10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2010; 6(5):465-474. doi:10.7150/ijbs.6.465 This issue Cite
Review
Skeletal Muscle Stem Cells from Animals I. Basic Cell Biology
1. Department of Animal Sciences, Washington State University, Pullman, WA 99164, USA
2. USDA-ARS, Richard B. Russell Agricultural Research Station, Athens, GA 30604, USA
3. Department of Agricultural, Food and Nutritional Science, University of Alberta, Edmonton, Alberta T6G 2P5, Canada
4. Department of Animal Science, University of Wyoming, Laramie, WY 82071, USA
5. Department of Pharmaceutical Sciences, University of Connecticut, Storrs, CT 06269, USA
6. The Coca-Cola Company, Research and Technology, Atlanta, GA 30313, USA
7. Agriculture and Agri-Food Canada Research Centre, Lethbridge T1J 4B1, Canada
8. Program in Cellular and Molecular Biosciences and Animal Sciences, Auburn University, AL 36849, USA
9. The Hartz Mountain Corporation, Secaucus, NJ 07003, USA
10. Department of Animal and Range Sciences, South Dakota State University, Brookings, SD 57007, USA
11. Department of Animal Sciences, University of Arizona, Tucson, AZ 85721, USA
12. Universidad Publica de Navarra, Campus Arrosadia, Pamplona 31006, Spain
13. Animal Sciences, Iowa State University, Ames, IA 50011, USA
14. Department of Animal Sciences, The Ohio State University/OARDC, Wooster, OH 44691, USA
Received 2010-7-21; Accepted 2010-8-27; Published 2010-8-31
Abstract
Skeletal muscle stem cells from food-producing animals are of interest to agricultural life scientists seeking to develop a better understanding of the molecular regulation of lean tissue (skeletal muscle protein hypertrophy) and intramuscular fat (marbling) development. Enhanced understanding of muscle stem cell biology and function is essential for developing technologies and strategies to augment the metabolic efficiency and muscle hypertrophy of growing animals potentially leading to greater efficiency and reduced environmental impacts of animal production, while concomitantly improving product uniformity and consumer acceptance and enjoyment of muscle foods.
Keywords: Skeletal muscle stem cells, Satellite cells, Adipocytes, Adipofibroblasts, Embryogenesis, Postnatal myogenesis.
Introduction
Stem cells, cells that maintain their ability to replicate and can differentiate into various cell types, have been important in understanding cell regulation. In addition, these cells are used therapeutically with continued research hoping to increase their therapeutic potential. Like many other organs, skeletal muscle contains various cell types and can give rise to both muscle-derived satellite cells and adipose tissue-derived adipocytes, both of which are important to animal agriculture. It is well-known that satellite cells are important to postnatal skeletal muscle growth [1] and skeletal muscle regeneration in adult skeletal muscle [2, 3]. Almost fifty years of research with isolated satellite cells has focused on the activation and inhibition of their proliferation [4], regulation of their activity in vitro [5], the interaction of these cells with other cells like angiogenic cells [6], the identification of their subpopulation potential [2, 7, 8], and their potential as vectors in genetic therapies [9]. More recently, it has become apparent that satellite cells exhibit more plasticity than was previous thought, since they can differentiate into cells with adipocyte features [10, 11]. Consideration of the multipotency of satellite cells to yield adipocytes has heightened interest in the regulation of these cells that might shed light on variables of disuse atrophy, senile muscular atrophy and the carcass composition variables that are important in meat products. Alternatively, adipocyte stem cells appear to be found in both the stromal vascular cell (SV) fraction [12], and the mature adipocyte fraction [13-15] of adipose tissue. While this observation was originally proposed in the mid 1970's [16, 17], it was not until recently that methods were developed to repeatedly study the dedifferentiation process of mature adipocytes in vitro [18, 19]. Presently, a variety of studies are being conducted on the dedifferentiated progeny of mature adipocytes (Figure 1), and applications are being developed for tissue regeneration/engineering purposes [15]. Since hundreds of papers have been published on the topic of muscle-derived (muscle and adipose) stem cells, and their potential use for a variety of medical and agricultural applications, this paper is designed to address practical aspects of contemporary skeletal muscle stem cell research with specific application to animal agriculture.
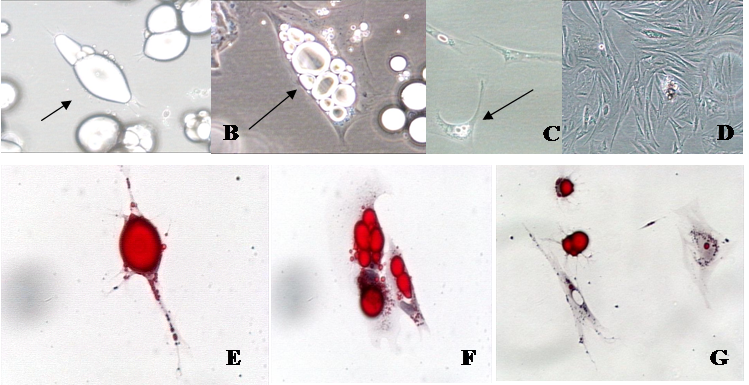
Phase contrast and oil-red-o photomicrographs of isolated fat cells in a variety of stages of development in vitro. A. Mature fat cells in ceiling culture (arrow; 20 X). B. Multilocular fat cell reverting to an adipofibroblast (arrow; 40 X). C. Adipofibroblasts that are beginning to proliferate (arrow; 20 X). D. Proliferating adipofibroblasts (10 X), E. Mature fat cell in ceiling culture (arrow; 40 X). F. Cells losing lipid at six days in culture (arrow; 40 X). G. Cells reverting to adipofibroblasts—note the lipid halo (red stain) around nuclei (20 X).
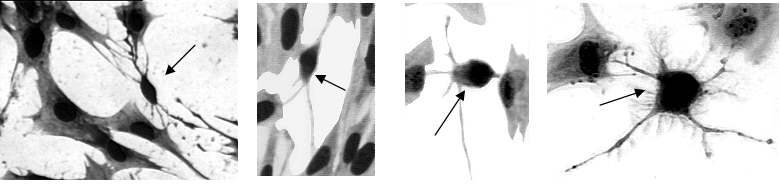
Photomicrographs showing the presence of morphologically dissimilar cells (small cells; arrows) to satellite cells (large cells) in vitro.
Involvement of Skeletal Muscle and Adipocyte Cells in Embryonic/Fetal Skeletal Muscle Development
Early molecular events underlying the commitment of embryonic stem cells to myogenic, adipogenic or fibrogenic lineage remain largely undefined. However, embryonic stem (ES) cells of proven quality have been isolated from a limited number of mammalian species. Most notably, ES cells were isolated from the laboratory mouse Mus musculus in 1981 [20, 21], and from nonhuman primates [22]. The pluripotency of mouse ES cells have been most thoroughly established with the birth of normal, live-born mice after injection into blastocysts and embryo transfer into surrogate female mice. Furthermore, the genome of mouse ES cells can be readily manipulated with the introduction of transgenes and through homologous recombination. The resulting engineered cells can undergo germline transmission to offspring. The pluripotency of human ES cell lines have also been well established, primarily by detailed analyses of pluripotency markers, and their ability to differentiate into a wide variety of cell types. Though considerable effort has been focused on developing germline-competent ES cells for agricultural species, efforts have been much less successful than with mouse and human. Several possibilities may contribute to this difficulty, including species-specific differences in the preimplantation developmental biology of agricultural species as compared to mice, an incomplete knowledge of the growth factors required to support the culture of the explanted inner cell mass of agricultural embyros, and a limited knowledge of useful pluripotency markers for agricultural species as compared to mice or humans. However, it seems likely that derivation methods and assays of pluripotency for ES cells from agricultural species will improve as knowledge from the rapidly-expanding stem cell field is obtained and applied. In fact, a unique opportunity exists for the development of ES cells from agricultural species since they can be assayed for germline competency by injecting them into embryos and implantation into surrogate mothers, an assay that is prohibited for human ES cells. In addition, it should be possible to use mouse ES cells (and their exquisite ability to be manipulated genetically), as a platform for basic research into satellite cell development and function. In the future, if germline competent ES cells from agricultural species are developed, knowledge from mouse ES cell research may be translated into applied research into the dynamics of skeletal muscle development in agricultural species.
In mammals, the majority of all skeletal muscle structures are finalized during the fetal stage of development. Primary myofibers are first formed in the embryonic stage, followed by the formation of secondary myofibers in the mid and late gestation in humans, and late and neonatal stages in mice [23, 24]. Myogenesis is regulated by a series of transcription factors, including Pax 3, Pax 7, Gli, and four myogenic regulatory factors including MyoD, Myf-5, myogenin and MRF-4 [25]. The formation of secondary myofibers overlaps with adipogenesis, and fibrogenesis, which are initiated at mid-gestation in humans, pigs, cattle and sheep, horses, chickens and late gestation in rodents. Myogenic, adipogenic and fibrogenic cells are derived from pools of embryonic stem cells (see below). Switching the commitment of these stem cells from myogenesis to adipogenesis may increase intramuscular fat, an event associated with muscle insulin resistance due to the paracrine effect of intramuscular adipocytes [26-28], and switching to fibrogenesis leads to impairment of skeletal muscle function including oxidative capacity [29]. A fibro/adipogenic progenitor cell may exist in skeletal muscle (Figure 2), having impacts on intramuscular fat accumulation as well as fibrosis in disease states. This cell population could be responsible for the massive fibrosis observed in the plantaris, but not the soleus muscle, of IL-6 null skeletal muscle that was subjected to work-overload [30]. In addition, the attenuation of myogenesis will reduce the muscle fiber density [31], exerting permanent negative effects on offspring muscle strength [32].
Both muscle cells and adipocytes are derived from mesenchymal stem cells which are abundant in the skeletal muscle at early developmental stages, especially during the fetal and neonatal stages. While most of the mesenchymal stem cells develop into myogenic cells, a small portion of these cells differentiate into adipocytes which are the basis for intramuscular fat accumulation [23]. A pivotal factor in the fate of the cells is the Wnt family of proteins, as these proteins are paracrine growth regulators that might have different functions at cell development: Wnt signals may cause cell proliferation, apoptosis, cell fate determination, differentiation, or precursor cell maintenance. The canonical Wnt pathway is β-catenin dependent: binding of Wnt to Frizzled proteins activates Disheveled (DSH) family proteins which inactivates glycogen synthase kinase 3 (GSK3), preventing it from phosphorylating β-catenin with subsequently increased degradation, leading to β-catenin accumulation [33]. Without Wnt stimulation, the axin/GSK-3β/APC complex promotes the degradation of β-catenin through its phosphorylation by GSK-3β [34]. Stabilized β-catenin enters the nucleus and interacts with members of the T cell factor/ Lymphoid enhancer factor (TCF/LEF) family of transcription factors to activate specific target genes [35]. Activation of the Wnt signaling pathway enhances myogenesis and inhibits adipogenesis in cultured mesenchymal stem cells derived from bone marrow [36]. Blocking the β-catenin pathway reduces the total number of myocytes [37, 38]. Wnt signals are also highly expressed in preadipocytes and have been shown to be inhibitors of adipogenesis [39] by blocking the induction of C/EBPα and PPARγ [40]. Stabilization of β-catenin is also associated with inhibition of adipogenesis in myoblasts and the age-related increase in adipogenic potential of muscle satellite cells [41].
Specific Skeletal Muscles vs Specific Adipose Depots
Skeletal muscle stem cells are resident in all skeletal muscles, but may possess varying proliferative/differentiative capacity, due to location and/or function. Postnatal skeletal muscle is extremely responsive to environmental and physiological cues and is able to modify growth and functional characteristics in accordance with the demands placed on it. For example, exercise, injury or trauma initiate regeneration and repair in skeletal muscle despite being largely composed of post-mitotic, multi-nucleated myofibers. The plasticity of skeletal muscle results, in large part, from a population of resident stem cells, often referred to as satellite cells. For most in vitro studies with rodents a collection of back and hind-limb muscles are used to isolate myogenic satellite cells. No distinction is given to the contribution of specific skeletal muscles in terms of numbers of satellite cells isolated. Recent studies describing the isolation and study of satellite cells from both ruminant and non-ruminant meat animals have described the specific skeletal muscles isolated but there are insufficient studies to determine if regulation of satellite cells isolated from different muscles differs. However, there are considerable reports that adipocyte behavior differs depending on the adipose depot from which the cells were isolated suggesting location may impact activity in cells of different tissues. For example, different adipose tissue depots possess unique growth, development and regulation properties [42-44], enzymatic activities [44-46] that are animal dependent [44, 45, 47-60]. These types of studies parallel recent ones using purified cultures of adipocyte stem cells at both the cell and molecular level [14, 61].
Postnatal Myogenesis
When needed, satellite cells proceed through a terminal differentiation program culminating in fusion competency. Interestingly, we are still identifying new growth factors (e.g. Wnt4) that influence satellite cell proliferation [62], which indicated that there are probably additional mechanisms yet to be identified. During muscle fiber hypertrophy or repair, satellite cells are able to fuse with the existing muscle fiber for nuclei donation. When muscle fibers are lost to damage, satellite cells fuse to each other for the formation of a nascent myotube and eventual muscle fiber replacement. Of course, skeletal muscle is a dynamic tissue composed of numerous elements including vascular, nervous and connective tissue. It is during skeletal muscle development and regeneration that these elements need to grow or repair in conjunction with the muscle fibers in order to produce a fully functional unit. This is supported by previous studies indicating that muscle regeneration involves the coordination of myogenesis, revascularization and neurogenesis in order to restore proper muscle function. Communication between myogenic and other cells seems plausible, especially given the number of growth factors and myokines produced by satellite cells leading to the question “do satellite cells play additional roles during skeletal muscle growth and repair aside from the traditional myogenic role?” Recently, investigators have begun to address this novel question and produce evidence in support of this idea. To characterize these interactions, an in vitro co-culture model composed of microvascular fragments (MVF) and satellite cells was developed [6]. In this system, isolated MVF suspended in collagen gel are cultured over a rat SC monolayer culture. In the presence of SC, MVF exhibit greater indices of angiogenesis than MVF cultured alone. Recent data by Christov et al. [63] indicates that satellite and endothelial cells are tightly juxtaposed in the muscle niche suggesting that direct contact may be an important means of cellular communication [63]. Collectively, these initial observations suggest that a previously unexplored aspect of satellite cell activation is the initiation of a pro-angiogenic program.
While a number of reports exist that document the extrinsic and intrinsic regulation of postnatal myogenic satellite cells, the plasticity of skeletal muscle is also exemplified by the capacity to produce and respond to various cytokines. Depending on the nature of the inflammatory event and cytokine profile present, skeletal muscle will respond in a catabolic or anabolic fashion. For example, skeletal muscle breakdown during periods of infection supports processes related to survival [64]. In contrast, myotrauma and inflammation following a bout of exercise ultimately leads to muscle hypertrophy [65]. To date, studies examining the effect of various cytokines on satellite cell activity have provided mixed results that may be related to cell type, dose and time of exposure. Regardless, early studies show that macrophage co-culture and monocyte conditioned medium have positive effects on satellite cell proliferation and that this effect may be mediated through interleukin (IL)-6 autocrine secretion by satellite cells. During work overload induced skeletal muscle hypertrophy, IL-6 expression is increased in a transitory manner [66]. Recently, it was demonstrated that IL-6 was necessary to keep fibrosis in check within the plantaris, but not the soleus muscle [30]. Investigators are extending these novel observations to include activated T cell function on satellite cells [67]. Despite such work, little is known about myogenic and white blood cell communication, an area that could provide much needed stimulus for therapies targeting skeletal muscle inflammation and regeneration.
Postnatal Adipogenesis
At the cellular level, two different physiological components are at play. The first, lipid metabolism, is the energy flow into or out of adipocytes (lipogenesis and lipolysis), respectively [68], and does not require stem cell activity. The second physiological component, termed adipogenesis, is (collectively) the discernable cellular transitions, through which a spindle-shaped stem-like precursor cell proceeds, first forming a preadipocyte devoid of lipid, then a multilocular adipocyte, and, finally, a mature (unilocular) adipocyte [12, 69]. Whereas countless scientific papers are published each year regarding both of these areas (lipid metabolism and adipogenesis), little gains have been made to either formulate an effective exogenous treatment for inducing an overall reduction in body lipid or for altering (decreasing) the cellular conversion to form adipocytes. Indeed, the majority of published articles in the adipogenesis field suggest that once a preadipocyte accumulates lipid, then the cell is a terminally differentiated adipocyte with a role in lipid metabolism from that point onward [reviewed in 13]. In most adipose depots, the number of adipocyte-like cells with the capability of lipid synthesis and storage does not appear fixed at birth. Rather, postnatal adipocyte growth is both hyperplastic and hypertrophic, the extent of each changing with depot location [45, 45, 53, 70-73]. It is interesting to note that, according to traditional thought, should additional adipocytes be required in specific adipose depots, and then the fibroblast-like cells that reside in the connective tissue fraction are converted into the requisite number of adipocytes.
In vitro studies demonstrate that peroxisome proliferator-activated receptor γ (PPARγ) and CCAAT-enhancer-binding proteins (C/EBPs) are crucial factors controlling adipogenesis. Their expression induces adipogenesis from embryonic stem cells [74]. Published evidence supports the notion that the mechanisms regulating adipogenesis in farm animals with human and rodents are somewhat similar. Adipogenesis initiates during the fetal stage, and around mid-gestation in ruminant animals [24, 75-78], and late gestation in pigs and rodents [78]. The difference in the initiation of adipogenesis is mainly due to the difference in maturity of neonatal animals at birth [23]. Adipogenesis is regulated by several key transcription factors, including PPARγ and C/EBPs [12]. C/EBPβ and-δ are first induced by adipogenic stimuli and followed by an increase in PPARγ and C/EBPα expression. C/EBPα and PPARγ re-enforce each other to turn on adipocyte-specific programs to promote adipogenesis [12, 79-81]. The adipocyte determination and differentiation-dependent factor-1/sterol regulatory element-binding protein-1 (ADD-1/SREBP-1) is another important protein induced during the early stages of adipogenesis that regulates genes involved in lipogenesis [82]. PPARγ is the master regulator of adipogenesis. PPARγ forms a heterodimer in partner with retinoid X receptor α (RXRα) and binds to peroxisome proliferator response elements (PPREs) on the promoters of targeted genes [83]. Therefore, retinoid acids affect adipogenesis via RXRα and its interaction with PPARγ [84, 85]. PPARγ is a ligand-activated transcriptional factor. In the inactive state, PPARγ is associated with co-repressors to silence its transcription activity. Binding of ligands leads to the replacement of co-repressors with co-activators possessing histone acetyl transferase activity such as cAMP response element binding protein binding protein (CBP/p300). Acetylation of histones leads to local chromatin decondensation and gene expression. Fatty acids are ligands for PPARγ [86, 87] and it appears that oxidized fatty acids activate PPARγ with higher potency compared to the native fatty acids [88].
Extracellular Matrix and Stem Cell Activity
Changes in the expression of extracellular matrix genes will affect muscle mass accretion impacting both meat yield and potentially meat quality. Indeed, communication between the extracellular matrix and skeletal muscle stem cells plays a pivotal role in the regulation of cellular events. In vitro studies have shown that the extracellular matrix is essential in the regulation of gene expression, cell proliferation, migration, adhesion, and differentiation, all of which are vital for muscle development and growth [89]. Specifically, the presence of the extracellular matrix is required for skeletal muscle satellite cells to respond to growth factors. Through interactions with growth factors such as transforming growth factor-β (TGF-β) [90], fibroblast growth factor 2 [91], myostatin [92, 93] and hepatocyte growth factor [94], the extracellular matrix can regulate the ability of skeletal muscle satellite cells to proliferate or differentiate. Differences in the expression of growth factor regulating extracellular matrix proteoglycans will alter satellite cell responsiveness to the growth factor. For example, the overexpression of the heparan sulfate proteoglycan, glypican-1, in satellite cells will increase the responsiveness of these cells to fibroblast growth factor 2, and underexpression reduced satellite cell proliferation and differentiation [95].
Dedifferentiation and Transdifferentiation
The reprogramming of somatic cells to an embryonic state has been achieved by three principal methods (1) somatic cell nuclear transfer [97, 97], (2) fusion mediated reprogramming, where ES cells are fused to somatic cells to yield pluripotent tetraploid lines with properties of ES cells [98], and (3) regulatory factor-induced pluripotency, a new method that yields induced pluripotent stem (iPS) cells [99]. Since it has been difficult to derive ES cells from preimplantation embryos of agricultural species, the use of iPS approach holds special promise. In the iPS procedure, combinations of key transcriptional regulatory factors (OCT4, SOX2, KLF4, and c-MYC) are introduced into fibroblasts by retroviral or lentiviral transduction. The expression of these factors then induce the pluripotent state in the recipient cells, possibly by inducing a transcriptional state that is quite similar to that found in ES cells. In addition, it is likely that extensive chromatin remodeling and attendant epigenetic changes also accompany the iPS change in developmental state. Use of the iPS approach offers an attractive strategy to produce ES-like cells for agricultural species, which are expected to function much like ES cells. The first success with iPS technology for agricultural species was recently reported in a study that shows that porcine iPS cells can be produced from pig mesenchymal stem cells [100]. The pluripotency of the porcine iPS cells was demonstrated by their ability to contribute to live-born chimeric offspring. In another recent report, mouse iPS cells have been differentiated into myogenic cells [101]. Therefore, it now seems highly likely that iPS approaches may yield porcine myogenic stem cells, thus opening the door to in vitro studies of muscle protein production with obvious avenues for future applied research.
Conclusions
Even though a number of lessons have been learned with respect to agricultural stem cells (Table 1), presently we are still struggling just to understand the basic concepts of in vitro culture and the developmental patterns of muscle satellite cells and intramuscular preadipocytes, stromal vascular cells and mature adipocytes. Completing this elementary line of research, however, will still provide a greater understanding of regulatory mechanisms controlling growth of these important tissues in production animals. Subsequently, defining the transcriptional signature and uncovering potential epigenetic network effects of these cell populations on the regulation of muscle development may result in future developments of new paradigms in animal production.
Lessons learned with respect to agricultural stem cell research.
| Lesson 1 - Research with stem cells must be novel | General cultures of muscle-derived satellite cells were initially isolated and examined for factors that regulated their proliferative and differentiative activity [5, 102]. However, it was determined that many of the cells that were in satellite cell isolates were likely heterogeneous in a variety of functional properties [3, 7, 8, 103]. Due to the thought that different cell types are co-isolated with muscle-derived satellite cells, it is logical to conclude that cell subpopulation dynamics may play a key role in subpopulation responsiveness to intrinsic and extrinsic regulatory signals. Moreover, if specific satellite cell subpopulations exist in different proportion/abundance at different developmental times, should we re-examine satellite cell subpopulation dynamics as a function of aging? The same is easily extended to muscle-derived adipose stem cells. Is the (past) research with adipose stem cells interpretable, considering the subpopulation dynamics of fractional contributions of cells during development? Questions like these will need to be addressed in the immediate future should agricultural stem cell research progress. |
| Lessons 2 and 3 - Research should be productive and applicable | For all agricultural research with muscle-derived stem cells, new principles and theories to address practical problems and questions must be added to justify the research to funding agencies. This may include an end-point whereby stem cell-based therapies to an animal health-related dysfunction are developed [104, 105], or for applications involving tissue engineering [3, 7, 8, 103] |
| Lesson 4 - Progress in research may be made even without the most up to date laboratory settings, complete experimental protocols and infrastructure | Better tools may need to be developed before mechanistic experiments can proceed. Whether the challenges are ill-defined growth media (culture environment), poorly designed cell cultureware, cell culture inserts, or analyses technologies, to make correct interpretations the system employed needs to be defined. Alternatively, if the tools and procedures to conduct research are not available, one should not be hesitant to devise and develop them. Any timely, new methods development will help numerous (other) laboratories. |
| Lessons 5 and 6 - Do not to give-up on a research problem with stem cells, and there are a variety of levels at which one can contribute effectively to the scientific arena | If the experimental system is relatively constant, environmental conditions are easily reproduced and short incubation periods are all that is needed to see a result, it is likely that substantial progress may be made with whatever cell is employed. However, for the most part this is not the case. Cells used are usually new and not easily categorized in terms of growth reagents needed to sustain them. Environmental conditions may need to be altered depending on the specific physiology being evaluated, and incubation condition may need to be changed as the cells age. These types of circumstances are normal when dealing with stem cells, and any alteration in any of the variables resulting in some difference in stem cell physiology may result in a new contribution to the scientific literature. |
| Lesson 7 - Develop viable research teams | Agricultural stem cell research is a broad area of scientific endeavor. It draws from a great many established disciplines, including developmental biology, cell biology, genetics, computational biology and bioinformatics, epigenetics, and others. Though there is a great deal of research activity focused on animal agricultural stem cell research, the field as a whole is still in its infancy. The specialist who is trained in one of the above disciplines can make good progress by applying his unique expertise to a team effort. For instance, a cell biologist that does not possess experience in molecular techniques might consider focusing on cells, cell behavior, cellular regulation and other aspects of cell physiology. By doing so, he brings the most strength to the research. Others might be recruited to conduct other aspects of the research effort. The development of research "teams" to solve mutually agreeable research projects results in a "divide and conquer" approach. In lean funding times, such a team effort will make scholarly efforts with skeletal muscle stem cells much more efficient [106]. |
| Lesson 8 - Publish all research, as it may influence other laboratories in different ways | All significant information related to the use of agriculturally-derived stem cells should be published. Someone, somewhere, might need the very information that you possess. What you may think is unimportant may be vitally important to others. The stem cell literature contains a surprisingly high volume of research papers that can best be described as "accounts of technical tinkering". Many of these methods and technology development papers describe improvements to cell culture methods for specific kinds of stem cells, the design and use of assays for cell type, and improved methods for the directed differentiation of stem cells of various kinds into a wide array of differentiated lineages. Many of the published methods work only partially. For instance, differentiation methods that produce desired cells with only marginal efficiency and purity are still readily publishable. Even negative results, which are notoriously difficult to publish at all in most disciplines, can still be published in the stem cell arena, provided that the experiments were well designed and controlled (though with negative outcomes). |
| Lesson 9 - Sometimes your research may be a bit ahead of its time | At times it may be necessary for you to venture onto a different aspect of the research and then return to your original model when conditions are more correct. |
| Lesson 10 - One must be adaptable, in all research outlets, while keeping the original research focus in light (Question yours and others results) | Also, only by questioning results can we make progress forward in our understanding of biological mechanisms. For example, why was fibrosis only observed in the plantaris and not the soleus in IL-6 null mice that were subjected to work overload? Why do satellite cells play a role in angiogenesis? Why do immune cells interact with muscle? These are just a couple of the myriad of questions that exist for agricultural stem cell researchers. The process of stem cell research is a dynamic one in which, even though you would like to control all aspects of the research pathway only in a few occasions do things really turn-out the way you planned. |
Conflict of Interests
The authors have declared that no conflict of interest exists.
References
1. Allen RE, Merkel RA, Young RB. Cellular aspects of muscle growth: myogenic cell proliferation. J Anim Sci. 1979;49:115-127
2. Sacco A, Doyonnas R, Kraft P. et al. Self-renewal and expansion of single transplanted muscle stem cells. Nature. 2008;456:502-506
3. Cosgrove BD, Sacco A, Gilbert PM. et al. A home away from home: Challenges and opportunities in engineering in vitro muscle satellite cell niches. Differentiation. 2009;78:185-194
4. Yamada M, Tatsumi R, Yamanouchi K. et al. High concentrations of HGF inhibit skeletal muscle satellite cell proliferation in vitro by inducing expression of myostatin: A possible mechanism for reestablishing satellite cell quiescence in vivo. Am J Physiol, Cell Physiol. 2010;298:C465-476
5. Rhoads R, Fernyhough ME, Velleman SG. et al. Invited review: Extrinsic regulation of domestic animal-derived satellite cells II. Domest Anim Endocrinol. 2009;36:111-126
6. Rhoads RP, Johnson RM, Rathbone CR. et al. Satellite cell-mediated angiogenesis in vitro coincides with a functional hypoxia-inducable factor pathway. Am J Physiol, Cell Physiol. 2009;296:c1321-1328
7. Molnar GR, Dodson MV. Satellite cells isolated from sheep skeletal muscle are heterogeneous. Basic Appl Myol. 1993;3:173-180
8. Blanton JRJr, Grant AL, McFarland DC. et al. Isolation of two populations of myoblasts from porcine skeletal muscle. Muscle Nerve. 1999;22:43-50
9. Blau HM. Cell therapies for muscular dystrophy. New England J Med. 2008;359:1403-1405
10. Grimaldi PA, Teboul L, Inadera H. et al. Trans-differentiation of myoblasts to adipoblasts: triggering effects of fatty acids and thiazoladinediones. Prostaglandins Leukot Essent Fatty Acids. 1997;57:71-75
11. Singh NK, Chae HS, Hwang IH. et al. Transdifferentiation of porcine satellite cells to adipoblasts with ciglitizone. J Anim Sci. 2007;85(5):1126-35
12. Hausman GJ, Dodson MV, Ajuwon K. et al. Board Invited Review: The biology and regulation of preadipocytes and adipocytes in meat animals. J Anim Sci. 2009;87:1218-1246
13. Fernyhough ME, Helterline DL, Vierck JL. et al. Dedifferentiation of mature adipocytes to form adipofibroblasts: More than just a possibility. Adipocytes. 2005;1:17-24
14. Dodson MV, Fernyhough ME. Mature adipocytes: Are there still novel things that we can learn from them? Tissue Cell. 2008;40:307-308
15. Fernyhough ME, Hausman GJ, Guan LL. et al. Mature adipocytes may be a source of stem cells for tissue engineering. Biochem Biophys Res Commun. 2008;368:455-457
16. Adebonojo FO. Monolayer cultures of disaggregated human adipocytes. In Vitro. 1975;11:50-54
17. Adebonojo FO. Studies on human adipose cells in culture: relation of cell size and multiplication to donor age. Yale J Biol Med. 1975;48:9-16
18. Fernyhough ME, Vierck JL, Hausman GJ. et al. Primary adipocyte culture: Adipocyte purification methods may lead to a new understanding of adipose tissue growth and development. Cytotechnology. 2004;46:163-172
19. Fernyhough ME, Bucci LR, Hausman GJ. et al. Gaining a solid grip on adipogenesis. Tissue Cell. 2005;37:335-338
20. Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154-156
21. Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981;78:7634-7638
22. Thomson JA, Marshall VS. Primate embryonic stem cells. Curr Top Dev Biol. 1998;38:133-165
23. Du M, Tong J, Zhao J. et al. Fetal programming of skeletal muscle development in ruminant animals. J Anim Sci. 2010;88:E51-60
24. Du M, Yan X, Tong JF. et al. Maternal Obesity, Inflammation, and Fetal Skeletal Muscle Development. Biol Reprod. 2010;82:4-12
25. Relaix F, Rocancourt D, Mansouri A. et al. A Pax3/Pax7-dependent population of skeletal muscle progenitor cells. Nature. 2005;435:948-953
26. Kim JK, Michael MD, Previs SF. et al. Redistribution of substrates to adipose tissue promotes obesity in mice with selective insulin resistance in muscle. J Clin Invest. 2000;105:1791-1797
27. Petersen KF, Shulman GI. Pathogenesis of skeletal muscle insulin resistance in type 2 diabetes mellitus. Am J Cardiol. 2002;90:11G-18G
28. Aguiari P, Leo S, Zavan B. et al. High glucose induces adipogenic differentiation of muscle-derived stem cells. Proc Natl Acad Sci U S A. 2008;105:1226-1231
29. Lahoute C, Sotiropoulos A, Favier M. et al. Premature aging in skeletal muscle lacking serum response factor. PLoS ONE. 2008;3:e3910
30. White JP, Reecy JM, Washington TA. et al. Overload-induced skeletal muscle extracellular matrix remodeling and myofiber growth in mice lacking IL-6. Acta Physiol. 2009;197:321-332
31. Tong JF, Yan X, Zhu MJ. et al. Maternal obesity downregulates myogenesis and beta-catenin signaling in fetal skeletal muscle. Am J Physiol Endocrinol Metab. 2009;296:E917-924
32. Bayol SA, Macharia R, Farrington SJ. et al. Evidence that a maternal "junk food" diet during pregnancy and lactation can reduce muscle force in offspring. Eur J Nutr. 2009;48:62-65
33. Novakofski J. Adipogenesis: Usefulness of in vitro and in vivo experimental models. J Anim Sci. 2005;82:905-915
34. Liu X, Rubin JS, Kimmel AR. Rapid, Wnt-induced changes in GSK3beta associations that regulate beta-catenin stabilization are mediated by Galpha proteins. Current Biology. 2005;15:1989-1997
35. Hecht A, Kemler R. Curbing the nuclear activities of beta-catenin. Control over Wnt target gene expression. EMBO Reports. 2000;1:24-28
36. Shang YC, Zhang C, Wang SH. et al. Activated β-catenin induces myogenesis and inhibits adipogenesis in BM-derived mesenchymal stromal cells. Cytotherapy. 2007;9:667-681
37. Pan W, Jia Y, Wang J. et al. Beta-catenin regulates myogenesis by relieving I-mfa-mediated suppression of myogenic regulatory factors in P19 cells. Proc. Natl. Acad. Sci. USA. 2005;102:17378- 17383
38. Yamanouchi K, Hosoyama T Murakami Y, Nishihara M. Myogenic and adipogenic properties of goat skeletal muscle stem cells. J Reprod Dev. 2007;53:51-58
39. Ross S E, Hemati N, Longo KA. et al. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289:950-953
40. Bennett CN, Ross SE, Longo KA. et al. Regulation of Wnt signaling during adipogenesis. J Biol Chem. 2002;277:30998-31004
41. Taylor-Jones J, McGehee M, Rando TA. et al. Activation of an adipogenic program in adult myoblasts with age. Mech Ageing Dev. 2002;123:649-661
42. Smith SB, Crouse JD. Relative contributions of acetate, lactate and glucose to lipogenesis in bovine intramuscular and subcutaneous adipose tissue. J Nutr. 1984;114:792-800
43. Cianzio DS, Topel DG. et al. Adipose tissue growth and cellularity: changes in bovine adipocyte size and number. J Anim Sci. 1985;60:970-976
44. Eguinoa P, Brocklehurst S, Arana A. et al. Lipogenic enzyme activities in different adipose depots of Pirenaican and Holstein bulls and heifers taking into account adipocyte size. J Anim Sci. 2003;81:432-440
45. May SG, Savell JW, Lunt DK. et al. Evidence for preadipocyte proliferation during culture of subcutaneous and intramuscular adipose tissues from Angus and Wagyu crossbred steers. J Anim Sci. 1994;72:3110-3117
46. Wu P, Sato K, Suzuta F. et al. Effects of lipid-related factors on adipocyte differentiation of bovine stromal-vascular cells in primary culture. J Vet Med Sci. 2000;62:933-939
47. Djian P, Roncari AK, Hollenberg CH. Influence of anatomic site and age on the replication and differentiation of rat adipocyte precursors in culture. J Clin Invest. 1983;72:1200-1208
48. Sztalryd C, Levacher C, Picon L. Acceleration by triiodothyronine of adipose conversion of rat preadipocytes from two anatomical locations. Cell Mol Biol. 1989;35:81-88
49. Kirkland JL, Hollenberg CH, Gillon WS. Age, anatomical site and the replication and differentiation of adipocyte precursors. Am J Physiol. 1990;258:C206-C210
50. Bjorntorp P. Adipose tissue distribution and function. Int J Obes. 1991;15:67-81
51. Pons CM, Mattacks CA, Sadler D. The effects of exercise and feeding on the activity of lipoprotein lipase in nine different adipose depots of guinea pigs. Int J Biochem. 1992;24:1825-1831
52. de la Hoz L, Vernon RG. Endocrine control of sheep adipose tissue fatty acid synthesis: Depot specific differences in response to lactation. Horm Metab Res. 1993;25:214-218
53. Huerta-Leidenz NO, Cross HR, Savell JW. et al. Fatty acid composition of subcutaneous adipose tissue from male calves at different stages of growth. J Anim Sci. 1996;74:1256-1264
54. Soret B, Lee HJ, Finley E. et al. Regulation of differentiation of sheep subcutaneous and abdominal preadipocytes in culture. J Endocrinol. 1999;161:517-524
55. Barber MC, Ward RJ, Richards SE. et al. Ovine adipose tissue monounsaturated fat content is correlated to depot-specific expression of stearoyl-CoA desaturase gene. J Anim Sci. 2000;78:62-68
56. Caserta F, Tchkonia T, Civelek VN. et al. Fat depot origin affects fatty acid handling in cultured rat and human preadipocytes. Am J Physiol Endocrinol Metab. 2001;280:E238-247
57. Wu P, Sato K, Yukawa S. et al. Differentiation of stromal-vascular cells isolated from canine adipose tissues in primary culture. J Vet Med Sci. 2001;63:17-23
58. Lelliot CJ, Logie L, Sewter CP. et al. Laminen expression in human adipose cells in relation to anatomical site and differentiation state. J Clin Endocrinol Metab. 2002;87:728-734
59. Shahparaki A, Grunder L, Soriski A. Comparison of human abdominal subcutaneous versus omental preadipocyte differentiation in primary culture. Metabolism. 2002;51:1211-1215
60. Altomonte J, Harbaran S, Richter A. et al. Fat depot-specific expression of adiponectin is impaired in Zucker fatty rats. Metabolism. 2003;52:958-963
61. Taniguchi M, Guan LL, Zhang B, Dodson MV. et al. Adipogenesis of bovine perimuscular adipocytes. Biochemical Biophysical Research Communications. 2008;366:54-59
62. Steelman CA, Recknor JC, Nettleton D. et al. Transcriptional profiling of myostatin-knockout mice implicates Wnt signaling in postnatal skeletal muscle growth and hypertrophy. FASEB J. 2006;20:580-582
63. Christov C, Chretien F, Abou-Khalil R. et al. Muscle satellite cells and endothelial cells: close neighbors and privileged partners. Mol Biol Cell. 2007;18:1397-1409
64. Frost RA, Lang CH. Regulation of muscle growth by pathogen-associated molecules. J Anim Sci. 2008; 86: E84-E93. 65. Vierck J, O'Reilly B, Hossner K, et al. Satellite cell regulation following myotrauma caused by resistance exercise. Cell Biol Int. 2000;24:263-272
65. Vierck JL, O'Reilly BA, Hossner K. et al. Satellite cell regulation following myotrauma caused by resistance exercise. Cell Biology International. 2000;24:263-272
66. Carson JA, Nettleton D, Reecy JM. Differential gene expression in the rat soleus muscle during early work overload-induced hypertrophy. FASEB J. 2002;16:207-209
67. Dumke BR, Lees SJ. Impact of activated T cells on rat skeletal muscle satellite cell function. FASEB J. 2010;24:997-913
68. Kokta TA, Dodson MV, Gertler A. et al. Intercellular signaling between adipose tissue and muscle tissue. Domest Anim Endocrinol. 2004;27:303-331
69. Ailhaud G, Grimaldi P, Negrel R. Cellular and molecular aspects of adipose tissue development. Annu Rev Nutr. 1992;12:207-233
70. Hirsch J, Han P. Cellularity of rat adipose tissue; effects of growth starvation and obesity. J Lipid Res. 1969;10:77-82
71. Greenwood M, Hirsch J. Postnatal development of adipocyte cellularity in the normal rat. J Lipid Res. 1974;15:474-483
72. Lee YB, Kauffman RG. Cellular and enzymatic changes with animal growth in porcine adipose tissue. J Anim Sci. 1974;38:532
73. Lee YB, Kauffman RG. Cellularity and lipogenic enzyme activities of porcine intramuscular adipose tissue. J Anim Sci. 1974;38:538-544
74. Rosen ED, Sarraf P, Troy AE. et al. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell. 1999;4:611-617
75. Feve B. Adipogenesis: cellular and molecular aspects. Best Pract Res Clin Endocrinol Metab. 2005;19:483-499
76. Gnanalingham MG, Mostyn A, Symonds ME. et al. Ontogeny and nutritional programming of adiposity in sheep: potential role of glucocorticoid action and uncoupling protein-2. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1407-1415
77. Muhlhausler BS, Duffield JA, McMillen IC. Increased maternal nutrition stimulates Peroxisome Proliferator Activated Receptor-γ (PPAR-γ), adiponectin and leptin mRNA expression in adipose tissue before birth. Endocrinology. 2006;148:878-885
78. Wright JT, Hausman GJ. Adipose tissue development in the fetal pig examined using monoclonal antibodies. J Anim Sci. 1990;68:1170-1175
79. Clarke SL, Robinson CE, Gimble JM. CAAT/enhancer binding proteins directly modulate transcription from the peroxisome proliferator-activated receptor gamma 2 promoter. Biochem Biophys Res Commun. 1997;240:99-103
80. Wu Z, Rosen ED, Brun R. et al. Cross-regulation of C/EBP alpha and PPAR gamma controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol Cell. 1999;3:151-158
81. Fernyhough ME, Okine E, Hausman G. et al. PPARγ and GLUT-4 expression as developmental regulators/markers for preadipocyte differentiation into an adipocyte. Domest Anim Endocrinol. 2007;33:367-378
82. Kim JB, Spiegelman BM. ADD1/SREBP1 promotes adipocyte differentiation and gene expression linked to fatty acid metabolism. Genes Dev. 1996;10:1096-1107
83. Ipenberg A, Jeannin E, Wahli W. et al. Polarity and specific sequence requirements of peroxisome proliferator-activated receptor (PPAR)/retinoid X receptor heterodimer binding to DNA. A functional analysis of the malic enzyme gene PPAR response element. J Biol Chem. 1997;272:20108-20117
84. Ziouzenkova O, Orasanu G, Sukhova G. et al. Asymmetric cleavage of beta-carotene yields a transcriptional repressor of retinoid X receptor and peroxisome proliferator-activated receptor responses. Mol Endocrinol. 2007;21:77-88
85. Ziouzenkova O, Plutzky J. Retinoid metabolism and nuclear receptor responses: New insights into coordinated regulation of the PPAR-RXR complex. FEBS Lett. 2008;582:32-38
86. Kliewer SA, Lenhard JM, Willson TM. et al. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor gamma and promotes adipocyte differentiation. Cell. 1995;83:813-819
87. Schopfer FJ, Lin Y, Baker PR. et al. Nitrolinoleic acid: an endogenous peroxisome proliferator-activated receptor gamma ligand. Proc Natl Acad Sci U S A. 2005;102:2340-2345
88. Nagy L, Tontonoz P, Alvarez JG. et al. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARgamma. Cell. 1998;93:229-240
89. Yanagishita M. Function of proteoglycans in the extracellular matrix. Acta Pathol Jpn. 1993;43:283-293
90. Yamaguchi Y, Mann DM, Ruoslahti E. Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature. 1990;346:281-284
91. Rapraeger AC, Krufka A, Olwin BB. Requirement of heparan sulfate for bFGF-mediated fibroblast growth and myoblast differentiation. Science. 1991;252:1705-1708
92. Miura T, Kishioka Y, Wakamatsu JI. et al. Decorin binds myostatin and modulates its activity to muscle cells. BBRC. 2006;340:675-680
93. Kishioka Y, Thomas M, Wakamatsu J-I. et al. Decorin enhances the proliferation and differentiation of myogenic cells through suppressing myostatin activity. J. Cell Physiol. 2008;215:856-867
94. Allen RE, Sheehan SM, Taylor RG. et al. Hepatocyte growth factor activates quiescent skeletal muscle satellite cells in vitro. J Cell Physiol. 1995;165:307-312
95. Zhang X, Liu C, Nestor KE. et al. The effect of glypican-1 glycosaminoglycan chains on turkey myogenic satellite cell proliferation, differentiation, and fibroblast growth factor 2 responsiveness. Poult Sci. 2007;86:2020-2028
96. Gurdon JB. Adult frogs derived from the nuclei of single somatic cells. Dev Biol. 1962;4:256-273
97. Wilmut I, Schnieke AE, McWhir J. et al. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997;385:810-813
98. Ambrosi DJ, Rasmussen TP. Reprogramming mediated by stem cell fusion. J Cell Mol Med. 2005;9:320-330
99. Takahashi K, Tanabe K, Ohnuki M. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861-872
100. West FD, Terlouw SL, Kwon DJ. et al. Porcine induced pluripotent stem cells produce chimeric offspring. Stem Cells Dev. 2010;19:1211-1220
101. Mizuno Y, Chang H, Umeda K. et al. Generation of skeletal muscle stem/progenitor cells from murine induced pluripotent stem cells. FASEB J. 2010;24:2245-2253
102. Dodson MV, McFarland DC, Grant AL. et al. Extrinsic regulation of domestic animal-derived satellite cells. Domest Anim Endocrinol. 1996;13:107-126
103. Feldman JL, DiMario JX, Stockdale FE. Developmental appearance of adult myoblasts in culture and adult myoblast transfer into embryonic avian limbs. Progress Clinical Biology Research. 1993;383:563-574
104. Plazek MR, Chung I-M, Macedo HM. et al. Stem cell bioprocessing: Fundamentals and principals. J R Soc Interface. 2009;6:209-232
105. Wagner J, Kean T, Young R. et al. Optimizing mesenchymal stem cell-based therapeutics. Curr Opin Biotechnol. 2009;20:531-536
106. Dodson MV, Guan LL, Fernyhough ME. et al. Perspectives on the formation of an interdisciplinary research team. Biochem Biophys Res Commun. 2010;391:1155-1157
Author contact
![]() Corresponding author: FAX +1 509 335 1082, E-mail: dodsonedu (M.V. Dodson)
Corresponding author: FAX +1 509 335 1082, E-mail: dodsonedu (M.V. Dodson)

 Global reach, higher impact
Global reach, higher impact