10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2010; 6(5):454-464. doi:10.7150/ijbs.6.454 This issue Cite
Research Paper
Expression of Peptidylarginine Deiminase Type 4 in Ovarian Tumors
1. Research Center for Medicinal Biotechnology, Shandong Academy of Medicinal Sciences. Key Laboratory for Biotech-Drugs Ministry of Health & Provincial Laboratory for Modern Medicine and Technology of Shandong. Jinan, Shandong, 250062. P. R. China.
2. 4th Peoples Hospital of Jinan. Jinan, Shandong. P. R. China.
Received 2010-3-30; Accepted 2010-8-3; Published 2010-8-27
Abstract
Peptidylarginine deiminase type 4 (PADI4) converts arginine residues into citrulline. The current study focused on the expression of PADI4 in various subtypes of ovary cancers, and this study investigated the effects of estrogen on PADI4 expression in SKOV-3 cells that originated from ovary tumors. We utilized immunohistochemistry, real-time PCR and western blotting to analyze the expression of PADI4 in the tumor tissues and in the cell line that were cultured with estrodial-17β. PADI4 was detected in serious cystadenocarcinoma (n=39, positivity=100%), clear cell cancer (n=7, positivity= 100%), mucinous cystadenocarcinoma (n=6, positivity=100%), dysgerminoma (n=6, positivity=100%), squamous cell tumor (n=6, positivity=100%), sibnet-ring cell carcinoma (n=6, positivity=100%), endodermal sinus tumor (n=6, positivity=100%), germ cell tumors (n=6, positivity=100%) and immature teratoma (n=6, positivity=100%). However, PADI4 was either not detected or detected at low levels in granulosa cell tumor (n=6), malignant thecoma (n=6), ovarian cystadenoma (n=5) and normal ovarian tissue (n=11). For serious cystadenocarcinoma, all of the samples with high PADI4 expression belonged to the T1 and T2 stages of pTMN, whereas all of the samples that exhibited weak or moderate PADI4 expression belonged to the T3 and T4 stages. PADI4 was evenly distributed in the cytoplasm of tumor cells of serious cystadenocarcinoma that were classified as being grade II and III by histopathological scoring. However, PADI4 showed granular cellular distribution in the tumor tissues that were isolated from grade I cystadenocarcinoma. In addition, the PADI4 level was positively related with the ages of the patients that presented with serious adenocarcinoma (p=0.029). Real-time PCR and western blot analyses confirmed that PADI4 was expressed at higher levels in ovarian adenocarcinoma (n=8) compared to ovarian cystadenoma (n=5) (p< 0.05). The study also detected an increased level of PADI4 in SKOV-3 cells that were incubated with estrodial-17β in the range of 10-12 to 10-4M. The results suggest an important role for PADI4 in the tumorigenesis of ovary cancers that are under the regulation of estrogen.
Keywords: Peptidylarginine deiminase type 4 (PADI4/PAD4), ovarian cancer (OCa), estrodial-17β.
1. Introduction
Peptidylarginine deiminase (PAD) conducts the post-translational modification of arginine into citrulline in the presence of Ca2+. PADI4, one of the four PAD isoforms, has been confirmed to be associated with rheumatoid arthritis (RA) in some populations [1, 2]. The enzyme is significantly expressed in synovial tissues of RA patients and was located in monocytes, macrophages, eosinophils and neutrophils of the affected tissue [3-7]. Some studies have indicated that PADI4 antagonizes the methylation of histones via deimination, which represses hormone (estrogen)-target transcription and interrupts cell apoptosis [8, 9].
Utilizing immunohistochemistry and western blot analysis, we detected significant levels of PADI4 expression in many types of adenocarcinomas, including colon, duodenum, esophagus, fallopian tube, gall gladder, lung, ovary, parotoid, pancreas, prostate, rectum, small intestine, stomach, thyroid and uterus adenocarcinomas [10].
Thus far, citrullination of histones, cytokeratin, antithrombin and fibronectin have been confirmed to be involved in abnormal apoptosis, high coagulation, and disordered cell proliferation and differentiation, all of which are main features of malignant tumors [8, 10, 11]. However, there is currently not enough data to clarify the pattern of PADI4 expression in various subtypes of tumors that have been isolated from the same organ, and there are no reports that reveal the relationship between the expression of PADI4 and the resultant clinical implications. All of these issues could facilitate the discovery of the mechanism underlying PADI4 activity in tumorigenesis.
Ovary cancer (OCa) is the fourth most frequent cause of cancer-related death among women [12].The incidence of OCa varies widely in frequency among different geographic regions and among ethnic groups. The incidence of OCa increases with age, as it is relatively rare in women younger than 30 years of age [13]. About two-thirds of patients with OCa show histological features that are consistent with stages III and IV (International Federation of Gynecology and Obstetrics, FIGO). These patients typically have widespread tumor dissemination within the abdominal cavity and varying degrees of pleural effusion [14]. The current study investigated the expression of PADI4 in various subtypes of OCa, which were categorized into different grades and stages according to their histological features, and explored how PADI4 expression is related to various aspects of clinical data. Previous studies have shown that estrogen may play an important role in OCa carcinogenesis [15]. To further understand the pathogenic mechanism of estrogen in the tumorigenesis of OCa, the present study also investigated the implications of estrodial-17β in PADI4 expression utilizing cultured SKOV-3 cells that originated from the tumors.
2. Materials and Methods
2.1. Anti-PADI4 antibody preparation
The PADI4 antibody was prepared by immunizing rabbits with an oligopeptide (FGDSCYPSNDSRQMH) that is specific for an amino acid sequence of PADI4, but not for the sequences of other PAD members. The immunospecificity of the antibody has been verified in our previous study [7].
2.2. Sample preparation
Samples of ovarian tumors, including 8 samples of ovarian adenocarcinoma and 5 samples of ovarian cystadenoma, were obtained during excision surgeries in the Shandong Tumor Hospital (Jinan, China) and The Fourth People's Hospital of Jinan (Jinan, China). In addition, tissue arrays were generated that contained 80 and 43 ovarian tissue sections were commercially obtained from Chaoying Bioscience (Shanxi, China) and Fanpu Bioscience (Guilin, China), respectively. The slides contained various tumor tissues including serous adenocarcinoma (n=39), clear cell cancer (n=7), musinous adenocarcinoma (n=6), teratoma (n=6), dysgerminoma (n=6), squamous cell tumor (n=6), sibnet-ring cell carcinoma (n=6), endodermal sinus tumor (n=6), germ cell tumors (n=6), normal ovarian tissue (n=11), granulosa cell tumor (n=6) and immature teratoma (n=6).
2.3. Total protein extraction and western blot analysis
Ovary adenocarcinoma (n=8) and ovary cystadenoma (n=5) were determined by histological methods, and the pathological classification was determined according to World Health Organization (WHO) classifications. These sample tissues were collected during excision surgery. Two hundred micrograms of the sample tissues were homogenized in Cell Lysis Solution (Sigma) and centrifuged at 16,000x g for 5 min at 4℃. The supernatant was collected after centrifugation, and the protein concentrations were determined using a BCA protein assay kit (Pierce). The extract of total protein was separated by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) and transblotted onto a nitrocellulose membrane (Amersham, USA). Western blot analysis was conducted using the anti-PADI4 antibody, with 1000-fold dilutions. All primary and secondary antibodies were diluted in 5% nonfat dry skim milk in TBST, pH 7.6, which contained 0.1% Tween 20. Immunoreactive signals were detected with alkalinephosphatase-conjugated secondary antibodies and visualized using a Western Blotting Luminol Reagent (Amsheram). The acquisition of ECL images was carried out with a Typhoon Trio (GE Healthcare, USA). The quantification of each sample was conducted using ImageQuant5.2 software. A second membrane was prepared using the same protocol as the primary blot, and this second membrane was hybridized with an anti-GAPDH antibody (Santa Cruz) to normalize the sample loading.
2.4. Cell culture
Human ovary cancer cells, SKOV-3, were cultured in RPMI 1640 media, which was phenol red-free (Hyclone, Thermo) and supplemented with 10% fetus ox blood serum, at 37℃ in a humidified atmosphere with 5% CO2. Estrodial-17β (Sigma) was added to the cultures at 10-4, 10-6, 10-8, 10-10 or 10-12 M final concentrations. Cells were incubated for 3 days. The cells were harvested and lysed using cell lysis buffer [20mM Tris PH7.5, 150mM NaCl, 1%Triton X-100, 2.5mM sodium pyrophosphate, 1mM EDTA, 1% Na3VO4, 0.5μg/ml leupeptin, 1mM phenylmethanesulfonyl fluoride (PMSF)]. The concentration of the total protein extracts was determined by a Bradford protein assay. Western blot analysis was performed as described above.
2.5. Immunohistochemistry
Tissue sections were de-paraffinized and re-hydrated using standard procedures. Before the anti-PADI4 antibody was applied, the tissue sections were heated at 95℃ for 10 min in Citrate Buffer Solution (Sigma) to repair the antigen. The tissue sections were then incubated with an Endogenous Peroxidase Inhibitor (MaixinBio, China) for 30 min at room temperature. After washing with PBS buffer (NaCl 7.75 g, K2HPO4 1.5 g, KH2PO4 0.2 g in 1 L distilled water, pH 7.6), the sections were incubated with the anti-PADI4 antibody overnight at 4℃. The immunoreaction was processed using the UltraSensitive TM S-P kit (Maixin-Bio, China) according to the manufacturer's instructions, and the immunoreactive signals were visualized using a DAB substrate. The DAB substrate stained the target protein yellow. Cell structures were counterstained with hematoxylin. A series of control slides were prepared as follows: (1) the slides were only incubated with the primary antibodies, (2) the slides were only incubated with the secondary antibodies or (3) the slides were incubated with normal rabbit serum.
2.6. Immunofluorescent Immunohistochemistry
The tissue sections were processed as described above. After washing the tissue sections three times with PBS buffer, the sections were treated with goat pre-immune serum (Maixin-Bio, China) for 30 min to elevate the specificity of the immunoreaction. The slides were incubated with the PADI4 antibody at 4℃ for 12 h and then washed with PBS. TRITC 5-goat anti rabbit IgG (ZSG-BIO, China) was added to the slide and incubated for 40 min at room temperature. The immunofluorescent results were observed with a fluorescent microscope (Nikon, Japan). In order to determine the antibody specificity and optimize the antibody dilution, a series of control slides were prepared as follows: (1) the slides were only incubated with the primary antibodies, (2) the slides were only incubated with the secondary antibodies or (3) the slides were incubated with normal rabbit serum and goat serum.
2.7. Quantitative Real-Time-PCR Analysis
The total RNA was isolated from the cultured SKOV-3 cells, ovary adenocarcinoma (n=8) and ovarian cystadenoma (n=5) by Trizol (Invitrogen) according to the manufacturers' protocols. The extracted RNA was reverse-transcribed in a final volume of 10 ul using a RNA PCR Kit (TaKaRa). Real-time PCR reactions were conducted using a LightCycler 2.0 Instrument (Roche Molecular Biochemicals) according to the manufacturer's protocols. PCR was performed in a 10 ul total volume that contained 1 ul of cDNA, 5ul of SYBR Green Real-time PCR Master Mix (ToYoBo, Japan) and 1 ul of each primer. PCR was conducted with the following conditions: 10 s at 95℃; 45 cycles of 5 s at 60℃ and 10 s at 72℃; 30 s at 65℃. The level of β-actin mRNA was used as a reference to normalize for sample loading. For each tumor sample, two reactions were performed at the same time: one reaction was to determine the mRNA level of the target gene and the second reaction was to determine the level of β-actin. All of the experiments with each sample were conducted in triplicate. The PCR products were confirmed by melting curve analysis. The relative expression of mRNA was calculated, using the comparative threshold cycle (Ct) method, by following formula: Ratio = 2-ΔΔCt = 2-ΔCt(sample), where ΔCt = Ct of target genes - Ct of endogenous control gene (β-actin) [16]. The relative target gene expression was normalized on the basis of its β-actin content. The primer sequences for the human PADI4 gene were as follows:
Forward primer for PADI4: 5'-GGCAAGTCTTCCAATGA-3';
Reverse primer for PADI4: 5'-CACATGCGATTGTATGC-3';
Forward primer for human β-actin: 5'-TGGCACCCAGCACAATGAA-3';
Reverse primer for human β-actin: 5'-CTAAGTCATAGTCCGCCTAGAAGCA-3.
3. Results
3.1. Immunodetection of PADI4 in ovarian tumor tissues
Immunohistochemistry and immunofluorescent labeling was utilized to determine the expression of PADI4 in various subtypes of OCa. The enzyme was detected in tumor cells in serous cystadenocarcinoma (39/39), clear cell cancer (7/7), mucinous cystadenocarcinoma (6/6), dysgerminoma (6/6), squamous cell tumor (6/6), sibnet-ring cell carcinoma (6/6), endodermal sinus tumor (6/6), germ cell tumors (6/6) and immature teratoma (6/6). However, PADI4 was not detected in tumor cells from granulosa cell tumor (0/6), malignant thecoma (0/6) and normal ovarian tissue (n=11).
In most cases, PADI4 was located in the cytoplasm of the tumor cells. For some samples, such as endodermal sinus tumor (6/6) and dysgerminoma (6/6), PADI4 was present in the nucleus of the tumor cells. The enzyme was not significantly detected in normal ovary tissues (n=11). No immuno-signal was observed in the negative controls that were incubated without the primary or secondary antibodies. The results of the immunohistochemistry are shown in Fig. 1.
The expression of PADI4 was also examined using the immunofluorescent method, and the results can be seen in Fig. 1. In order to evaluate the level of PADI4 expression, a semi-quantitative scoring system, which was based on the protocol of Koensgen's study [17], was used to analyze the results of the immunofluorescent labeling. The scoring system considers the following percentages: 0% positive staining cells = 0, <10% = 1, 10-50% = 2, 51-80% = 3, and >80% = 4. The scoring system also accounts for the intensity of the staining as follows: negative staining = 0, low staining = 1, moderate staining = 2 and strong staining = 3. The expression level was determined by multiplying these two scoring indices and the expression level was grouped into the following categories: 0 (no), 1-4 (low), 5-8 (moderate) and 9-12 (strong). The semi-quantitative analysis of PADI4 expression in tumor tissues is shown in Table 1.
According to the clinical prognosis, serous cystadenocarcinoma (n=39) were classified into either T1 (n=13), T2 (n=10), T3 (n=14) or T4 (n=2) stages based on the standard of Pathological Tumor-Node-Metastasis (pTNM). All 8 of the samples that had high expression of PADI4 (score ≥9) were classified as belonging to the T1 (n=4) or T2 (n=4) stages, whereas 16 samples that were classified as belonging to the T3 (n=14) or T4 (n=2) stages exhibited weak (score ≤4) or moderate (4<score ≤8) PADI4 expression. Based on the histopathological features, the serous cystadenocarcinoma samples were classified into grade I (n=6), grade II (n=12) and grade III (n=22). The expression of PADI4 was evenly distributed in the cytoplasm of tumor cells from 14 samples, 12 of which were classified as the grade III and 2 samples were classified as grade II. The expression of PADI4 in the other 25 samples, which were classified as grade I, showed a granular shape of cellular distribution. The immunosignal of PADI4 in the sections that were grades II and III (score ≥9) were significantly stronger than the immunosignal from sections that were grade I (1<score ≤8). The immunohistochemical results are shown in Fig. 2. In addition, PADI4 levels were positively related to the ages of the patients with serous adenocarcinomas (p=0.029). The PADI4 expression level in serous cystadenocarcinomas is shown in Table 2, and the data suggests that the expression level and cellular distribution of PADI4 are related to the clinical outcomes and histopathologic grades of the tumor. There was no significant association between the PADI4 level and either lymph node metastasis or distance metastasis, which are two parameters that are associated with the clinical prognosis related to the tumors.
Expression of PADI4 in various subtypes of ovarian cancers
| Tissue type\Expression level | No Score (0) | Low Score (1-4) | Moderate Score (5-8) | Strong Score (9-12) | Total |
|---|---|---|---|---|---|
| serous adenocarcinoma (n=39) | 0 | 12 | 19 | 8 | 39 |
| clear cell cancer (n=7) | 0 | 7 | 0 | 0 | 7 |
| musinous adenocarcinoma (n=6) | 0 | 0 | 4 | 2 | 6 |
| teratoma (n=6) | 0 | 4 | 2 | 0 | 6 |
| dysgerminoma (n=6) | 0 | 0 | 5 | 1 | 6 |
| squamous cell tumor (n=6) | 0 | 0 | 2 | 4 | 6 |
| endodermal sinus tumor (n=6) | 0 | 5 | 1 | 0 | 6 |
| sibnet-ring cell carcinoma (n=6) | 0 | 0 | 4 | 2 | 6 |
| germ cell tumors (n=6) | 0 | 6 | 0 | 0 | 6 |
| granulosa cell tumor (n=6) | 6 | 0 | 0 | 0 | 6 |
| malignant thecoma (n=6) | 6 | 0 | 0 | 0 | 6 |
| normal ovarian tissue (n=11) | 11 | 0 | 0 | 0 | 11 |
| Total | 23 | 37 | 41 | 22 | 123 |
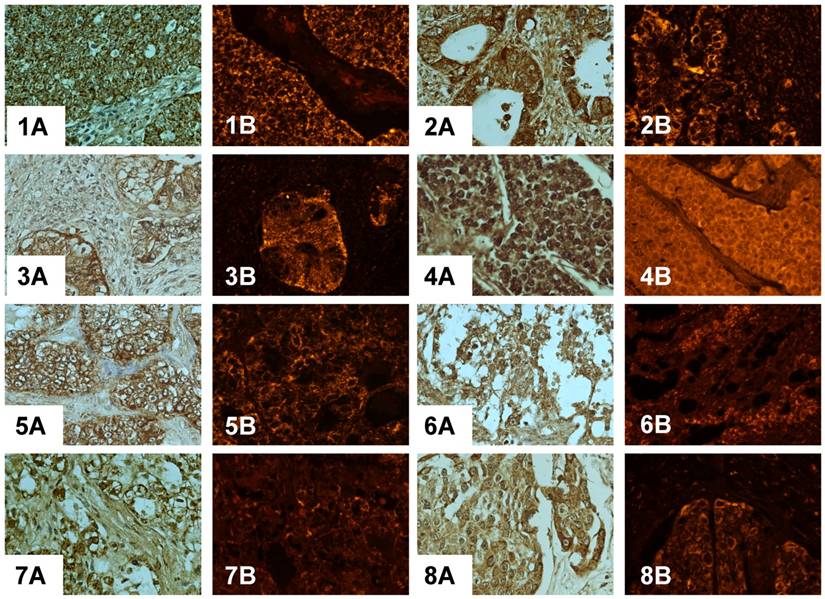
Immunodetection of PADI4 in various kinds of ovarian tumors by immunohistochemistry (A) and immunofluorescent labeling (B). 1 serous adenocarcinoma, 2 mucinous adenocarcinoma, 3 immature teratoma, 4 dysgerminoma, 5 clear cell cancer, 6 endodermal sinus tumor, 7 sibnet-ring cell carcinoma, 8 squamous cell tumor, 9 malignant thecoma, 10 granulosa cell tumor, 11-12 ovary cystadenoma. (SP×400)
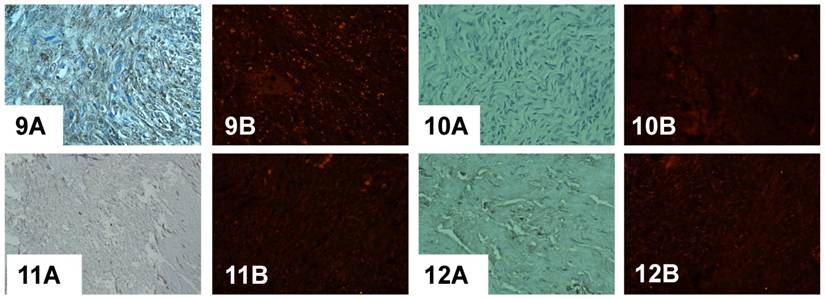
Immunodetection of PADI4 in a serous adenocarcinoma that was found to be different based on the histopathological classification by immunohistochemistry (A) and immunofluorescent labeling (B). PADI4 showed an even cellular distribution in tumor cells from a serous adenocarcinoma that were classified as being grade II (1) and III (2), and PADI4 was found to have a granular shape of cellular distribution in tumor cells from a serous adenocarcinoma that was grade I (3, 4). PADI4 expression was significantly stronger in sections 1 and 2 compared to the expression in sections 3 and 4. (SP×400)
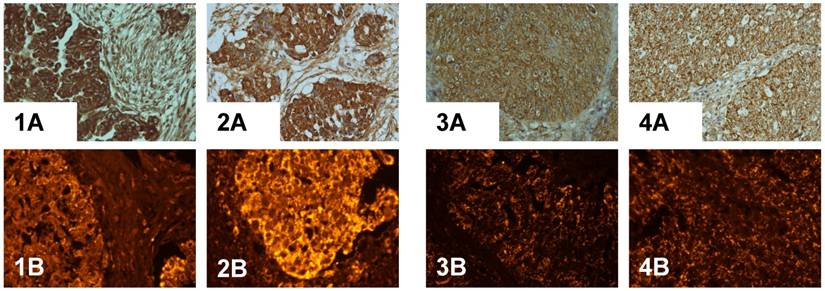
The expression of PADI4 in serous cystadenocarcinoma
| Patients (n=39) | Age (years) | Tumor Stage (FIGO) | pTNM (T1, T2, T3, T4) | pTNM (N0, N1) | pTNM (M0, M1) | Expression Level of PADI4 |
|---|---|---|---|---|---|---|
| 1 | 57 | Ⅱ | T1 | N0 | M0 | 6 |
| 2 | 57 | Ⅱ | T3 | N0 | M0 | 8 |
| 3 | 43 | Ⅲ | T3 | N1 | M0 | 6 |
| 4 | 54 | Ⅰ | T1 | N0 | M0 | 2 |
| 5 | 63 | Ⅲ | T2 | N1 | M1 | 4 |
| 6 | 46 | Ⅲ | T3 | N0 | M0 | 6 |
| 7 | 54 | Ⅲ | T3 | N0 | M0 | 4 |
| 8 | 49 | Ⅲ | T2 | N0 | M0 | 12 |
| 9 | 39 | Ⅲ | T4 | N1 | M1 | 6 |
| 10 | 42 | Ⅲ | T2 | N0 | M0 | 4 |
| 11 | 50 | Ⅲ | T1 | N0 | M0 | 9 |
| 12 | 49 | Ⅲ | T3 | N1 | M0 | 8 |
| 13 | 62 | Ⅲ | T2 | N0 | M0 | 9 |
| 14 | 26 | Ⅰ | T1 | N0 | M0 | 2 |
| 15 | 47 | Ⅰ | T1 | N0 | M0 | 6 |
| 16 | 47 | Ⅲ | T1 | N0 | M0 | 12 |
| 17 | 51 | Ⅰ | T3 | N1 | M0 | 8 |
| 18 | 55 | Ⅲ | T2 | N0 | M0 | 12 |
| 19 | 46 | Ⅲ | T2 | N1 | M0 | 6 |
| 20 | 57 | Ⅲ | T3 | N1 | M0 | 8 |
| 21 | 75 | Ⅲ | T3 | N1 | M0 | 8 |
| 22 | 69 | Ⅲ | T1 | N0 | M0 | 9 |
| 23 | 30 | Ⅲ | T1 | N0 | M0 | 2 |
| 24 | 42 | Ⅱ | T3 | N1 | M0 | 2 |
| 25 | 22 | Ⅰ | T2 | N0 | M0 | 6 |
| 26 | 32 | Ⅱ | T1 | N0 | M0 | 4 |
| 27 | 48 | Ⅱ | T1 | N0 | M0 | 4 |
| 28 | 65 | Ⅲ | T3 | N1 | M0 | 6 |
| 29 | 38 | Ⅲ | T3 | N1 | M0 | 8 |
| 30 | 51 | Ⅲ | T2 | N0 | M0 | 12 |
| 31 | 65 | Ⅱ | T3 | N0 | M0 | 12 |
| 32 | 26 | Ⅱ | T3 | N1 | M0 | 8 |
| 33 | 55 | Ⅱ | T1 | N0 | M0 | 6 |
| 34 | 49 | Ⅲ | T2 | N0 | M0 | 3 |
| 35 | 46 | Ⅲ | T3 | N1 | M0 | 8 |
| 36 | 63 | Ⅲ | T2 | N0 | M0 | 8 |
| 37 | 37 | Ⅱ | T4 | N1 | M1 | 4 |
| 38 | 55 | Ⅱ | T1 | N0 | M0 | 6 |
| 39 | 55 | Ⅱ | T1 | N0 | M0 | 4 |
*: T1: tumor limited to the ovaries; T2: tumor involved with one or both ovaries with pelvic extension; T3: tumor involved with one or both ovaries with microscopically confirmed peritoneal metastasis outside of the pelvis; T4: peritoneal metastasis that extended beyond the pelvis by more than 2 cm in its greatest dimension.
*: N0: no lymph node metastasis; N1: regional lymph node metastasis
*: M0: no distant metastasis; M1: distant metastasis (excludes peritoneal metastasis)
3.2. Quantification of PADI4 expression in ovarian tumors by western blotting analysis and real-time PCR
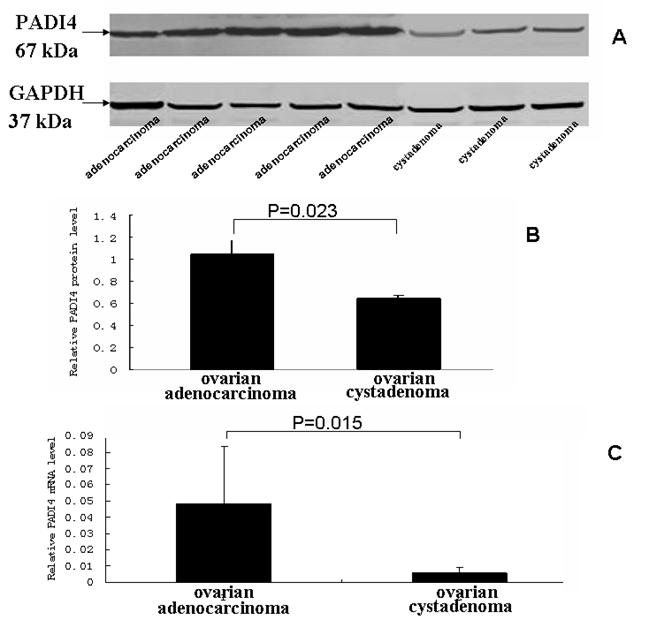
The level of PADI4 in the ovary tumors was determined by western blotting analysis. We analyzed 8 samples of ovarian adenocarcinoma and 5 samples of ovary cystadenoma. Western blot detected an increase in the level of PADI4, which had a molecular weight of 67 kDa, in OCa compared to levels detected in the ovarian cystadenoma (p <0.05). The results are shown in Fig. 3A and 3B.
Transcription of PADI4 was quantified by real-time quantitative PCR. All of the OCa samples exhibited a higher degree of PADI4 expression, compared with the level of PADI4 expression observed in the ovary cystadenomas. We did detect a low level of PADI4 expression in 5 samples from the ovarian cystadenoma (p <0.05). The results are shown in Fig. 3C.
(A) The levels of PADI4 in ovarian adenocarcinoma and ovarian cystadenoma were analyzed by western blotting. (B) The expression of PADI4 in ovarian adenocarcinomas (lane 1, n=8) was normalized to the expression of GAPDH, and compared with the expression of PADI4 in ovarian cystadenoma (lane 2, n=5). The results indicated that PADI4 expression was significantly increased in ovarian adenocarcinoma compared to ovarian cystadenoma. (p=0.023< 0.05). (C) Real-time PCR was used to measure the levels of PADI4 mRNA in ovarian adenocarcinoma (lane 1, n=8) and ovarian cystadenoma (lane 2, n=5). The expression of PADI4 was normalized to the expression of β-actin. The results indicated that PADI4 expression was significantly increased in ovarian adenocarcinoma compared to the expression in ovarian cystadenoma (p=0.015< 0.05).
3.3. Expression of PADI4 in cultured human SKOV-3 cells that were cultured with estrogen
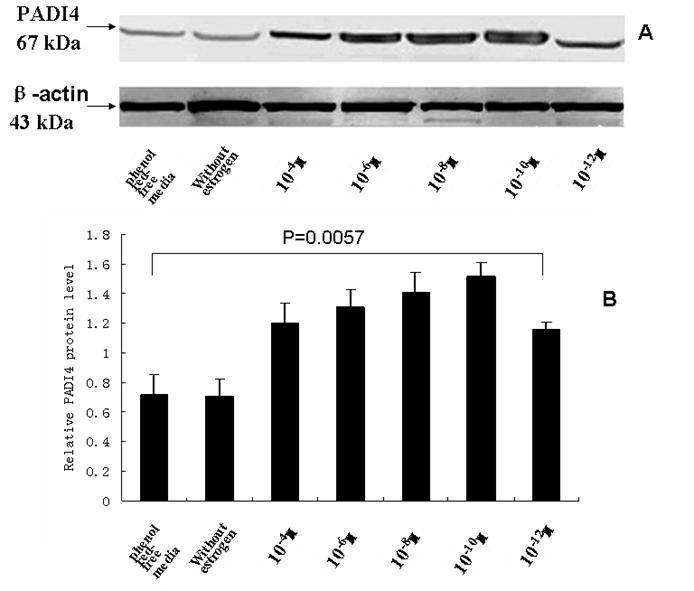
The total protein was extracted from SKOV-3 cells that were incubated with estrodial-17β and the PADI4 expression was analyzed by western blotting using an anti-PADI4 antibody. A band of PADI4 protein, which was 67 kDa, was detected in the cultured cells in presence of estrogen. This band was also present in the controls that did not receive estrogen treatment and were maintained in phenol red-free media. The expression of β-actin was used as a reference. The level of PADI4 was significantly increased with dilutions of estrodial-17β at 10-4, 10-6, 10-8, 10-10 and 10-12 M concentrations. The level declined when the concentration of estrogen was diluted to 10-12 M, but the level of PADI4 expression was still higher than the levels observed in the cells that did not receive estrogen treatment (p =0.034). The results are shown in Fig. 4.
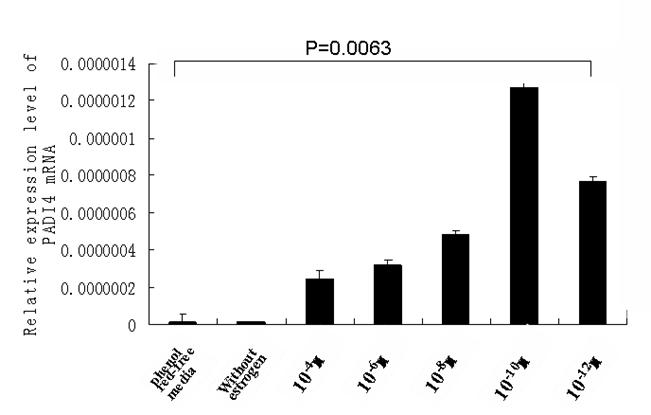
The transcription of PADI4 in cultured cells was qualified by real-time quantitative PCR. The transcription of β-actin was used as a reference. The mRNA level of PADI4 was increased following treatments with estrogen, which corresponded with the results from the western blot analysis. The results of the quantitative PCR are shown in Fig. 5.
Western blotting was used to analyze PADI4 expression in SKOV-3 cells that were cultured with either estrodial-17 (10-4, 10-6, 10-8, 10-10, 10-12 M) or controls that were cultured without estrogen treatment in phenol red-free media. The expression of PADI4 in SKOV-3 was normalized to the expression of β-actin. A shows the western blotting results and B shows the relative levels of PADI4 in the cultured cells. The results indicate that PADI4 expression is increased with dilutions of estrogen that range from 10-4 M to 10-12 M compared to the PADI4 expression levels in the control cells (without estrogen treatment / phenol red-free media) (p=0.0057).
Real-time PCR measured the level of PADI4 mRNA in SKOV3 cells that were cultured with estrodial-17β at concentrations of 10-4, 10-6, 10-8, 10-10 and 10-12 M and control cells that were cultured without treatments of estrogen in phenol red-free media. The results indicate that PADI4 had increased expression with dilutions of estrogen that ranged between 10-4 M and 10-12 M, compared to the expression of PADI4 in the control cells (without estrogen treatment in phenol red-free media) (p=0.0063< 0.01).
4. Discussion
In the present study, PADI4 expression was analyzed using immunohistochemistry and immunofluorescent labeling in a cohort of ovarian tumor forms. In comparison to benign and normal ovary tissues, the expression of PADI4 was significantly increased in tumor cells from most of the OCa subtypes, including serous cystadenocarcinoma, clear cell cancer, metastatic adenocarcinoma, metastatic sibnet-ring cell carcinoma, dysgerminoma, mucinous cystadenocarcinoma, endodermal sinus tumor, squamous cell tumor, germ cell tumors and immature teratoma. However, PADI4 expression was either not detected or detected at low levels in granulosa cell tumor, malignant thecoma, ovarian cystadenoma and normal ovary tissues. Our real-time PCR and western blot analysis confirmed that PADI4 was significantly expressed in ovary adenocarcinoma but maintained a low level of expression in ovary cystadenoma. All of these findings suggest that PADI4 may play a role in the tumorigenic process, could be considered an immunohistochemical barometer to distinguish tumor cells from non-tumor cells, and PADI4 could be utilized to define the tissue structure of tumors.
The effect of estrogen on tumorigenesis in OCa cells has aroused attention in the past few years. With MCF-7 cells, which were originated from human breast cancer tissue, Dong et al. found that estrogen enhanced PADI4 expression through both classical and non-classical transcription factor pathways. This group also reported that activator protein-1, Sp1/Sp3 and nuclear factor-Y are the cis-acting factors that are responsible for the basal and the E2-induced transcription of PADI4 in an ER-dependent manner [18]. With cultured SKOV-3 cells, which originated from OCa, we found that PADI4 had increased expression that corresponded with a decline in the concentrations of estrodial-17β within the range of 10-4 to 10-10 M. These findings suggest that estrogen, at these concentrations, may significantly elevate PADI4 expression in OCa. The physiological level of estrodial-17β in healthy women ranges between 10-8 and 10-10 M [19]. The level of estrogen declines in most postmenopausal women. Previous publications have shown that the level of estrogen can decline to 10-10 M in women during their postmenopausal stage of life [20]. Our correlation analysis indicated that a significant association existed between the PADI4 level and the age of the patients, which corresponds to the results shown in our cell culture studies and suggests that elderly patients may synthesize higher levels of PADI4 compared to younger patients. The dose-dependent reverse correlation of β-estrodial concentrations and PADI4 expression indicated that PADI4 may participate in tumorigenesis, under the regulation of estrogen, as the estrogen levels decline with age. This may be especially true in postmenopausal women. PADI4 expression may account for the age factor in Oca. The highest level of PADI4, which was stimulated at the 10-10 M concentration of estrogen, may contribute to the observation that postmenopausal women are most susceptible to Oca. The function of estrogen is very complicated, in vivo. Thus, estradiol-17β at the different concentrations used in the current study, has revised the effects on PADI4 expression. Further studies are needed to study the regulatory mechanism of estrogen on tumorigenesis in Oca.
Ovary cancers were categorized, based on their embryonic development, into three subgroups including epithelial tumors, germocyte tumors and sex cord mesenchyma tumors. Serous cystadenocarcinoma, musinous cystadenocarcinoma and clear cell cancer belong to the epithelial tumor category. Dysgerminoma, immature teratoma and endodermal sinus tumor belong to the germocyte tumor category. Granulosa cell tumor and malignant thecoma belong to the sex cord mesenchyma tumor category. In the present study, all of the OCa subtypes, except granulosa cell tumors and malignant thecomas, exhibited significant expression of PADI4. In the ovaries, granulosa cells are the main source of estrogen [21], although malignant thecomas also have the ability to synthesize estrogen [22]. These facts suggested that PADI4 might be expressed in tumors that originated from the epithelial cells and germcytes, but not in estrogen-producing cells. This analysis is consistent with our observation that estrogen, at the low concentrations tested, may significantly stimulate PADI4 expression in the cultured SKOV-3 cells.
Other studies have also found that PADI4 was related to the tumorigenic process, although the prior studies did not report an over-expression of PADI4 in the various tumor tissues. Cuthbert et al. and Wang et al. found that citrullination of PADI4 prevented arginine methylation of histone H3 and H4 during transcriptional activation of estrogen-responsive genes [8, 9]. Yao et al. and Li et al. reported that PADI4 repressed the expression of p53-mediated genes, including OKL38, p21, CIP1 and WAF1, by deiminasing the methylated arginine sites in histone H3. This consequently interrupted apoptotic processes and the cell cycle [23 24]. Disrupted apoptosis and disruptions to the cell cycle are the main features of tumor cells [25]. When our results and the previous findings of others are combined, the cumulative results suggest that PADI4 may be involved in OCa tumorigenesis by antagonizing the regulation of p53, which results in the disruption of tumor suppressor genes that are mediated by estrogen.
To summarize, the present study using immunohistochemistry, western blot analysis, real-time PCR and cell culture, identified an increase in the expression of PADI4 in many types of OCa. The expression level of PADI4 in ovary tumor tissues was related to the age of the patient, the histopathological classification of their respective tumors and some of their clinical outcomes when the patients were affected by serous adenocarcinomas. Our study also indicated that low concentrations of estrogen can stimulate the expression of PADI4 in ovary tumor tissues. The above results suggest an important role for PADI4 in OCa tumorigenesis that is under the regulation of estrogen.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (NTFC) (30972720), the National Basic Research Program of China (2010CB529105), the Shandong Taishan Scholarship, and Scientific and Technological Project of Shandong Province (2009ZHZX1A1004).
Abbreviations
GAPDH: glyceraldehyde-3-phosphate dehydrogenase; PADI4/PAD4: Peptidylarginine deiminase type 4; Oca: ovarian cancer; RA: rheumatoid arthritis; CK: cytokeratin; SDS-PAGE: sodium dodecyl sulphate polyacrylamide gel electrophoresis; PMSF: phenylmethanesulfonyl fluoride.
Conflict of Interest
The authors have declared that no conflict of interest exists.
References
1. Cornélis F, Fauré S, Martinez M. et al. New susceptibility locus for rheumatoid arthritis suggested by a genome-wide linkage study. Proc Natl Acad Sci USA. 1998;95:10746-50
2. Shiozawa S, Hayashi S, Tsukamoto Y. et al. Identification of the gene loci that predispose to rheumatoid arthritis. Int Immunol. 1998;10:1891-5
3. Asaga H, Nakashima K, Senshu T. et al. Immunocytochemical localization of peptidylarginine deiminase in human eosinophils and neutrophils. J Leukoc Biol. 2001;70:46-51
4. Hagiwara T, Nakashima K, Hirano H. et al. Deimination of arginine residues in nucleophosmin/B23 and histones in HL-60 granulocytes. Biochem Biophys Res Commun. 2002;290:979-83
5. Nakashima K, Hagiwara T, Yamada M. Nuclear localization of peptidylarginine deiminase V and histone deimination in granulocytes. J Biol Chem. 2002;277:49562-8
6. Vossenaar ER, Radstake TR, van der Heijden A. et al. Expression and activity of citrullinating peptidylarginine deiminase enzymes in monocytes and macrophages. Ann Rheum Dis. 2004;63:373-81
7. Chang X, Yamada R, Suzuki A. et al. Localization of peptidylarginine deiminase 4 (PADI4) and citrullinated protein in synovial tissue of rheumatoid arthritis. Rheumatology (Oxford). 2005;44:40-50
8. Wang Y, Wysocka J, Sayegh J. et al. Human PAD4 regulates histone arginine methylation levels via demethylimination. Science. 2004;306:279-283
9. Cuthbert GL, Daujat S, Snowden AW. et al. Histone deimination antagonizes arginine methylation. Cell. 2004;118:545-53
10. Chang X, Han J. Expression of Peptidylarginine Deiminase Type 4 (PAD4) in Various Tumors. Mol Carcinog. 2006;45:183-96
11. Chang X, Han J, Pang L. et al. PADI4 has increased expression in blood and tissues of malignant tumors. BMC cancer. 2009;9:40
12. Rodriguez C, Calle EE, Coates RJ. et al. Estrogenreplacement therapy and fatal ovarian cancer. Am J Epidemiol. 1995;141:828-35
13. Mant JW, Vessey MP. Ovarian and endometrial cancers. Cancer Surv. 1994;19-20:287-307
14. Riman T, Persson I, Nilsson S. Hormonal aspects of epithelial ovarian cancer: review of epidemiological evidence. Clin Endocrinol (Oxf). 1998;49:695-707
15. Greiser CM, Greiser EM, Dören M. Menopausal hormone therapy and risk of ovarian cancer: systematic review and meta-analysis. Hum Reprod Update. 2007;13:453-463
16. Michael WP. A new mathematical model for relative quantification in realtime RT-PCR. Nucleic Acids Res USA. 2001;29:e45
17. Koensgen D, Mustea A, Klaman I. et al. Expression analysis and RNA localization of PAI-RBP1 (SERBP1) in epithelial ovarian cancer: Association with tumor progression. Gynecol Oncol. 2007;107:266-73
18. Dong S, Zhang Z, Takahara H. Estrogen-enhanced peptidylarginine deiminase type IV gene (PADI4) expression in MCF-7 cells is mediated by estrogen receptor-alpha-promoted transfactors activator protein-1, nuclear factor-Y, and Sp1. Mol Endocrinol. 2007;21:1617-29
19. Prior JC. Perimenopause: the complex endocrinology of the menopausal transition. Endocr Rev. 1998;19:397-428
20. Prior JC. Ovarian aging and the perimenopausal transition: the paradox of endogenous ovarian hyperstimulation. Endocrine. 2005;26:297-300
21. Schumer ST, Cannistra SA. Granulosa cell tumor of the ovary. J Clin Oncol. 2003;21:1180-9
22. Björkholm E, Silfverswärd C. Theca-cell tumors. Clinical features and prognosis. Acta Radiol Oncol. 1980;19:241-4
23. Yao H, Li P, Venters BJ, Zheng S. et al. Histone Arg modifications and p53 regulate the expression of OKL38, a mediator of apoptosis. J Biol Chem. 2008;283:20060-8
24. Liu GY, Liao YF, Chang WH. et al. Over-expression of peptidylarginine deiminase IV features in apoptosis of haematopoietic cells. Apoptosis. 2006;11:183-96
25. Sandal T. Molecular aspects of the mammalian cell cycle and cancer. Oncologist. 2002;7:73-81
Author contact
![]() Corresponding author: Xiaotian Chang, Mail address: Research Center for Medicinal Biotechnology, Shandong Academy of Medicinal Sciences. Jingshi Road 18877, Jinan, Shandong, P. R. China. E-mail: changxtcom. Telephone: +86-531-82919606; Fax: +86-531-82951586.
Corresponding author: Xiaotian Chang, Mail address: Research Center for Medicinal Biotechnology, Shandong Academy of Medicinal Sciences. Jingshi Road 18877, Jinan, Shandong, P. R. China. E-mail: changxtcom. Telephone: +86-531-82919606; Fax: +86-531-82951586.

 Global reach, higher impact
Global reach, higher impact