10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2010; 6(5):475-490. doi:10.7150/ijbs.6.475 This issue Cite
Research Paper
Smaller cardiac cell size and reduced extra-cellular collagen might be beneficial for hearts of Ames dwarf mice
1. Donald W. Reynolds Department of Geriatrics, The University of Arkansas for Medical Sciences and Geriatric Research, Education, and Clinical Center, Little Rock, AR, USA
2. Central Arkansas Veterans Healthcare System, Geriatric and Extended Care, Little Rock, AR, USA
3. Geriatrics Research, Department of Internal Medicine, Southern Illinois University School of Medicine, Springfield, IL, USA
4. Central Arkansas Veterans Healthcare System, Little Rock, AR, USA
* Both authors contributed equally to this paper.
Received 2010-3-18; Accepted 2010-8-12; Published 2010-9-1
Abstract
Purpose: To test the hypothesis that cardiac morphologic differences between Ames dwarf and wild-type littermates might correlate with the increased longevity observed in the Ames dwarf mice.
Methods: Hearts removed from young adult (5-7 mo) and old (24-28 mo) Ames dwarf and wild-type littermates underwent histological and morphometric analysis. Measurements of cell size, nuclear size, and collagen content were made using computerized color deconvolution and particle analysis methodology.
Results: In the young mice at six months of age, mean cardiomyocyte area was 46% less in Ames dwarf than in wild-type mice (p<0.0001). Cardiomyocyte size increased with age by about 52% in the wild-type mice and 44% in the Ames dwarf mice (p<0.001). There was no difference in nuclear size of the cardiomyocytes between the young adult wild-type and Ames dwarf mice. There was an age-associated increase in the cardiomyocyte nuclear size by approximately 50% in both the Ames and wild-type mice (p<0.001). The older Ames dwarf mice had slightly larger cardiomyocyte nuclei compared to wild-type (2%, p<0.05). The collagen content of the hearts in young adult Ames dwarf mice was estimated to be 57% less compared to wild-type littermates (p<0.05). Although collagen content of both Ames dwarf and wild-type mouse hearts increased with age, there was no significant difference at 24 months.
Conclusions: In wild-type and Ames dwarf mice, nuclear size, cardiomyocyte size, and collagen content increased with advancing age. While cardiomyocyte size was much reduced in young and old Ames dwarf mice compared with wild-type, collagen content was reduced only in the young adult mice. Taken together, these findings suggest that Ames dwarf mice may receive some longevity benefit from the reduced cardiomyocyte cell size and a period of reduced collagen content in the heart during adulthood.
Keywords: Ames dwarf, collagen, hypertrophy.
Introduction
Death due to heart disease accounted for 27% of all causes combined in the U.S., and remains the number one cause of mortality (1,2). It is imperative therefore, that a discussion of longevity and aging-associated diseases address the underlying mechanisms of cardiovascular disorders (2). There is no large database for documenting natural causes of deaths in mice secondary to cardiac events. To date most of the mouse histopathological data is reported as gross morphological abnormalities, such as tumors. Nevertheless, the mouse has been used as a model to understand aspects of the cardiac pathophysiologly in humans. Arrythymogenic death in mice would be difficult to detect but an indication that it might be important is provided by recent studies of arrhythmias induced in mouse hearts with fibrosis (Stein et al, 2010). The Ames mice with their extended life-expectancy might also have a diminished tendency to develop cardiac pathology which contributes to early morbidity (2-4).
Long-lived Ames dwarf mice are homozygous for the deficient function “Prophet of pituitary factor 1” (Prop 1) allele, resulting indirectly in a version of combined pituitary hormone deficiency (CPHD) in which growth hormone (GH), prolactin, and thyroid stimulating hormone (TSH) are deficient (5). The combined chronic deficiency of these 3 hormones on cardiac structure and morphology is of interest as these deficiencies especially of growth and thyroid hormone have an increased prevalence in the aging population.
GH production over a sustained period of time usually results in a positive energy balance and an increased insulin and IGF-1 production. While 75% of circulating IGF-1 is produced in the liver in response to GH stimulation, the remaining 25% is produced locally and operates in a paracrine- and autocrine-fashion (7). Acute-term GH secretion results in lipolysis and increased levels of free fatty acids (FFA), as well as insulin resistance (6). There are several mouse models of chronic deficiency of growth hormone (7-14). In this study, we have focused on describing the morphologic cardiac features of Ames dwarf mice that have significantly reduced GH, plasma IGF, insulin and glucose compared to wild-type litter-mates.
Acute alteration of thyroid hormones mainly affects cardiac rhythm but chronic alterations in thyroid hormone levels, whether hypo or hyperthyroidism, can produce cardiomyopathies with significant morbidity and mortality if left untreated (15-18). Interestingly, the Ames dwarf mice do not appear to suffer any deleterious cardiac effects from the thyroid hormone deficiency.
The effect of prolactin on the heart has been less well studied and so this study will discuss the cardiac morphology of the Ames dwarf mice mainly in the context of chronic deficiencies of growth and thyroid hormones. However, prolactin does increase IGFR phosphorylation (19-20).
The longevity benefit of the Ames dwarf mice is complex to understand because of the multiple hormonal deficits. The present study therefore investigated the impact of these combined hormonal changes on the heart and correlated it with possible longevity of Ames mice based on the cumulative knowledge about positive cardiac morphological alterations.
Methods
The young adult (4-7mo) and old (24-28mo) male Ames dwarf mice used in these studies, as well as their wild-type littermates, were from a closed heterogeneous (with respect to Prop1) colony of greater than 20 years' duration located at Southern Illinois University (30,31). Animals were group-housed by gender and genotype at 22±2°C, with a 12 hr light / 12 hr dark cycle, and fed non-autoclaved “Rodent Laboratory Chow 5001”, (PMI Feeds in St. Louis MO, USA). Major murine pathogens were monitored using co-housed sentinel animals. Ames dwarf pups were produced by mating homozygous dwarf males with heterozygous females. There are no known phenotypic differences between heterozygous Prop1+/df and homozygous wild-type mice. For this reason, both types of littermates were used as wild-type controls for comparison with Ames dwarf (Prop1df/df) mice. Only male mice were used to reduce the potential for variability in data resulting from gender-related differences in cardiac aging. Mice were killed by cervical dislocation, and their hearts, livers, brains, and hindlimbs were removed. The animal protocols in this study were approved by the laboratory animal care and use committees of Southern Illinois University and the University of Arkansas for Medical Sciences, and were conducted in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals.
Experimental Animals
Histological Preparation
After sacrifice, mouse hearts were removed immediately, weighed, and placed in 25mM KCl & PBS relaxing buffer, then stored in 10% neutral-buffered formalin at 4°C. Hematoxylin and eosin (H&E) and Masson Trichrome (MT) stained sections (~4μm) were prepared at the level of the papillary muscles, so as to compare the cardiac dimensions at the same level for each heart, using standard procedures.
Cell Size Distribution
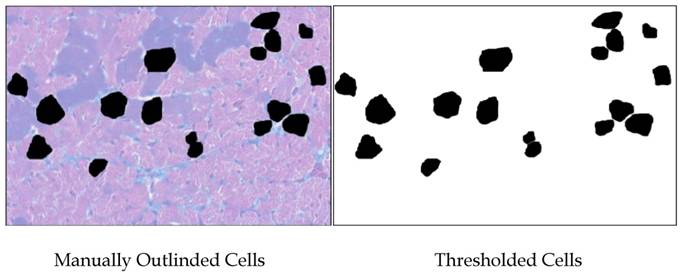
Color images were made of H&E-stained sections of Ames dwarf and wild-type left ventricles at 200x total magnification using a Nikon Eclipse E600 equipped with CoolSNAP-ES digital camera and MetaVue 6.2 software. Microscope lighting, focus, and field selection were optimized for distinction of cell boundaries. In order to increase the probability of cardiomyocytes being visualized in appropriate cross-section and not tangentially, fields were chosen so as to include the papillary muscles and the vertically aligned muscle layers adjacent to them. Cardiomyocytes were outlined manually and bucket-filled-in Gimp. images were opened in ImageJ and after setting the threshold, analyzed(Fig. S1). Major and minor transverse axes and cross-sectional area were measured, and cardiomyocytes with an aspect ratio of ≥1.2 were excluded. This helped eliminate cells sectioned tangentially. Data from all the fields were combined and analyzed in Minitab14®. As relative hypertrophic compensation or decompensation of the heart can result in differences in the ratio of length to diameter of the cardiomyocytes, the volume of the cardiomyocytes was not assessed.
Nuclear Size Distribution
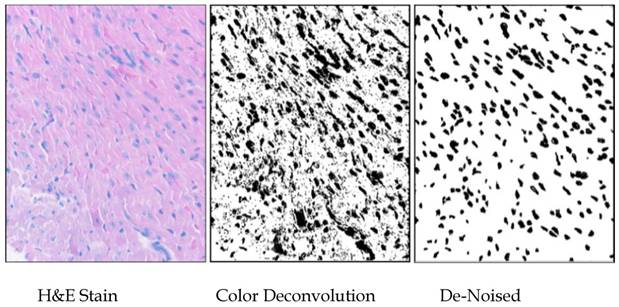
Images of nuclei were separated from background using the ImageJ v1.39 (Wayne Rasband, NIH) plugin “Color Deconvolution” (using the H&E setting) written by Gabriel Landini based on code by A.C. Ruifrok (21). The threshold for the images was set by having all positive pixels set to maximum darkness, using ImageJ, and the darkened “particles” were analyzed after manual de-noising. The areas of the nuclei were standardized to square micrometers (in this case, 1mm2 = [(80 mm)/(345 pixel sides) and the measurements from about thirty fields from each of three hearts per group were compiled into lists, which were statistically analyzed using Minitab14®. Particles of less than one square micrometer were not included in the analysis (Fig. S2).
Collagen Distribution
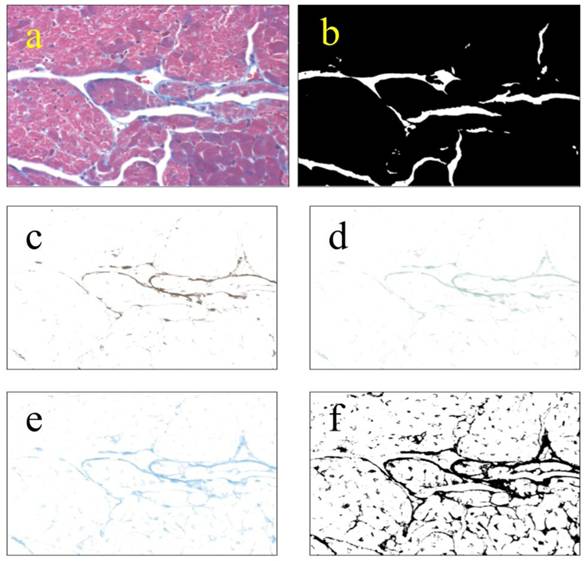
Images were obtained as in Cell Size Distribution above. Microscope lighting, focus, and field selection were optimized to distinguish collagen. Color deconvolution was performed using the “Haematoxylin and DAB (H DAB)”, “Haematoxylin, Eosin and DAB (H&E DAB)”, and “Alcian blue & Haematoxylin” software filters in ImageJ. The images generated were then opened as layers in Gimp 2.6, flattened, and saved. The same flattened images were then opened in ImageJ, thresholded and then particle analyzed (Fig S3). The analyses were saved as “.xls” files, and the lists of particles were summed for each field. The fraction of collagen per field was determined by dividing the total area of collagen per field by the total area of the tissue field, leaving out voids. 25 non-overlapping fields were taken from each of three left ventricles from each group. Lists of collagen fractions were analyzed using Minitab14®.
Sarcomere length
Length measurements of sarcomeres. Fifty measurements were taken of twenty sarcomeres apiece in each of three sections from each group of young adult or old, wild-type or Ames dwarf, for a total of six hundred measurements and twelve thousand sarcomeres. Lengths of sarcomeres were determined from using GIMP and ImageJ, with conversion to micrometers being done after measurement.
Tibial lengths
Tibial lengths of young and old Ames and wild-type mice were measured using a PIXImus system (GE-Lunar).
Statistical Analyses
Cardimyocyte cell size, nuclear size, and collagen fraction measurements were determined by the Anderson-Darling (AD) method to be insufficiently normal in distribution for parametric analysis. For this reason, Mann-Whitney-Wilcoxon (MWW) tests were performed to determine significance of differences between groups of mice with respect to these variables. The nuclear size data was re-sampled to accommodate software limitations by assignment of random numbers followed by non-inclusion of values to which higher numbers had been assigned. The cut-off for significance was p<0.05.
Results
Heart and Body Weight
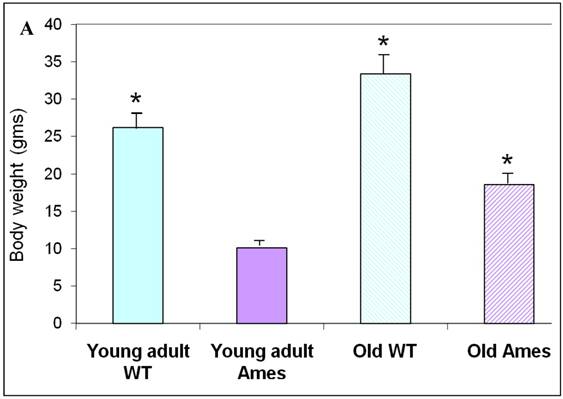
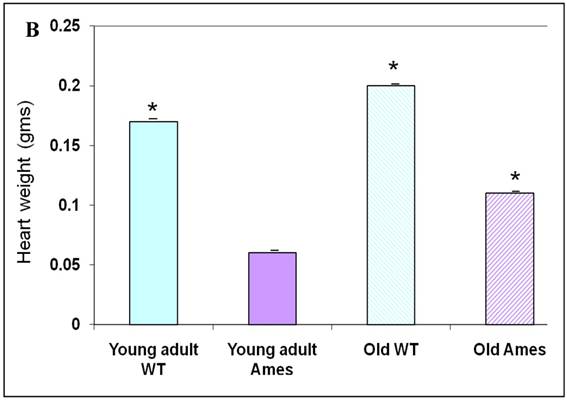
The body weight of each mouse was recorded before sacrifice. Hearts were weighed after removal of excess blood. On the whole, wild-type young adult and old mice were about 72% and 45% heavier, respectively, than age-matched Ames littermates (Fig. 1A) The hearts of wild-type young adult and old mice were 65% and 48% heavier than those of age-matched Ames littermates (Fig. 1B). The old Ames had a slightly reduced HW/BW ratio (0.61) compared with the old wild-type (0.65) which is likely due to the relative adiposity of older Ames dwarf mice. The HW/BW ratios did not change significantly with aging in the Ames or wild-type mice.
Heart Weight/Tibial Size
The use of tibia length for normalization has been previously demonstrated to be more reliable than those based on body weight (22-23). Thus, in conditions in which body weight differences may occur, the heart size and /or cardiac hypertrophy can be more accurately quantified by relating the heart weight to tibial length.
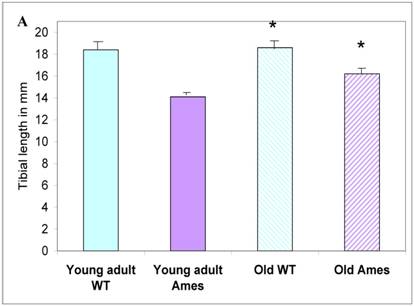
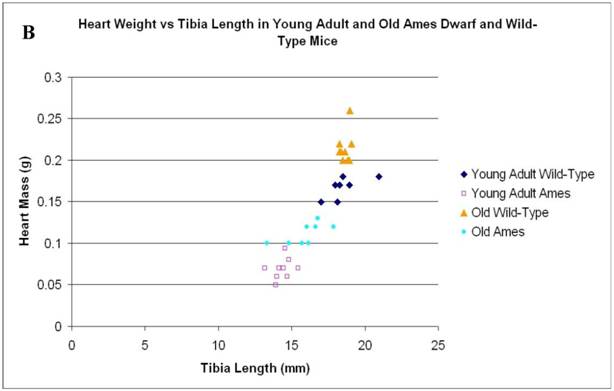
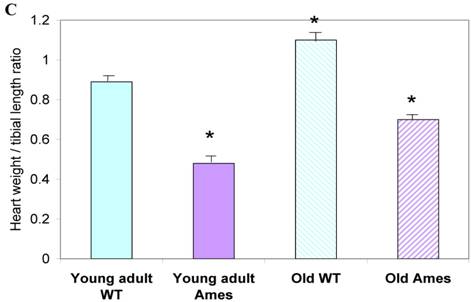
In the present study we found that although the tibial length did not increase with age in wild-type mice, its length increased slightly in the adult Ames mice with age (Fig. 2A). In addition, in contrast to the minor differences in HW/BW ratios, the HW/tibial size ratios were significantly reduced in the Ames dwarf mice at both young adult and older age (Fig. 2B, 2C).
A) Bar Graph showing body weights of young adult and old, wild-type and Ames dwarf mice. *p<0.01. B) Bar Graphs showing heart weights of young adult and old, wild-type and Ames dwarf mice. Young adult = 4-5 months; Old =24-28 months, *p<0.05.
A) Bar graphs showing tibial length of adult and old, wild-type and Ames dwarf mice. Young adult = 4-5 months; Old =24-28 months. *p<0.05. Tibiae were measured using a PIXImus system (GE-Lunar). B) Scatter-plot of heart weight vs tibial length. Note that the tibiae of wild-type mice did not grow post-maturation. However, the tibae of Ames mice continued to grow beyond 6 months of age. C) Heart weight/Tibial length ratios. HW/tibial length ratios were significantly reduced in the Ames dwarf mice at both young adult and older age versus age-matched wild-type *p<0.05.
Cell Size measurements
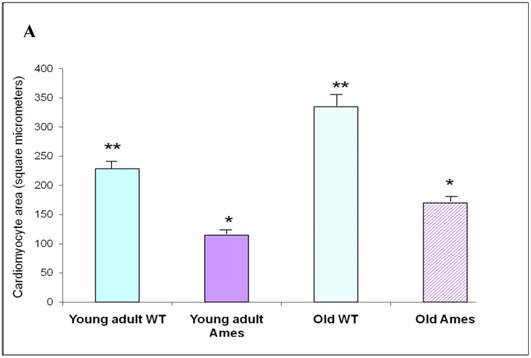
Cross-sectional Area: The mean cardiomyocyte area was 46% less in the 6month old Ames dwarf hearts than in wild-type mice (p<0.0001). Cardiomyocyte size increased with age by about 52% in the wild-type mice and 44% in the Ames dwarf mice (p<0.001). Interestingly, the cardiac myocytes of old Ames dwarf mice were 22% smaller than those of young adult wild-type. Comparisons of cardiomyocyte cross-sections are based on median values, and via MWW tests showed significant differences at p<0.01 (old Ames vs. young wild-type) and p<0.0001 for the others (Fig 3A).
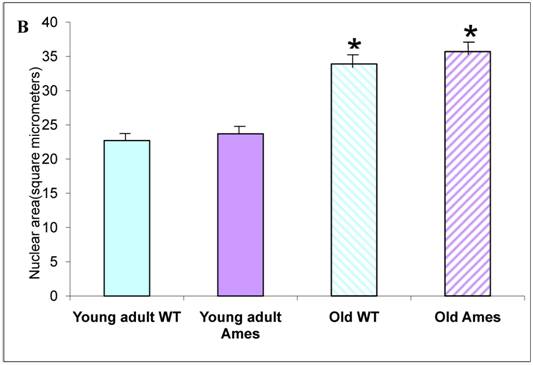
Nuclear Size: There was no significant difference in nuclear cross-sectional area between wild-type and Ames dwarf in the young adult mice, but in the older mice, the Ames cardiomyocyte nuclei were slightly larger (1.02, p<0.05) than in their wild-type littermates. In both genotypes, nuclear size increased about 40-50% between 6 and 26 months of age (Fig. 3B).
A) Bar graph of Cardiomyocyte cross-sectional area. Areas of 100 cells per group (~33/heart) were measured using ImageJ. Both Ames and wild-type cardiomyocytes underwent hypertrophy (51% and 50%, respectively) with advancing age. Not only were Ames cardiomyocytes smaller than those of their wild-type littermates, but the old Ames cardiomyocytes were smaller than those of the young adult wild-type (* p<0.001). B) Bar graphs of Nuclear Area. Nuclei of Ames dwarf and wild-type cardiomyocytes increased in size with advancing age (49% and 44%, respectively, * p<0.001). There was no significant difference in nuclear cross-sectional area between young wild-type and Ames nuclei at the same age.
Cardiomyocyte/Nuclear ratio: The cardiomyocyte/nuclear area ratio was significantly reduced in the Ames Dwarf mice compared with the wild-type (Table 1).
Cell size/nuclear size ratio The cardiomyocyte cells are larger in wild-type than Ames in young and old. The cell size increased significantly more in the wild-type hearts with a relatively greater cell to nuclear ratio than in the Ames hearts.
| Heart specimens | Cardiomyocyte area (μm²) | Nuclear area (μm²) | Ratio Cardiomyocyte/nuclear area |
|---|---|---|---|
| Young adult wild-type | 228.0 | 22.81 | 10.0 |
| Young adult Ames | 122.8 | 23.45 | 5.24 |
| Old Wild-type | 346.2 | 33.74 | 10.3 |
| Old Ames | 177.2 | 36.14 | 4.9 |
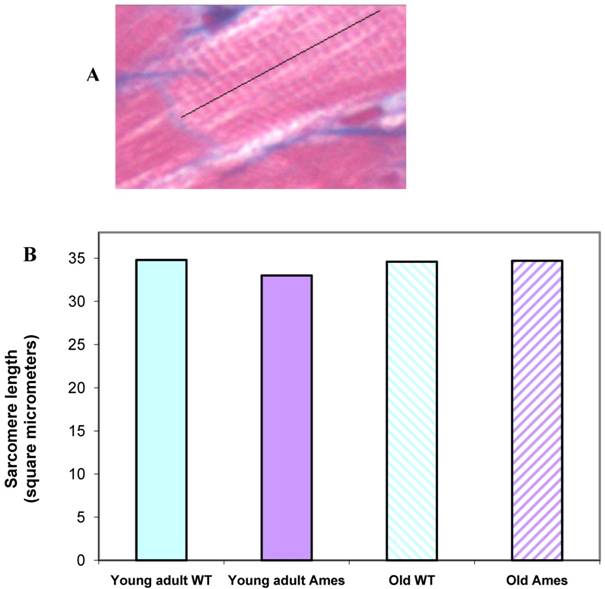
Sarcomere length: Extensive measurements were made of sarcomere length in young and old in the Ames and wild-type groups. There were no significant differences in the sarcomere lengths in the different groups, hence minimizing any potential errors in measurements due to different contractile states of the hearts (Fig. 4A, 4B).
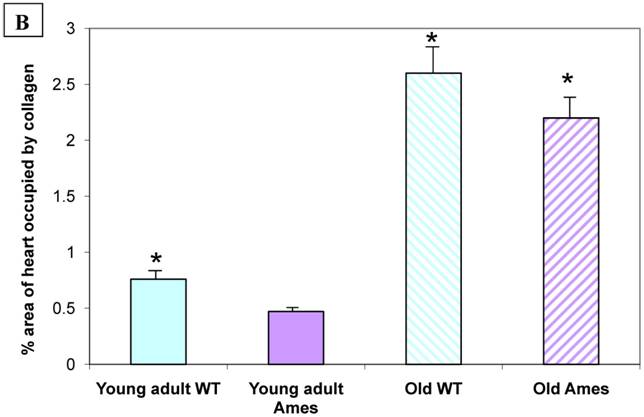
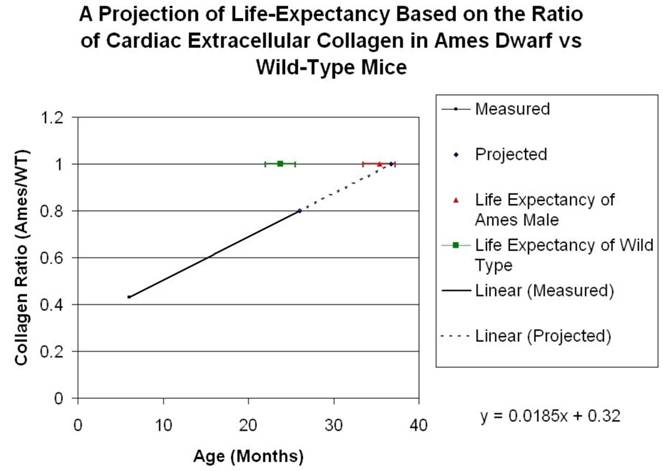
Collagen quantitation: Young adult Ames dwarf hearts contained about 1/3 of the collagen (0.2% vs. 0.7% of area) in age-matched wild-type, but at 26mo, about 1.5% of the sectional areas of both were accounted for by collagen (Fig. 5A, 5B). We did a projection of life expectancy of the Ames dwarf mice based of the ratio of collagen in the hearts compared to wild-type mice. When the line of collagen ratio was extrapolated, it fell within the range of Ames dwarf life expectancy (Fig. 6).
Table S2 summarizes all the measurements made on the Ames dwarf and wild-type mice.
A) Cardiac muscle under 40X magnification showing sarcomeres. The black line illustrates the line of measurement used for data collection. B) Bar graph of sarcomere length. Sarcomere length measurements were not significantly different (n=50 measurements of 20 sarcomeres each per heart) between groups, indicating that differences in cell size cannot be attributed to differences in contractile state.
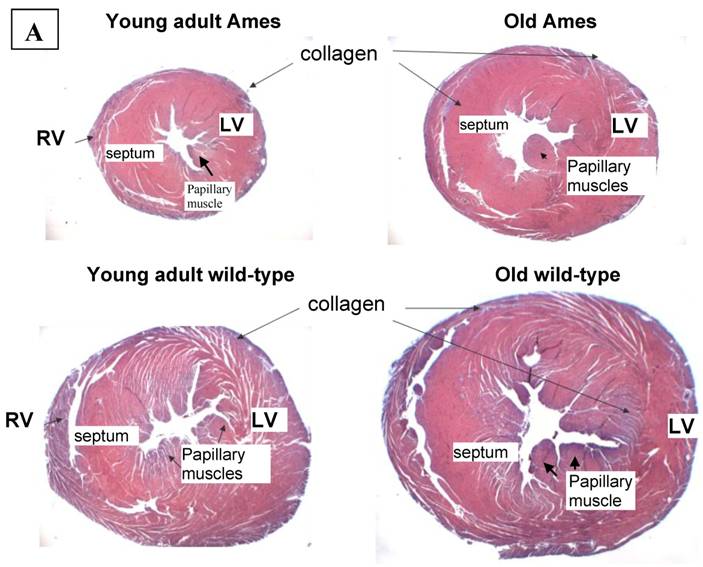
A) Representative cross-sections of the hearts of young adult Ames and age-matched wild-type (4-7mo) and old Ames and age-matched wild-type (24-28 mo) at the level of the papillary muscles, stained with Masson Trichome. The collagen is stained blue. B) Collagen Distribution in the aging of both Ames and wild-type mice. Collagen area was measured in 75 fields per group using ImageJ. Young adult wild-type hearts had 87% more median collagen by area than did the young adult Ames (* p<0.05). With advancing age, however, the percentage of tissue attributed to collagen increased more rapidly in Ames dwarf than in wild-type hearts to the point that, at 26mo, there were was no significant difference.
Projection of Life Expectancy in Ames Based on Ratio of Collagen Fraction of Ames Hearts vs. Wild Type. When the ratios of the collagen fraction (the mean portion of a tissue section which is accounted for by collagen) of Ames dwarf mice to those of wild-type littermates are plotted over time, the resultant curve may be linearly projected to a fair approximation of life expectancy.
Discussion
This paper has four major findings. First, the heart weight/tibial length ratio was significantly reduced in the Ames mice versus the wild-type. Second, the hearts of old Ames dwarf mice had a significantly reduced cardiomyocyte size versus the age-matched and young adult wild-type hearts. Third, young adult Ames dwarf mouse hearts had reduced collagen compared with their wild-type littermates. Fourth, with senescence, the Ames dwarf mice lost the advantage of having a reduced cardiac collagen content.
Heart weight/tibial length(HW/TL)
The Ames dwarf mice have smaller tibia and hearts, hence, the reduction in the HW/TL ratio in the Ames mice compared to the wild-type is not by itself surprising (24). However, there appears to be an age-associated increase in the HW/TL ratio in the Ames between 4-7 months to 24-28 months, which is not evident in the age-matched wild-type. This is due to an increase in the tibial length with age, post-maturation, which could be explained partially by the growth promoting effect of compensatory increase in other factors or cytokines such as parathyroid hormone-related protein, fibroblast growth factor, vascular endothelial growth factor and bone morphogenetic proteins (23 -27). Since GH has lipolytic activity, it was not surprising that the percent body weight gained by the Ames dwarf mice was greater than the wild-type. This has also been observed in GH knock-out mice in which body composition analyses showed that these mice were relatively obese in comparison to littermate controls (28). The Ames mice are also “fat”, but perhaps this adiposity is offset by the benefit of an increased HW/TL ratio in the Ames vs. the wild-type. Obesity is usually associated with impaired insulin sensitivity, cardiovascular disease, metabolic syndrome and increased morbidity and mortality (29). However, there currently exists the paradox of reverse epidemiology, in which at an older age increased body weight and BMI are considered good prognostic indicators (29-31). Thus, the Ames and the other long-lived GH deficient mice offer an opportunity to scientists to study and explain the impact of increased fat content on longevity. It would be of interest to calculate the HW/TL length ratio in healthy overweight versus age-matched normal and under-weight elders in longevity studies.
Changes in Cardiomyocyte and Nuclear Size
There have a few studies of cardiac cellular structure in the Ames dwarf mice or dwarf rats, but reduced cardiomyocyte cell size has been documented with GH deficiency as well as an impaired remodeling and hypertrophic response after myocardial ischemia in dwarf rats (32-35). During the process of normal aging, cardiomyocyte tends to increase with age, whereas in the closely related tissue, the striated skeletal muscle, cell size decreases. Just as cardiac hypertrophy can have detrimental consequences, an age-related reduction in skeletal muscle fiber number and size (sarcopenia) can result in impaired mechanical muscle performance even in trained athletes (36-37). In our study the cardiomyocyte size of both wild-type and Ames dwarf mice showed an age-associated increase, although interestingly, the cardiomyocytes of the old Ames were even smaller than those of the young adult wild-type mice. Also, while nuclear size increased by about 50% in both wild-type and Ames dwarf mice, there was no significant difference in nuclear size between genotypes within age groups. Given the large difference in cardiomyocyte size between Ames and wild-type mice, and referring to Gerdes et al (38), in which nuclear volume was found to increase with cell volume, the fact that the nuclear size did not proportionately increase with cell size in the wild-type mice, was notable. There are two main mechanisms of cardiomyopathy associated with increase in nuclear size: one involves polyploidy, and the other shows a strong correlation with expression of PCNA, SC-35, and p70S6K (39-43). Both mechanisms, however, result in concomitant nuclear and cellular size increase. More notable than either cell size or nuclear size was the ratio of the nuclear to cell size, which was significantly reduced in Ames heart at young and old age. Since the nucleus appeared normal in the Ames dwarf, a reduced nuclear to cell ratio could signify more biosynthetic function and better governance of cellular functions and integrity (44). This hypothesis will require further elucidation.
Potential Significance of Cardiac Fibrosis
In clinical conditions of excess growth hormone such as acromegaly, both cardiac hypertrophy and fibrosis are typically present (45). Increased collagen concentration of the heart causes increased ventricular stiffness, reduced ventricular compliance and impaired diastolic function of the heart, which is a very common problem in the elderly (2-4, 46-50). There have been few studies of cardiac function in the Ames dwarf mice. A study of young Ames mice using dobutamine demonstrated that Ames hearts were functionally better than wild-type and resistant to the stress (51). These results of better cardiac function in young Ames mice were similar to the preserved cardiac function using dobutamine in 17 month old Little mice with about 1% of normal circulating GH (52). On the other hand, a study using cardiomyocytes from hearts of old Ames mice suggested that there may be greater impairment of contraction and relaxation in cardiomyocytes from the older Ames dwarf mice versus the wild-type (53). Heterozygous IGF 1 deficient dwarf mice have a better preserved cardiac function one week after myocardial infarction and demonstrate a blunted hypertrophic response with less remodeling (54). The data from previous studies on Ames hearts could suggest that significantly reduced collagen content in the Ames hearts could be important in maintaining cardiac compliance and consequently a preserved cardiac response to stress. Collagen increase is important humans in the pathogenesis of diastolic heart failure and various cardiac pathological states with reduced cardiac compliance (55-61). Some studies have demonstrated that increase in the degree of collagen cross-linking correlates better with diastolic dysfunction than collagen concentration per sec (57). We did not evaluate collagen cross-linking in our study but Flurkey et al found that the degree of increase in collagen cross-linking associated with aging from 4-8mo to 16-19mo was reduced in Snell dwarf (Pit1dw/dwJ) mice compared to age-matched wild-type mice (62). It is possible that Ames mice will also have reduced collagen cross-linking, but this will require systematic study. Another factor promoting collagen-cross-linking is advanced glycation end products (AGE) which has not been studied in the dwarf mice (63).
In humans, chronic growth hormone excess produces cardiac fibrosis, and one might postulate that chronic growth hormone deficiency would result in reduced fibrosis (64). To date, collagen content of hearts from people with chronic growth hormone deficiency has not been analyzed. However, in the current study, the collagen area fractions were reduced by about two-thirds in young adult Ames hearts compared to the age-matched wild-type, but there was no difference in the collagen content between the genotypes in the older mice. So even though the Ames dwarf mouse hearts had significantly less collagen when young, they accumulated approximately the same amount of collagen as the wild-type mice by 24-26 months of age. Why this is so, is not clear, but it is possible that the Ames dwarf mice secrete increased amounts of growth promoting factors and cytokines relative to their body/mass index as they become older, which could enhance accelerated cardiac fibrosis (Table S1). Contrary to humans where growth hormone is associated with cardiac fibrosis, in studies done in rats treated with growth hormone, the collagen content of the heart was found to be reduced in Western blots (65-66). However, the variation in the collagen content in rat studies could have been due to different isoforms of collagen being evaluated and or due to the dose and duration of the growth hormone treatment.
Another factor that could influence fibrosis is the level of the thyroid hormone, with typically increased collagen accumulation occurring in hypothyroidism (67). The prevalence of hypothyroidism in humans increases with age, with approximately 10% of the elderly being clinically hypothyroid (68-69). It is not clear if a similar process of thyroid insufficiency develops in older rodents but assuming it does, the increase in fibrosis with aging in the Ames dwarf mice may be related to a further age-reduced reduction of their thyroid hormone levels. Thyroid hormone is also important in controlling the SERCA2 cardiac pump and hence contractility (70). This effect has been observed in the FOG2 transgenic mice, where FOG2 by binding T3, reduced SERCA2 activity, resulting in congestive heart failure, and early death (70-71). In-vitro function in the Ames dwarf mice demonstrated reduced cardiomyocyte size and impaired excitation-contraction coupling of the myocytes (53). However, the negative functional effects of hypothyroidism do not appear to be present in the Ames hearts. This might be due to a complimentary reduction in GH with a balanced growth and metabolism reduction. This hypothesis will need to be tested with varying ratios of replacement of thyroid and GH and measuring the effect on heart and longevity (72).
Although results from our study suggest that longevity of the Ames dwarf mice might be linked to the reduced collagen and structural changes in the heart, there have been two reports of negative regulation of the cardiovascular system in dwarf mice. One study of aorta of Ames mice demonstrated increased endothelial O2•− and H2O2 production, mitochondrial reactive oxygen generation and reduced expression of antioxidant enzymes, and nitric oxide (NO) production in aortas of Ames dwarf versus wild-type control (73). The second study using mice with mutant IGF-1 gene expression exhibited elevated blood pressure and enhanced cardiac contractility, but these mice had high plasma insulin and GH levels (69). However, a number of recent studies have shown preserved cardiac function in response to stress in Ames dwarf mice (46,48). These differences in cardiac health might be due to varying autocrine/paracrine effects of IGF, and it has been shown previously that circulating IGF-I and tissue IGF-I levels do not always correlate (71-72). There also appear to be tissue specific optimal levels of IGF-I which is why the effects of Prop1df/df genotype differ from other long lived dwarf mice. Moreover, the Ames mice have a significant advantage of increased insulin sensitivity compared to wild-type (11). It has been reported in several studies that insulin sensitivity might be more important than GH levels for longevity because there was no increase in life-span in transgenic mice over-expressing GH antagonist, which have reduced IGF but no alteration of insulin sensitivity (12). In addition, Masternak et al recently demonstrated that growth hormone (GH) treatment in long-lived Ames dwarf mice stimulated somatic growth of the Ames dwarf mice by increased production of IGF1 but decreased the insulin sensitivity of the mice, negatively affecting both their healthspan and longevity (8). In the current study, based on our observation of significantly better cardiac morphology in the Ames, it may be that the particular combination of chronically reduced levels of growth hormone and thyroid hormone may contribute to the longevity of these mice. Future studies to characterize in-vivo cardiac function in animals with chronically low GH and thyroid hormone levels will be of interest. Apart from growth and thyroid hormones, there are many other pro and anti-fibrotic factors in the heart that could potentially have altered the balance in favor of less collagen in the Ames hearts (Table S1). Undoubtedly, these factors will also be of interest to elucidate in hearts of Ames versus wild-type.
There is no large database for documenting natural causes of deaths in mice with details of various cardiac events. Nevertheless, the mouse has been used as a model to understand many aspects of cardiac pathophysiologly in humans. Spontaneous hypertensive rat and mouse models have increased amount of cardiac collagen and fibrosis which correlates with a shortened life-span and higher mortality. It has recently been determined that fibrosis of the hearts in mice can predispose to greater arrythymogenicity, probably via destroying the conducting pathways in the heart (77-79). Hence, the importance of collagen in cardiac pathophysiology cannot be understated.
The role of IGF itself in longevity continues to be investigated. A study by Li and Ren IGF1 transgenic mice showed that they had a slightly longer life-span than the FVB wild-type (80). However these mice were heterogyzgous and the percent elevation of IGF transgene in these mice was not defined in the study. It is quite possible that a mild elevation of IGF can provide some beneficial effect and increase lifespan especially in the generally short-lived FVB strain. In the Ames mice, the combined deficiency of 3 hormones might afford better protection than one hormone alone.
The Ames dwarf mice and caloric restricted mice both have increased insulin sensitivity with activation of the insulin signaling cascade (IR/IRS/PI3K/AKT). In fact caloric restriction of the Ames mice has been shown to further increase their insulin sensitivity and extend life-span (81). Long lived mice that do not exhibit a direct link to GH/IGF-1/insulin signaling have been shown to have resistance to stress. For example, transgenic mice over-expressing mitochondrial catalase (MCAT) live 17-21% longer than wild-type mice (82). TRX-Tg(,thioredoxin) mice have antioxidant, anti-inflammatory and anti-apoptotic, properties and live 22-35% longer (83). Dhahbi et al have shown smaller cardiomyocytes and reduced collagen accumulation in caloric restricted mice (84). Interestingly, reduced expression levels of pro-collagen genes in caloric restricted mice have also been shown by other investigators (85).
Our study explores this issue in the Ames dwarf mice because age-related morphological changes can substantially influence target organs, which can ultimately determine morbidity and life-span. A reduction in fibrosis and remodeling is very important in cardiac health and many clinical trials have been devoted to this area with a significant impact on morbidity (86-87). When comparing collagen fractions in Ames dwarf compared with wild-type, both in young adult and old mice, we found that when the line was extrapolated it fell within the range of Ames dwarf life expectancy (Fig. 6). Hence, it appears plausible that a smaller cardiac cell size and reduced extracellular collagen might also contribute towards improved cardiac health and longevity of the Ames dwarf mice. The mechanistic pathways for reduced age-related collagen accumulation and cell size in the Ames dwarf hearts will need to be further elucidated.
Acknowledgements
We are deeply grateful to Dr. Andrzej Bartke for providing the mice. This work was supported in part by HHS grants AG018388 and AG026091 and by the Veterans Healthcare System.
Conflict of Interests
The authors have declared that no conflict of interest exists.
References
1. Hsiang-Ching K, Hoyert DL. et al. Deaths: Final Data for 2005. National Vital Statistics Reports. 2008;56(10):5
2. Wei JY. Age and the cardiovascular system. N Engl J Med. 1992;327:1735-1739
3. Pugh KG, Wei JY. Clinical Implications of Physiological Changes in the Aging Heart. Drugs & Aging. 2001;18:263-276
4. Wei JY. Understanding the Aging Cardiovascular System. Geriatrics and Gerontology International. 2004;4:S298-S303
5. Wu W, Cogan JD. et al. Mutations in PROP1 cause familial combined pituitary hormone deficiency. Nature Genetics. 1998;18(2):147-9
6. White HK, Petrie C. et al. Effects of an Oral Growth Hormone Secretagogue Capromorelin in Older Adults. JCEM. 2009;94(4):1198-206
7. Sjögren K, Liu JL. et al. (1999) Liver-derived insulin-like growth factor I (IGF-I) is the principal source of IGF-I in blood but is not required for postnatal body growth in mice. Proc Natl Acad Sci U S A. 1999;96(12):7088-92
8. Masternak MM, Panici JA. et al. The effects of growth hormone (GH) treatment on GH and insulin/IGF-1 signaling in long-lived Ames dwarf mice. J Gerontol A Biol Sci Med Sci. 2010;65(1):24-30
9. Egecioglu E, Andersson IJ. et al. Growth hormone receptor deficiency in mice results in reduced systolic blood pressure and plasma renin, increased aortic eNOS expression, and altered cardiovascular structure and function. Am J Physiol Endocrinol Metab. 2007;292(5):E1418-25
10. Koedam JA, Hoogerbrugge CM. et al. Insulin-like growth factor-binding protein-3 protease activity in Snell normal and Pit-1 deficient dwarf mice. J Endocrinol. 1998;157(2):295-303
11. Bartke A. Impact of reduced insulin-like growth factor-1/insulin signaling on aging in mammals: novel findings. Aging Cell. 2008;7(3):285-90
12. Coschigano KT, Holland AN. et al. Flyvbjergs, insulin, and insulin A, Kopchick JJ Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin, and insulin-like growth factor I levels and increased life span. Endocrinology. 2003;144:3799-3810
13. Rutishauser J, Schmid C. et al. Growth hormone, but not insulin-like growth factor I, induces a serum protease activity for insulin-like growth factor binding protein-3 in hypophysectomized rats in vivo. FEBS Lett. 1993;334(1):23-6
14. Koedam JA, Hoogerbrugge CM. et al. Insulin-like growth factor-binding protein-3 protease activity in Snell normal and Pit-1 deficient dwarf mice. J Endocrinol. 1998;157(2):295-303
15. Jiang M, Xu A. et al. Thyroid hormone downregulates the expression and function of sarcoplasmic reticulum-associated CaM kinase II in the rabbit heart. Am J Physiol Heart Circ Physiol. 2006;291(3):H1384-94
16. Kenessey A, Ojamaa K. Thyroid hormone stimulates protein synthesis in the cardiomyocyte by activating the Akt-mTOR and p70S6K pathways. J Biol Chem. 2006;281(30):20666-72
17. Stănescu C, Branidou K. et al. Heart failure and dilated cardiomyopathy associated with severe longstanding untreated hypothyroidism. Rom J Intern Med. 2007;45(1):77-83
18. Rouf R, Greytak S. et al. Increased FOG-2 in failing myocardium disrupts thyroid hormone-dependent SERCA2 gene transcription. Circ Res. 2008;103(5):493-501
19. Kelly PA, Binart N. et al. Prolactin receptor signal transduction pathways and actions determined in prolactin receptor knockout mice. Biochem Soc Trans. 2001;29(Pt 2):48-52
20. Goffin V, Binart N. et al. Prolactin: the new biology of an old hormone. Annu Rev Physiol. 2002;64:47-67
21. Ruifrok AC, Johnston DA. Quantification of histochemical staining by color deconvolution. Anal Quant Cytol Histol. 2001;23:291-299
22. Wei JY, Spurgeon HA, Lakatta EG. Excitation-contraction in rat myocardium: alterations with adult aging. Am J Physiol. 1984Jun;246(6 Pt 2):H784-91
23. Yin FC, Spurgeon HA, Rakusan K, Weisfeldt ML, Lakatta EG. Use of tibial length to quantify cardiac hypertrophy: application in the aging rat. Am J Physiol. 1982Dec;243(6):H941-7
24. Bokov AF, Lindsey ML. et al. Long-lived Ames dwarf mice are resistant to chemical stressors. J Gerontol A Biol Sci Med Sci. 2009;64(8):819-27
25. Gurkan UA, Gargac J. et al. The Sequential Production Profiles of Growth Factors and Their Relations to Bone Volume in Ossifying Bone Marrow Explants. Tissue Eng Part A. 2010 [Epub ahead of print]
26. Rochira V, Zirilli L. et al. Tall Stature without Growth Hormone: Four Male Patients with Aromatase Deficiency. J Clin Endocrinol Metab. 2010 [Epub ahead of print]
27. Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423:332-336
28. Berryman DE, List EO. et al. Two-year body composition analyses of long-lived GHR null mice. J Gerontol A Biol Sci Med Sci. 2010;65(1):31-40
29. Horwich TB, Fonarow GC. Glucose, obesity, metabolic syndrome, and diabetes relevance to incidence of heart failure. J Am Coll Cardiol. 2010;55(4):283-93
30. Influence of obesity on outcomes in atrial fibrillation. yet another obesity paradox. Badheka AO, Rathod A, Kizilbash MA et al. Am J Med. 2010;123(7):646-51
31. Oreopoulos A, Kalantar-Zadeh K. et al. The obesity paradox in the elderly: potential mechanisms and clinical implications. Clin Geriatr Med. 2009;25(4):643-59
32. Ren J, Brown-Borg HM. Impaired cardiac excitation-contraction coupling in ventricular myocytes from Ames dwarf mice with IGF-1 deficiency. Growth Hormone and IGF Research. 2002;12:99-105
33. Cittadini A, Strömer H. et al. Differential cardiac effects of growth hormone and insulin-like growth factor-1 in the rat. A combined in vivo and in vitro evaluation. Circulation. 1996;93(4):800-9
34. Cittadini A, Strömer H. et al. Consequences of growth hormone deficiency on cardiac structure, function, and beta-adrenergic pathway: studies in mutant dwarf rats. Endocrinology. 1997;138(12):5161-9
35. Palmen M, Daemen MJAP. et al. Cardiac remodeling after myocardial infarction is impaired in IGF-1 deficient mice. Cardiovascular Research. 2001;50:516-524
36. Aagaard P, Suetta C. et al. Role of the nervous system in sarcopenia and muscle atrophy with aging: strength training as a countermeasure. Scand J Med Sci Sports. 2010;20(1):49-64
37. Frontera WR, Hughes VA. et al. Aging of skeletal muscle: a 12-yr longitudinal study. J Appl Physiol. 2000;88(4):1321-6
38. Gerdes AM, Liu Z. et al. Changes in nuclear size of cardiac myocytes during the development and progression of hypertrophy in rats. Cardioscience. 1994;5(3):203-8
39. McMullen JR, Sherwood MC. et al. Inhibition of mTOR signaling with rapamycin regresses established cardiac hypertrophy induced by pressure overload. Circulation. 2004;109(24):3050-5
40. Sadoshima J, Izumo S. Rapamycin selectively inhibits angiotensin II-induced increase in protein synthesis in cardiac myocytes in vitro. Potential role of 70-kD S6 kinase in angiotensin II-induced cardiac hypertrophy. Circ Res. 1995;77(6):1040-52
41. Feng Z, Hu W. et al. The regulation of AMPK beta1, TSC2, and PTEN expression by p53: stress, cell and tissue specificity, and the role of these gene products in modulating the IGF-1-AKT-mTOR pathways. Cancer Res. 2007;67(7):3043-53
42. Hayashi AA, Proud CG. The rapid activation of protein synthesis by growth hormone requires signaling through mTOR. Am J Physiol Endocrinol Metab. 2007;292(6):E1647-55
43. Boluyt MO, Zheng JS. et al. Rapamycin inhibits alpha 1-adrenergic receptor-stimulated cardiac myocyte hypertrophy but not activation of hypertrophy-associated genes. Evidence for involvement of p70 S6 kinase. Circ Res. 1997;81(2):176-86
44. Koda M, Takemura G. et al. Nuclear hypertrophy reflects increased biosynthetic activities in myocytes of human hypertrophic hearts. Circ J. 2006;70(6):710-8
45. Lombardi G, Galdiero M. et al. Acromegaly and the cardiovascular system. Neuroendocrinology. 2006;83(3-4):211-7
46. Isoyama S, Grossman W. et al. Effect of age on myocardial adaptation to volume overload in the rat. J Clin Invest. 1988;81:1850-1857
47. Isoyama S, Wei JY. et al. Effect of age on the development of cardiac hypertrophy produced by aortic constriction in the rat. Circ Res. 1987;61:337-345
48. Isoyama S, Grossman W. et al. Effect of age on myocardial adaptation to volume overload in the rat. J Clin Invest. 1988;81:1850-1857
49. Lipsitz LA, Jonsson PV. et al. Reduced supine cardiac volumes and diastolic filling rates in elderly patients with chronic medical conditions. JAGS. 1990;38:103-7
50. Zhang X, Azhar G. et al. Model of functional cardiac aging: young adult mice with mild overexpression of serum response factor. Am J Physiol Regul Integr Comp Physiol. 2003;285(3):R552-60
51. Bokov AF, Lindsey ML. et al. Long-lived ames dwarf mice are resistant to chemical stressors. Gerontol A Biol Sci Med Sci. 2009;64(8):819-27
52. Reddy AK, Amador-Noguez D. et al. Cardiac function in young and old Little mice. J Gerontol A Biol Sci Med Sci. 2007;62(12):1319-25
53. Ren J, Brown-Borg HM. Impaired cardiac excitation-contraction coupling in ventricular myocytes from Ames dwarf mice with IGF-I deficiency. Growth Horm IGF Res. 2002;12(2):99-105
54. Palmen M, Daemen MJ. et al. Cardiac remodeling after myocardial infarction is impaired in IGF-1 deficient mice. Cardiovasc Res. 2001;50(3):516-24
55. Janicki JS, Brower GL. The role of myocardial fibrillar collagen in ventricular remodeling and function. J Card Fail. 2002;8(6 Suppl):S319-25
56. Díez J. Diagnosis and treatment of myocardial fibrosis in hypertensive heart disease. Circ J. 2008;72(Suppl A):A8-12
57. Brower GL, Gardner JD. et al. The relationship between myocardial extracellular matrix remodeling and ventricular function. Eur J Cardiothorac Surg. 2006;30(4):604-10
58. López Salazar B, Ravassa Albéniz S. et al. Altered fibrillar collagen metabolism in hypertensive heart failure. Current understanding and future prospects Rev Esp Cardiol. 2006;59(10):1047-57
59. Mak GJ, Ledwidge MT. et al. Natural history of markers of collagen turnover in patients with early diastolic dysfunction and impact of eplerenone. J Am Coll Cardiol. 2009;54(18):1674-82
60. Baicu CF, Stroud JD. et al. Changes in extracellular collagen matrix alter myocardial systolic performance. Am J Physiol Heart Circ Physiol. 2003;284(1):H122-32
61. Cox MA, Kortsmit J. et al. Tissue-Engineered Heart Valves Develop Native-Like Collagen Fiber Architecture. Tissue Eng Part A. 2010May;16(5):1527-37
62. Flurkey K, Papaconstantinou J. et al. Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc Natl Acad Sci U S A. 2001;98(12):6736-41
63. Fujimoto E, Kobayashi T. et al. AGE-modified collagens I and III induce keratinocyte terminal differentiation through AGE receptor CD36: epidermal-dermal interaction in acquired perforating dermatosis. J Invest Dermatol. 2010;130(2):405-14
64. Brüel A, Oxlund H. The effect of growth hormone on rat myocardial collagen. Growth Horm IGF Res. 1999;9(2):123-30
65. Brüel A, Oxlund H. The effect of growth hormone on rat myocardial collagen. Growth Horm IGF Res. 1999;9(2):123-30
66. Drobnik J, Ciosek J. et al. Experimental hypothyroidism increases content of collagen and glycosaminoglycans in the heart. J Physiol Pharmacol. 2009;60(3):57-62
67. Simonsick EM, Newman AB. et al. Health ABC Study Subclinical hypothyroidism and functional mobility in older adults. Arch Intern Med. 2009;169(21):2011-7
68. Wei JY, Genecin A. et al. Coronary spasm with ventricular fibrillation during thyrotoxicosis: response to attaining euthyroid state. Am J Cardiol. 1979;43:335-339
69. Yao J, Eghbali M. Decreased collagen gene expression and absence of fibrosis in thyroid hormone-induced myocardial hypertrophy. Response of cardiac fibroblasts to thyroid hormone in vitro. Circ Res. 1992;71(4):831-9
70. Ghose Roy S, Mishra S. et al. Thyroid hormone induces myocardial matrix degradation by activating matrix metalloproteinase-1. Matrix Biol. 2007;26(4):269-79
71. Zhou B, Ma Q. et al. Fog2 is critical for cardiac function and maintenance of coronary vasculature in the adult mouse heart. J Clin Invest. 2009;119(6):1462-76
72. Louis A, Bartke A. et al. Effects of Growth Hormone and Thyroxine Replacement Therapy on Insulin Signaling in Ames Dwarf Mice. J Gerontol A Biol Sci Med Sci. 2010 [Epub ahead of print]
73. Csiszar A, Labinskyy N. et al. Endothelial function and vascular oxidative stress in long-lived GH/IGF-deficient Ames dwarf mice. Am J Physiol Heart Circ Physiol. 2008;295(5):H1882-94
74. Lembo G, Rockman H. et al. Elevated Blood Pressure and enhanced myocardial contractility in mice with severe IGF-1 deficiency. J Clinical Invest. 1996;98(11):2648-2655
75. Sun LY, Al-Regaiey K. et al. Local expression of GH and IGF-I in the hippocampus of GH-deficient long-lived mice. Neurobiol Aging. 2005;26:929-937
76. Masternak MM, Al-Regaiey KA. et al. Caloric restriction and growth hormone receptor knockout: effects on expression of genes involved in insulin action in the heart. Exp Gerontol. 2006;41:417-429
77. Stein M, Boulaksil M. et al. Reduction of fibrosis related arrthythmias by chronic renin-angiotensin-aldosterone-system inhibitors in an aged mouse model. AJP. 2010 in press
78. Akar FG, Spargg DD. et al. Mechanisms of underlying conduction slowing and arrhythmogenesis in non-ischemic dilated cardiomyopathy. Circ Res. 2004;95:717-725
79. Stein M, Noorman M. et al. Dominant arrhythmia vulnerability of the right ventricle in senescent mice. Heart Rhythm. 2008;5:438-448
80. Li and Ren. Influence of cardiac-specific over-expression of insulin-like growth factor on life-span and aging associated changes in cardiac intracellular Ca+ homeostasis, protein damage and apoptotic protein expression. Aging Cell. 2007;6:799-806
81. Barzilai N, Bartke A. Biological approaches to mechanistically understand the healthy life span extension achieved by calorie restriction and modulation of hormones. J Gerontol A Biol Sci Med Sci. 2009;64(2):187-91
82. Schriner SE, Linford NJ. et al. Extension of murine lifespan by overexpression of catalase targeted to mitochondria. Science. 2005;208:1909-1911
83. Mitsui A, Hamuro J. et al. Overexpression of human of human thioredoxin in transgenic mice controls oxidative stress and life span. Antioxid Redox Signal. 2002;4(4):693-6
84. Dhahbi JM, Tsuchiya T. et al. Gene expression and physiologic responses of the heart to the initiation and withdrawal of caloric restriction. J Gerontol A Biol Sci Med Sci. 2006Mar;61(3):218-31
85. References and further reading may be available for this article. To view references and further reading you must purchase Swindell WR. Genes regulated by caloric restriction have unique roles within transcriptional networks. Mechanisms of Ageing and Development. 2008;129:580-592
86. Anand K, Mooss AN. et al. Aldosterone inhibition reduces the risk of sudden cardiac death in patients with heart failure. Renin Angiotensin Aldosterone Syst. 2006;7(1):15-9
87. Werner C, Pöss J. et al. Optimal antagonism of the Renin-Angiotensin-aldosterone system: do we need dual or triple therapy? Drugs. 2010;70(10):1215-30
Tables and Figures
Factors influencing cardiac fibrosis.
| Profibrotic molecules | |
| Cytokines | Cardiotrophin 1, IL6, Endothelin |
| Vasoactive mediators | Angiotensin II, catecholamines |
| Growth factors | TGF-ß, CTGF, PDGF, IGF |
| Adhesion factors | ICAM-1, VCAM-1 |
| Others | Osteopontin, Thrombospondin, PAI-1, ROS, desoxycorticosterone |
| Anti-fibrotic molecules | |
| Cytokines | IL1 ß, TNF-α |
| Vasoactive mediators | bradykinin, prostacylin, nitric oxide, natriuretic peptides |
| Adrenal hormones | glucocorticoids |
| Others | N-acetyl-aspartly-lysyl-proline, estrogens, endogenous ligands of PPAR-α. |
Table summarizing all the measurements acquired in the study.
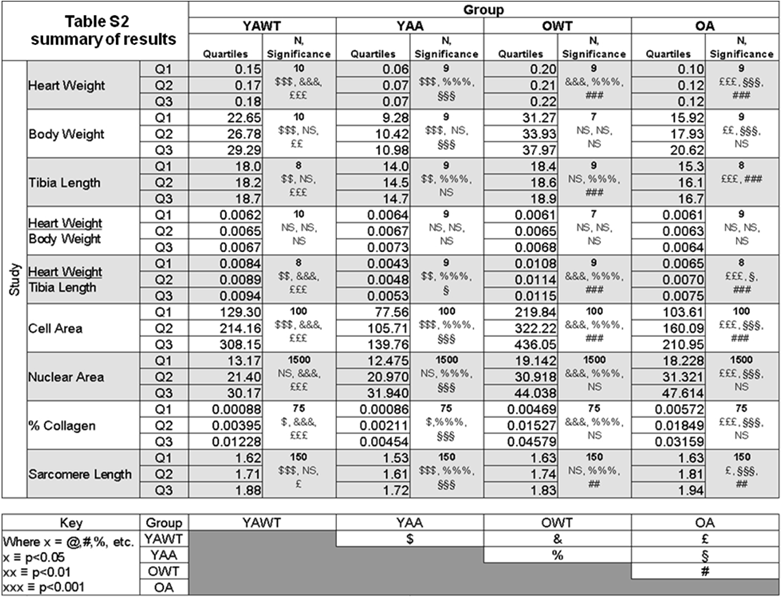
YAWT = Young adult wild-type
YAA = Young adult Ames
OWT = Old wild-type
OA = Old Ames
Methodology used for measuring cardiomyocytes. Areas of 100 cells per group (~33 per heart) were outlined and bucket-filled in Gimp and analyzed in ImageJ. Where cells were adjacent, care was taken to manually make a clean and minimal-width division of their respective areas. Data from all the fields were combined and analyzed in Minitab14®.
Methodology used for measuring nuclei. Areas of 1500 nuclei per group were determined by color-deconvolution of H&E-stained heart sections in ImajeJ, manual de-noising in Gimp, and particle analysis in ImageJ. Areas of nuclei were measured and tabulated in ImageJ, and size distributions were analyzed using Minitab14.
Methodology used for measuring collagen. Collagen was measured by color-deconvolution of images from Masson-trichrome-stained heart sections. TIFF images obtained at 200x total magnification (a) were thresholded to obtain the area of tissue leaving out voids (b). Original images were color-deconvolved using the “Haematoxylin Eosin & DAB” (c), “Haematoxylin & DAB” (4d), and “Alcian Blue & Haematoxylin” (4e) filters in ImageJ. The deconvolved images were combined in Gimp and thresholded in ImageJ (f). The resulting thresholded images were particle-analyzed in ImageJ. Area percentage of collagen was obtained by division of the combined image (f) by the total tissue image. Lists of collagen percentage were analyzed using Minitab14®.
Author contact
![]() Corresponding author: Email: AzharGoharedu
Corresponding author: Email: AzharGoharedu

 Global reach, higher impact
Global reach, higher impact