Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2010; 6(3):282-293. doi:10.7150/ijbs.6.282 This issue Cite
Research Paper
Melatonin Plays a Protective Role in Postburn Rodent Gut Pathophysiology
1. Department of Biological Sciences, Chicago State University, Chicago, IL, USA
2. Department of Pharmaceutical Sciences, College of Pharmacy, Chicago State University, Chicago, IL, USA
Received 2009-12-29; Accepted 2010-5-11; Published 2010-5-17
Abstract
Melatonin is a possible protective agent in postburn gut pathophysiological dynamics. We investigated the role of endogenously-produced versus exogenously-administered melatonin in a major thermal injury rat model with well-characterized gut inflammatory complications. Our rationale is that understanding in vivo melatonin mechanisms in control and inflamed tissues will improve our understanding of its potential as a safe anti-inflammatory/antioxidant therapeutic alternative. Towards this end, we tested the hypothesis that the gut is both a source and a target for melatonin and that mesenteric melatonin plays an anti-inflammatory role following major thermal injury in rats with 3rd degree hot water scald over 30% TBSA. Our methods for assessing the gut as a source of melatonin included plasma melatonin ELISA measurements in systemic and mesenteric circulation as well as rtPCR measurement of jejunum and terminal ileum expression of the melatonin synthesizing enzymes arylalkylamine N-acetyltransferase (AA-NAT) and 5-hydroxyindole-O-methyltransferase (HIOMT) in sham versus day-3 postburn rats. Our melatonin ELISA results revealed that mesenteric circulation has much higher melatonin than systemic circulation and that both mesenteric and systemic melatonin levels are increased three days following major thermal injury. Our rtPCR results complemented the ELISA data in showing that the melatonin synthesizing enzymes AA-NAT and HIOMT are expressed in the ileum and jejunum and that this expression is increased three days following major thermal injury. Interestingly, the rtPCR data also revealed negative feedback by melatonin as exogenous melatonin supplementation at a dose of 7.43 mg (32 μmole/kg), but not 1.86 mg/kg (8 μmole/kg) drastically suppressed AA-NAT mRNA expression. Our methods also included an assessment of the gut as a target for melatonin utilizing computerized immunohistochemical measurements to quantify the effects of exogenous melatonin supplementation on postburn gut mucosa barrier inflammatory profiles. Here, our results revealed that daily postburn intraperitoneal melatonin administration at a dose of 1.86 mg/kg (8 μmole/kg) significantly suppressed both neutrophil infiltration and tyrosine nitrosylation as revealed by Gr-1 and nitrotyrosine immunohistochemistry, respectively. In conclusion, our results provide support for high mesenteric melatonin levels and dynamic de novo gut melatonin production, both of which increase endogenously in response to major thermal injury, but appear to fall short of abrogating the excessive postburn hyper-inflammation. Moreover, supplementation by exogenous melatonin significantly suppresses gut inflammation, thus confirming that melatonin is protective against postburn inflammation.
Keywords: Melatonin, burn, gut barrier, nitrotyrosine, inflammation, sepsis, ELISA, rtPCR, HIOMT, AA-NAT, neutrophils, extravasation, ileum, jejunum, mesenteric, immunohistochemistry, GR1.
1. Introduction
Intestinal barrier dysfunction plays a major role in the pathophysiology of many disorders including postburn intestinal sepsis where the gut has been described as a “motor” that drives sepsis [1-7]. Central to this role is a dynamic and delicate homeostatic balance that involves a complex web of intricate internal mechanisms that span paracrine, endocrine, nervous, as well as innate and adaptive immune responses alongside external environmental parameters that include circadian synchronization, gut microbiota and digestion-related agents [1-9]. The complexity of these interlinking factors masks the importance of individual players in the overall, integrated, whole organism-level responses to major injury. This complexity makes it difficult to explain individual clinical variations often encountered in patient or animal models subjected to the same traumatic insult. Indeed, our own interest in pursuing sources of such variations was triggered by the persistent observation of small but remarkable subsets of experimental subjects that survived major thermal injury with modest, if any, signs of intestinal inflammation, where the trend in the majority of their closely-related and identically-raised, -housed and -treated littermates was to exhibit severe distress. Here, we focus on mesenteric melatonin as a possible protective player among the myriad of other possible sources of variation in gut inflammatory responses [8-12].
To date, there is mounting evidence for extrapineal, non-circadian, protective roles for gastrointestinal melatonin [13-18]. In spite of being lipophilic, melatonin has been shown to be localized at relatively high concentrations in various body compartments that include bile, ovary, bone and cerebrospinal fluid [15-17, 19-23]. Indeed, daytime melatonin has been reported at 20 to 30 times higher concentration in bile than in the general blood circulation in many mammals, including rats and humans [23]. The persistence of such high melatonin levels, at times of low melatonin synthesis by pineal synthesizing enzymes arylalkylamine N-acetyltransferase (AA-NAT) and 5-hydroxyindole-O-methyltransferase (HIOMT), points to extrapineal melatonin synthesis and/or to the presence of melatonin binding proteins that aid in compartmentalizing melatonin [15-19,22]. Once excreted, bile melatonin is thought to play a protective antioxidant/anti-inflammatory role against gut epithelial damage where it could also be reabsorbed and recycled in the bile [13,14,21-30]. Moreover, evidence exists for de novo melatonin synthesis and high tissue melatonin levels in the gut [13,14]. Taken together, these observations suggest the gut as a source for melatonin with a persistently high extrapineal mesenteric melatonin pool, independent of diurnal systemic melatonin fluctuations. Additionally, mounting evidence suggests that the gut mucosa barrier tissue is a target for melatonin's protective actions [13-18]. Evidence for anti-inflammatory/antioxidant melatonin actions has been reported in several tissue targets [22-30] and melatonin has been shown to be beneficial for burn inflected subjects [31,32].
These observations point to a great therapeutic potential, especially in relation to gut barrier inflammatory abnormalities associated with major thermal injury and postburn intestinal sepsis pathogenesis [1-3]. Here, we begin to closely explore this potential by examining melatonin's postburn gut pathophysiological dynamics. Specifically, we utilize a rodent animal model of major thermal injury with well-characterized postburn gut barrier abnormalities to test the hypothesis that the gut is a source and a target for melatonin. Towards this end, we assess gut melatonin dynamics by comparing daytime mesenteric versus systemic blood melatonin levels, and by examining intestinal tissue AA-NAT and HIOMT mRNA changes in response to exogenous melatonin injection or to major thermal injury. We also investigate the inflammatory/antioxidant effects of melatonin by assessing neutrophil infiltration and protein nitrosylation in the gut mucosa milieu in the aftermath of major thermal injury, in the presence and absence of exogenous melatonin supplementation.
2. Materials and Methods
Thermal Injury Protocol
Male Harlan Sprague-Dawley rats weighing 250-300 g (Indianapolis, IN) were used in this investigation. All animal housing, experimentation and treatment were based on previously published protocols approved by the Chicago State University Animal Care and Use Committee (IACUC) and in accordance with NIH guidelines [1-3]. All rats (1) were acclimatized in the animal facility for at least one week before being subjected to any treatment; (2) were given free access to water and standard lab rat chow ad libitum and kept in a 12:12 light-dark from time of arrival till sacrifice; and (3) unless otherwise noted, all treatments including major thermal injury (TI) were carried out between zeitgeber time (ZT) 6 and 8, where ZT 0 represents the onset of the light period of the L-D cycle at 6 a.m. Major thermal injury protocols were previously described and shown to be associated with full thickness 3rd degree skin burns over ~30% of the total body surface associated with fairly consistent inflammation-linked immune as well as gut barrier abnormalities [1-3]. Accordingly, upon verification of deep anesthesia with sodium pentobarbital (40-50 mg/kg intraperitoneal, i.p.), as determined by absence of any signs of awareness including response to hind limb pinch, rats were subjected to the following steps in quick succession: (1) the back fur was shaved off (~30% TBSA), (2) rats were securely placed in an appropriately-sized bottomless plastic mold that allows the shaved area of the skin on the back to be immersed in water while the rest of the body is protected, (3) the back was immersed in a hot water bath (95-97 ºC) for 10 seconds, (4) excess hot water was immediately wiped off to avoid an additional injury, and (5) 10 ml of normal saline was injected intraperitoneally for resuscitation.
Experimental groups and melatonin treatment
To assess circulating and mesenteric plasma melatonin levels by ELISA, three groups were compared, namely, sham, 3-day-postburn, and sham rats sacrificed 2 hours after exogenous melatonin (Sham+mel; 2mM, 1 ml/250 g b.wt., i.p.) injection. To assess the effect of thermal injury and exogenous melatonin on gut AA-NAT and HIOMT mRNA expression, four groups were compared, namely: sham, 3-day-postburn, and 3-day-postburn rats sacrificed 2 hours after exogenous melatonin injection at a dose of 2 or 8 mM at 1ml/250 g b.wt. i.p.
To determine the anti-inflammatory/antioxidant effects of thermal injury and those of melatonin, four matched groups were run in parallel: sham, thermal injury (TI), sham plus melatonin (sham+mel), and thermal injury plus melatonin (TI+mel). All groups received identical anesthesia and treatments as above, except that (1) sham and sham+mel were immersed in 25 ºC instead of 95-97 ºC water (step 3 above) and (2) melatonin treatment rats, namely, sham+mel and TI+mel, received daily melatonin (Sigma-Aldrich) injections (1.86 mg/kg b.wt. dissolved in ~ 1ml saline, i.p.) at ZT 6-8 starting just before thermal injury and continuing until sacrifice.
Blood and Tissue Collection
All rats were sacrificed under deep anesthesia (pentobarbital 40-60 mg/kg) between ZT 6 and 8 on day 3 after thermal injury, at which time: (a) mesenteric veins draining from the lower bowel into the hepatic portal vein were microscopically identified and punctured; because this is a terminal procedure, we found this approach to be a simple and straightforward alternative for sampling mesenteric blood without cannulating the mesenteric and/or portal veins and the complications/trauma and other variables they may introduce [19, 33,34], (b) systemic circulating blood was obtained by transcardial puncture, and (c) jejunum and terminal ileum samples were immediately dissected. Blood samples were collected in heparinized syringes and tubes and immediately preserved on ice and quickly centrifuged to obtain plasma which was immediately stored under -80 ºC until use. Plasma and terminal ileum as well as jejunum samples were snap frozen in liquid nitrogen and stored in a -80 ºC deep freezer until use for rtPCR methods. Additional terminal ileum and jejunum samples were fixed in 4% paraformaldehyde and preserved at 4 ºC until being cut for immunohistochemistry into 30 μm frozen sections using a cryostat (HM525 Clinical/ Research Cryostat, Richard-Allan Scientific HM 550).
Melatonin ELISA
Melatonin levels were compared in mesenteric and systemic general blood circulation of sham versus experimental day-3 major thermal injury rats. Mesenteric plasma was obtained from blood pooled in the peritoneal cavity upon puncturing the lower mesenteric microvasculature draining the small and large bowel and leading to the hepatic portal vein. Systemic plasma was collected from blood drawn transcardially through the left ventricle wall. In both cases, blood was collected at ~ZT 8 upon terminal deep anesthesia with sodium pentobarbital (40-60 mg/kg intraperitoneal, i.p.).
Plasma melatonin levels were assessed utilizing a commercial melatonin ELISA kit (MP Biomedicals' Inc., Irvin CA). Data was collected and analyzed using manufacturer's instructions and standards. Briefly, 50 μl of samples, controls, and standards were micropipetted in their designated ELISA plate wells, followed by adding 50 μl of melatonin-biotin, then 50 μl melatonin antiserum mixes, before incubating overnight at 4 ºC. Next day, 3 washes with buffer were followed by incubation with 150 μl conjugate for 2 hours at room temperature, then washing 3 times with buffer, before incubation with 200 μl PNPP substance for 20-40 minutes at room temperature, then adding stop solution. ELISA analyzer absorbance readings were taken at 405 nm and sample melatonin concentrations were calculated using their average optical densities based on kit standard curves.
RT-PCR
To determine whether the gut naturally expresses the key enzymes for melatonin synthesis during the light hours of the day, AA-NAT and HIOMT mRNA expression was tested in RNA isolated from ileum and jejunum tissue specimens that were freshly dissected from all rat groups at ZT 8 and stored in RNAlater (Qiagen, Valencia, CA). To test the effect of exogenous melatonin on gut melatonin expression, AA-NAT mRNA was compared four hours after ~1 ml i.p. injection of 1.86 mg (8 μmole) or 7.43 mg (32 μmole) /kg b.wt. exogenous melatonin (Sigma-Aldrich, St Louis) dissolved in normal saline.
RNA isolation: RNA isolation was performed utilizing Qiagen's RNeasy Fibrous Tissue kit. Briefly, ileum and jejunum tissue segments weighting ≤ 30 mg were homogenized and lysed, then treated with proteinase K solution in order to remove protein, connective tissues, and collagen, as well as DNase to remove DNA. Samples were then microcentrifuged to remove debris before washing, eluting, then deep freezing high quality pure RNA until use for rtPCR.
Reverse transcription: RNA isolated from ileum and jejunum tissues was processed for RT-PCR using the Qiagen One-Step RT-PCR kit (Qiagen, Valencia, CA) following manufacturer's instructions. Briefly, master mix was prepared on ice by adding 10 μl 5X RT-PCR Buffer, 1 μl dNTP mix, 1 μl Qiagen RT-PCR enzyme mix, 1.50 μl forward primer, 1.50 μl reverse primer and 10 μl RNase-free water. Master mix (10 ul) was mixed in sterile tubes with template RNA (10 μl ; 10 ng/μl ) solution and run on a PCR Sprint Thermal Cycler (Thermo Electron Company, Milford MA) set according to thermal cycler protocol as follows: Stage 1: reverse transcriptase (cDNA) at 50 °C for 30 minutes, Stage 2: initial PCR activation at 95 °C for 15 minutes, Stage 3: denaturation at 94 °C for 45 seconds, annealing at 50-68 °C for 45 seconds, then extension 72 °C for 1 minute, and Stage 4: final extension at 72 °C for 10 minutes. The kit allows both reverse transcription and PCR amplification to take place in what is commonly referred to as a one-step reaction. The kit contains OmniscriptReverse Transcriptase (ORT), Sensiscript Reverse Transcriptase (SRT), and HotStar Taq DNA polymerase (HDP) and has a reverse transcription target RNA detection range of 1 pg to 2 μg.
Primers AA-NAT (F: 5- TGA GCG CGA AGC CTT TAT CTC AGT; R: 3- TGT GGC ACC GTA AGG AAC ATT GCA) and HIOMT (F: 5-GGTAGCTCCGTGTGTGTCTT-3'; R: 5'-AGTGGCCAGGTTGCGGTAGT-3') were designed in our lab based on Genbank and oligoprimer analysis software (OligoPerfect™ Designer, Invitrogen) and synthesized commercially by Invitrogen. Housekeeping gene, GAPDH (F: AGA TGG TGA AGG TCG GTG TC; R: ATT GAA CTT GCC GTG GGT AG) was used as a control to ensure validity, reliability, and proper pipette technique.
rtPCR product visualization and densitometry. rtPCR amplification product (10 μl product mixture + 2 μl dye) were loaded in triplicates in parallel with DNA marker (100 bp) using a 2.5 % agarose gel prepared with TAE buffer. Gel was run (Gel apparatus model 250, GIBCO BRL Life Technologies Inc, Gaithersburg , MD) for 45 to 60 minutes with voltage set at 105 V then developed in ethidium bromide solution for 15 minutes before imaging with a Fluor-S MultiImager (Bio-Rad). The PCR bands were digitally captured then AA-NAT/GADPH and HIOMT/GADPH mRNA transcript density ratios were quantified utilizing Metamorph (Universal Imaging) densitometry software.
Inflammation assessment with H&E and Gr-1 and nitrotyrosine immunohistochemistry [1-3, 35-37]
All staining was performed on 30 μm thick ileum and jejunum cross sections cut by cryostat and thaw-mounted on superfrost-plus slides. H&E staining colors basophilic structures with blue-purple hue with hematoxylin and eosinophilic structures bright pink with eosin Y, thus making it fairly easy to spot sites of inflammation marked by leukocyte infiltration with a light microscope. Inflammation was also confirmed by granulocyte-1 (Gr-1) as well as nitrotyrosine immunohistochemistry (IHC) examined by fluorescent microscopy, whereby site-specific immunolabeling corresponds to neutrophil infiltration (Gr-1 IHC) or protein nitrosylation (nitrotyrosine IHC).
For immunohistochemistry, sections from all experimental groups were treated as follows: thawed (10 min, room temp), surrounded with a repellent circle using a Pap Pen, fixed with 4% formaldehyde (10 min, room temp), washed with PBS, incubated with 0.1% diluted goat serum in serum in PBS (30 min, room temp) to block nonspecific binding, incubated inside a humid chamber with rabbit anti-Gr-1 or mouse anti-nitrotyrosine primary antibody (1:1000 in PBS, 4 ºC, overnight), washed with PBS, incubated with FITC goat anti-rabbit IgG secondary antibody (for Gr-1) and goat-anti mouse IgG (for nitrotyrosin; Upstate Biotechnology, Lake Placid, NY; 1:1000 in PBS, 45 min, room temp), washed with PBS, cover-slipped using Crystal Mount, then dried and analyzed.
Immunolabeling density was assessed using computerized digital imaging with Metamorph software (Universal Imaging) and fluorescent microscopy with a TE2000-S Inverted Fluorescence Microscope with CFI Plan Fluor objectives. Densitometric measurements for granulocyte-1 (Gr-1) as well as nitrotyrosine immunolabeling intensity reflect the overall degree of tissue inflammation. Cell counts were also performed for Gr-1 immunopositive cells to assess level of gut mucosa infiltration by Gr-1 labeled cells from extracellular and background labeling.
Statistical analysis
Quantitative ELISA, rtPCR, and microscopic cell count as well as densitometry measurements were tabulated and plotted as mean ± SE in MS Excel and analyzed using the Sigma Statistical program (SPSS, version 2.0; SigmaStat). T-tests and ANOVA as well as Tukey's post-HOC analyses were used to compare groups and P values less than 0.05 were considered statistically significant.
3. Results
Melatonin concentration in mesenteric versus general circulation and the effects of major thermal injury
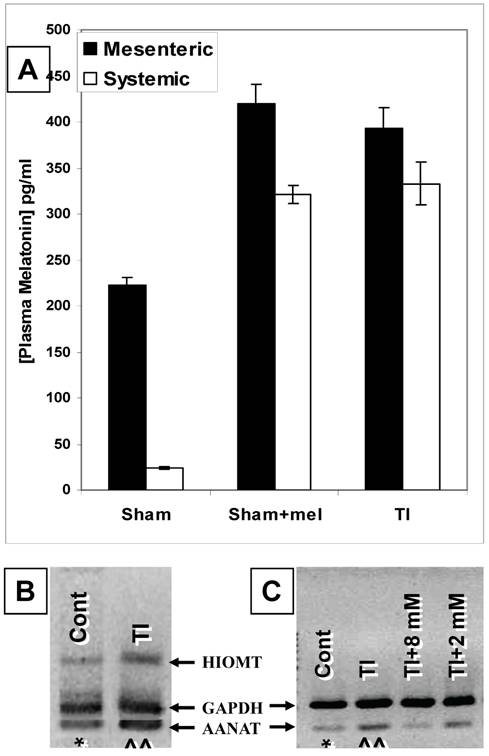
Our ELISA melatonin measurements revealed that plasma melatonin levels in the mesenteric blood draining the intestines (221.9±9.3 pg/ml) were over 9 times those found in the general circulation (24.2±1.4 pg/ml) (p <0.01) (Fig. 1). Major thermal injury was associated with an almost two-fold increase in mesenteric (from 221.9±9.3 to 393.2±22.6 pg/ml) and over than 13-fold increase in general circulation (from 24.2±1.4 to 333.1±22.9 pg/ml) plasma melatonin (p<0.001) (Fig. 1). Exogenous melatonin administration in sham rats (single i.p. injection; 1.86 mg/kg b.wt.) resulted in an increase in mesenteric as well as systemic melatonin to 419.9±21.4 pg/ml and 321.5±9.8 pg/ml, respectively. Interestingly, this is a very close match to circulating and mesenteric plasma melatonin levels seen in TI rats (Fig. 1A). These levels returned to normal levels in non-treated sham rats within 7 hours (mesenteric plasma, 243.7±38.4 pg/ml, systemic circulation, 33.9±1.1 pg/ml; data not graphed).
The gut is a source of melatonin and levels of mesenteric melatonin and gut melatonin synthesizing enzymes are influenced by major themal injury as well as exogenous melatonin. A. In sham rats, ELISA melatonin measurements revealed that melatonin levels in mesenteric blood were around 9 times higher than those found in the general circulation (Far left bars). Contrariwise, plasma melatonin levels were profoundly increased in both mesenteric and general circulation in rats with a 3rd degree burn over 30% TBSA 3 days after thermal injury (TI, far right, p<0.01). Interestingly, these mesenteric and general circulation plasma melatonin levels were a very close match to circulating and mesenteric plasma melatonin levels found in sham rats 2 hours after exogenous melatonin (Sham+mel; 2mM, 1 ml/250 g b.wt., i.p.) injection. B. Gut AANAT and HIOMT mRNA expression levels were higher (^^) in thermally injured (TI) than sham control (*) ileum.C. AANAT mRNA expression in TI subjects was more suppressed by a higher (8 mM) than a lower (2 mM) dose of exogenous melatonin administered by IP injection. rtPCR products appear at 109 bp (AANAT), 260bp (HIOMT) and 222 bp (housekeeping GAPDH). All measurements were performed on blood samples and tissues obtained at ~ZT 8 of a 12:12 light cycle at resting conditions with free access to chow feed.
AA-NAT and HIOMT expression and the effect of thermal injury and exogenous melatonin administration
Our rtPCR data demonstrated that AA-NAT and HIOMT mRNAs are expressed in ileum (Fig. 1B&C) and duodenum (data not shown) tissues. The expression of these two enzymes supports the possibility of de novo melatonin synthesis in the gut that would contribute to the higher melatonin levels in mesenteric relative to systemic blood melatonin during the light-day hours (Z.T. 8) when pineal melatonin production is suppressed.
Our data also revealed that major thermal injury results in robust increases in AA-NAT and HIOMT mRNA levels in the terminal ileum (Fig. 1B&C) 3 days after major thermal injury, thus supporting a possible contribution of increased de novo melatonin synthesis in the gut. In parallel with qualitative and computerized densitometry analysis of rtPCR bands, our QrtPCR experiments confirmed that AA-NAT and HIOMT rtPCR products emerged in 29 to 32 cycles, whereas the housekeeping gene GAPDH rtPCR product appeared around cycle 26, the AA-NAT/GADPH mRNA transcript ratio increased by about 9% (0.99±0.007, N=3 to 1.08±0.04, N=5) and HIOMT/GADPH ratios increased by about 9.5% (0.81±0.011, N=3 to 0.89±0.03, N=5). We also observed that AA-NAT mRNA decreases with exogenous melatonin administration (Fig.1B), thus raising the possibility that gut melatonin may be subject to negative feedback.
To further assess whether melatonin exerts feedback on its own production, we compared the effects of boosting melatonin levels by administering exogenous melatonin by i.p. injection of 1ml/250g b.wt. doses at 2 or 8 mM concentrations two hours before measuring the ileum expression levels of the melatonin synthesizing enzyme mRNA. Our data revealed that the 8 mM (1ml/250g b.wt., i.p.) dose diminished AA-NAT mRNA expression to about ~5-15% of its postburn expression whereas the 2 mM (1ml/250g b.wt., i.p.) dose decreased it only to 70-80% its postburn expression (Fig. 1C).
Melatonin decreases intestinal tissue inflammation
To test whether the postburn increase in melatonin (Fig. 1) contributes to postburn intestinal injury or represents an insufficient attempt at truncating it, we examined the effect of reinforcing endogenous melatonin production by an exogenous injection. Towards this end, a dose of 1.86 mg/kg, i.p. was chosen because it increases both mesenteric and systemic blood melatonin levels (Fig. 1a) without drastically decreasing levels of gut AA-NAT mRNA expression (Fig. 1c). The effects of such an injection are presented in Figs. 2-4.
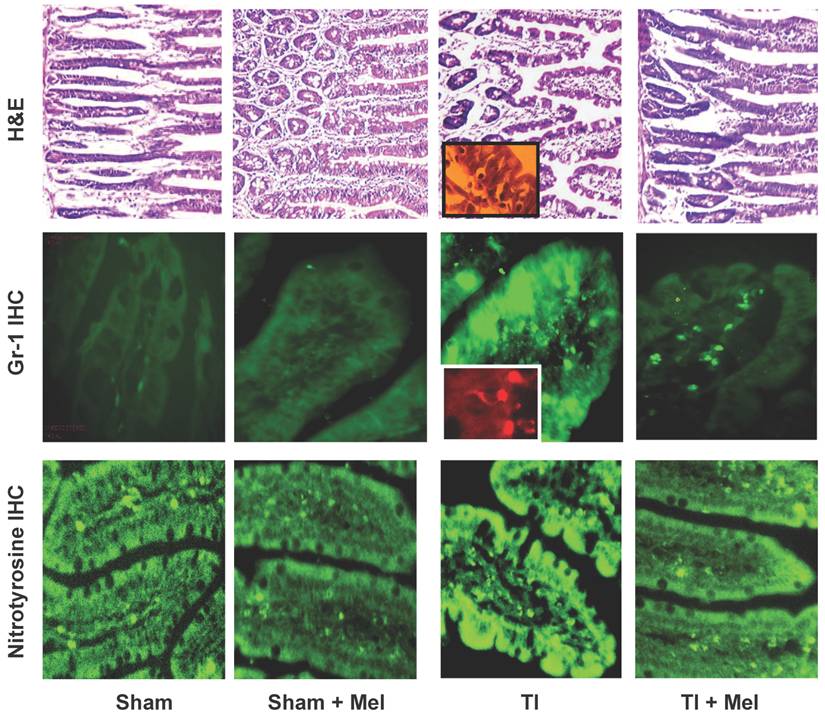
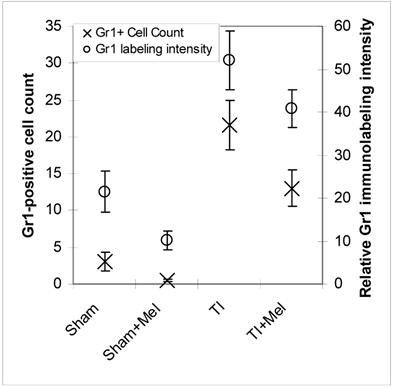
Microscopic analysis of H&E stained terminal ileum and duodenum tissues revealed robust signs of inflammation, especially in the form of mucosa neutrophil influx and extravasation (Fig. 2, top row, TI inset) on day 3 of major thermal injury. Such signs of inflammation were profoundly decreased in specimens from melatonin-treated thermal injury rats, far lower in sham, and virtually absent in sham plus melatonin. A similar inflammation pattern was confirmed using Gr-1 immunohistochemistry with robust neutrophil influx and extravasation in the terminal ileum and jejunum mucosa (Fig. 2, middle row, TI inset). Here, we quantified and statistically analyzed the degree of inflammation and found approximately 40% drop in Gr-1 immunopositive cell counts and over 20% decrease in Gr-1 labeling intensity (Fig. 3) both of which were statistically significant (p<0.01, N=3 to 7). The decrease in Gr-1-positive cell count, which showed a larger effect for melatonin, is indeed a better indicator of the robust anti-inflammatory actions of melatonin than overall labeling intensity, as the former reflects the actual change in the total number of infiltrating Gr-1 immunopositive cells (presumably neutrophils) while the latter averages overall color intensity of cellular, non-cellular, and background labeling.
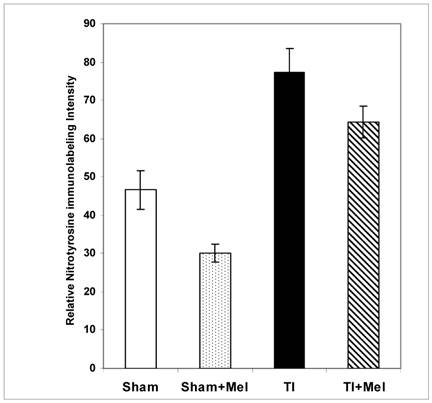
Melatonin-mediated decreases in gut mucosa neutrophil infiltration and inflammation were also further supported by a parallel decrease in one of their down stream manifestations, namely, tissue protein nitrosylation as determined by nitrotyrosine immunohistochemistry. This was clear in Fig. 2 (bottom row) where nitrotyrosine immunoreactivity was very high in TI tissue but not in TI+melatonin. This effect is depicted quantitatively in Fig. 4, where relative density of nitrotyrosine labeling is in the order TI > TI+mel > sham > sham+mel, with melatonin suppressing about 15% of tissue nitrosylation in melatonin-treated relative to TI rats (p<0.05).
Melatonin decreases intestinal tissue inflammation as visualized by hematoxylin and eosin (H&E) staining as well as Gr-1 and nitrotyrosine immunohistrochemistry (IHC). Inflammation was highest in ileum tissue of thermally injured rats (TI, column 3) as evidenced by tissue infiltration with extravasated neutrophils (H&E, top row; and Gr-1 IHC, middle row; see insets) as well nitrotyrosine immunohidtrovhemistry (bottom row). These markers were markedly decreased in melatonin treated rats, be they sham with melatonin (sham+Mel; second column) or thermally inured (TI+Mel; 4th column). All measurements were performed on samples obtained ~ZT 8 of a 12:12 light cycle at resting conditions with free access to chow feed.
Levels of postburn inflammation in the terminal ileum. Tissue inflammation as assessed using both relative Gr-1 immunofluorescence intensity (open circles) and Gr-1 immunopositive cell counting was significantly higher in thermally injured (TI) subjects relative to sham controls (p<0.01). The signal was significantly decreased in both control (p<0.01) and burn (p<0.05) subjects treated with daily IP melatonin injections (Sham+Mel and TI+Mel). Notably, variability in labeling intensity was least evident among melatonin treated sham subjects. All measurements were performed on samples obtained ~ZT 8 of a 12:12 light cycle at resting conditions with free access to chow feed.
Levels of postburn nitrosylation in the terminal ileum. Tissue nitrosylation as assessed using quantitative nitrotyrosine immunohistochemistry appeared significantly higher in thermally injured (TI) subjects relative to sham controls (p<0.01). The signal was significantly decreased in both control (p<0.01) and burn (p<0.05) subjects treated with daily IP melatonin injections (Sham+Mel and TI+Mel). Notably, variability in labeling intensity was least evident among melatonin treated sham subjects. All measurements were performed on samples obtained ~ZT 8 of a 12:12 light cycle at resting conditions with free access to chow feed.
4. Discussion
Our data shows that the gut acts a source for melatonin as mesenteric melatonin levels appear much higher than in the systemic circulation (Fig. 1A) and AA-NAT and HIOMT mRNAs are expressed in the ileum (Fig. 1B and 1C). Gut melatonin is dynamically adjusted as both mesenteric and systemic melatonin levels are increased three days following major thermal injury (Fig. 1A), AA-NAT and HIOMT mRNA expression is increased three days following major thermal injury (Fig. 1B and 1C), and exogenous melatonin suppresses melatonin synthesizing enzyme mRNA expression (Fig. 1C). Our data also demonstrates that gut barrier tissue is a target for melatonin anti-inflammatory/antioxidant effects as exogenous melatonin supplementation suppresses postburn neutrophil infiltration as well as tyrosine nitrosylation (Figs. 2-4). Importantly, these observations were demonstrated in Harlan Sprague Dawley rats (and reproduced in BALB/c mice; data not shown) during the light time of the day when pineal melatonin production is known to be suppressed.
The gut as a source for melatonin
The presence of nine times as much melatonin in mesenteric as in general blood circulation during the light day hours is in accordance with previous reports of high gut tissue as well as bile melatonin levels [11-19, 22,23]. To our knowledge, this is the first time rat mesenteric melatonin levels have been reported in sham and thermally injured rats although physiological gut tissue melatonin levels have been previously published [15-18]. AA-NAT and HIOMT mRNA expression in the ileum and jejunum also supports previous reports of extrapineal de novo melatonin synthesis in the gut [15-18]. Indeed, high levels of mesenteric blood melatonin could be attributed to inputs from de novo gut melatonin synthesis as previous reports have demonstrated high gut melatonin levels in pinealectomized animals [16]. As such, our data support previous proposed models of a compartmentalized, gut-derived melatonin pool that is maintained by de novo synthesis in the gut and partial recycling through entero-hepatic circulation [16]. Converging evidence points to melatonin production by gut enterochromaffin cells, where it acts in a paracrine fashion in gut tissue as an antioxidant/anti-inflammatory agent and gets absorbed through mesenteric circulation, to be passed on through the hepatic portal circulation to the liver, where it gets recycled through bile or catabolized and excreted through the kidneys [15-19, 22, 23, 38]. Other melatonin sources such as T cells in the lamina propria and gut associated lymph tissues are also possible as it has been recently demonstrated that human T cells can produce melatonin at levels as high as five times that found in nocturnal systemic plasma [39].
Taken together, our observations support the existence of a circadian-independent mesenteric blood melatonin compartment that intersects the bile compartment. Metabolically, the two compartments complement each other so that mesenteric blood receives reabsorbed excess bile melatonin and shunts it back to the liver and bile along with de novo gut tissue-synthesized melatonin. The steady-state outcome of such dynamics is a relatively small but very high concentration (20 to 30 fold systemic blood) bile compartment [23] balanced by the relatively large but lower concentration (~9 times systemic blood) mesenteric blood compartment (Fig. 1A). Ultimately, such metabolic dynamics account for a sustained, robust non-circadian extra-pineal melatonin compartment that is largely sheltered from systemic circulation by recycling/excretion with bile [15-19, 22, 23]. Additional factors that may account for a high mesenteric relative to systemic melatonin levels include protein binding, rapid 6-hydroxylation and conjugation in the liver and excretion as 6-hydroxy conjugates and 6-sulfatoxymelatonin by the kidneys [15-19, 22, 23]. Interestingly, our data shows that doubling the mesenteric melatonin levels (due to thermal injury or by i.p. exogenous 1.86 mg/kg melatonin injection) is associated with a drastic rise in systemic melatonin (Fig. 1A). These data suggest an upper saturation limit for the mechanisms responsible for compartmentalizing or restricting gut melatonin to mesenteric/entero-hepatic circulation.
In thermally injured rats, the large increase in systemic melatonin is attributable to multiple factors that include increased melatonin synthesizing enzyme expression (Fig. 1B, C). As discussed below this endogenous melatonin increase is evidently not sufficient to counteract postburn gut barrier inflammatory complications. Indeed, it is quite possible that negative feedback (Fig. 1C) may put a cap on how much melatonin synthesizing enzyme expression can be ratcheted up in response to major thermal injury. Remarkably, melatonin synthesizing enzyme mRNA expression appears to undergo suppression in sham rats following exogenous intraperitoneal melatonin injection possibly starting at concentrations as low as those seen postburn (2 mM; Fig. 1). Additionally, doubling of mesenteric melatonin levels, be it in association with major thermal injury or exogenous injection, appears to be associated with a dramatic increase in systemic melatonin (Fig. 1A). Whereas such spillover effect could have the benefit of tapping into mesenteric melatonin for systemic use, it has the side effect of limiting its availability for local use. This is not to say that the increase in systemic postburn melatonin is entirely due to increased mesenteric melatonin. Other possible factors include a postburn change in dynamics outside the entero-hepatic pool and/or a postburn change in liver melatonin handling, namely, metabolism, and bile excretion [15-19, 22, 23].
The gut as a target for melatonin
Our data provides support for a strong anti-inflammatory/antioxidant role for melatonin in the gut when administered intraperitoneally (1.86 mg/kg, i.p.) (Figs. 2 and 3). These data are in agreement with several recent reports indicating an anti-inflammatory/antioxidant role for melatonin, as well as data linking extra-pineal melatonin imbalance to various pathologies [10-14, 24-32, 40-42], including reports that specifically examined gut pathophysiology [10-14] and/or complications of burn injury [40-42]. Our data and those of others suggest that melatonin actions include anti-inflammatory mechanisms that involve blocking neutrophil extravasation, lymphocyte melatonin production, and/or NFκB activation [25,27,39,42]. Our results also show that melatonin drastically suppresses but does not completely abrogate postburn gut inflammation which leaves the door open for other mechanisms such as those involving molecular circadian clock disturbances [8,9,43-45].
Regardless of its trigger, our data suggest a gut-mediated, compensatory anti-inflammatory response to major thermal injury associated with increased gut melatonin expression and mesenteric melatonin levels (Fig. 1). Interestingly, this rise in gut melatonin is associated with a sharp increase in systemic melatonin (Fig. 1A) which may be associated with lethargy and sleepiness seen in thermally injured rats. This could be beneficial for channeling body energy towards tissue repair. However, the increase in mesenteric melatonin falls short in terms of its ability to prevent gut tissue inflammation as evidenced by the beneficial effects of exogenous melatonin supplementation (Figs. 2-4). Three possible mechanisms through which melatonin could decrease inflammation are: (A) decrease leukocyte adhesion and extravasation and nitrotyrosine formation as reported in the current work (Fig. 2,3), (B) chemical quenching of oxygen radical production [26,30], and (C) decreased NFκB activation [27]. These mechanisms, which might be interlinked, could play a major role in melatonin-mediated decreases in intestinal tissue neutrophil infiltration and protein nitrosylation and thus protection against gut barrier damage.
In summary, the gut appears to possess the ability to produce melatonin and to pathophysiologically adjust mesenteric melatonin levels independent of circadian pineal fluctuations. Endogenous mesenteric as well as systemic melatonin levels are increased in response to major thermal injury but fall short of abrogating the massive wave of postburn inflammation manifested in the gut by neutrophil infiltration, tissue oxidation and nitrosylation and gut barrier damage. Supplementation by exogenous melatonin significantly suppresses gut inflammation thus confirming that melatonin is protective against postburn inflammation. These observations support our hypothesis that the gut is both a source and a target for melatonin. Furthermore, melatonin could be a major dynamic player in tipping the balance of gut homeostasis on the inflammation scale and henceforth contribute to setting the stage for individual variations in the degrees of susceptibility to gut inflammation and/or responsiveness to exogenous anti-inflammatory intervention.
Acknowledgements
Special thanks to Rush University Medical Center, Department of Gastroenterology and Chicago State University for the sabbatical opportunity that facilitated the analysis and writing phase of this work. This work was supported by grant funding (to Dr. Walid Al-Ghoul) from the: (1) Extramural Associates Research Development Award (#52611, EARDA)- National Institutes of Health - National Institute of Child Health and Development (NIH-NICHD) (2) National Institute of Health (NIH) Minority Biomedical Research Support (MBRS) Grant # 3 S06 GM008043-34S1 project # 6, and (3) NSF BIO-MRI Award# DBI-0520869. Thanks to Abdelrahman Elarja (Chicago State University, Chicago), Anne Vahey, Sharon Wagner, Dr. McGivney and Judy Meldas (Department of Pathology, MacNeal Hospital, Chicago) for their technical assistance.
Conflict of Interest
The authors declare that they do not have any conflict of interest in this study.
References
1. Al-Ghoul WM, Khan M, Fazal N. et al. Mechanisms of postburn intestinal barrier dysfunction in the rat: roles of epithelial cell renewal, E-cadherin, and neutrophil extravasation. Crit Care Med. 2004;32(8):1730-9
2. Fazal N, Al-Ghoul WM. Thermal injury-plus-sepsis contributes to a substantial deletion of intestinal mesenteric lymph node CD4 T cell via apoptosis. Int J Biol Sci. 2007;3(6):393-401
3. Fazal N, Raziuddin S, Khan M. et al. Antigen presenting cells (APCs) from thermally injured and/or septic rats modulate CD4+ T cell responses of naive rat. Biochim Biophys Acta. 2006;1762(1):46-53
4. Choudhry MA, Chaudry IH. Alcohol, burn injury, and the intestine. J Emerg Trauma Shock. 2008;1(2):81-7
5. Li X, Schwacha MG, Chaudry IH. et al. Heme oxygenase-1 protects against neutrophil-mediated intestinal damage by down-regulation of neutrophil p47phox and p67phox activity and O2- production in a two-hit model of alcohol intoxication and burn injury. J Immunol. 2008;180(10):6933-40
6. Alexander JW, Boyce ST, Babcock GF. et al. The process of microbial translocation. Ann Surg. 1990;212:496-512
7. Swank GM, Deitch EA. Role of the gut in multiple organ failure: bacterial translocation and permeability changes. World J Surg. 1996;20(4):411-7
8. Tang Y, Preuss F, Turek FW. et al. Sleep deprivation worsens inflammation and delays recovery in a mouse model of colitis. Sleep Med. 2009;10(6):597-603
9. Preuss F, Tang Y, Laposky AD. et al. Adverse effects of chronic circadian desynchronization in animals in a "challenging" environment. Am J Physiol Regul Integr Comp Physiol. 2008;295(6):R2034-40
10. Wichmann MW, Haisken JM, Ayala A. et al. Melatonin administration following hemorrhagic shock decreases mortality from subsequent septic challenge. J Surg Res. 1996;65(2):109-14
11. Ates B, Yilmaz I, Geckil H. et al. Protective role of melatonin given either before ischemia or prior to reperfusion on intestinal ischemia-reperfusion damage. J Pineal Res. 2004;37:149-152
12. Carrillo-Vico A, Guerrero JM, Lardone PJ. et al. A review of the multiple actions of melatonin on the immune system. Endocrine. 2005;27(2):189-200
13. Reiter RJ, Tan DX, Mayo JC. et al. Neurally-mediated and neurally-independent beneficial actions of melatonin in the gastrointestinal tract. J Physio and Pharmac. 2003;54(Suppl 4):113-125
14. Sewerynek E, Reiter RJ Melchiorri D. et al. Oxidative damage in the liver induced by ischemia-reperfusion: Protection by melatonin. Hepatogastroenterology. 1996;43:898-905
15. Messner M, Huether G Lorf T. et al. Presence of melatonin in the human hepatobiliary-gastrointestinal tract. Life Sciences. 2001;69:543-551
16. Konturek SJ, Konturek PC, Brzozowska I. et al. Localization and biological activities of melatonin in intact and diseased gastrointestinal tract (GIT). J Physiol Pharmacol. 2007;58(3):381-405
17. Huether G. The contribution of extrapineal sites of melatonin synthesis to circulating melatonin levels in higher vertebrates. Experientia. 1993;49:665-670
18. Stefulj J, Hörtner M, Ghosh M. et al. Gene expression of the key enzymes of melatonin synthesis in extrapineal tissues of the rat. J. Pineal Res. 2001;30:243-7
19. Bubenik GA, Pang SF, Cockshut JR. et al. Circadian variation of portal, arterial and venous blood levels of melatonin in pigs and its relationship to food intake and sleep. J Pineal Res. 2000;28:9-15
20. Tan DX, Manchester LC, Reiter RJ. et al. Identification of highly elevated levels of melatonin in bone marrow: its origin and significance. Biochimica et Biophysica Acta. 1999;1472:206-214
21. Reiter RJ, Calvo JR, Karbownik M. et al. Melatonin and its relation to the immune system and inflammation. Ann NY Acad Sci. 2000;917:376-86
22. Reiter RJ, Tan DX. What constitutes a physiological concentration of melatonin? J Pineal Res. 2003;34(1):79-80
23. Tan DX, Manchester LC, Reiter RJ. et al. High physiological levels in the bile of mammals. Life Sci. 1999;65(23):2523-9
24. Sener G, Tosun O, Sehirli AO. et al. Melatonin and N-acetylcysteine have beneficial effects during hepatic ischemia and reperfusion. Life Sci. 2003;72:2707-2718
25. Cuzzocrea S, Tan DX Costantino G. et al. The protective role of endogenous melatonin in carrageenan-induced pleurisy in the rat. FASEB J. 1999;13:1930-8
26. Daniels WM, Reiter RJ, Melchiorri D. et al. Melatonin counteracts lipid peroxidation induced by carbon tetrachloride but does not restore glucose-6-phosphatase activity. J Pineal Res. 1995;19:1-6
27. Li JH, Yu JP, Yu HG. et al. Melatonin reduces inflammatory injury through inhibiting NF-κB activation in rats with colitis. Mediators Inflamm. 2005;4:185-193
28. Padillo FJ, Cruz A, Navarrete C. et al. Melatonin prevents oxidative stress and hepatocyte cell death induced by experimental cholestasis. Free Radic Res. 2004;38:697-704
29. Rodriguez-Reynoso S, Leal C, Portilla E. et al. Effect of exogenous melatonin on hepatic energetic status during ischemia reperfusion: possible role of tumor necrosis factor-alpha and nitric oxide. J Surg Res. 2001;100:141-149
30. Peyrot F, Ducrocq C. Potential role of tryptophan derivatives in stress responses characterized by the generation of reactive oxygen and nitrogen species. J Pineal Res. 2008;45:235-246
31. Bekyarova G, Tancheva S, Hristova M. Protective effect of melatonin against oxidative hepatic injury after experimental thermal trauma. Methods Find Exp Clin Pharmacol. 2009;31(1):11-4
32. Maldonado MD, Francisco MC, Calvo JR. et al. Melatonin as pharmacologic support in burn patients: A proposed solution to thermal injury-related lymphocytopenia and oxidative damage. Crit Care Med. 2007;35(4):1177-1188
33. Chojkier M, Groszmann RJ. Measurement of portal-systemic shunting in the rat by using γ-labeled microspheres. Am. J. Physiol. 1981;240(5):G371-5
34. Walker WF, Homberger DG. Anatomy & Dissection of the Rat. USA: WH Freeman and Company. 1997
35. Al-Ghoul WM, Herman MD, Dubocovich ML. Melatonin receptor subtype (MT1 and MT2) expression in human cerebellum. Neuroreport. 1998;9:4063-68
36. Dubocovich ML, Yun K, Al-Ghoul WM. et al. Melatonin phase shifts circadian rhythms by MT2 receptor activation. FASEB J. 1998;12:1211-1220
37. Hunt AE, Al-Ghoul WM, Gillette MU. et al. Activation of MT (2) melatonin receptors in rat suprachiasmatic nucleus phase advances the circadian clock. Am J Physiol Cell Physiol. 2001;280(1):C110-C118
38. Raikhlin NT, Kvetnoy IM, Tolkachev VN. Melatonin may be synthesised in enterochromaffin cells. Nature. 1975;255:344- 345
39. Carrillo-Vico A, Calvo JR, Abreu P. et al. Evidence of melatonin synthesis by human lymphocytes and its physiological significance: possible role as intracrine, autocrine, and/or paracrine substance. FASEB J. 2004;18:537-539
40. Peschke E, Wolgast S, Bazwinsky I. et al. Increased melatonin synthesis in pineal glands of rats in streptozotocin induced type 1 diabetes. J Pineal Res. 2008;45(4):439-48
41. Pandi-Perumal SR, Srinivasan V, Maestroni GJ. et al. Melatonin: Nature's most versatile biological signal? FEBS J. 2006;273(13):2813-38
42. Akcan A, Kucuk C, Sozuer E. et al. Melatonin reduces bacterial translocation and apoptosis in trinitrobenzene sulphonic acid-induced colitis of rats. World J Gastroenterol. 2008;14(6):918-924
43. Klerman EB. Clinical Aspects of Human Circadian Rhythms. J Biol Rhythms. 2005;20(4):375-86
44. Laposky AD, Bass J, Kohsaka A. et al. Sleep and circadian rhythms: key components in the regulation of energy metabolism. FEBS Lett. 2008;582(1):142-51
45. Turek FW, Joshu C, Kohsaka A. et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308(5724):1043-5
Author Biography
Walid M. Al-Ghoul (PhD) is an associate professor at Chicago State University. He earned his joint PhD degree from Rutgers, the State University of New Jersey and University of Medicine and Dentistry of New Jersey (1991). He has extensive research training at the postdoctoral and research assistant professor levels in the areas of pathophysiology of spinal cord injury and epilepsy (University of North Carolina, 1990-97), molecular pharmacology of circadian function (Northwestern University, 1997-00), the pathophysiology of burn injury and postburn intestinal sepsis (Loyola University-Chicago, 2000-03), gastrointestinal inflammation and gut barrier pathophysiology as they relate to circadian and microbiota abnormalities (Rush University Medical Center, 2009). He currently holds an Associate Professor position at Chicago State Un iversity with a joint appointment at Rush University Medical Center's Division of Digestive Disease of the Department of internal medicine. In addition to his teaching responsibilities at Chicago State University, Dr Al-Ghoul has an active research program in the investigation of inflammatory drug targets in intestinal sepsis and stress-mediated gut mucosa pathophysiology. In addition to MBRS grant support, Dr Al-Ghoul's research has also been supported by the National Science Foundation Major Research Instrumentation (NIH-MRI) for acquiring a state-of-the-art Fluorescent Activated Cell Sorter (FACS) that is one of a few such powerful cell sorting instruments in the Midwest. He is a member of American Gastroenterological Association (AGA), The Shock Society, Society of Critical Care Medicine (SCCM), American Physiological Society (APS), and The American Association for the Advancement of Science (AAAS).
Nadeem Fazal (MD, PhD) is an Associate Professor, Department of Pharmaceutical Sciences at Chicago State University College of Pharmacy. He was educated at King Edward Medical University, Lahore, and The University of Birmingham, England. He completed his Post-doctoral training, Fellowship and later served as faculty at Burn and Shock Trauma Institute, Stritch School of Medicine at Loyola University Chicago. He is a member of American Society for Microbiology (ASM) and American Association of Immunologists (AAI).
Steven Abu-Shaqra (MSc, MT, ASCP) is currently an adjunct professor at Moraine Valley Community College (Palos Hills, Illinois 2007- Current) and a laboratory supervisor at Advocate Health Care, Lutheran General Hospital (Park Ridge, Illinois). He held a laboratory supervisor position at MacNeal Hospital (Berwyn, Illinois, 2002-2007) after working as a general Medical Technologist in the Chicago area (1997-2002). He earned a Master of Science in Applied Physiology with honor, Magna Cum Laude, Chicago State University (Chicago, Illinois, 2007) and Bachelor of Science in Medical Technology, Northern Illinois University (DeKalb, Illinois, 1997) after earning an Associate of Science (Pre-Med) with high honor, Harold Washington College (Chicago, Illinois, 1994).
Byeong Gyu Park (MSc) is a part-time adjunct faculty and lab support specialist at Chicago State University. He completed his Master of Science in Microbiology, Chicago State University (2005). Master of Science in Biology (1998) and Bachelor of Science in Biology (1996) at Kyung Nam University, South Korea.
![]() Corresponding author: Walid M. Al-Ghoul, Department of Biological Sciences, Sci Bldg Rm#310, Chicago State University, 9501 S. King Drive, Chicago, IL 60628. Tel: 773-995-2443; Email: walghouledu
Corresponding author: Walid M. Al-Ghoul, Department of Biological Sciences, Sci Bldg Rm#310, Chicago State University, 9501 S. King Drive, Chicago, IL 60628. Tel: 773-995-2443; Email: walghouledu

 Global reach, higher impact
Global reach, higher impact