Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2010; 6(1):80-88. doi:10.7150/ijbs.6.80 This issue Cite
Short Research Communication
Cellular and molecular comparison of redifferentiation of intramuscular- and visceral-adipocyte derived progeny cells
1. College of Animal Science and Technology, Nanjing Agricultural University, Nanjing 210095, China
2. Department of Animal Sciences, Washington State University, Pullman, WA 99164-6351, USA
Received 2009-12-4; Accepted 2010-1-19; Published 2010-1-21
Abstract
In the present study, mature adipocytes from pig-derived visceral and intramuscular adipose depots were isolated, purified, and allowed to undergo dedifferentiation and redifferentiation in vitro. During the redifferentiation process at days 1, 2, 4, 6, and 8, we observed that both visceral- and intramuscular adipose-derived progeny cells possessed a similar capacity to accumulate lipid. However, at days 10, 12, 14, and 16, the latter progeny cells accumulated lipid much faster--the content almost doubled at day 16 (P < 0.05). Such faster potential of lipid accumulation in the intramuscular adipose-derived progeny cells was then supported by higher expressions of CCAAT/enhancer binding protein-α (CEBP-α) and peroxisome proliferator-activated receptor-γ (PPAR-γ) at all these nine time points, and diacylglycerol O-acyltransferase homolog 1 (DGAT1), fatty acid binding protein 4 (FABP4) and fatty acid synthase (FASN) at some time points (P < 0.05). These preliminary data suggest that adipose depot differences exist with respect to ability of purified cells of the adipose lineage to redifferentiate and form viable lipid-assimilating cells in vitro. Therefore, our present study might provide a foundation to develop tools for biomedical and agricultural applications, as well as to determine the regulation of depot-specific cells of the adipose lineage. Further studies with more animals will validate and expand our results.
Keywords: Visceral adipocytes, Intramuscular adipocytes, Dedifferentiation, Redifferentiation, Gene expression dynamics.
1. Introduction
Adipose tissue serves as a major source of energy during periods of under-nutrition [1]. However, adipocytes provide for other regulatory roles, including the secretion of hormones, growth factors and cytokines, as well as proteins that are involved in the immune system and vascular functions [1,2]. By forming endocrine, paracrine, and autocrine signaling networks, adipocytes participate in the regulation of energy homeostasis, host defense mechanisms and reproduction. However, possessing too much body fat often results in obesity, diabetes, heart disease, stroke, arthritis and some cancers in humans [3]. Moreover, a vascular risk in humans is correlated with visceral adiposity in obese subjects [4].
In domestic animals the lipid deposited in the marbling fat depot has been widely recognized as one of the most economically important traits in livestock production [5]. This adipose depot contributes significantly to the quality, appearance, texture, flavor, tenderness, caloric value and shelf-life of red meat products [5]. Therefore, understanding variables regarding the regulation, cellularity, growth and/or development of the intramuscular adipose depot is important [1,5].
Studies utilizing meat animals have suggested that adipocyte number and volume is achieved most rapidly in the visceral adipose depot and least rapidly in the intramuscular depot [6], and that both depots appear to preferentially accumulate different forms of lipid [1,7]. However, many other cellular and molecular characteristics associated with these physiological differences between body and intramuscular depots remain ill-defined. One reason for this is that most cellular studies utilize stromal vascular (SV) cell preparations, which consist of numerous cell types that vary from preparation to preparation [reviewed in 8].
Recently, methods were devised to isolate pure populations of mature adipocytes from adipose depots, and generate homogeneous cell populations of proliferative-competent progeny cells [9] that possess ability to redifferentiate into lipid-assimilating adipocytes [10]. Although the process of adipocyte dedifferentiation and progeny cell redifferentiation may be different between animals [11,12], and between SV cells [13,14] this cell system may also be of value for comparative studies between similar cells isolated among adipose depots. Therefore, the objective of this study was to examine the redifferentiation potential, lipid accumulation capability and gene regulation dynamics using progeny cell cultures from two different adipose depots (visceral and intramuscular) in pigs. The hypothesis was that proliferative-competent progeny cells would redifferentiate in a different manner due to the adipose depot that the maternal adipocytes were initially isolated.
2. Materials and Methods
2.1. Animal/tissue sampling
Semitendinosus muscle and visceral (perirenal) fat samples were collected from one castrated, finished male Yorkshire/Landrace pig in the Washington State University (WSU) meats laboratory. The WSU Animal Care and Use Committee screened the use of animals in this research, and tissue isolation procedures met the standards imposed by the United States Department of Agriculture and Public Health Service. Adipose tissue samples were immediately transported to the cell culture laboratory in sterile 37 °C HBSS supplemented with antibiotics and antimycotics [8]. Complete methods for cell isolation, initial culture and propagation were reported previously [9].
2.2. Isolation of mature adipocytes
Adipocytes were isolated by the method described previously [8] with slight modifications [11,12]. Flasks containing mature adipocytes were inverted, which allowed the floating adipocytes to attach to the top inner ceiling surface of the flask while remaining fibroblast-like cells sink to the bottom and placed into a 37 °C with 5% CO2 and 95% air environment/incubator. Lipid-laden fat cells were purified with two (quite efficient) serial differential plating steps within 4 d of initial isolation [11,12].
2.3. Dedifferentiation of mature adipocytes
After confirming no contamination of any culture by fibroblast-like cells, mature adipocytes were left undisturbed in a 12.5 cm2 cell culture dish with DMEM (Invitrogen Inc., Carlsbad, CA) + 10% FBS (Invitrogen Inc., Carlsbad, CA) for 4-5 d, after which the culture flask with adherent mature adipocytes was turned right side up [9]. The media was changed every 2 d. Mature adipocytes were allowed to dedifferentiate [8] into proliferative-competent cells in vitro. After reaching about 200 cells each adipocyte clone was trypsinized and subcultured into progressively larger cell culture dishes until the final 150 mm culture dishes. The plated cells were maintained in DMEM + 10% FBS, and the medium was changed every 2 d. The cells of the second passage (PN2) were used for all studies of this project.
2.4. Spontaneous redifferentiation of dedifferentiated adipocytes
Dedifferentiated progeny cells were seeded in 24-well culture plates at a density of 2 × 104 cells/well, and allowed to redifferentiate (no chemical induction/conversion treatment was required). All cultures were exposed to DMEM + 10% FBS, and the medium was changed every 4 d. Difference of redifferentiation between intramuscular adipose- and visceral adipose-derived progeny cells was compared. Wells from progeny cell cultures originating from both adipose depots were collected at days 1, 2, 4, 6, 8, 10, 12, 14 and 16 from initiation of culture. At every time point, 6 wells of cells were harvested for analysis, of which 3 were used for examining lipid content and another 3 were for extracting RNA.
2.5. Determination of cellular lipid content
Cultures were washed with PBS and then fixed with 10% formalin for 10 min, followed by 30 s washing with 60 % isopropanol. The cells were then stained with Oil-red-O (ORO) as described [15], solvent allowed to evaporate/dry, and the dye from the stained cultures was then extracted by adding 200 μl 60% isopropanol for 30 min to be measured spectrophotometrically at 510 nm [16]. The cultures were then digested with 200 ul PBS/EDTA+0.25% Trypsin at 37 ℃ for 24 h. After centrifuged at > 8000 g for 15 s, the digestion was measured spectrophotometrically at 260 nm. The ORO absorbance values were standardized to reflect culture DNA [spectrophotometric absorbance at 260 nm].
2.6. Real-time reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted from cells with QIAGEN RNeasy Mini Kit (QIAGEN Inc, Valencia, CA) and digested with DNase I (Fermentas Inc., Glen Burnie, MD). Integrity of RNA was verified by electrophoresis, using a 2.2 M formaldehyde, 1.5% agarose gel stained with ethidium bromide. Total RNA was reverse transcribed into cDNA with Invitrogen SuperScript III First-Strand Synthesis System for RT-PCR (Invitrogen Inc., Carlsbad, CA). Expression levels were analyzed for six lipogenic genes: PPAR-γ, CEBP-α, SREBF1, FABP4, DGAT1 and FASN. β-actin was used as an internal standard marker. The primers were designed to span genomic regions with sequences of 5'-TGC TGT GGG GAT GTC TCA TA-3'(forward)/5'- TGT CTG TGG TCT TTC CTG TCA-3' (reverse) for PPAR-γ, 5'- ACA CGA AGC ACA ATC AGT CG-3 (forward)/5'- AGC AAC ACC CAC ACA AAA CA-3' (reverse) for CEBP-α, 5'- TTC TTC GTG GAT GGG GAC T-3' (forward)/5'- GTG CTG GAG GCA ATG GAG-3' (reverse) for SREBF1, 5'- AAT TGG GCC AGG AAT TTG AT-3' (forward)/5'- ATT CTG GTA GCC GTG ACA CC-3' (reverse) for FABP4, 5'- ACA AGG ACA AGG ACG GAC AC-3' (forward)/5'- TTG CTC AAG ACC AGC ATG AC-3' (reverse) for DGAT1, 5'- CTG CTG AAG CCT AAC TCC TCG-3' (forward)/5'- TGC TCC TGC ACG TCT CCC-3' (reverse) for FASN, and 5'- GAC ATC CGC AAG GAC CTC TA-3' (forward)/5'- ACA TCT GCT GGA AGG TGG AC-3' (reverse) for β-actin, respectively.
PCR reaction system was 20 μl, containing 5.0 μl sterile water, 1.5 μl of 1.0 μmol/l sense primer, 1.5 μl of 1.0 μmol/l antisense primer, 2.0 ul of cDNA, 10 μl of 2 × SYBR Green Mix (BioRad Inc., Hercules, CA). PCR condition was as follows: 95°C for 2 min 30 s, 50 cycles of 95°C for 30 s, 54°C for 30 s, 72°C 30 s. PCR amplification was performed in iCycler thermal cycler (BioRad Inc.). Relative mRNA level was analyzed with 2-ΔΔCT method [17] with β-actin as the endogenous control. The mean CT of visceral adipose-derived dedifferentiated cells at day 1 was set as calibrator. In addition, as for the real time PCR, its efficiency was determined by running a serial dilution of cDNA from a control sample with each primer pair. The data are presented as the fold change in target gene mRNA level normalized to that of β-actin gene and relative to the calibrator.
2.7. Statistical analysis
Statistical analysis was performed with SPSS 16.0 software (SPSS Inc., Chicago, IL). Means at different days of redifferentiation were examined by One-Way ANOVA. Differences between visceral and intramuscular adipose-derived cells at the same day were analyzed by Independent-Samples T Test. Differences were considered statistically significant at (P < 0.05).
3. Results
3.1. Isolation of mature adipocytes from adipose and muscle tissues
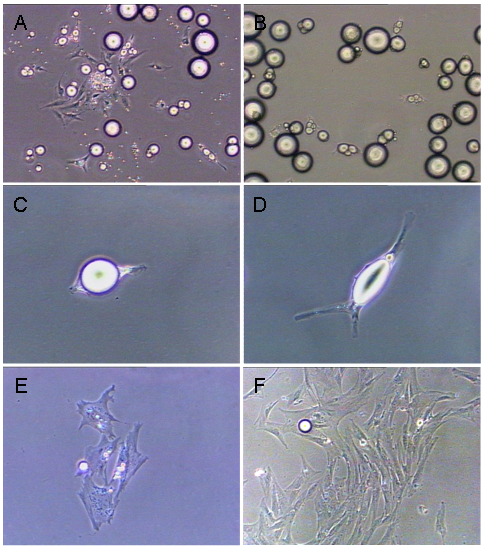
Adipose tissue derived from visceral and intramuscular depots were dispersed, separated from SV-type cells and placed in ceiling culture as a single-cell suspension of mature adipocytes (approximately 100 cells per flask). This allowed the buoyant mature adipocytes to physically attach to the ceiling surface of the culture flask. After 48 h of the first ceiling culture, mature adipocytes began attaching to the ceiling surface (Fig. 1A). To eliminate potential contamination of fibroblast-like cells [8], two serial differential plating steps were performed which allowed the buoyant mature adipocytes to separate from any fibroblast-like cells that might have been present on the ceiling surface [11,12]. After the second differential plating, a pure mature adipocyte culture without contamination of fibroblast-like cells was obtained (Fig. 1B). Mature adipocytes then extended their cytoplasm (Fig. 1C, D) and started to proliferate at 3- 4 d of initiating ceiling culture (Fig 1E). The daughter cells gradually extruded lipid droplets [11, 12] and changed their morphology to a proliferative-competent progeny cell. By days 7-10, these daughter cells formed colonies (Fig 1F). No significant morphological difference was observed during the initial dedifferentiation process among adipocyte types.
3.2 Spontaneous redifferentiation of dedifferentiated adipocytes
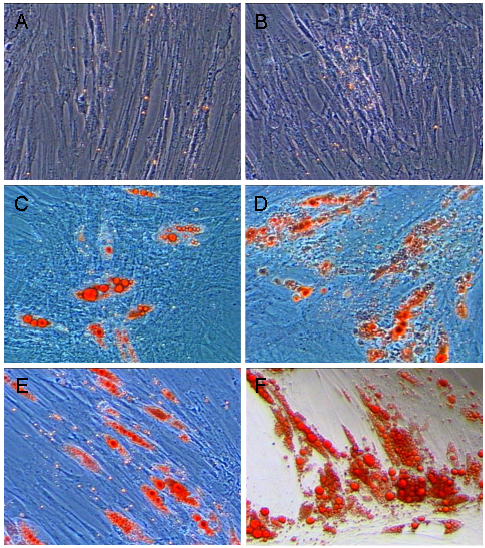
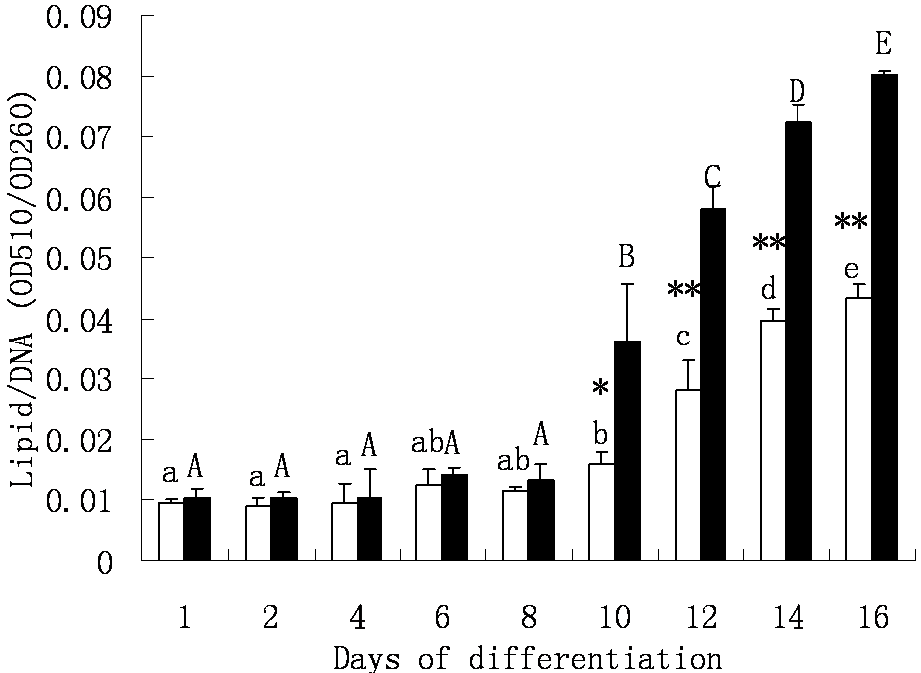
Our previous work had revealed that dedifferentiated adipocytes had the capability to redifferentiate spontaneously into lipid-laden cells in the absence of any traditional inducing reagents [10]. The cellular lipid accumulation processes of the two type cells are shown in Fig. 3. At day 6 of plating, tiny lipid droplets were observed in cultures of progeny cells derived from mature adipocytes from both the visceral and intramuscular adipose depots (Fig. 2A, B), and the lipid assimilation by both cell fractions did not change significantly before day 10. At day 10, however, both intramuscular adipose- and visceral adipose-derived progeny cells started to accumulate large lipid droplets (Fig. 2C, D), and this kept increasing until day 16 (Fig. 3D, E). Nevertheless, intramuscular adipose-derived progeny cells accumulated lipid at significantly higher speed than visceral adipose-derived cells after day 10 to 16. At the end of the differentiation period, the average lipid amount in intramuscular adipose-derived cells was nearly one-fold more than in visceral adipose-derived cells (Fig. 3).
Morphological changes of intramuscular mature adipocytes in ceiling cultures (A) Mature adipocytes along with fibroblast-like cells start attaching to the ceiling surface after 48 h of the first ceiling culture. (B) Pure mature adipocyte cultures were obtained after two serial differential plating regimens. (C, D) Mature adipocytes extended their cytoplasm after attaching. (E) Mature adipocytes started to proliferate at 3- 4 d of initiating ceiling culture. (F) The daughter cells formed colonies by d 7-10.
Cellular lipid accumulation of visceral adipose and intramuscular adipose-derived dedifferentiated progeny cells. Mature adipocytes (Figure 1) were allowed to dedifferentiate and resultant progeny cells proliferated to generate cells for experimentation of the re-differentiation potential of the daughter cells. (A), (C), (E) Visceral adipose-derived progeny cells re-differentiating at d 6, 10 and 16. (B), (D), (F) intramuscular adipose-derived progeny cells re-differentiating at d 6, 10 and 16.
Cellular lipid accumulation in the visceral adipose and intramuscular adipose-derived dedifferentiated progeny cells. □ Visceral adipose-derived progeny cells. ■ Intramuscular adipose-derived progeny cells. The measurement was estimated as average lipid content normalized to DNA content of triplicates per time point. Averages with different letters (lowercase for visceral adipose-derived progeny cells, uppercase for intramuscular adipose-derived progeny cells) are significantly different (P < 0.05) between days of differentiation within a progeny cell type. Averages that are significantly different between cell types at a specific day of differentiation are indicated by * (P < 0.05) and ** (P < 0.01).
3.3 Lipogenic gene expression during redifferentiation of dedifferentiated adipocytes
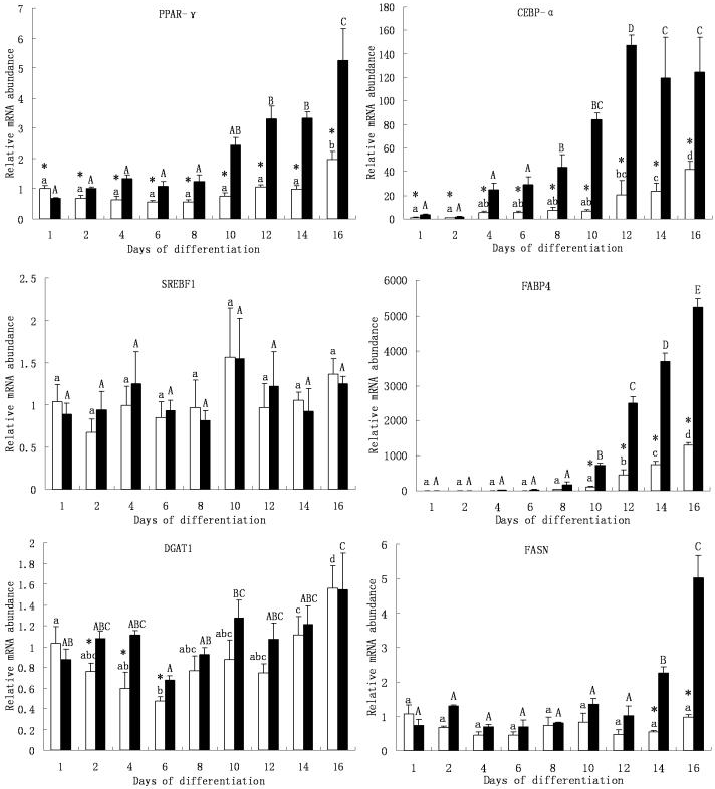
Six lipogenic genes were analyzed for their mRNA abundance dynamics during the redifferentiation timeframe (Figure 4). These genes can be classified into two clusters. Cluster 1 consists of PPAR-γ, CEBP-α and SREBF1 genes, which have been proved to be important lipogenic transcription factors. Cluster 2 includes FABP4, DGAT1 and FASN genes, which are involved in lipid metabolism. In cluster 1, PPAR-γ and CEBP-α genes mRNA abundance changed along differentiation, while SREBF-1 gene mRNA abundance remained stable during the differentiation in both the visceral and intramuscular adipose-derived cells. The PPAR-γ mRNA level was stable at the early stages of differentiation both in visceral and intramuscular adipose-derived cells, but started to increase to a higher level in intramuscular adipose-derived cells from day 12, while in visceral adipose-derived cells it increased at day 16. Intramuscular adipose-derived cells expressed higher level of PPAR-γ mRNA compared to visceral adipose-derived cells except at day 1. At the end of differentiation (day 16), the PPAR-γ mRNA level was 7.6 fold higher than day 1 in intramuscular adipose-derived cells (5.26 ± 1.06 vs. 0.69 ± 0.02), while it increased only 1.9 fold in visceral adipose-derived cells (1.97 ± 0.26 vs. 1.01 ± 0.10). CEBP-α gene was up-regulated from day 8 to 12 and decreased from day 12 to 16 in intramuscular adipose-derived cells, while in visceral adipose-derived cells the mRNA level increased from day 12 to 16. The mRNA level in intramuscular adipose-derived cells remained higher than that in visceral adipose-derived cells during differentiation.
In cluster 2, FABP4 gene mRNA level changed significantly during the differentiation period both in intramuscular and visceral adipose-derived cells. The mRNA level was unchanged from day 1 to 8 in intramuscular adipose-derived cells, and kept increasing from day 10 to 16. In visceral adipose-derived cells, the mRNA level was stable until day 10, and then increased from day 12 to 16. The mRNA level at day 16 was about 1260 fold higher than that of day 1 in visceral adipose-derived cells (1.03 ± 0.17 vs. 1295.71 ± 81.18), while nearly 2520 fold in intramuscular adipose-derived cells (2.08 ± 0.55 vs. 5243.26 ± 230.32). Intramuscular adipose-derived cells displayed higher mRNA level than visceral adipose-derived cells from day 10 to 16. The DGAT1 mRNA at day 16 was 1.52 (1.03 ± 0.16 vs 1.56 ± 0.22) and 1.76 (0.88 ± 0.10 vs 1.54 ± 0.35) fold higher than that at day 1 in visceral and intramuscular adipose-derived cells, respectively. However, it had a decreasing trend from day 1 to 6 in visceral adipose-derived cells, and then increased from day 8 to 16. DGAT1 mRNA reached lowest level at day 6 in both types of cells (0.48 ± 0.06 and 0.68 ± 0.04). There was no change of FASN mRNA level in visceral adipose-derived cells during differentiation. No change of FASN mRNA level was observed in intramuscular adipose-derived cells from day 1 to 12. However, it increased from day 14 to 16. The mRNA level had no difference between two type cells from day 1 to 12, while intramuscular adipose-derived cells expressed higher mRNA level than visceral adipose-derived cells from day 14 to 16. At day 16, the mRNA level in intramuscular adipose-derived cells was 5.08 fold higher than that in visceral adipose-derived cells (5.01 ± 0.66 vs 0.99 ± 0.05).
Lipogenic gene relative mRNA abundance during redifferentiation of visceral adipose and intramuscular adipose-derived dedifferentiated progeny cells. □ Visceral adipose-derived progeny cells. ■ Intramuscular adipose-derived progeny cells. The mRNA abundance was normalized to b-actin and the expression level of day 1 was used as a calibrator. Averages with different letters (lowercase for visceral adipose-derived progeny cells, uppercase for intramuscular adipose-derived progeny cells) are significantly different (P < 0.05) between days of differentiation within a progeny cell type. Averages that are significantly different (P < 0.05) between cell types at a specific day of differentiation are indicated by *.
4. Discussion
The focus of this paper was to determine potential differences in redifferentiation between proliferative-competent progeny cells, originally derived from the dedifferentiation of mature adipocytes from two different adipose depots (visceral and intramuscular adipose depots) from pigs. Two concrete items had to be in place-- a mechanism to generate purified and repeatable cell systems and methods to determine that differentiation has occurred. Over time, we have developed the cell isolation and culture procedures to obtain the requisite cell systems [8,11,12]. Moreover, in this communication we have used procedures to define the amount of ORO through the use of minute samples, as well as the use of traditional marker definition methods.
Recent studies have provided evidence that terminally differentiated mature adipocytes are able to dedifferentiate into proliferative-competent progeny cells in vitro [1,8,9,18], although the plasticity of such progeny cells has yet to be determined [9,19]. In the present study, we obtained pure mature adipocytes from pig visceral fat and skeletal muscle tissues by using the same isolation methods as previously described [8,9,11,12]. When maternal adipocytes were subjected to ceiling culture, both visceral-derived and intramuscular-derived adipocytes were capable of re-entering the cell cycle and dedifferentiating into proliferative-competent progeny cells in the absence of any special regulatory factors (other than what might exist in the serum). Also, there were no morphological differences between these two type cells at the beginning of the redifferentiation experiments.
Our present study revealed that both visceral adipose-derived and intramuscular adipose-derived proliferative-competent progeny cells may redifferentiate to lipid-laden adipocytes without any differentiation-induction chemicals. These results suggest that this cell system may be different to SV-cells and other preadipocyte cultures, and offer a more natural/holistic test system for future use. Moreover, the present results are consistent with our previous observations that some regulatory signals may not necessarily be required for these progeny cells to undergo redifferentiation [14]. At the first 8 d, the lipid content appeared no difference between two the cell types. From the 10th d onwards, however, both visceral adipose-derived and intramuscular adipose-derived cells started to accumulate different amounts of cellular lipid. Moreover, the progeny cells derived from the intramuscular adipose depot were more active and inclined to accumulate cellular lipid than in cultures of progeny cells originating from visceral adipose depots. To further explain the molecular mechanisms underlying the cellular difference between these two cell types, we studied the expression patterns of several important adipogenic genes during redifferentiation.
A number of molecular markers exist to define the progression of cell differentiation into lipid-assimilating adipocytes [20]. We expected the progeny cells to undergo a similar cascade of intracellular signals, including the expression of several transcription factors like CEBP-α, CEBP-β, CEBP-δ and PPAR-γ [21]. Based primarily on studies using SV cells, PPAR-γ and CEBP-δ are expressed at low levels at the early stages of adipocyte differentiation [22, 23], while the differentiated state of adipocytes is achieved and maintained via a cycle of positive cross-regulation between PPAR-γ and CEBP-α. CEBP-α is also required for the control of insulin action in mature adipocytes in non-ruminants [24], but not necessarily ruminants [21]. Adipocyte lipid accretion and PPAR-γ levels were strongly linked in fetal and adult pig SV cultures [25-28], and regulation of adipogenesis may ultimately depend on PPAR-γ expression and nuclear localization [21]. Our data revealed that PPAR-γ and CEBP-α mRNA levels were relatively low at the early stage of differentiation, and both increased to higher level at later times. Additionally, PPAR-γ and CEBP-α mRNA level were higher in intramuscular adipose- than in visceral adipose-derived cells along differentiation. These results suggest that both PPAR-γ and CEBP-α play important roles in the differentiation of two cell types in pigs [14], but that intramuscular adipose-derived cells may be more active in adipogenesis than visceral adipose-derived cells.
The sterol regulatory element binding transcription factor 1 (SREBF1) belongs to the transcription factor family that regulates genes involved in lipid metabolism. In the present study, we observed that this gene is expressed consistently from day 1 to day 16 and shows no significant difference between the visceral adipose- and intramuscular adipose-derived dedifferentiated progeny cells (Figure 4). Previous studies have shown that the SREBF1 expression is usually induced in adipose tissue by glucose/insulin and by fasting/refeeding, thus playing a critical role in nutritional regulation of lipogenic gene expression [29]. Therefore, it is not particularly surprising for us to see that the SREBF1 gene was not affected throughout the differentiation process because there are no such glucose/insulin or nutritional stimulations in our experiments conducted in the present study. In addition, the human SREBF1 gene encodes three different isoforms, SREBF-1a, -1c and -1ac, which are expressed at varying levels in different tissues and cultured cells and exhibit common and distinct functions [30]. Whether the porcine SREBF1 gene has different splicing forms and if so, what isoforms might be associated with the differentiation process need to be addressed in future experiments.
Enlargement of adipocytes might arise from increased lipogenesis, either from fatty acids (FAs) taken up from plasma or from those synthesized de novo [29]. We examined two key genes implicated in these two physiological processes, i.e. FABP4 and FASN. Cytoplasmic FABP4 is involved in improvement of FFA solubility and transport of FFA to specific enzymes and cellular compartments. It plays key role in the import, storage and export of FFA [30,31,32,33], whereas cytosolic fatty acid synthase (FASN) is primarily responsible for de novo FA biosynthesis from acetyl-coenzyme A (CoA) and malonyl-CoA [34]. Our data revealed that FABP4 mRNA level was upregulated in both visceral adipose-derived and intramuscular adipose-derived cells from day 10, which is well in accordance with the increasing of cellular lipid amount. However, FASN mRNA level increased only at the later stage of differentiation in intramuscular adipose-derived cells. This might be due to the fact that cellular lipid accumulation, in pig-derived adipocytes, is a result of plasma FA uptake rather than FA de novo biosynthesis. FABP4 and FASN mRNA levels were higher in intramuscular- than in visceral adipose-derived cells from the 10th and 14th d onwards, respectively. Our preliminary data suggest that both FA uptake and de novo synthesis are more active in intramuscular adipose-derived cells than in visceral adipose-derived cells.
In the present study, mature adipocytes from two adipose depots in pigs (visceral and intramuscular) readily dedifferentiated to form proliferative-competent progeny cells in vitro. Proliferative-competent progeny cells from both depots appeared morphologically similar, but redifferentiated to form lipid assimilating adipocytes at a different rate and with different molecular markers. These differences might provide us with useful clues on how to manipulate intramuscular and visceral adipose depots in the future. However, many more animals and repeated studies will be required in order to concretely develop this mechanism for exploitation.
Acknowledgements
This study was supported by National Natural Science Foundation of China (No. 30800780), and by an Emerging Research Issues Internal Competitive Grant from the Agricultural Research Center, College of Agricultural, Human, and Natural Resource Sciences, Washington State University.
Conflict of Interest
The authors have declared that no conflict of interest exists.
References
1. Hausman GJ, Dodson MV, Ajuwon K. et al. Board Invited Review: The biology and regulation of preadipocytes and adipocytes in meat animals. Journal of Animal Sciences. 2009;87:1218-1246
2. Kather H. Adipose tissue and obesity. Ther Umsch. 2000;57(8):488-492
3. Jiang Z, Pappu SS, Rothschild MF. Hitting the jackpot twice: identifying and patenting gene tests related to muscle lipid accumulation for meat quality in animals and type 2 diabetes/obesity in humans. Recent Pat DNA Gene Seq. 2007;1(2):100-111
4. Ohman MK, Wright AP, Wickenheiser KJ. et al. Visceral adipose tissue and atherosclerosis. Curr Vasc Pharmacol. 2009;7(2):169-179
5. Dodson MV, Jiang Z, Chen J. et al. Allied industry approaches to alter intramuscular fat content and composition in beef animals. Journal of Food Science. 2010;75:R1-8
6. Allen CE. Cellularity of adipose tissue in meat animals. Fed Proc. 1976;35(11):2302-2307
7. Smith SB, Kawachi H, Choi CB. et al. Cellular regulation of bovine intramuscular adipose tissue development and composition. Journal of Animal Science. 2009;87:72-82
8. Fernyhough ME, Helterline DI, Vierck JL. et al. Dedifferentiation of mature adipocytes to form adipofibroblasts: More than a possibility. Adipocytes. 2005;1(1):17-24
9. Fernyhough ME, Vierck JL, Hausman GJ. et al. Primary adipocyte culture: Adipocyte purification methods may lead to a new understanding of adipose tissue growth and development. Cytotechnology. 2005;46:163-172
10. Fernyhough ME, Hausman GJ, Dodson MV. Progeny from dedifferentiated adipocytes display protracted adipogenesis. Cells, Tissues, Organs. 2008;188:359-372
11. Chen J, Guridi M, Fernyhough ME. et al. Initial differences in lipid processing leading to pig- and beef-derived mature adipocyte dedifferentiation. Basic and Applied Myology (European Journal of Translational Myology). 2009;19(5):243-246
12. Chen J, Guridi M, Fernyhough ME. et al. Clonal mature adipocyte production of proliferative-competent daughter cells requires lipid export prior to cell division. International Journal of Stem Cells. 2009;2:76-79
13. Taniguchi M, Guan LL, Zhang B. et al. Gene expression patterns of bovine perimuscular adipocytes during adipogenesis. Biochemical Biophysical Research Communications. 2008;366:346-351
14. Taniguchi M, Guan LL, Zhang B. et al. Adipogenesis of bovine perimuscular adipocytes. Biochemical Biophysical Research Communications. 2008;366:54-59
15. Kinkel AD, Fernyhough ME, Helterline DL. et al. Oil red-O stains non-adipogenic cells: A precautionary note. Cytotechnology. 2005;46:49-56
16. Ramírez-Zacarías JL, Castro-Muñozledo F, Kuri-Harcuch W. Quantitation of adipose conversion and triglycerides by staining intracytoplasmic lipids with Oil red O. Histochemistry. 1992;97:493-497
17. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402-408
18. Matsumoto T, Kano K, Kondo D. et al. Mature adipocyte-derived dedifferentiated fat cells exhibit multilineage potential. J Cell Physiol. 2008;215:210-222
19. Dodson MV, Fernyhough ME. Mature adipocytes: Are there still novel things that we can learn from them? Tissue & Cell. 2008;40:307-308
20. Kokta T, Dodson MV, Gertler A. et al. Intercellular signaling between adipose tissue and muscle tissue. Domestic Animal Endocrinology. 2004;27:303-331
21. Fernyhough ME, Okine E, Hausman G. et al. PPARgamma and GLUT-4 expression as developmental regulators/markers for preadipocyte differentiation into an adipocyte. Domest Anim Endocrinol. 2007;33:367-378
22. Cao Z, Umek RM, McKnight SL. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 1991;5:1538-1552
23. Yeh WC, Cao Z, Classon M. et al. Cascade regulation of terminal adipocyte differentiation by three members of the C/EBP family of leucine zipper proteins. Genes Dev. 1995;9:168-181
24. Wu Z, Rosen ED, Brun R. et al. Cross-regulation of C/EBP alpha and PPAR gamma controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol Cell. 1999;3:151-158
25. Kiec-Wilk B, Dembinska-Kiec A, Olszanecka A. et al. The selected pathophysiological aspectsof PPARs activation. J Physiol Pharmacol. 2005;56:149-162
26. Gurnell M. Peroxisome proliferator-activated receptor gamma and the regulation of adipocyte function: lessons from humangenetic studies. Best Pract Res Clin Endocrinol Metab. 2005;19:501-523
27. Montague CT. Adipose depot-specific effects of PPAR gamma agonists: a consequence of differential expression of PPAR gamma in adipose tissue depots? Diabetes Obes Metab. 2002;4:356-361
28. Hausman GJ. Dexamethasone induced preadipocyte recruitment and expression of CCAAT/enhancing binding protein alpha and peroxisome proliferator activated receptor-gamma proteins in porcine stromal-vascular (S-V) cell cultures obtained before and after the onset of fetal adipogenesis. Gen Comp Endocrinol. 2003;133:61-70
29. Yang YA, Morin PJ, Han WF. et al. Regulation of fatty acid synthase expression in breast cancer by sterol regulatory element binding protein-1c. Exp Cell Res. 2003;282:132-137
30. Felder TK, Klein K, Patsch W. et al. A novel SREBP-1 splice variant: tissue abundance and transactivation potency. Biochim Biophys Acta. 2005;1731:41-47
31. Roberts R, Hodson L, Dennis AL. et al. Markers of de novo lipogenesis in adipose tissue: associations with smalladipocytes and insulin sensitivity in humans. Diabetologia. 2009;52(5):882-890
32. Krusinová E, Pelikánová T. Fatty acid binding proteins in adipose tissue: a promising link between metabolic syndrome and atherosclerosis? Diabetes Res Clin Pract. 2008;82:S127-134
33. Chmurzyńska A. The multigene family of fatty acidbinding proteins (FABPs): function, structure and polymorphism. J. Appl. Genet. 2006;47:39-48
34. Zimmerman AW, Veerkamp JH. New insights into the structure and function of fatty acid-binding proteins. Cell Mol Life Sci. 2002;59:1096-1116
Author contact
![]() Corresponding author: jiech1119edu.cn (Jie Chen) or jiangzedu (Zhihua Jiang).
Corresponding author: jiech1119edu.cn (Jie Chen) or jiangzedu (Zhihua Jiang).

 Global reach, higher impact
Global reach, higher impact