reducing buffer (5mM Tris/HCl pH 7.4, 4% (w/v) SDS, 20% (v/v) glycerol, 10% (v/v) mercaptoethanol, 0.2% (w/v) bromophenol blue), resolved on 12% polyacrylamide gels, electroblotted onto nitrocellulose membranes. The antibodies used were monoclonal anti-Rb, p53, p21, CDK6, BAX, and Bcl-2 antibody. After probing with the appropriate secondary antibody, nitrocellulose membranes were developed using Supersignal horseradish peroxidase according to the manufacturer's instructions (Pierce Thermo Scientific, IL, USA) on Gel Doc (BioRad, CA, USA). Equal loading of the protein samples was assessed by re-probing the membrane with a 1:2000 dilution of β-actin antibody. All antibodies were supplied by Santa Cruz Biotechnology, CA, USA except anti-Rb antibody was supplied by BD Pharmingen, CA, USA.
Animal Studies. This study was approved by the local ethical committee. Healthy adult male albino rats (Rattus rattus; 120±5g) were housed and maintained on 12 hours light/dark cycle under a constant temperature of 25±1oC with free access to food and drinking water. Rats were acclimatized to laboratory conditions for one week prior to experiments. Animals were randomly divided into four groups (n=10 per group) and treated for a period of 12 months as follows: 1) Group 1 (Untreated control group): animals were fed on a standard diet and given water ad. libitum for 12 months; 2) Group 2 (SP-treated group): animals were fed on a standard diet mixed with 1% SP powder, and given water ad. libtium for 12 months; 3) Group 3 (DBN-treated group): were fed on the same standard diet and given DBN precursors in their drinking water as described above, for 6 months followed by normal drinking water ( next 6 months) until the termination of the experiment; and 4) Group 4 (DBN + SP-treated group): In addition to DBN-treatment (similar to Group 3), these animals were fed on a standard diet mixed with 1% SP powder throughout the experimental period (12 months). Body weight of the animal was recorded every month, and at the end of the 12 months treatment, animals were sacrificed, liver tissues were collected, fixed in 10% formalin and then paraffin embedded for histology and for tumor examination.
Histology and Immunohistochemistry (IHC). Four µm sections of paraffin-embedded liver tissue were analyzed for expression of p53 and PCNA by immunohistochemistry as described previously (14). Tissue sections were incubated with primary anti-p53 and anti-PCNA antibodies (Zymed Laboratories, USA). Incubation with appropriate secondary antibody was followed by direct diaminobenzidine staining and light counterstaining with hematoxylin. As a negative control, tissue sections were incubated with PBS replacing the primary antibody.
Electron Microscopy. The liver tissue was fixed, for 2 hours, in 2.5% glutaraldehyde buffered in 0.1 M cacodylate buffer (pH 7.2) at 4oC and post fixed in 1% cold osmium tetraoxide in 0.1M cacodylate at pH 7.2, for 3 hours. Ultrathin sections were obtained from specimens embedded in Lowicryl K4M resin after dehydration through graded ethanol series, substitution and polymerization at -200C. Ultrathin sections were obtained using an Ultracut S microtome (Leica, Vienna, Austria). Sections were mounted on 400-mesh collodion-carbon-coated nickel grids and examined with a Joel Electron Microscope (JAPAN) operating at 60 kV.
Statistical analysis. Data was presented as Mean ± S.E.M. of at least triplicates or replicates from three experiments and the data were analyzed statistically using Newman-Keuls multiple comparison test and Student's t-test using GraphPad Prism 2.01. Differences with P <0.05 were considered significant. All the experiments have been performed thrice and have obtained consistent results.
Results
In vitro studies
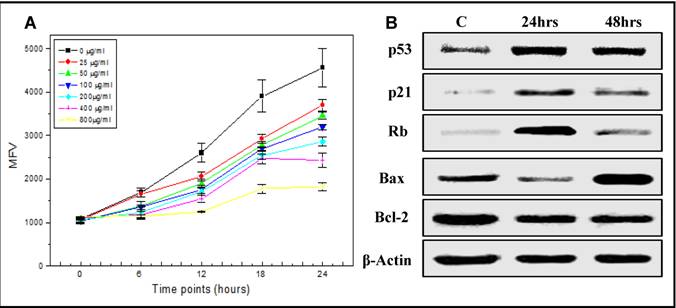
Effect of SP on Hep-G2 cell proliferation. Alamar blue cell proliferation assay was used to examine the cytotoxic effect of increasing doses of SP on Hep-G2 cells. The cells in the control group, treated only with the vehicle, reduced the dye in a linear time dependent manner (Figure 1A) over a 24 hr period. However, increasing concentrations of SP treatment reduced the metabolism (fluorescence)/proliferation rate in time and dose-dependent manner (Figure 1A), suggesting an induction of apoptosis and inhibition of cell proliferation in HepG2. However, no effect of SP was observed in MSC control cells. Based on these results, the concentration of 200µg of SP extract was used to treat HepG2 cells, and further elucidate the molecular mechanisms of action of SP involved in the regulation of cell cycle and apoptosis.
Effect of SP extract on cell cycle and apoptosis-related proteins. HepG2 cells were treated with 200 μg/ml of SP extract. Protein lysates collected at 24 and 48hrs post-treatment were analyzed by western blot to examine the effect of SP extract on the expression of proteins associated with cell cycle regulation (p53, p21Waf1/Cip1 and Rb) and apoptosis (p53, bcl-2 and Bax). Expression of p53 increased in response to SP and was maximal by 24 hrs followed by an increase of its direct downstream target, the cell cycle inhibitor, p21Waf1/Cip1 (Figure 1B). In contrast, the expression of Rb, which was increased by 24hrs, dropped significantly by 48hrs, suggesting cell cycle arrest and inhibition of cell proliferation by SP (Figure 1B). Furthermore, 48hrs post-SP treatment, the proapoptotic Bax was upregulated and the antiapoptotic bcl-2 downregulated leading to an increased Bax/bcl-2 ratio, indicating induction of apoptosis of HepG2 cells by SP (Figure 1B).
In vivo studies
The in vitro results described above suggest that SP inhibits growth and induces apoptosis of the hepatocellular carcinoma cell line HepG2. DBN-induced rat liver carcinogenesis model was used to test this observation in vivo.
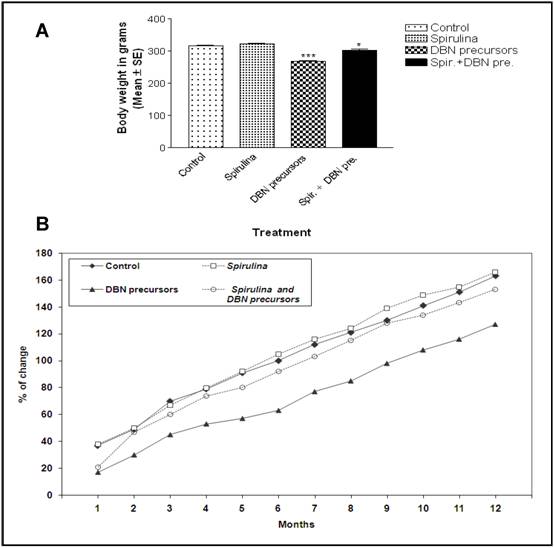
Changes in the body weight gain and tumor incidence. The body weight of each animal was recorded every month during the whole period of the experiment. While rats fed on SP containing diet had similar final body weight (Figure 2A) to controls, DBN-treated rats showed a significant reduction (p< 0.01). In addition, there was an increasing percentage of change in the body weight gain of the animal with time in all the groups described in this study (Figure 2B). However, DBN-treatment slowed significantly the change in the body weight gain as compared to other groups. SP supplementation with DBN overcame the loss in body weight loss, suggesting a protective effect of SP in these animals.
Effects of spirulina (SP) on proliferation (A) of the human hepatocellular carcinoma cells, HepG2, and on the expression (B) of genes associated with cell cycle and apoptosis mechanisms. A) Alamar blue cell proliferation assay was used to assess the cytotoxic effect of increasing doses of SP (0-800 µg/ml) on HepG2 cells. As described in materials and methods, the dye was reduced over a period of time and the fluorescence intensity was measured as mean fluorescence value (MFV). The cells in the control group, treated only with the vehicle, reduced the dye in a linear time dependent manner over a 24 hr period. Increasing concentrations of SP treatment reduced the metabolism (fluorescence)/proliferation rate in time and dose-dependent manner. B) A representative western blot analysis of expression of genes associated with cell cycle (p53, p21Waf1/Cip1, and Rb) and apoptosis (p53, Bax and Bcl-2) mechanisms after treatment of HepG2 with 200 µg/ml of SP for 24 and 48 hrs (C: untreated cells). Equal protein loading was confirmed by re-probing the membrane for β-actin expression.
Spirulina prevents the inhibitory effect of DBN on the final body weight (A) and the percentage of body weight change (B). A) The final body weight of each animal by the end of the duration of the experimental period (12 months) is given as mean ± SE. DBN-treatment reduced the final body weight, but spirulina supplementation partially reversed the decrease in the body weight induced by DBN (p< 0.01)*. (Spir: Spirulina). B) The body weight of each animal was recorded every month, mean values were calculated and the percentage of body weight change was determined.
The animals were also examined for DBN-induced tumors in the liver. While none of the rats from either control or SP groups developed liver tumors, 80% of the rats showed DBN-induced liver tumors (Table 1). However, SP treatment reduced liver tumor incidence to 20% in the DBN+SP group (Table 1). These results suggest that SP can prevent DBN-initiated tumor development in the rat liver.
Chemoprevention of Spirulina against liver tumorigenesis
| Groups (n=10) | % of animals with liver tumors |
|---|---|
| Control | 0% |
| Spirulina | 0% |
| DBN | 80% |
| DBN+Spirulina | 20% |
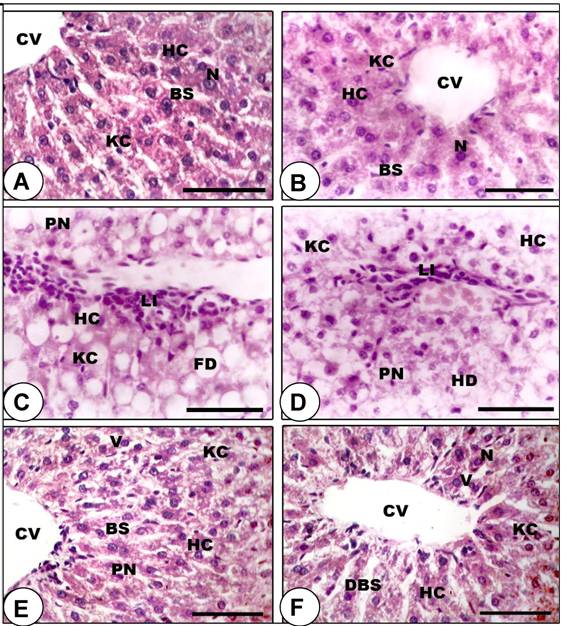
Histopathological analysis of the liver tissue. Animals were sacrificed, liver tissues were collected, fixed in 10% formalin and then paraffin embedded for histology. Light microscopic investigations revealed that control (Figure 3A) and SP-fed (Figure 3B) rats demonstrated normal polyhedral hepatocytes and the boundaries of the sinusoids showed a single layer of fenestrated endothelial cells and kupffer cells. However, the liver of rats treated with DBN showed damaged hepatocytes with manifestations of extensive cytoplasmic vacuolization, hydropic degeneration (oedema), and hyperchromatic nuclei (Figures 3C and 3D). SP-DBN treated rats (Figures 3E and 3F) demonstrated dilatation in blood sinusoids, slight vacuolization in the cytoplasm of the hepatocytes, few pyknotic nuclei and active kupffer cells in the liver. This data indicate a partial prevention of the effects of DBN by SP.
Prevention of DBN-induced histopathological abnormalities in rat liver by spirulina supplementation. A-B: Liver sections of control (A) and spirulina-treated (B) rats showing normal histological appearance of the liver, including central vein (CV), blood sinusoids (BS), hepatic cells (HC), kupffer cell (KC) and centrally located nuclei (N). C-D: Section of rat liver treated with DBN precursors revealed considerable number of damaged HC that have lost their characteristic appearance, with manifestations of hydropic degeneration (HD), appearance of pyknotic nuclei (PN), fatty infiltrations (FD), inflammatory leukocyte infiltrations (Li) and activated KC. E-F: Liver section of the rat treated with DBN supplemented with spirulina demonstrated restoration of normal arrangement of hepatocytes, although dilatation in blood sinusoids (DBS) and cytoplasmic vacuoles (V) were observed. (Scale bar = 75µm).
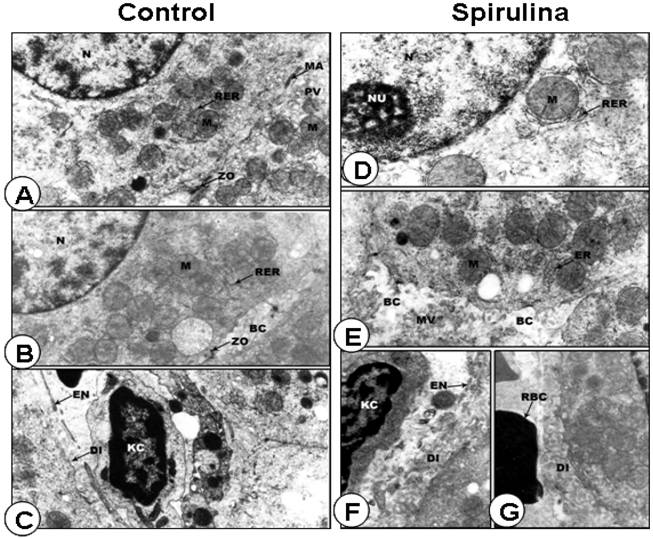
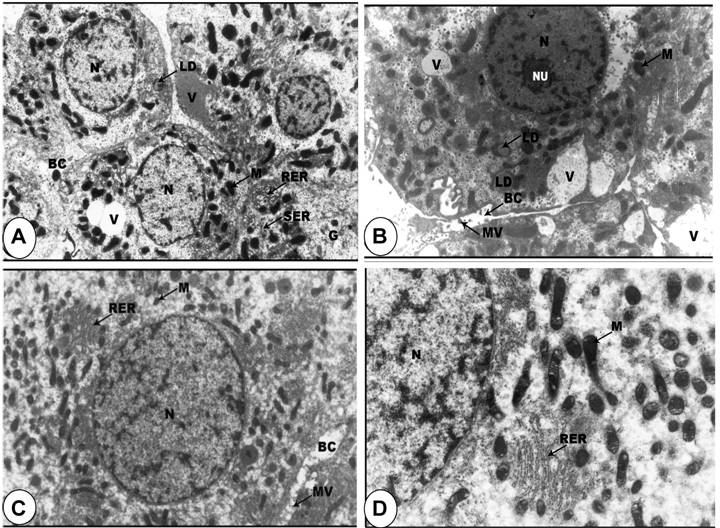
Ultrastructural analysis of the livers. In electron microscope preparations, the cell surface of the hepatic cells from control group was smooth, with large spherical nucleus and nucleoli showing fibrilo-granular network structure. The cytoplasm showed a granular appearance. There were profuse amount of rough endoplasmic reticulum especially around the nuclear envelope and between the mitochondria. The hepatic sinusoids were thin-walled with discontinuous layer of endothelial and Kupffer cells. The endothelial cells were extremely thin with an electron-lucent cytoplasm. The Kupffer cells were macrophages lining the sinusoids, with the endothelial cells (Figures 4A, 4B and 4C). In animals treated with SP, the hepatic cell surfaces showed lateral interdigitations. The nuclei were found to contain a large amount of electron-lucent euchromatin, scattered areas of heterochromatin, and distinct nucleolus (Figure 4D). The cytoplasm of the hepatic cells contained a fairly large amount of rough and smooth endoplasmic reticulum with many rounded mitochondria (Figures 4D and 4E). The hepatic sinusoids were found to contain only one discontinuous layer of lining cells. The endothelial cells were extremely thin with an electron-lucent cytoplasm and contained different varieties of vacuoles (Figures 4F and 4G).
Normal ultrastructural appearance of rat liver cells of rat fed on standard diet containing spirulina. A-C: Representative electron micrograph of hepatocytes (A-B) and blood sinusoid cells (C) from control rat liver. D-G: Representative electron micrographs of rat liver cells treated with SP exhibiting the normal appearance of hepatocyte (D) as well as adjacent hepatic cell borders (E-G). N, nucleus; M, mitochondria; RER, rough endoplasmic reticulum; PV, pinocytic vesicle; ZO), zonulae occludents belt; MA, maculae adherens; BC, bile canaliculus; EN, endothelial cell; KC, Kuppfer cell; Di, space of Disse; NU, nucleolus; MV, microvilli; ER, endoplasmic reticulum; RBC, red blood cells. Original magnification, x 15,000.
However, in DBN treated rats, most tumor cells were poorly differentiated due to rapid multiplication, with characteristic large rounded nuclei and large nucleoli (Figures 5A and 5B). When rats treated with DBN supplemented with SP, remarkable changes were observed, with differentiation of the hepatic cells rather than multiplication. The nuclei of all the hepatic cells contained small masses of condensed heterochromatin distributed throughout the nucleus and the cells showed appearance of large number of mitochondria. The cytoplasmic vacuoles were sparse and the fat droplets disappeared (Figures 5C and 5D).
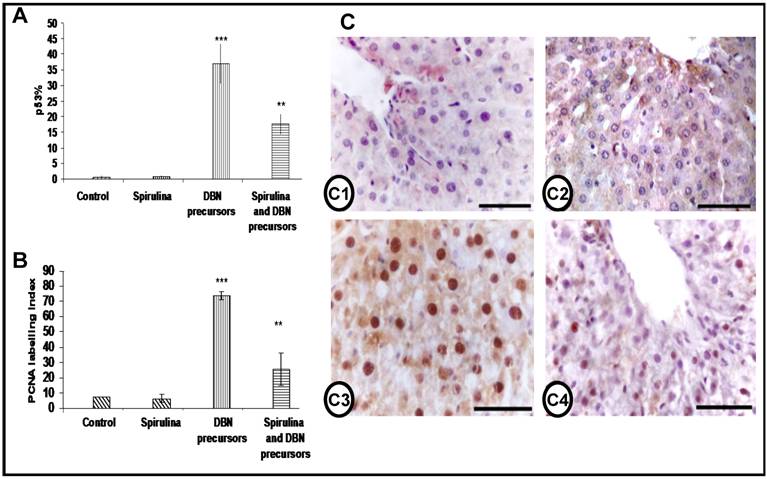
Expression levels of p53 and PCNA in the liver. The expression of the tumor suppressor gene p53 and the cell proliferation marker PCNA was examined by IHC in the rat liver of all the groups. While, the expression of both p53 and PCNA were not affected by SP supplementation, DBN treatment led to strong increase in p53- and PCNA-positive cells; these expression levels were significantly reduced by SP supplementation (Figures 6A and 6B). The liver sections of control and SP-treated rats stained for PCNA showed few nuclear positive cells as seen in Figure 6 C1 and C2. However, DBN-treatment increased dramatically both the intensity of the immunostaining as well as the number of PCNA-positive hepatocytes (Figure 6 C3), which were significantly reduced by SP supplementation (Figure 6 C4).
Ultrastructural evidence of DBN-induced pathological abnormalities in rat liver cells and its prevention by spirulina supplementation. A-B: Representative electron micrograph of rat liver cells treated with DBN precursors showing nucleus (N) with marginated heterochromatin, large nucleolus (NU), condensed mitochondria (M), lipid droplets (LD), glycogen particles (G), vacuoles (V), very large bile canaliculi (BC) with few microvilli (MV) and rough (RER) and smooth (SER) endoplasmic reticulum (x 6,000). C-D: Electron micrograph of hepatic cells in rats treated with DBN precursors and spirulina showing the normal appearance of the nuclei (N), large number of mitochondria (M) and also mitochondria with balloony of its cristae, electrolucent cytosol and rough endoplasmic reticulum (RER) and bile canaliculus (BC) with degenerative microvilli (MV). Original magnification, X 10,000.
Inhibition of the expression of p53 and the cell proliferation marker PCNA by spirulina in DBN-induced rat liver injury. Graphical representation of expression of the percentage of p53 positive cells (A) and the proliferating cell nuclear antigen (PCNA) labeling index (B) in the livers of all the groups studied. Both the percentage of p53 and the PCNA labeling index were significantly higher in DBN-treated rats (***p< 0.001), which were significantly decreased in DBN+Spirulina treatment (**p< 0.001). p53% is a value expressed to denote the percentage number of positive p53 nuclear stained cells. PCNA labeling index is the percentage number of positive cells stained for PCNA in each study group. C) Immunohistochemistry of PCNA in the liver section from all the groups studied. Few weakly stained nuclei were detected in the hepatocytes of normal control rats (C1). The liver sections of rats fed with spirulina also showed PCNA immunostaining similar to background (C2). Liver sections of rats treated with DBN showed prominent and distinctly stained nuclei in the hepatocytes (C3). The number of PCNA-stained nuclei was reduced when DBN-treated rats are supplemented with spirulina diet (C4). (Scale bar = 75µm).
Discussion
The present investigation is the first report of the in vivo chemopreventive effect of SP against liver carcinogenesis induced by DBN. SP inhibited the incidence of liver carcinogenesis and prevented DBN-induced hepatotoxicity which was demonstrated by light and electron microscopy. The expression levels of proliferating cell nuclear antigen (PCNA) and p53 were highly elevated in the liver of DBN-treated rats, but were significantly reduced by SP supplementation. Investigation into the molecular mechanisms of action of SP in the hepatocellular carcinoma cells HepG2 revealed that SP induced p53, which in turn upregulated the cell cycle inhibitor p21 to inhibit cell proliferation. In addition, SP-induced p53 was followed by an increase of Bax and a decrease of Bcl-2 expression, coinciding with induction of apoptosis (data not shown).
SP has been traditionally used for nutrition worldwide by people from Mexico, Africa and Asia. It is being widely studied for its possible antioxidant, antibacterial, and antiparasitic properties, and for several medical conditions such as allergies, ulcers, anemia, heavy-metal poisoning, and radiation poisoning (4, 17). SP or its extracts can prevent or inhibit cancer in humans and animals (1-3, 10, 11, 18-21).
In the present study, SP alone did not cause any side effects or organ toxicity as previously described (1, 8, 22), but it was remarkably effective in reducing the incidence of liver tumors, suggesting its potential therapeutic effect in our model. Mathew et al. reported a chemopreventive role of SP against oral cancer (19). It has been suggested that the ability of SP to inhibit carcinogenesis is due to its anti-oxidant properties that protect tissues from cell damage (23). The potential hepatoprotective role of SP may be associated with its antioxidant constituents such as selenium, chlorophyll, carotene, gamma-linolenic acids, tocopherol, phenolic compounds content and vitamin E and C working individually or in synergy (24-27). SP has been shown to be effective against free radical induced cellular transformation (6, 8). In addition, phycocyanin, the main pigment present in SP, can inhibit cytochrome P450 mediated reactions involved in the formation of reactive metabolites of the hepatotoxins (7). Mittal et al. showed that SP significantly reduced the hepatic cytochrome P-450 content and significantly induced the hepatic glutathione S-transferase activity (28).
The liver of rats treated with DBN showed damaged hepatocytes with manifestations of extensive cytoplasmic vacuolization, hydropic degeneration (oedema) and hyperchromatic nuclei, which was partially resolved by SP. At ultrasctructural level, the cytoplasm of the hepatocytes in this study showed different sizes of vacuoles. The cytoplasmic vacuolization implies increased permeability of cell membranes, leading to an increase in intracellular water (29). Also, the hepatic cytoplasm of rats administered with DBN precursors displayed increased number of mitochondria, ballooning of the mitochondrial cristae (30). The pathological effect of DBN in the liver mainly due to the degradation products, either the carbonium ion or the diazoalkane. These two reactive metabolites may react with some vital compounds of the liver, such as DNA or proteins, by alkylation (31). Moreover, the liver had been found to be the main site of nitrosamine metabolism that was mainly confined to the microsomal fraction in the cells (32). In vitro studies revealed that polysaccharides of SP enhanced cell nucleus enzyme activity and DNA repair mechanisms, which are known to be closely associated with chemoprevention properties of natural products. The most striking feature in these rats was the appearance of a large number of mitochondria and ample RER around the nucleus. Rats treated with diethyl nitrosamine and protected with red ginseng, showed hepatocytes with normal nucleus, large number of mitochondria and well developed RER indicating liver regeneration; extensive RER reflected the activity of the cell to produce high amount of proteins needed for normal differentiation (33).
In the present study, liver cells of both control and SP did not express p53 protein. The wild-type p53 protein cannot be detected by conventional immunohistochemical technique due to its shorter half life and hence does not accumulate in tumor cells (34-36). However, liver sections of rats treated with DBN precursors showed significant increase in p53 protein, which was reduced by SP supplementation. This might be attributed to the anti-mutagenic effect of SP which minimized DNA damage caused by DBN precursors. Another plausible explanation is that SP might have prevented high levels of wild-type p53, produced in response to SP, from being transformed into mutant p53 (37). The wild-type p53 is a tumor suppressor gene involved in several different mechanisms including gene transcription, DNA synthesis, and repair, and programmed cell death (38-42). Mutation in and loss of the p53 gene are the most common genetic defects in human malignant tumors, including hepatocellular carcinoma (HCC) (40, 43). Oxidative stress and chronic inflammation are closely associated with increased risk of cancer (44). High concentrations of nitric oxide (NO) products generated by DBN precursors can cause DNA damage, either directly or through secondary molecules, by nitroso-active deamination, DNA strand breakage, and DNA modifications (45). NO-induced DNA damage can lead to p53 accumulation and p53-mediated apoptosis (46, 47). Moreover, the higher the rate of p53 expression, the higher the degree of HCC malignancy (48).
In addition to p53, PCNA is closely associated with cell cycle, and its expression increases at the end of G1 phase, reaches its maximum in S-phase, declines during G2 phase and is absent during the mitotic phase and in quiescent cells (42, 49, 50). In the present study, the liver of both control and SP-treated rats showed few PCNA-positive cells in the proliferating stage. In contrast, liver sections of DBN-treated rats showed significant increase in the number of PCNA-positive cells, which was significantly reduced by SP supplementation. Previous studies have demonstrated that PCNA labeling index increased sequentially from normal tissue through premalignant stage to carcinoma of various tumors (51, 52). On the other hand, the bioactive pigment, C-PC (C-phycocyanin), and other proteins of SP had inhibitory activity on proliferation of cancer cells (10, 53).
Our in vitro studies revealed an increase in Bax/Bcl-2 ratio, which was accompanied by induction of apoptosis as previously reported using C-PC instead of the whole extract of SP (54, 55). These data suggest that C-PC is the main active ingredient of SP in inducing cancer cell death, and a potential anti-cancer agent. The decreased expression of the phoshoprotein, Rb, involved in regulating progression through the cell cycle and a concomitant increase in p21 expression indicate that these proteins along with p53 are important for SP-driven inhibition of cell proliferation (56). This study is the first to report p53/p21/Rb and p53/bax/Bcl-2 as potential pathways regulating SP-induced cell cycle inhibition and apoptosis, respectively.
In summary, our study is the first to show that DBN-induced severe liver injury and carcinogenesis in rat liver were prevented by SP supplementation, suggesting that SP is a protective phyto-antioxidant against liver toxicity and an anti-tumor agent. Further pre-clinical and clinical trials are warranted to characterize the efficacy of SP in combination with existing therapeutics for chemoprevention and chemotherapy of hepatocellular carcinoma.
Acknowledgements
Grant support. Dr. Ouhtit is supported by the Louisiana Cancer Research Consortium in New Orleans. Mohamed Abdraboh is supported by a fellowship from the Egyptian Ministry of Higher Education.
Conflict of Interest
The authors have declared that no conflict of interest exists.
References
1. Chamorro G, Salazar M, Favila L. et al. Pharmacology and toxicology of Spirulina alga. Rev Invest Clin. 1996;48:389-399
2. Piñero Estrada JE, Bermejo Bescós P, Villar del Fresno AM. Antioxidant activity of different fractions of spirulina platensis protean extract. Farmaco. 2001;56:497-500
3. Chamorro G, Salazar M, Araújo KG. et al. Update on the pharmacology of Spirulina (Arthrospira), an unconventional food. Arch Latinoam Nutr. 2002;52:232-40
4. Hirahashi T, Matsumoto M, Hazeki K. et al. Activation of the human innate immune system by Spirulina: augmentation of interferon production and NK cytotoxicity by oral administration of hot water extract of Spirulina platensis. Int Immunopharmacol. 2002;2:423-434
5. Grawish ME. Effects of Spirulina platensis extract on Syrian hamster cheek pouch mucosa painted with 7,12-dimethylbenz[a]anthracene. Oral Oncol. 2008;44:956-962
6. Romay C, Armesto J, Remirez D. et al. Antioxidant and anti-inflammatory properties of C-phycocyanin from blue-green algae. Inflamm Res. 1998;47:36-41
7. Vadiraja BB, Gaikwad NW, Madyastha KM. Hepatoprotective effect of C-phycocyanin: protection for carbon tetrachloride and R-(+)-pulegone-mediated hepatotoxicty in rats. Biochem Biophys Res Commun. 1998;249:428-431
8. Upasani CD, Khera A, Balaraman R. Effect of lead with vitamin E, C, or Spirulina on malondialdehyde, conjugated dienes and hydroperoxides in rats. Indian J Exp Biol. 2001;39:70-74
9. Premkumar K, Abraham SK, Santhiya ST. et al. Protective effect of Spirulina fusiformis on chemical-induced genotoxicity in mice. Fitoterapia. 2004;75:24-31
10. Kaji T, Fujiwara Y, Inomata Y. et al. Repair of wounded monolayers of cultured bovine aortic endothelial cells is inhibited by calcium spirulan, a novel sulfated polysaccharide isolated from Spirulina platensis. Hayashi T. Life Sci. 2002;70:1841-8
11. Pang QS, Guo BJ, Ruan JH. Enhancement of endonuclease activity and repair DNA synthesis by polysaccharide of Spirulina platensis. Yi Chuan Xue Bao. 1988;15:374-381
12. Andrade MR, Costa JA. Outdoor and indoor cultivation of Spirulina platensis in the extreme south of Brazil. Z Naturforsch. 2008;63:85-90
13. Raj M, Abd Elmageed Z, Zhou J. et al. Synergistic action of dietary phyto-antioxidants on survival and proliferation of ovarian cancer cells. Gynecol Oncol. 2008;110:432-438
14. Ouhtit A, Muller HK, Davis DW. et al. Temporal events in skin injury and the early adaptive responses in ultraviolet-irradiated mouse skin. Am J Pathol. 2000;156:201-207
15. Blinkova LP, Gorobets OB, Baturo AP. Biological activity of Spirulina. Zh Mikrobiol Epidemiol Immunobiol. 2001;2:114-118
16. Otles S, Pire R. Fatty acid composition of Chlorella and Spirulina microalgae species. J AOAC Int. 2001;84:1708-1714
17. Al-Batshan HA, Al-Mufarrej SI, Al-Homaidan AA. et al. Enhancement of chicken macrophage phagocytic function and nitrite production by dietary Spirulina platensis. Immunopharmacol Immunotoxicol. 2001;23:281-289
18. Schwartz J, Shklar G. Regression of experimental hamster cancer by beta carotene and algae extracts. J Oral Maxillofac Surg. 1987;45:510-515
19. Mathew B, Sankaranarayanan R, Nair PP. et al. Evaluation of chemoprevention of oral cancer with Spirulina fusiformis. Nutr Cancer. 1995;24:197-202
20. Qureshi MA, Ali RA. Spirulina platensis exposure enhances macrophage phagocytic function in cats. Immunopharmacol Immunotoxicol. 1996;18:457-463
21. Qureshi MA, Garlich JD, Kidd MT. Dietary Spirulina platensis enhances humoral and cell-mediated immune functions in chickens. Immunopharmacol Immunotoxicol. 1996;18:465-476
22. Krishnakumari MK, Ramesh HP, Venkataram L. Food safety evaluation: Acute oral and dermal effects of the algae Senedesmus acutus and SP platensis on albino rats. Journal of Food Protection. 1982;44:934-935
23. Khan Z, Bhadouria P, Bisen PS. Nutritional and therapeutic potential of Spirulina. Curr Pharm Biotechnol. 2005;6:373-379
24. Kay RA. Microalgae as food and supplement. Critical Reviews in Food Science and Nutrition. 1991;30:555-573
25. García-Martínez D, Rupérez FJ, Ugarte P. et al. Tocopherol fate in plasma and liver of streptozotocin-treated rats that orally received antioxidants and Spirulina extracts. Int J Vitam Nutr Res. 2007;77:263-71
26. Torres-Duran PV, Miranda-Zamora R, Paredes-Carbajal MC. et al. Studies on the preventive effect of Spirulina maxima on fatty liver development induced by carbon tetrachloride, in the rat. J Ethnopharmacol. 1999;64:141-147
27. Kaushik T, Shyam R, Vats P. et al. Glutathione metabolism in rats exposed to high fluoride water and effect of SP treatment. Fluoride. 2001;34:132-138
28. Mittal A, Kumar PV, Banerjee S. et al. Modulatory potential of Spirulina fusiformis on carcinogen metabolizing enzymes in Swiss albino mice. Phytother Res. 1999;13:111-114
29. Wilhelm Filho D. Reactive oxygen species, antioxidants and fish mitochondria. Front Biosci. 2007;12:1229-37
30. Shimizu S, Eguchi Y, Kamiike W. et al. Involvement of ICE family proteases in apoptosis induced by reoxygenation of hypoxic hepatocytes. Am J Physiol. 1996;271:G949-58
31. Singer B. N-nitrosoalkylating agents. Formation and persistence of alkyl derivatives in mammalian nucleic acids and contributing factors in carcinogenesis. J. Natl. Cancer Inst. 1979;62:1329
32. Ramakrishnan G, Raghavendran HR, Vinodhkumar R. et al. Suppression of N-nitrosodiethylamine induced hepatocarcinogenesis by silymarin in rats. Chem Biol Interact. 2006;161:104-14
33. Wu XG, Zhu DH, Li X. Anticarcinogenic effect of red ginseng on the development of liver cancer induced by diethylnitrosamine in rats. J Korean Med Sci. 2001;16(Suppl):S61-65
34. Lane DP. p53 and human cancers. Br Med Bull. 1994;50:582-599
35. Rodriguez-Alonso A, Pita-Fernandez S, Gonzalez-Carrero J, Nogueira-March JL. p53 and ki67 expression as prognostic factors for cancer-related survival in stage T1 transitional cell bladder carcinoma. Eur Urol. 2002;41:182-188
36. Wang ZF, Peng ZY, Huang LJ. et al. Physico-chemical properties and biological activities of a glycoconjugate SPPA-1 from Spirulina platensis. Yao Xue Xue Bao. 2001;36:112-115
37. Moon NS, Di Stefano L, Morris EJ. et al. E2F and p53 induce apoptosis independently during Drosophila development but intersect in the context of DNA damage. PLoS Genet. 2008;4:e1000153
38. Kastan MB, Onyekwere O, Sidransky D. et al. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991;51:6304-6311
39. Lane DP. Cancer. p53, guardian of the genome. Nature. 1992;358:15-16
40. Vogelstein B, Kinzler KW. Carcinogens leave fingerprints. Nature. 1992;355:209-210
41. Harris CC, Hollstein M. Clinical implications of the p53 tumor-suppressor gene. N Engl J Med. 1993;329:1318-1327
42. Wang C, Liu Z. Arabidopsis ribonucleotide reductases are critical for cell cycle progression, DNA damage repair, and plant development. Plant Cell. 2006;18:350-365
43. Anzola M, Burgos JJ. Hepatocellular carcinoma: molecular interactions between hepatitis C virus and p53 in hepatocarcinogenesis. Expert Rev Mol Med. 2003;5:1-16
44. Wang XW, Hussain SP, Huo TI. et al. Molecular pathogenesis of human hepatocellular carcinoma. Toxicology. 2002;181:43-47
45. Ambs S, Hussain SP, Harris CC. Interactive effects of nitric oxide and the p53 tumor suppressor gene in carcinogenesis and tumor progression. FASEB J. 1997;11:443-448
46. Forrester K, Ambs S, Lupold SE. et al. Nitric oxide-induced p53 accumulation and regulation of inducible nitric oxide synthase expression by wild-type p53. Proc Natl Acad Sci U S A. 1996;93:2442-2447
47. Messmer UK, Brune B. Nitric oxide (NO) in apoptotic versus necrotic RAW 264.7 macrophage cell death: the role of NO-donor exposure, NAD+ content, and p53 accumulation. Arch Biochem Biophys. 1996;327:1-10
48. Nakano A, Watanabe N, Nishizaki Y. et al. Immunohisto-chemical studies on the expression of P-glycoprotein and p53 in relation to histological differentiation and cell proliferation in hepatocellular carcinoma. Hepatol Res. 2003;25:158-165
49. Esposito V, Baldi A, De Luca A. et al. Role of PCNA in differentiating between malignant mesothelioma and mesothelial hyperplasia. prognostic considerations. Anticancer Res. 1997;17:601-604
50. Dworakowska D, Gozdz S, Jassem E. et al. Prognostic relevance of proliferating cell nuclear antigen and p53 expression in non-small cell lung cancer. Lung Cancer. 2002;35:35-41
51. Theunissen PH, Leers MP, Bollen EC. Proliferating cell nuclear antigen (PCNA) expression in formalin-fixed tissue of non-small cell lung carcinoma. Histopathology. 1992;20:251-255
52. Fukumasu H, da Silva TC, Avanzo JL. et al. Chemopreventive effects of Paullinia cupana Mart var. sorbilis, the guarana, on mouse hepatocarcinogenesis. Cancer Lett. 2006;233:158-164
53. Guan Y, Guo B. Inhibition activity of spirulina platensis proteins photo-immobilization biomaterial on proliferation of cancer cells. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2002;19:1-3
54. Roy KR, Arunasree KM, Reddy NP. et al. Alteration of mitochondrial membrane potential by Spirulina platensis C-phycocyanin induces apoptosis in the doxorubicinresistant human hepatocellular-carcinoma cell line HepG2. Biotechnol Appl Biochem. 2007;47:159-167
55. Subhashini J, Mahipal SV, Reddy MC. et al. Molecular mechanisms in C-Phycocyanin induced apoptosis in human chronic myeloid leukemia cell line-K562. Biochem Pharmacol. 2004;68:453-462
56. Sheahan S, Bellamy CO, Harland SN. et al. TGFbeta induces apoptosis and EMT in primary mouse hepatocytes independently of p53, p21Cip1 or Rb status. BMC Cancer. 2008;8:191-201
Author contact
![]() Correspondence to: Allal Ouhtit, M Ph, Ph D, Stanley S. Scott Cancer Center, Louisiana State University Health Science Center, CSRB Building, Room# 748C, 533 Bolivar Street, New Orleans, LA 70112. Ph: 1-504-568-2896 (Office); Fax: 1-504-568-2932; aouhtiedu
Correspondence to: Allal Ouhtit, M Ph, Ph D, Stanley S. Scott Cancer Center, Louisiana State University Health Science Center, CSRB Building, Room# 748C, 533 Bolivar Street, New Orleans, LA 70112. Ph: 1-504-568-2896 (Office); Fax: 1-504-568-2932; aouhtiedu

 Global reach, higher impact
Global reach, higher impact