Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2010; 6(1):107-115. doi:10.7150/ijbs.6.107 This issue Cite
Research Paper
High prevalence of multiple paternity in the invasive crayfish species, Procambarus clarkii
1. Molecular Population Genetics Group, Temasek Life Sciences Laboratory, 1 Research Link, National University of Singapore, 117604 Republic of Singapore
2. Aquaculture Division, E-Institute of Shanghai Universities, College of Aquatic Life Sciences and Technology, Shanghai Ocean University, Shanghai 201306, China
3. College of Animal Science and Technology, Nanjing Agriculture University, Nanjing, China
Received 2009-10-3; Accepted 2010-2-12; Published 2010-2-17
Abstract
Reproductive strategy is a central feature of the ecology of invasive species as it determines the potential for population increase and range expansion. The red swamp crayfish, Procambarus clarkii, has invaded many countries and caused serious problems in freshwater ecosystems. However, little is known about the effects of environmental conditions on crayfish paternity and offspring traits in the wild. We studied these reproductive characteristics of P. clarkii in wild populations from two different habitats (ponds and ditches) in three locations with different environmental conditions in China. Genotyping of 1,436 offspring and 30 mothers of 30 broods was conducted by using four microsatellites. An analysis of genotyping results revealed that gravid females were the exclusive mother of the progeny they tended. Twenty-nine of 30 mothers had mated with multiple (2-4) males, each of which contributed differently to the number of offspring in a brood. The average number of fathers per brood and the number of offspring per brood were similar (P > 0.05) among six sampling sites, indicating that in P. clarkii multiple paternity and offspring number per brood are independent of environmental conditions studied. Indirect benefits from increasing the genetic diversity of broods, male and sperm competition, and cryptic female choice are a possible explanation for the high level multiple paternity and different contribution of fathers to offspring in this species.
Keywords: Decapod, allochthonous species, microsatellite, mating system, multiple paternity Running title: Multiple paternity in red swamp crayfish
1. Introduction
Reproductive strategy is a central feature of the ecology of invasive species as it determines the potential for population increase and range expansion [1]. Sexual reproduction and high fecundity levels are some of the characteristics most frequently cited when compiling criteria promoting invasion [2]. The recent development and application of polymorphic DNA markers (e.g. microsatellites and SNPs) [3, 4] and data analysis tools [5] to parentage studies in natural populations has initiated several important paradigm shifts in the field of reproductive biology [6]. Among the most prominent of these shifts is the realization that females of most animal species, even those believed to be socially monogamous, copulate routinely with multiple males and often produce broods composed of both full and half-sibs [7]. Recent studies have shown that multiple paternity is frequent in both invertebrates [8] and vertebrates [9]. The general evolutionary significance of multiple paternity and the factors which cause it to vary in frequency among related taxa continue to be debated vigorously [10].
The red swamp crayfish, Procambarus clarkii (Girard 1852), native to south-central United States and north-eastern Mexico, has been introduced to Europe, Africa, central and South America and Southeast Asia [11]. This species was introduced to Nanjing, China from Japan in 1929 [12] and has rapidly spread to most provinces of China and has established dense populations [13]. Procambarus clarkii has caused serious problems to local fish, crustaceans, aquatic plants and freshwater ecosystems [13]. Extensive studies on reproduction [14, 15] and population dynamics [15-17] have been carried out. The red swamp crayfish reaches maturity in approximately three months, and in warm climates it may produce two generations per year [18]. Mating period of P. clarkii varies according to the hydrographic period and the environmental conditions, recruitment, as well as male/female maturation [14]. Size at reproduction is related to water temperature, population density, and the length of the hydroperiod. The number of eggs per female is size dependent, with reproductive output per unit of weight being highest in large females from permanent water bodies [14]. Large and healthy females typically produce over 200 viable young [19]. It is generally believed that some life history traits, such as rapid growth, high fecundity, polytrophism, resistance to diseases, pollution and extreme environmental conditions, make P. clarkii an invincible invasive species [20]. We applied four polymorphic microsatellite markers to investigate the patterns of multiple paternity in P. clarkii in six sampling sites (i.e. two different freshwater habitats in three locations in China) with different environmental conditions. The study of multiple paternity and effects of environmental factors on multiple paternity will help to understand the mechanisms underlying the successful invasion of the red swamp crayfish.
2. Methods
Selection of study sites and measuring parameters of water quality
Three locations were selected for collecting crayfish samples taking into account the different environmental conditions. In each location, samples were collected in two different habitats (pond and ditch), therefore there are a total of 6 sampling sites (Table 1). These three locations are Jiangning (31°15'16'' N, 118°7'49'' E) in Jiangsu Province, Huzhou (30°22' 29'' N, 119°44'38'' E) and Tongxiang (30°38′18'' N, 120°17′38'′ E) in Zhejiang Province, China. The climate in three locations is slightly different [21]. In Jiangning (JN) the annual average temperature is 15.3oC and annual precipitation of is 1106.5 mm. In Tongxiang (TX), the annual average temperature is 15.8oC and the annual average rainfall is 1193 mm. Huzhou's (HZ) annual average temperature is 16.9°C and an annual average rainfall of is 1126 mm. In all of these locations, water bodies (i.e. rivers, ponds, ditches and reservoirs) are very abundant. Ponds are connected to rivers and ditches only during the rainy season. Ditches are connected to rivers and flowing water is abundant during raining and agricultural seasons due to the high level of water. Ditches are connected to rivers, and water in ditches is flowing during raining and agricultural seasons. Food resources (e.g. detritus, water plants, macroinvertebrates and snails) are more abundant in ponds than in ditches, and density of crayfish is higher in ponds than in ditches [22]. Water quality parameters (pH, chemical oxygen demand, dissolved oxygen, total nitrogen and total phosphorus) of ponds and ditches from the three locations were measured according to guidelines of State Environmental Protection Administration, China [23]. Statistic analysis of the difference of water quality parameters among six sampling sites (two habitats from each of three locations) was conducted using F-test for analyses of variance (one way ANOVA) with the SAS program (SAS Institute) due to the fact that multiple comparisons are required.
Collection of samples
Samples from the studied populations of wild red swamp crayfish were collected, from May to September 2005, at the two habitats in each of the three locations (see Table 1) using baited nets. All crayfish individuals were sexed as previously described [24]. We measured the total body length of females (from tip of the rostrum to tip of the telson) and counted the total number of hatchlings per ovigerous female. Statistical analysis of the difference of body length of females and the total number of hatchlings per ovigerous female in six sampling sites (two habitats from each of three locations) was conducted using one way ANOVA with the SAS program.
We collected a small part of the third pleopod of each adult female bearing hatchlings and all the hatchlings, and stored them in absolute ethanol. The details of sample number and sampling locations are listed in Table 2.
Parameters of water quality in ponds and ditches from three sampling locations
| Location | Jiangning | Huzhou | Tongxiang | |||
|---|---|---|---|---|---|---|
| Site | Pond | Ditch | Pond | Ditch | Pond | Ditch |
| pH | 6.96 ± 0.03 a | 7.07 ± 0.03 a | 6.76 ± 0.04 a | 7.14 ± 0.14 a | 6.88 ± 0.08 a | 7.07 ± 0.07 a |
| COD (mg/ml) | 14.76 ± 0.32 b | 5.29 ± 0.18 cd | 5.73 ± 0.09 c | 3.51 ± 0.13 e | 27.41 ± 0.63 a | 4.63 ± 0.21 d |
| DO (mg/ml) | 4.80 ± 0.10 e | 11.59 ± 0.21 b | 7.63 ± 0.17 d | 12.20 ± 0.20 a | 3.73 ± 0.03 f | 10.69 ± 0.11 c |
| TN (mg/ml) | 1.31 ± 0.05 b | 0.51 ± 0.02 a | 0.68 ± 0.01 c | 0.42 ± 0.10 de | 1.51 ± 0.04 a | 0.35 ± 0.03 e |
| TP (mg/ml) | 0.75 ± 0.01 b | 0.11 ± 0.01 d | 0.20 ± 0.01 c | 0.09 ± 0.00 e | 0.86 ± 0.10 a | 0.07 ± 0.01 e |
COD: Chemical oxygen demand; DO: Dissolved oxygen; TN: Total nitrogen; TP: Total phosphorus; M: Male and F: Female; Significant differences (P < 0.05) are shown using different letters in rows.
Sampling location, environment, sample sizes, total body length (BL) of dams and inferred genetic paternity in 30 Procambarus clarkii broods
| Location | Site | Dam's ID | BL (cm) | Number of offspring | Number of sires | |
|---|---|---|---|---|---|---|
| genotyped | total | |||||
| TX | ditch | M01 | 10.5 | 48 | 351 | 4 |
| M02 | 9.8 | 48 | 220 | 2 | ||
| M03 | 9.7 | 48 | 356 | 2 | ||
| M04 | 9.0 | 48 | 219 | 3 | ||
| pond | M05 | 10.5 | 46* | 318 | 4 | |
| M06 | 10.9 | 48 | 310 | 2 | ||
| M07 | 9.4 | 47* | 236 | 2 | ||
| M08 | 11.6 | 48 | 331 | 4 | ||
| M09 | 10.1 | 48 | 220 | 3 | ||
| M10 | 10.2 | 48 | 316 | 3 | ||
| JN | ditch | M11 | 8.7 | 48 | 209 | 2 |
| M12 | 11 | 48 | 308 | 2 | ||
| M13 | 11.2 | 48 | 325 | 4 | ||
| M14 | 7.4 | 48 | 136 | 3 | ||
| pond | M15 | 10.7 | 48 | 321 | 3 | |
| M16 | 10.0 | 48 | 270 | 2 | ||
| M17 | 10.8 | 48 | 356 | 2 | ||
| M18 | 10.1 | 48 | 219 | 3 | ||
| M19 | 11.6 | 48 | 318 | 2 | ||
| HZ | ditch | M20 | 11.5 | 48 | 330 | 2 |
| M21 | 7.4 | 48 | 136 | 3 | ||
| M22 | 10.7 | 47* | 321 | 2 | ||
| M23 | 11.8 | 48 | 362 | 3 | ||
| M24 | 10.4 | 48 | 256 | 2 | ||
| pond | M25 | 10.0 | 48 | 219 | 4 | |
| M26 | 11.9 | 48 | 318 | 1 | ||
| M27 | 11.8 | 48 | 361 | 2 | ||
| M28 | 8.6 | 48 | 188 | 2 | ||
| M29 | 7.5 | 48 | 136 | 4 | ||
| M30 | 11.0 | 48 | 321 | 2 | ||
TX : Tongxiang, Zhejiang province; JN: Jiangning, Jiangsu province and HZ: Huzhou, Zhejiang province. * In these broods, 48 offspring were genotyped, whereas genotypes of 1-2 offspring were not clear due to low quality of DNA.
DNA isolation and microsatellite genotyping
DNA of mothers and 48 hatchlings of each brood were isolated using a method that we developed [25] and were arrayed onto 96-well plates for PCR. Four microsatellites (PCLG03, PCLG09, PCL02 and PCL017) described previously [26, 27] were selected for genotyping samples due to their high polymorphism, independent inheritance, ease of PCR and no appearance of null alleles. One primer of each pair was labelled with a fluorescent dye (6-Fam or Hex). Each microsatellite locus was amplified in a 25 µL total reaction volume containing 1 x PCR buffer (Finnzymes) with 1.5 mM MgCl2, 40 ng genomic DNA, 200 nM of each primer, 200 μM dNTPs and one unit DNA polymerase (Finnzymes). The following program was used for PCR: 94 oC for 2 min, followed by 35 cycles of 94 oC for 30 sec, 55 oC for 30 sec and 72 oC for 45 sec, and then a final extension at 72 oC for 5 min. The PCR products of 1 µL were detected on an ABI3730xl capillary DNA sequencer (Applied Biosystems). Fragment sizes of alleles were calculated against the size standard GS-500-ROX using GeneMapper (Applied Biosystems). A genotype table was exported for analysis of allele number, size range, and expected and observed heterozygosity using the software GDA [28]. Mean expected paternal exclusion probabilities were estimated across the four loci using the GERUD [29].
Parentage analysis
The genotypes of all mothers sampled were determined directly by genotyping the microsatellite loci, whereas the paternal alleles were inferred from offspring genotypes after the maternal alleles were accounted for. We determined the minimum number of fathers in each brood using GERUD version 1.0 [29]. This program reconstructs all possible multilocus genotypes of fathers and searches for the minimum number of fathers that can explain the offspring genotypes. If multiple solutions of father genotypes are obtained for a given minimum number of fathers, GERUD can rank them based on the Mendelian segregation of alleles and the allele frequencies in the population. The contributions of individual fathers can likewise be calculated in GERUD. Since technical limitations prevent the resolution of five or more males, the last two procedures can only be conducted for broods with up to four males. For each brood, we tested whether the male contributions departed from equality using χ2-tests.
Analysis of factors associated with the frequency of multiple paternity
We performed an ordinal logistic regression analysis to assess whether the number of detected fathers depends on the body size of females, and the association between the number of father and the number of hatchlings in broods, designating the number of detected fathers as the dependent variable. An ordinal scaling was chosen for the number of detected fathers since the paternity analysis did not allow differentiating between five or more fathering males (see above). The analysis was carried out with the Logistic Procedure of the SAS program (SAS Institute). We also conducted a regression analysis to assess the association between the total body length of the mother and the number of hatchlings in broods using the linear regression function with the program JMP (SAS Institute).
We compared the average number of fathers and offspring in broods in six sampling sites (two habitats from each of three locations) using one way ANOVA with the SAS program. To further determine if habitat, location and their interaction affected the number of fathers in broods and the number of offspring in broods, we conducted statistical analysis (split-plot design) using the GLM procedure of the SAS program (SAS Institute):
Yijk = μ +Li + Ej + (LE)ij+ εijk
Where, Yijk is the kth observation of the jth habitat in the ith location, μ is the mean value of in all broods, Li is the mean value of the ith location, Ej is the mean value of the jth habitat, (LE)ij is the interaction between location and habitat and εijk is the residual error.
3 Results
Water quality parameters in ponds and ditches at three sampling locations
Five parameters (pH, chemical oxygen demand, dissolved oxygen, total nitrogen and total phosphorus) related to water quality were measured (Table 1). Besides pH values that showed no significant difference (one way ANOVA, F = 1.23, d.f. = 5, P > 0.05) in six sampling sites (i.e. ditches and ponds at each of the three locations), all other parameters of water quality: chemical oxygen demand (COD), dissolved oxygen (DO), total nitrogen (TN) and total phosphorus (TP) differed significantly among six sampling sites (one way ANOVA, d.f. = 5, F = 272.2~ 2707.6, P < 0.05), These differences suggest different environmental conditions in six sampling sites Water quality was poorest in the pond in Tongxiang (TX), whereas the best quality of water was obtained in the ditch in Huzhou (HZ).
The number of offspring per brood and parentage analysis
We collected samples of mothers and offspring from 30 broods of red swamp crayfish and amplified 30 adult females and 1,440 of their offspring using four polymorphic microsatellites. The average offspring number per brood was 276.3 ± 12.9 (Table 2). There were no significant differences of number of offspring/brood among the six sampling sites (one way ANOVA, d.f. = 5, F = 0.526, P > 0.05) (Table 3).
With the exception of four offspring, all samples were successfully genotyped with the four microsatellites. In these 30 mothers, the allele number ranged from 5 for the locus PCLG03 to 10 for the locus PCLG10 with an average of 7.5 alleles/locus. The average expected heterozygosity of these markers was 0.77, whereas the observed heterozygosity was 0.63. Paternity exclusion probability for all four loci combined was 0.959. Alleles at all four loci in each brood were inherited in simple Mendelian way. All 1,436 offspring from the 30 broods showed alleles consistent with maternal genotypes.
Multiple paternity was found in 29 of 30 broods and six broods had a minimum of four different fathers (Table 2). In all 29 multiple sired broods, male parental contributions deviated significantly from equality (goodness-of-fit χ2-tests, P < 0.05; Fig. 1).
Multiple comparisons of the body length of mothers, number of offspring and number of fathers in 30 Procambarus clarkii broods among habitats within location *
| Location | Habitat | N | Body length of mothers (cm) | No. of offspring | No. of sirs | |||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |||
| HZ | ditch | 5 | 10.36 | 1.75 | 281.0 | 89.7 | 2.4 | 0.5 |
| pond | 6 | 10.13 | 1.79 | 257.2 | 88.9 | 2.5 | 1.2 | |
| NJ | ditch | 4 | 9.57 | 1.84 | 244.5 | 88.6 | 2.8 | 1.0 |
| pond | 5 | 10.64 | 0.64 | 296.8 | 53.2 | 2.4 | 0.5 | |
| TX | ditch | 4 | 9.75 | 0.61 | 286.5 | 77.4 | 2.8 | 1.0 |
| pond | 6 | 10.45 | 0.75 | 288.5 | 47.6 | 3.0 | 0.9 | |
* Differences among environments within location were not significant.
Relative contribution of fathers to broods sired by multiple paternity in 30 broods of red swamp crayfish (Procambarus clarkii). F1: the first father; F2: the second father; F3: the third father and F4: the fourth father.
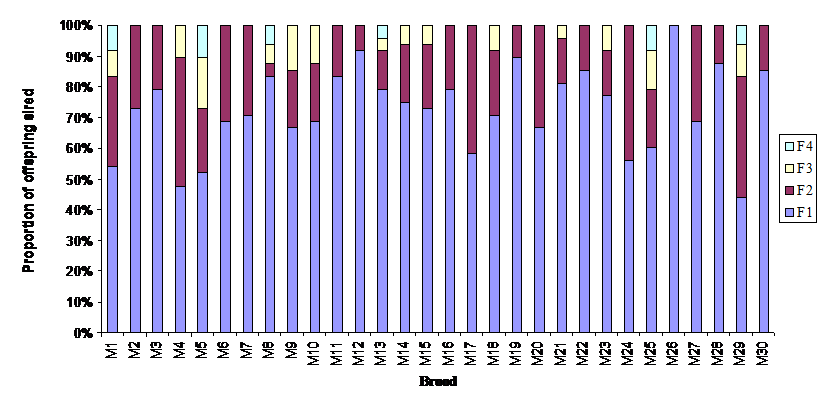
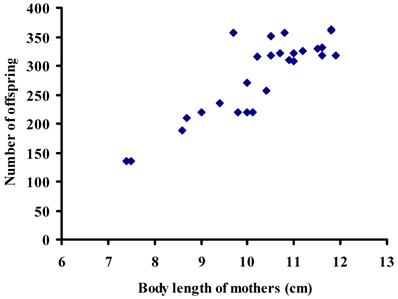
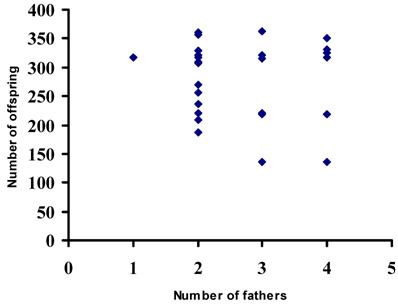
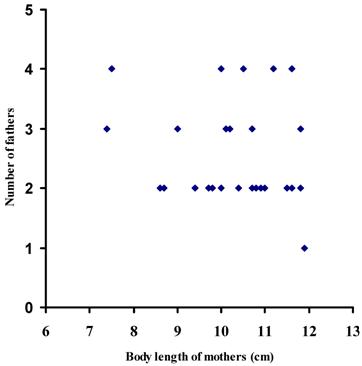
The average body length of mothers collected in the six sampling sites (one way ANOVA, d.f. = 5, F = 0.416, P > 0.05) was similar (Table 3). The body size of the mothers was significantly associated with the number of offspring (r2 = 0.77; d.f. = 29; P < 0.01) (Fig. 2), whereas the number of offspring in broods was not correlated with the number of fathers sired in broods (χ2 = 0.77; d.f. = 1; P > 0.05) (Fig. 3). The ordinal logistic regression analysis revealed that the body size of mothers was not associated with the number of males sired in broods (χ2 = 1.07; d.f. = 1; P > 0.05) (Fig. 4). Multiple paternity appeared in all three locations studied (Table 2 and Figure 1). The average number of fathers and the average number of offspring in broods were not statistically different (one way ANOVA, d.f. = 5, F = 0.344-0.526, P > 0.05) among six sampling sites (Table 3). The effects of location, habitat and the interaction between them on the number of fathers in broods and the number of offspring in broods were not statistically significant (Split-plot design, d.f. for locations =2, d.f. for habitats = 1, d.f. for interaction between habitat and locations = 2, P > 0.05).
The number of offspring per brood plotted against female body size in 30 broods of red swamp crayfish (Procambarus clarkii)
The number of offspring in 30 broods plotted against the number of fathers in 30 broods of red swamp crayfish (Procambarus clarkii)
The number of fathers detected in 30 broods plotted against female body length in 30 broods of red swamp crayfish (Procambarus clarkii)
4 Discussion
Little is known about the reproductive strategies of P. clarkii in different natural ecosystems, although reproductive performances [14, 16, 18] and population dynamics [15, 17] have been extensively studied recently. In this study, we presented the data on paternity analysis using polymorphic microsatellites that could provide new insights into the mating strategies of P. clarkii under different environmental conditions. Two major conclusions could be drawn from this study: (1) multiple paternity was common in different habitats and locations with significantly different environmental conditions. The average number of fathers per brood and the number of offspring per female were not statistically different in habitats and locations with dissimilar environmental conditions; (2) the contribution of each father to the number of offspring in each brood was significantly different. Most offspring were sired by one primary sire per brood; the gravid female was the exclusive mother of the progeny it tended.
Multiple paternity
We discovered that the majority (96.7%) of mothers had mated with multiple (2-4) males. In crayfish, during copulation, a spermatophore is transferred to an opening of the female's seminal receptacle. This leads to a sperm storage vessel [30] that, after mating, may be sealed by a sperm plug [11]. However, in P. clarkii, our study showed that even if sperm plugs were inserted, they were not effective to prevent paternity by other males. Sperm plugs may function to hold sperm in place [31], rather than block later insemination. We found that the frequency (96.7%) of multiple paternity was much higher than that (60.0%) reported for other crayfish species Orconectes placidus [32], the American lobster, Homarus americanus (13% of 108 brooders) [33], the Norway lobster, Nephrops norvegicus (54.6% of 11 brooders) [8] and the porcelain crab, Petrolisthes cinctipes (80% of 10 brooders) [34]. It is likely that multiple paternity supplies a chance for females to ensure fertilization of more eggs or to select high quality sperm for fertilization, thus improving the number and quality of offspring. A previous study [35] revealed that broods of adders (Vipera berus) and sand lizards (Lacerta agilis) with multiple sires have higher embryonic survival, fewer deformities and, in L. agilis, the offspring is heavier and present higher survival rates during the first year of life. Although there is dispute on whether polyandry increases the effective population size [36], it is generally believed that polyandry is likely to increase the effective population size due to genetic diversity [37, 38]. To ensure long-term success of survival, the maintenance of genetic variation is very important [37]. Reduced genetic variability as a result of genetic drift limits the opportunity for future expansion. Therefore, high frequency of multiple paternity in P. clarkii may be an important factor ensuring the production of high number and quality of offspring, and maintaining effective population size, and this in turn promotes its ability to expand to new habitats.
Skew contribution of males to the offspring
In this study, fathers in 29 of 30 broods contributed with different numbers of offspring to a brood, i.e. one of the inferred sires was assigned to a large proportion of offspring in each brood. Similar results have been reported in another crayfish species Orconectes placidus [32]. This kind of skewness may arise from many factors. One factor is the mating order. In some insect species where internal fertilization was similar to that in crayfish, fertilization success is biased toward the last-mating male [39], whereas in mammals, fertilization success is biased toward the first-mating male [40]. However, the current study is not able to answer the question about the order of sperm use and the length of time that sperm may survive stored in the female. To address this question, additional experiments need to be conducted. Another factor affecting the skewness of male contribution to the offspring is the body size of males and females. Under laboratory conditions, large body size is selected by both sexes [41]. However, in this study, we found that female size did not have an effect on the frequency of multiple paternity. Male and sperm competition are typical for many polyandrous mating systems [7, 42, 43] resulting in male reproductive skewness. Multiple paternity may supply the chance for females to select different males to copulate or select the best sperm for fertilization of their eggs, thus resulting in male reproductive skew and improving the fitness of offspring. Skew of multiple paternity is believed to be a major driving mechanism of evolution [44]. Therefore, in P. clarkii skewed male contribution to the offspring may be related to sperm competition and enhanced progeny fitness.
Effect of environmental conditions on multiple paternity
Many factors can greatly influence multiple paternity and reproductive performance. Previous studies have demonstrated that environmental conditions could influence multiple paternity [45] and reproductive performance of animals [6]. Some species can only reproduce in specific habitats, while others can reproduce in a wide range of environmental conditions. Successful reproduction is precondition of alien species to survive and expand in new environments. In this study, in the three locations and two types of habitats, the environmental conditions were different, while the reproductive performance (i.e. the number of offspring) of the females and the frequency of multiple paternity were similar in different environmental conditions. In the pond in Tongxiang, the water quality was quite bad, P. clarkii could not only survive, but also reproduce successfully. Our results indicate that reproductive performance of females is independent of environmental conditions examined in this study. This independence may be related to their high ability to mate with several males (multiple paternity). However, previous studies showed variation in the reproductive performance of P. clarkii under different environmental conditions [14, 15]. In the future it would be interesting to further study the reproductive performance of females and males living in more diversified environments.
Several factors have been related to the incidence of multiple paternity: body size of females [33, 46], sperm number of males [47], population density and environmental conditions [48]. Laboratory experiments showed that both males and females favoured large sized individuals for mating in P. clarkii [41]. However, in this study, we demonstrated that the number of males sired was not associated with the body size of the females, suggesting that mating behaviour is somewhat different in the laboratory than in the field. In some species, sperm of males is limited. In this case, to ensure fertilization of all eggs, multiple paternity is required [42]. However, in this study, we found that the number of offspring per brood was not associated with the number of fathers per brood, suggesting that sperm limitation was not a factor causing multiple paternity. In the two habitats (ponds and ditches), the density of crayfish was quite different, while the frequency of multiple paternity and number of fathers/brood were almost the same, suggesting population density is not a factor promoting multiple paternity. We also showed that the number of fathers sired in the broods collected from different locations and habitats with different environmental conditions was similar, indicating that multiple paternity in this species is independent of environmental conditions. This allows females to produce large number and high quality offspring in different environments, which in turn permits this species to thrive in less favourable conditions. In the future, it is necessary to study paternity in more habitats to examine whether we can generalize our conclusion that the reproductive performances of females and multiple paternity in P. clarkii are independent of environmental conditions.
Acknowledgements
This study was supported by the internal funds of the Temasek Life Sciences Laboratory and Aquaculture Division, E-Institute of Shanghai Universities.
Conflict of interest
The authors have declared that no conflict of interest exists.
References
1. Ruiz GM, Carlton JT. Invasive Species. London: Island Press. 2003
2. Cox GW. Alien Species and Evolution-The Evolutionary Ecology of Exotic Plants, Animals, Microbes, and Interacting Native Species. London: Island Press. 2004
3. Zhang DX, Hewitt GM. Nuclear DNA analyses in genetic studies of populations: practice, problems and prospects. Molecular Ecology. 2003;12(3):563-584
4. Selkoe KA, Toonen RJ. Microsatellites for ecologists: a practical guide to using and evaluating microsatellite markers. Ecology Letters. 2006;9:615-629
5. Jones AG, Ardren WR. Methods of parentage analysis in natural populations. Molecular Ecology. 2003;12(10):2511-2523
6. Avise JC, Jones AG, Walker D, DeWoody JA. Genetic mating systems and reproductive natural histories of fishes: Lessons for ecology and evolution. Annual Review of Genetics. 2002;36:19-45
7. Birkhead T, Moller AP. Sperm Competition and Sexual Selection. London: Academic Press. 1998
8. Streiff R, Mira S, Castro M, Cancela ML. Multiple paternity in Norway lobster (Nephrops norvegicus L.) assessed with microsatellite markers. Marine Biotechnology. 2004;6(1):60-66
9. Uller T, Olsson M. Multiple paternity in reptiles: patterns and processes. Molecular Ecology. 2008;17(11):2566-2580
10. Pearse DE, Avise JC. Turtle mating systems: behaviour, sperm storage, and genetic paternity. Journal of Heredity. 2001;92:206-211
11. Huner JV. Procambarus in North America and elsewhere. Freshwater Crayfish: Biology, Management and Exploitation. Edited by Holdich DM, Lowery RS. Portland: Timber Press. 1988:239-261
12. Li JL, Dong ZG, Li YS, Wang CH. Invasive Aquatic Species in China. Shanghai: Shanghai Science and Technology Publisher. 2007
13. Li SC, Xu YX, Du LQ, Yi XL, Men XD, Xie JY. Investigation on and analysis of alien invasions in Chinese farming industry. Chinese Agricultural Science Bulletin. 2005;21(6):156-159
14. Alcorlo P, Geiger W, Otero M. Reproductive biology and life cycle of the invasive crayfish Procambarus clarkii (Crustacea: Decapoda) in diverse aquatic habitats of South-Western Spain: Implications for population. Fundamental and Applied Limnology. 2008;173:197-212
15. Anastacio PM, Marques JC. Population biology and production of the red swamp crayfish Procambarus clarkia (Girard) in the lower Mondego river valley, Portugal. Journal of Crustacean Biology. 1995;15:156-168
16. Anastacio PM, Marques JC. Crayfish (Procambarus clarkii) condition throughout the year in the lower Mondego River Valley, Portugal. Crustaceana. 1998;71:593-602
17. Anastacio PM, Leitao AS, Boavida MJ. Population dynamics of the invasive crayfish (Procambarus clarkii Girard, 1852) at two marshes with differing hydroperiods. Annales de Limnologie - International Journal of Limnology. 2009;45:247-256
18. Dorr AJM, La Porta G, Pedicillo G, Lorenzoni M. Biology of Procambarus clarkii (Girard, 1852) in Lake Trasimeno. Bulletin Francais De La Peche Et De La Pisciculture. 2006;380(81):1155-1167
19. Barnes R. Invertebrate Zoology. Philadelphia, PA: WB Saunders Company. 1974
20. Barbaresi S, Santini G, Tricarico E, Gherardi F. Ranging behaviour of the invasive crayfish, Procambarus clarkii (Girard). Journal of Natural History. 2004;38(22):2821-2832
21. Liu DD. China National Geography-South and East (in Chinese). Shanghai: China PUB Com. 2007
22. Gao Y, Yuan CH. Friends or enemies: a survey of invasive alien species in China. National Geography of China. 2003;8:84-99
23. SEPA C. Methods for water and wastewater monitoring and analysis, 4th ed. Beijing: China Environmental Science Press. 2002
24. Yue GH, Wang GL, Zhu BQ, Wang CH, Zhu ZY, Lo LC. Discovery of four natural clones in a crayfish species Procambarus clarkii. International Journal of Biological Science. 2008;4:279-282
25. Yue GH, Orban L. A simple and affordable method for high throughput DNA extraction from animal tissues for PCR. Electrophoresis. 2005;26:3081-3083
26. Belfiore NM, May B. Variable microsatellite loci in red swamp crayfish, Procambarus clarkii, and their characterization in other crayfish taxa. Molecular Ecology. 2000;9(12):2231-2234
27. Zhu ZY, Yue GH. Polymorphic microsatellites from the genome of red swamp crayfish (Procambarus clarkii). Molecular Ecology Resources. 2008;8:796-798
28. Genetic Data Analysis. Recent access in 2010 Lewis PO, Zaykin D. http://hydrodictyon.eeb.uconn.edu/people/plewis/software.php
29. Jones AG. GERUD1.0: A computer program for the reconstruction of parental genotypes from progeny arrays using multi-locus DNA data. Molecular Ecology Notes. 2001;1:215-218
30. Holdich DM, Reeve ID. Functional morphology and anatomy. Freshwater crayfish: Biology, Management, and exploitation. Edited by Hoidicg.D.M, Lowery RS. London: Groomhelm. 1988:11-51
31. Berrill M, Arsenault M. The breeding behavior of a northern temperate Orconectid crayfish. Animal Behaviour. 1984;32:333-339
32. Walker D, Porter BA, Avise JC. Genetic parentage assessment in the crayfish Orconectes placidus, a high-fecundity invertebrate with extended maternal brood care. Molecular Ecology. 2002;11(10):2115-2122
33. Gosselin T, Sainte-Marie B, Bernatchez L. Geographic variation of multiple paternity in the American lobster, Homarus americanus. Molecular Ecology. 2005;14(5):1517-1525
34. Toonen RJ. Genetic evidence of multiple paternity of broods in the intertidal crab Petrolisthes cinctipes. Marine Ecology Progress Series. 2004;270:259-263
35. Olsson M, Madsen T. Sperm Competition and Sexual Selection. London: Academic Press. 1998
36. Karl SA. The effect of multiple paternity on the genetically effective size of a population. Molecular Ecology Notes. 2008;17:3973-3977
37. Hedrick PW. A standardized genetic differentiation measure. Evolution. 2005;59(8):1633-1638
38. Garcia-Vazquez E, Moran P, Martinez JL, Perez J, de Gaudemar B, Beall E. Alternative mating strategies in Atlantic salmon and brown trout. Journal of Heredity. 2001;92(2):146-149
39. Parker GA. Sperm competition and its evolutionary consequences in insects. Biological Reviews. 1970;45:525- 567
40. Lacey EA, Wieczorek JR, Tucker PK. Male mating behaviour and patterns of sperm precedence in Arctic ground squirre. Animal Behaviour. 1997;53:767-779
41. Aquiloni L, Gherardi F. Evidence of female cryptic choice in crayfish. Biology Letters. 2008;4(2):163-165
42. Simmons LW, Beveridge M, Kennington WJ. Polyandry in the wild: temporal changes in female mating frequency and sperm competition intensity in natural populations of the tettigoniid Requena verticalis. Molecular Ecology. 2007;16(21):4613-4623
43. Fitzsimmons NN. Single paternity of clutches and sperm storage in the promiscuous green turtle (Chelonia mydas). Molecular Ecology. 1998;7(5):575-584
44. Ross KG. Molecular ecology of social behaviour: analyses of breeding systems and genetic structure. Molecular Ecology. 2001;10(2):265-284
45. Williams GC. Natural selection, the costs of reproduction, and a refinement of Lack's principle. The American Naturists. 1966;100:687
46. Zbinden JA, Largiader CR, Leippert F, Margaritoulis D, Arlettaz R. High frequency of multiple paternity in the largest rookery of Mediterranean loggerhead sea turtles. Molecular Ecology. 2007;16(17):3703-3711
47. Firman RC, Simmons LW. Polyandry facilitates postcopulatory inbreeding avoidance in house mice. Evolution. 2008;62(3):603-611
48. Matthews IM, Evans JP, Magurran AE. Male display rate reveals ejaculate characteristics in the Trinidadian guppy Poecilia reticulata. Proceedings: Biological Sciences. 1997;264:695-700
Author Biography
YGH is the Principal Investigator of the molecular population genetics group at the Temasek Life Sciences Laboratory, Singapore. His research interests are population genetics, DNA markers, marker-assisted breeding and molecular ecology. LJL is a professor at Shanghai Ocean University working on genetic resources and breeding of aquatic animals. WCM and XJH are Research Fellow in YGH's group. WGL is a Professor at Nanjing Agricultural University, China, working on animal reproductive biology. FJB is a lecturer at Shanghai Ocean University working on genetic resources and breeding of aquatic animals.
![]() Corresponding author: Dr. Gen Hua Yue, Molecular Population Genetics Group, Temasek Life Sciences Laboratory, 1 Research Link, National University of Singapore, 117604 Republic of Singapore. Tel: 65-68727405; Fax: 65-68727007; Email: genhuaorg.sg
Corresponding author: Dr. Gen Hua Yue, Molecular Population Genetics Group, Temasek Life Sciences Laboratory, 1 Research Link, National University of Singapore, 117604 Republic of Singapore. Tel: 65-68727405; Fax: 65-68727007; Email: genhuaorg.sg

 Global reach, higher impact
Global reach, higher impact