Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2010; 6(4):316-326. doi:10.7150/ijbs.6.316 This issue Cite
Research Paper
Cloning, characterization, and expression analysis of goat (Capra hircus) phospholipid hydroperoxide glutathione peroxidase (PHGPx)
1. College of Animal Science and Veterinary Medicines, Shanxi Agricultural University, Taigu, Shanxi 030801, China
2. College of Animal Science and Veterinary Medicine; Taiyuan Shanxi 030024, China
3. Licheng sheep Breeding Base of Shanxi Agricultural University, Licheng Shanxi 047600, China
Received 2010-4-8; Accepted 2010-6-6; Published 2010-6-10
Abstract
Phospholipid hydroperoxide glutathione peroxidase (PHGPx), as a ubiquitous antioxidant enzyme in the glutathione peroxidases (GPx) family, plays multiple roles in organisms. However, there is very little information on PHGPx in goats (Capra hircus). In this study, a full-length cDNA was cloned and characterized from Taihang black goat testes. The 844 bp cDNA contains an open reading frame (ORF) of 597 bp. The goat PHGPx nucleotide sequence contains a selenocysteine (sec) codon TGA244-246, two potential start codons ATG20-22 and ATG108-110, a polyadenylation signal AATAAA813-818 and selenocysteine insertion sequence (SECIS) motif AUGA688-691, UGA729-731 and AAA703-705. As a selenoprotein, the active-site motifs and GPx family signature motifs LAFPCNQF101-108 and WNFEK165-170 were also found. The order of PHGPx mRNA expression levels was: testes >> heart > brain > epididymis > kidney > liver > lung > spleen > muscle. Real-time PCR and immunohistochemistry results revealed similar expression differences in different age testes, with high expression levels during adolescence. Immunofluorescence results suggested that PHGPx mainly expressed in Leydig cells and spermatids in mature goat testes.
Keywords: goat, phospholipid hydroperoxide glutathione peroxidase, real-time PCR, expression, immunohistochemistry.
1. Introduction
Selenium (Se) is an essential micronutrient for animals and humans that exerts its biological functions through selenoproteins. These proteins contain Se in the form of selenocysteine (Sec), the 21st naturally amino acid in the genetic code [1, 2]. Phospholipid hydroperoxide glutathione peroxidase (PHGPx or GPx4, E.C. 1.11.1.12) is characterized by the presence of selenocysteine at the active site, and belongs to the important family of glutathione peroxidases (GPx). Since the discovery of PHGPx, a number of studies have demonstrated that this seleno-enzyme is essential to organisms. PHGPx differs from the other GPx in terms of its monomeric structure and specificity. PHGPx reduces phospholipid hydroperoxides, lipid hydroperoxides, cholesterol and cholesterol esters produced in cell membranes, which cannot be reduced by other GPx [3-8]. Furthermore, it has also been postulated that PHGPx plays an important role in signal transduction, inflammation and apoptosis [9].
In mammals, PHGPx has been described as a single copy gene with multiple transcription start sites. Three PHGPx isoforms have been identified with differing N-terminal amino acid sequences: a long form which contains a mitochondrial targeting sequence exists in mitochondria (mPHGPx), a short form without a mitochondrial targeting sequence is expressed in the cytosol (cPHGPx), and a third form with a nuclear targeting signal is expressed in sperm nuclei (nPHGPx) [10]. mPHGPx plays an important role in protecting mitochondrial ATP generation against oxidative, attenuating ischemia/reperfusion (IR) injury, and in regulating the apoptogenic protein released from mitochondria [8, 11, 12]. cPHGPx is responsible for catalyzing lipid hydroperoxides, fatty acid hydroperoxides, cholesterol and phospholipids peroxides in the cytosol and biological membranes. nPHGPx is associated with male fertility and embryonic development [13, 14].
So far, PHGPx has been identified in many organisms including mammals [15, 16], insects [17, 18], plants [19, 20], fish [9] etc. However, little is known about PHGPx in goats, and tissue specific expression patterns of PHGPx in goat have not been examined. Additionally, we know that this enzyme is the molecular target for selenium in male germ cells and considered essential for male fertility in livestock and experimental rodents [21-25]. It's also interesting to note that PHGPx activity changes during the life-span in testes and epididymis [26]. Thus, the current experiment was designed to identify the PHGPx gene of the goat, and to identity relative expression levels of PHGPx mRNA in various tissues and in different developmental stages of the testis in order to provide useful molecular information and determine the expression patterns of this widely-distributed seleno-enzyme. Also, the cellular locations of PHGPx in different developmental stages of the testis from bucks were investigated.
2. Materials and methods
2.1. Animals and tissue collection
The male Taihang black goats that were used in this study came from Licheng Sheep Breeding Center in Shanxi Province, China. For the cloning and tissue expression profile of goat PHGPx, 5 adult bucks were killed by exsanguinations, and the heart, kidneys, liver, lung, muscle, testis, brain, epididymis and spleen were collected, frozen in liquid nitrogen, and stored at -80 ℃ until use. For age-dependent expression analysis of PHGPx, 1-, 2-, 4-, 6-, 8- and 12-week-old male bucks were castrated. The left testes was frozen in liquid nitrogen for total RNA extraction, and the same portion of the right testes was dissected into 0.5 cm × 0.5 cm ×0.2 cm and fixed in a 4 % paraformaldehyde/PBS solution at 4 ℃ for 24 h. There were five replicates for each age and three replicates for each tissue.
2.2. Total RNA isolation and clean up
Total RNA was extracted from adult goat testes, different age testes, and various tissues using a TRIzol Reagent (Invitrogen, USA) according to the manufacturers' manual. The RNA was confirmed by 1 % formaldehyde agarose gel electrophoresis and quantified by a spectrophotometer (NanoDrop ND-1000, USA). Total RNA was cleaned up from contaminating DNA using RNeasy Mini Kit (Invitrogen, USA) and mRNA was purified using the mRNA purification kit (Amersham Pharmacia, USA) following the manufacturer's instructions.
2.3. Cloning the full-length cDNA of goat testes PHGPx
Two microgram testicular total RNA was used to synthesize cDNA by PrimeScriptTM RT reagent kit (Takara, Japan) according to the instructions of the manufacturer. Specific primers (SP1F/SP1R, SP2F/SP2R) (showed in Table 1) were designed in a conserved region by comparing all known PHGPx sequences using the Blastn program and used to amplify the partial cDNA of goat PHGPx. PCR reaction was performed at 94 ℃ for 3 min; 35 cycles of 94 ℃ for 30 s, 59 ℃ for 30 s, 72 ℃ for 30 s, and finally 72 ℃ for 10 min. The amplified product was recovered, cloned into pMD 18-T vector (Takara, Japan) and sequenced.
The 5'-and 3'-segments of PHGPx cDNA were cloned using the SMART RACE cDNA amplification kit (Clontech, USA). Gene specific primers (GSP) (showed in Table 1) were designed based on the above partial sequence. The 3'-end was cloned by using GSP-PHGPx1 and 10× Universal Primer A Mix (UPM). PCR reaction was performed at 94 ℃ for 2 min; ten cycles of 94 ℃for 30 s, 65 ℃for 30 s, 72 ℃ for 2 min; 30 cycles of 94 ℃for 30 s, 60 ℃for 30 s, 72 ℃ for 2 min; and finally 72 ℃ for 10 min. The 5'-end amplified with UPM and GSP-PHGPx2. PCR reaction was performed at 94 ℃ for 2 min; ten cycles of 94 ℃for 30 s, 65 ℃for 30 s, 72 ℃ for 2 min; 30 cycles of 94 ℃for 30 s, 60 ℃for 30 s, 72 ℃ for 2 min; and finally 72 ℃ for 10 min. PCR products were purified, cloned into pMD 18-T vector, and sequenced. The PHGPx sequences obtained after 5'-and 3'-RACE were assembled using DNAStar Lasergene 7.1 software to obtain full-length cDNA.
Sequences of primers used in the study.
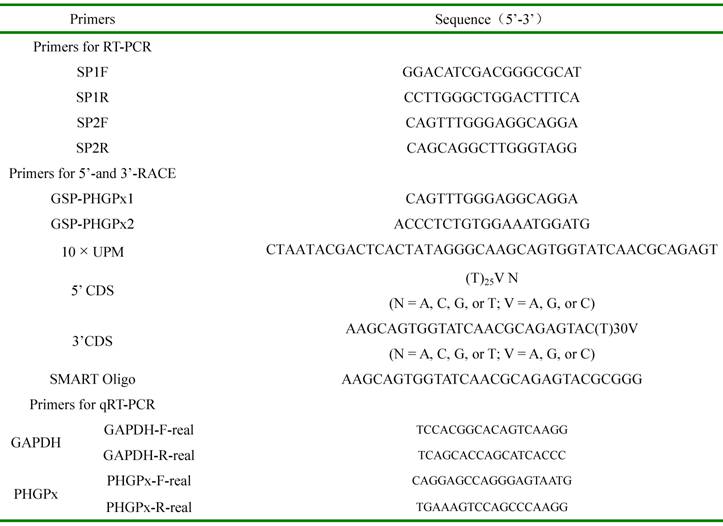
2.4. Nucleotide sequence analysis
Nucleic acid and deduced amino acid sequences of PHGPx cDNA were analyzed using the DNAMAN 5.2.2 sequence analysis programs. The prediction of the signal peptide was predicted by the SignalP program (http://www.cbs.dtu.dk/services/SignalP/). Motif sequences were identified using the software of InterPro Scan (http://www.ebi.ac.uk/InterProScan/). The SECIS element was analyzed using the SECISearch 2.19 program (http://genome.unl.edu/SECISearch.html). Potential N-glycosylation sites were predicted by NetNGlyc 1.0 (http://www.cbs.dtu.dk/services/NetNGlyc/). Multiple sequence alignment of different species accessible from NCBI database was performed using DNAStar Lasergene 7.1 software MegAlign program.
2.5. Analysis of mRNA expression profile in various tissues and different age of testes
The mRNA expression of PHGPx in different samples was performed using Mx3000P real-time PCR system (Stratagene, USA) and SYBR® Permix Ex TaqTM kit (Takara, Japan) following the manufacturers' instructions. One microgram total RNA from different samples treated with RNase-free DNase and was used to synthesize cDNA using PrimeScriptTM RT reagent kit. Primer was design based on the sequence of goat PHGPx cDNA , GAPDH as a housekeeping gene based on goat GAPDH by using Primer Premier 5.0 software. The reaction was performed at 95 ℃ for 10 s; 40 cycles of 95 ℃for 10 s, 62 ℃for 20 s, and a following cycle of 62 ℃for 30 s and 95 ℃for 15 s to obtain the dissociation curves. The standard curves testing derived from a series of diluted cDNA (10-fold serial dilution with six levels: 106×, 105×, 104×, 103×, 102×, 101×, respectively for GAPDH and PHGPx). PCR efficiencies of the two genes were between 100 and 105 %, R2 values of standard curves were between 0.998 and 0.999, and dissociation curves showed a single peak. Relative ratios of PHGPx to GAPDH mRNA were calculated using the standard curves confirm that the real-time PCR data is trustworthy. Data of real-time PCR analysis subjected to ANOVA and t-test was used to determine the difference in mean values with SPSS software. The P value for significance was set at P ≤0.05.
2.6. Immunohistochemistry assay
Immunohistochemistry was carried out for PHGPx localization and expression in different ages using commercial immunostaining kit (Boster, China). Fixed testes samples were dehydrated in gradient ethanol, decolorized in xylene, and finally embedded in paraffin wax. Serial paraffin sections (5 μm) were deparaffinized in xylene and rehydrated in decreasing concentrations of ethanol. Endogenous peroxidase activity was inhibited in 0.3 % H2O2 for 10 min. To prevent background staining, sections were incubated in normal goat serum at room temperature for 20 min after being washed in phosphate buffer saline (PBS, 0.01 M pH 7.2). Polyclonal rabbit anti-PHGPx primary antibody (Abcam, USA) was diluted 1:100 in PBS buffer (0.01 M, pH 7.2) and incubated overnight at 4 ℃. After being washed in a PBS buffer, sections were treated in the polymerized HRP conjugated mouse-anti-rabbit IgG and streptavidin-biotin-complex (SABC) under the instruction of manufacturer. Immunoreaction products were visualized using 3, 3'-diaminobenzidine (DAB). For immunofluorescence analysis, sections were treated in the green-fluorescein isothiocyanate (FITC) or red-Cy3 conjugated mouse-anti-rabbit IgG (Boster, China) after incubated in primary antibody. Negative controls were performed in parallel under identical conditions, but either omitting the primary antibody or by replacing it with normal rabbit serum. Finally, the sections were imaged using a Leica DMIRB microscope (Leica, Germany). Immunohistochemical results are described as negative (no signal, NS), weak (weak signal, WS) and positive (strong signal, SS).
3. Results and Discussion
3.1. Cloning and analysis of PHGPx cDNA
The fragment of PHGPx partial cDNA, 5' and 3' segments of RACE-PCR products were assembled using DNAStar Lasergene 7.1 software to obtain goat PHGPx cDNA. The 844 bp PHGPx cDNA (Fig. 1) (GeneBank accession No. GQ302986) includes a 597 bp opening reading frame (ORF) of 199 aa with two potential start sites, ATG20-22, ATG108-110, and a TGA244-246 codon corresponding to selenocysteine residue [19, 27]. The two ATG, as translation initiation codons, were necessary as mitochondrial targeting sequences in testes. 3' UTR was 226 bp, containing stop codon TAG619-621 and the polyadenylation signal AATAAA813-818. A conserved stem-loop structure in 3'UTR, SECIS, has been reported in selenoprotein mRNA [28, 29], and the recoding of UGA to translate as Sec instead of a stop codon requires this specific structure. In the present study, the predicted selenocysteine insertion sequence (SECIS) in 3'-UTR with AUGA688-691, UGA729-731 and AAA703-705 motifs was also observed, with the same structure and function of SECIS of other species [17, 30, 31].
The deduced amino acids contained the potential mitochondrial targeting sequence (MTS) and Se-GPx signature motif (S-GPx-M) LAFPCNQF101-108, active site motif (ASM) WNFEKF 165-170, two active-sites Gln111 and Trp166, which interact with Sec76. A putative signal peptide of 23 aa (identified by SignaIP software), and N-glycosylation site NAE116-118, NGD139-141 suggested that PHGPx is a glycoprotein which associated with the membrane. The computer analyses of goat PHGPx seemed to predict that the protein is devoid of a transmembrane region. Therefore, PHGPx is most likely a cytosolic or membrane-bound enzyme, which is in agreement with the conclusion of Li [20] and Yeh [31]. Previous studies have shown that the 5' N-terminal sequence of PHGPx is conserved across species [32]. However, the amino acid sequence deduced from goat PHGPx cDNA reveals that the 23-amino acid signal peptide and the 30-amino acid potential mitochondrial targeting signal varies greatly. The two motifs and two amino acid residues responsible for glutathion peroxidase activity are conserved in all PHGPx suggesting their possible specialized structure and function.
3.2. Expression analysis of goat PHGPx mRNA in different tissue
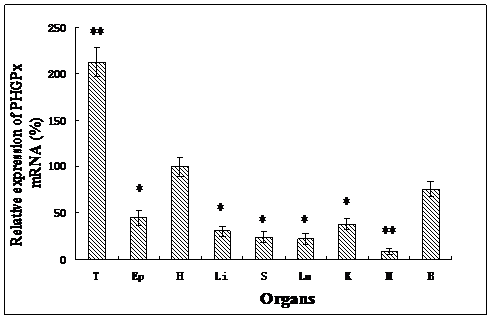
As determined by quantitative real-time PCR, the expression of PHGPx mRNA was detected in different tissues investigated in this study (Fig. 2). The order of PHGPx mRNA expression in different tissues was: testes >> heart > brain > epididymis > kidney > liver > lung > spleen > muscle. The highest rate of transcription was observed in testes (P<0.001), but much lower rates were found in the other gonad. Very low expression was detected in the muscle (P<0.001). This is consistent with observation in humans and mice [33, 34]. It suggests that PHGPx in testes have more than just an antioxidant function, they also may play an important role in male fertility. The expression profile of goat PHGPx mRNA in heart, brain and epididymis is similar with the results reported by Baek [35]. The different mRNA expression levels are considered to be associated with the different functions. Oxidative stresses play a major role in neuronal disease in brain regions and myocardial ischemia/reperfusion (I/R) injury. Schenider studied the PHGPx mRNA expression in mouse brain and Dabkowski confirmed that PHGPx provides protection to cardiac contractile function following myocardial I/R insult [12, 36]. PHPGx mRNA has a relatively high expression level in the heart and brain in our results and this suggests that PHGPx may be associated with antioxidative and protective functions in the brain and heart.
Nucleotide and the deduced amino acid sequences of goat PHGPx. Two potential start codon ATG, stop codon TAG and polyadenylation AATAAA are in the boxes. The potential mitochondrial targeting sequence in testis is shaded in gray, and the signal peptide is under waved. The active-site Gln (Q) and Trp (W), interact with selenocysteine (Sec) are shown in bold italics. Two putative N-glycosylation are in red, and two Arg (R) residues for catalytic substrate are in blue. The active-site motif and GPx family signature motif are underlined. The predicted selenocysteine insertion sequence (SECIS) in 3'-UTR is double underlined, and conserved ATGA, AAA and TGA motif are in green.

Relative PHGPx mRNA expression levels in various tissues, which normalized with GAPDH mRNA in each tissue. All expression data were determined and shown as means ± SD (n=5). Results that are significantly different (P<0.001) from heart are indicated with **, and significantly different (P<0.05) from heart are indicated with *. Testes (T), Epididymis (Ep), Heart (H), Liver (Li), Spleen (S), Lung (Lu), Kidney (K), Muscle (M), Brain (B).
3.3 Expression profile of PHGPx in testes of different age
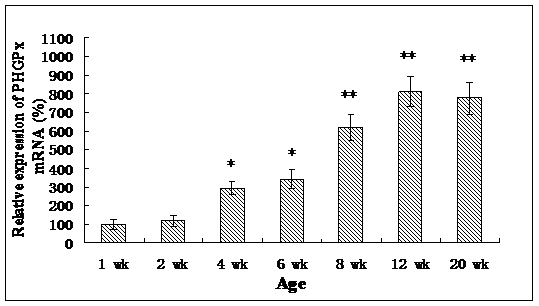
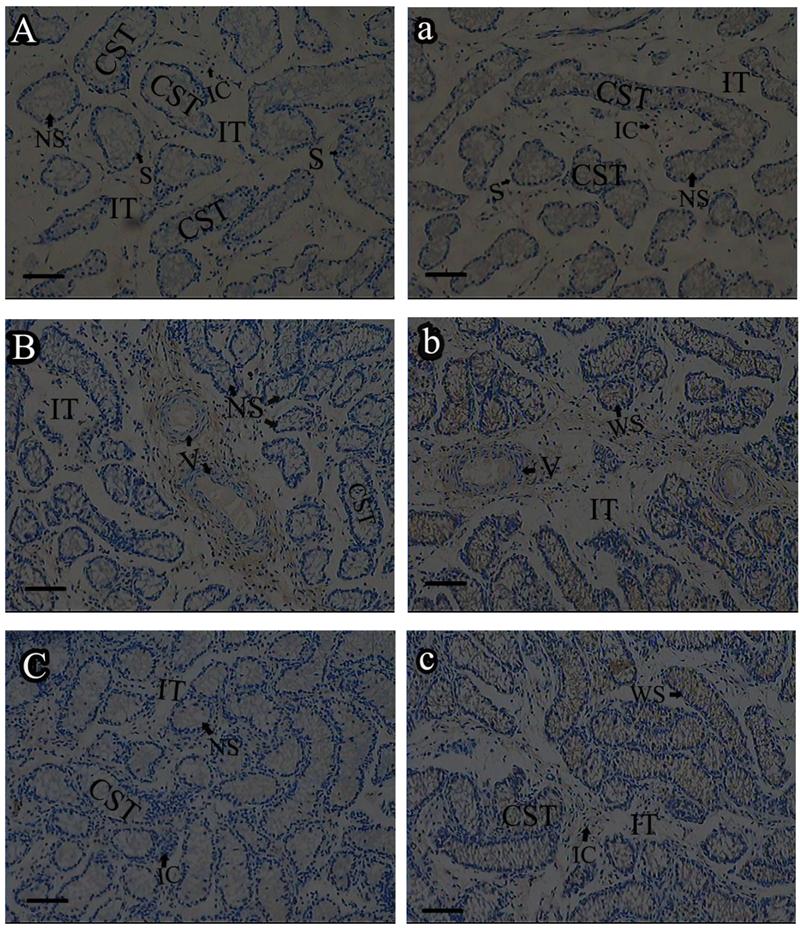
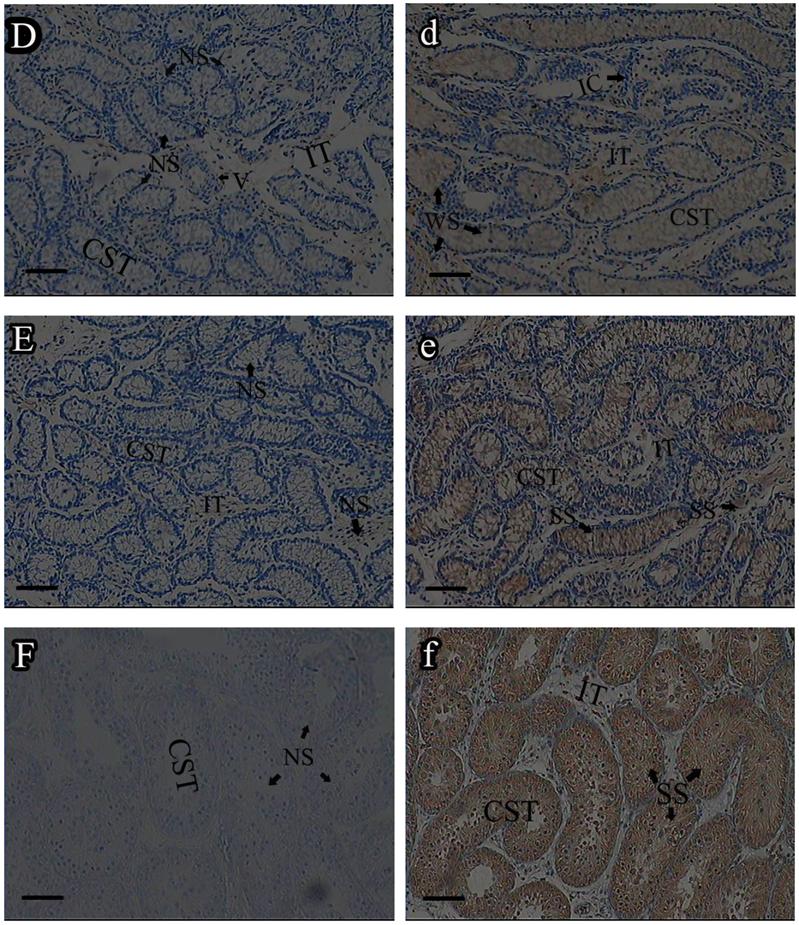
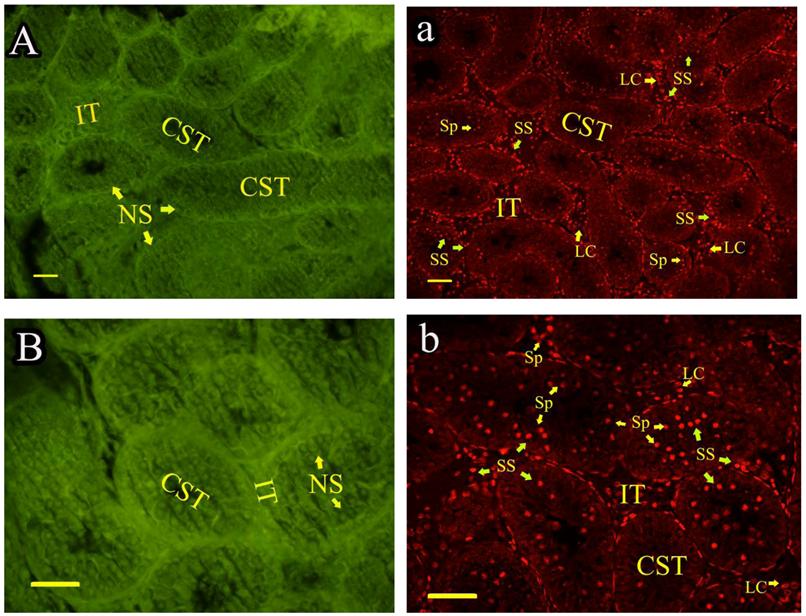
Roveri indicated that PHGPx was only expressed after puberty in the testes [37], Nam pointed out PHGPx was expressed stage-specifically [38], and Tramer also found PHGPx was age-dependent [26]. In our study, the relative mRNA expression levels of PHGPx showed no difference between 1- and 2-wk-old testes, increased in 4- and 6-wk-old testes (P<0.05), reached the highest level in 8-and 12-wk-old testes (P<0.001), and then maintained a constant level in 20-wk-old testes (Fig. 3). In the immunohistochemistry analysis, goat PHGPx also showed a similar pattern of expression in testis of different ages. A strong staining was detected on the sections of 8- and 12-wk-old goat testes (Fig. 4e, f), while a weak signal was detectable in 2-, 4- and 6-wk-old testes (Fig. 4b, c, d). Almost no signal was observed in sections of the 1-wk-old testes section (Fig. 4a). Besides, no signal was visible in the negative control sections which were treated with normal rabbit serum (Fig. 4A-F), thus indicating that the polyclone rabbit anti-PHGPx antibody is specific. The cellular locations of PHGPx in mature buck testes (20-wk-old) were studied by immunofluorescence. PHGPx (labled by red fluorescence) was detected in Leydig cells and the spermatids distributed in the interstitial tissue and convoluted seminiferous tubules (Fig. 5a, b). These observations match with Baek's in situ hybridization results in mouse [35]. PHGPx protein in testes was mainly expressed after 8 weeks, when the goat began adolescence but weak expression was also observed in 1- and 6-week old testes. That may be associated with goat growth and development. In mice, the highest PHGPx activity also has been reported in sexually mature testes [26, 39, 40].
In conclusion, a 844 bp PHGPx cDNA was cloned and identified from the goat Capra hircus. PHGPx mRNA was expressed in all goat tissues studied. High levels of mRNA expression in the testes, heart and brain suggest that PHGPx plays an antioxidative and reproductive-related role in goats. In addition, we found that the age-specific expression patterns of PHGPx exist that may be associated with hormone and gene regulation. The mechanisms need further investigations.
Relative PHGPx mRNA expression levels of goat testes in different ages, and normalized with GAPDH in each age. Date are means ± SD (n=5). Results that are significantly different (P<0.001) from 1-wk-old are indicated with **, and significantly different (P<0.05) from 1-wk-old are indicated with *.
Immunohistochemistry analysis of PHGPx in goat testes of different age. PHGPx staining was visualized using DAB (brown), and slides were counterstained with hematoxylin, 1-wk-old (A, a), 2-wk-old (B, b), 4-wk-old (C, c), 6-wk-old (D, d), 8-wk-old (E, e) and 12-wk-old (F, f). a-f were reacted with anti-PHGPx antibody, for controls A-F reacted with normal serum, respectively. The antibody reacted prominently within CST and IT in 8-and 12-wk-old testes section (e, f), weak immunoreaction was detected during 2-to 6-wk-old (b, c and d).The control exhibited no positive signals (A-F). NS indicated no signal, WS indicated weak signal, and SS indicated strong signal. Bars represent 15 μm for A(a)-E(e) and 25 μm for F(f). Convoluted seminiferous tubules (CST), Interstitial tissue (IT), Interstitial cells (IC), Vein (V), Spermatogonia (S).
Immunofluorescence analysis of PHGPx in sexually mature goat testes. PHGPx staining was visualized using FITC (green fluorescence) and Cy3 (red fluorescence). The section a and b were reacted with anti-PHGPx antibody. PHGPx protein expressed in Leydig cells and spermatids (red signal), and the negative control A and B were detected no signal (green fluorescence). NS indicated no signal, and SS indicated strong signal. All scale bars represent 25 μm. Convoluted seminiferous tubules (CST), Interstitial tissue (IT), Spermatids (Sp), Leydig cell (LC).
Acknowledgements
This work was supported by a grant from National Natural Science Foundation of China (No.30871794). The author would like to thank the staff of the Key Laboratory of Animal Breeding and Genetics & Reproduction, Shanxi Agriculture University.
Conflict of Interest
The authors have declared that no conflict of interest exists.
References
1. Burk RF, Hill KE. Regulation of selenoproteins. Annu Rev Nutr. 1993;13:65-81
2. Hatfield DL, Gladyshev VN. How selenium has altered our understanding of the genetic code. Mol Cell Biol. 2007;22:3565-3576
3. Ursini F, Maiorino M, Valente M, Ferri L, Gregolin C. Purification from pig liver of a protein which protects liposomes and biomembranes from peroxidative degradation and exhibits glutathione peroxidase activity on phosphatidylcholine hydroperoxides. Biochim Biophys Acta. 1982;710:197-211
4. Ursini F, Maiorino M, Gregolin C. The selenoenzyme phospholipid hydroperoxide glutathione peroxidase. Biochim Biophys Acta. 1985;839:62-70
5. Ursini F, Maiorno M, Brigelius-Flohé R, Aumann KD, Roveri A, Schomburg D, Flohé L. Diversity of glutathione peroxidases. Methods Enzymol. 1995;252:38-53
6. Imai H, Nakagawa Y. Biological significance of phospholipid hydroperoxide glutathione peroxidase (PhGPx, GPx4) in mammalian cells. Free Radic Biol Med. 2003;34:145-169
7. Thomas JP, Maiorino M, Ursini F, Girotti AW. Protective action of phospholipid hydroperoxide glutathione peroxidase against membranedamaging lipid peroxidation: in situ reduction of phospholipid and cholesterol hydroperoxides. J Biol Chem. 1990;265:454-461
8. Liang HY, Remmen HV, Frohlich V, Lechleiter J, Richardson A, Ran Q. Gpx4 protects mitochondrial ATP generation against oxidative damage. Biochem Biophys Res Commun. 2007;356:893-898
9. Hermesz E, Ferencz A. Identification of two phospholipid hydroperoxide glutathione peroxidase (gpx4) genes in common carp. Comp Biochem Physiol C. 2009;150:101-106
10. Puglisi P, Bevilacqua A, Carlomagno G, Lenzi A, Gandini L, Stefanini M, Mangia F, Boitani C. Mice overexpressing the mitochondrial phospholipid hydroperoxide glutathione peroxidase in male germ cells show abnormal spermatogenesis and reduced fertility. Endocrinology. 2007;148:4302-4309
11. Liang HY, Ran Q, Jang YC, Holstein D, Lechleiter J, McDonald-Marsh T, Musatov A, Song W, Remmen HV, Richardson A. Glutathione peroxidase 4 differentially regulates the release of apoptogenic proteins from mitochondria. Free Radic Biol Med. 2009;47:312-320
12. Schneider M, Vogt Weisenhorn DM, Seiler A, Bornkamm GW, Brielmeier M. Embryonic expression profile of phospholipid hydroperoxide glutathione peroxidase. Gene Expr Pattern. 2006;6:489-494
13. Godeas C, Tramer F, Micali F, Roveri A, Maiorino M, Nisii C, Sandri G, Panfili E. Phospholipid hydroperoxide glutathione peroxidase (PHGPx) in rat testis nuclei is bound to chromatin. Biochem Mol Med. 1996;59:118-124
14. Borchert A, Wang CC, Ufer C, Schiebel H, Savaskan NE, Kuhn H. The role of phospholipid hydroperoxide glutathione peroxidase isoforms in murine embryogenesis. J Biol Chem. 2006;281:19655-19664
15. Esworthy RS, Doan K, Doroshow KH, Chu FF. Cloning and sequencing of the cDNA encoding a human testis phospholipid hydroperoxide glutathione peroxidase. Gene. 1994;144:317-318
16. Nam SY, Maeda S, Ogawa K, Kurohmaru M, Hayashi Y. Expression pattern of the mitochondrial capsule selenoprotein mRNA in the mouse testis after puberty; in situ hybridization study. J Vet Med Sci. 1997;59:983-988
17. Cossío-Bayúgara R, Miranda E, Holman PG. Molecular cloning of a phospholipid hydroperoxide glutathione peroxidase gene from the tick, Boophilus microplus (Acari: Ixodidae). Insect Biochem Mol Biol. 2005;35:1378-1387
18. Hu Z, Lee KS, Choo YM, Yoon HJ, Kim L, Wei YD, Gui ZZ, Zhang GZ, Sohn HD, Jin BR. Molecular characterization of a phospholipid hydroperoxide glutathione peroxidase from the bumblebee Bombus ignitus. Comp Biochem Physiol B. 2010;155:54-61
19. Sugimoto M, Takeda K. Structure and function of a phospholipid hydroperoxide glutathione peroxidase-like protein from barley. J Mol Catal B Enzym. 2003;23:397-403
20. Li WJ, Feng H, Fan JH, Zhang RQ, Zhao NM, Liu JY. Molecular cloning and expression of a phospholipid hydroperoxide glutathione peroxidase homolog in Oryza sativa. Biochim Biophys Acta. 2000;1493:225-230
21. Puglisi R, Tramer F, Carlomagno G, Gandini L, Panfili E, Stefanini M, Lenzi A, Mangia F, Boitani C. PHGPx in spermatogenesis: how many functions? Contraception. 2005;72:291-293
22. Ursini F, Heim S, Kiess M, Maiorino M, Roveri A, Wissing J, Flohé L. Dual function of the selenoprotein PHGPx during sperm maturation. Science. 1999;285:1393-1396
23. Maiorino M, Mauri P, Roveri A, Benazzi L, Toppo S, Bosello V, Ursini F. Primary structure of the nuclear forms of phospholipid hydroperoxide glutathione peroxidase (PHGPx) in rat spermatozoa. FEBS Lett. 2005;579:667-670
24. Schneider M, Förster H, Boersma A, Seiler A, Wehnes H, Sinowatz F, Neumüller C, Deutsch MJ, Walch A, Hrabé de Angelis M, Wurst W, Ursini F, Roveri A, Maleszewski M, Maiorino M, Conrad M. Mitochondrial glutathione peroxidase 4 disruption causes male infertility. FASEB J. 2009;23:3233-3242
25. Stradaioli G, Sylla L, Monaci M, Maiorino M. Phospholipid hydroperoxide glutathione peroxidase in bull spermatozoa provides a unique marker in the quest for semen quality analysis. Theriogenology. 2009;72:91-98
26. Tramer F, Micali F, Sandri G, Bertoni A, Lenzi A, Gandini L, Panfili E. Enzymatic and immunochemical evaluation of phospholipid hydroperoxide glutathione peroxidase (PHGPx) in testes and epididymal spermatozoa of rats of different ages. Int J Androl. 2002;25:72-83
27. Bae YA, Cai GB, Kim SH, Zo YG, Kong Y. Molecular evolution of glutathione peroxidase genes in association with different biochemical properties of their encoded proteins in invertebrate animals. BMC Evol Biol. 2009;9:72
28. Shen Q, Chu FF, Newburger PE. Sequences in the 3'untranslated region of the human cellular glutathione peroxidase gene are necessary and sufficient for selenocysteine incorporation at the UGA codon. J Biol Chem. 1993;268:11463-11469
29. Grundner-Culemann E, Martin GW, Harney JW, Berry MJ. Two distinct SECIS structures capable of directing selenocysteine incorporation in eukaryotes. RNA. 1995;5:625-635
30. Cai GB, Bae YA, Kim SH, Sohn WM, Lee YS, Jiang MS, Kim TS, Kong Y. Vitellocyte-specific expression of phospholipid hydroperoxide glutathione peroxidases in Clonorchis sinensis. Int J Parasitol. 2008;38:1613-1623
31. Yeh SP, Liu KF, Chiu ST, Jian SJ, Cheng W, Liu CH. Identification and cloning of a selenium dependent glutathione peroxidase from giant freshwater prawn. Macrobrachium rosenbergii, Fish Shellfish Immunol. 2009;27:181-191
32. Pushpa-Rekha TR, Burdsall AL, Oleksa LM, Chisolm GM, Driscoll DM. Rat phospholipid-hydroperoxide glutathione peroxidase; cDNA cloning and identification of multiple transcription and translation start sites. J Biol Chem. 1995;270:26993-26999
33. Imai H, Sumi D, Hanamoto A, Arai M, Sugiyama A. Molecular cloning and functional expression of a cDNA for rat phospholipid hydroperoxide glutathione peroxidase: 3'-untranslated region of the gene is necessary for functional expression. J Biochem. 1995;118:1061-1067
34. Diaconu M, Tangat Y, Böhm D, Kühn H, Michelmann HW, Schreiber G, Haidl G, Glander HJ, Engel W, Nayernia K. Failure of phospholipid hydroperoxide glutathione peroxidase expression in oligoasthenozoospermia and mutations in the PHGPx gene. Andrologia. 2006;38:152-157
35. Baek IJ, Seo DS, Yon JM, Lee SR, Jin Y, Nahm SS, Jeong JH, Choo YK, Kang JK, Lee BJ, Yun YW, Nam SY. Tissue expression and cellular localization of phospholipid hydroperoxide glutathione peroxidase (PHGPx) mRNA in male mice. J Mol Histol. 2007;38:237-244
36. Dabkowski ER, Williamson CL, Hollander JM. Mitochondria-specific transgenic overexpression of phospholipid hydroperoxide glutathione peroxidase (GPx4) attenuates ischemia/reperfusion-associated cardiac dysfunction. Free Radic Biol Med. 2008;45:855-865
37. Roveri A, Casasco A, Maiorino M, Dalan P, Calligaro A, Ursini F. Phospholipid hydroperoxide glutathione peroxidase of rat testis. Gonadotropin dependence and immunocytochemical identification. J Biol Chem. 1992;267:6142-6146
38. Nam SY, Fujisawa M, Kim JS, Kurohmaru M, Hayashi Y. Expression pattern of phospholipid hydroperoxide glutathione peroxidase messenger ribonucleic acid in mouse testis. Biol Reprod. 1998;58:1272-1276
39. Godeas C, Sandri G, Panfili E. Distribution of phospholipid hydroperoxide glutathione peroxidase (PHGPx) in rat testis mitochondria. Biochim Biophys Acta. 1994;1191:147-150
40. Roveri A, Maiorino M, Ursini F. Enzymatic and immunological measurements of soluble and membrane bound PHGPx. Methods Enzymol. 1994;233:202-212
Author contact
![]() Corresponding author: College of Animal Science and Veterinary Medicines, Shanxi Agricultural University, Taigu, Shanxi 030801, China. Tel: 86-354-6288205 E-mail: ywbsxaucom
Corresponding author: College of Animal Science and Veterinary Medicines, Shanxi Agricultural University, Taigu, Shanxi 030801, China. Tel: 86-354-6288205 E-mail: ywbsxaucom

 Global reach, higher impact
Global reach, higher impact