10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2010; 6(5):499-512. doi:10.7150/ijbs.6.499 This issue Cite
Research Paper
Increased Proliferation and Analysis of Differential Gene Expression in Human Wharton's Jelly-derived Mesenchymal Stromal Cells under Hypoxia
1. Stempeutics Research Pvt. Ltd, Manipal Hospital, Bangalore, India.
2. Stempeutics Research Malaysia Sdn Bhd, Kuala Lumpur, Malaysia.
3. Manipal Institute of Regenerative Medicine, Manipal University, Bangalore, India.
Received 2010-5-5; Accepted 2010-8-27; Published 2010-9-9
Abstract
Multipotent mesenchymal stromal cells (MSCs) from Wharton's jelly (WJ) of umbilical cord bear higher proliferation rate and self-renewal capacity than adult tissue-derived MSCs and are a primitive stromal cell population. Stem cell niche or physiological microenvironment plays a crucial role in maintenance of stem cell properties and oxygen concentration is an important component of the stem cell niche. Low oxygen tension or hypoxia is prevalent in the microenvironment of embryonic stem cells and many adult stem cells at early stages of development. Again, in vivo, MSCs are known to home specifically to hypoxic events following tissue injuries. Here we examined the effect of hypoxia on proliferation and in vitro differentiation potential of WJ-MSCs. Under hypoxia, WJ-MSCs exhibited improved proliferative potential while maintaining multi-lineage differentiation potential and surface marker expression. Hypoxic WJ-MSCs expressed higher mRNA levels of hypoxia inducible factors, notch receptors and notch downstream gene HES1. Gene expression profile of WJ-MSCs exposed to hypoxia and normoxia was compared and we identified a differential gene expression pattern where several stem cells markers and early mesodermal/endothelial genes such as DESMIN, CD34, ACTC were upregulated under hypoxia, suggesting that in vitro culturing of WJ-MSCs under hypoxic conditions leads to adoption of a mesodermal/endothelial fate. Thus, we demonstrate for the first time the effect of hypoxia on gene expression and growth kinetics of WJ-MSCs. Finally, although WJ-MSCs do not induce teratomas, under stressful and long-term culture conditions, MSCs can occasionally undergo transformation. Though there were no chromosomal abnormalities, certain transformation markers were upregulated in a few of the samples of WJ-MSCs under hypoxia.
Keywords: Hypoxia, Wharton's jelly, Mesenchymal stem cells (MSCs), Transcription, Transformation markers, Cell proliferation.
INTRODUCTION
Mesenchymal stem cells or multipotent mesenchymal stromal cells (MSCs) comprise a promising tool for regenerative stem cell therapy due to their ability to self renew, differentiate into multiple tissues [1] and immunomodulatory properties [2]. The umbilical cord, which is discarded after birth, can provide an inexhaustible and non-controversial source of stem cells for therapy. MSCs have been isolated from different compartments of the umbilical cord, and Wharton's jelly (WJ) is the embryonic mucous connective tissue lying between the amniotic epithelium and the umbilical vessels. WJ-MSCs bear higher proliferation rate and self-renewal capacity than adult tissue-derived MSCs and are a primitive stromal cell population [3].
Stem cell niche or physiological microenvironment plays a crucial role in maintenance of stem cell properties and oxygen concentration is an important component of the stem cell niche. Low oxygen environment (hypoxia) is physiologic for most mammalian embryos and in fact, human embryonic stem cells (hESC) derived from early stage blastocysts are also exposed to the low oxygen environment in vivo. Hypoxia has been shown to maintain hESC pluripotency and minimize spontaneous differentiation [4, 5]. Although, MSCs are typically cultured at 21% oxygen under in vitro conditions, physiological niches of MSCs have much lower oxygen tension than ambient [6]. There is a probable link between hypoxia and maintenance of stemness and hence, oxygen tension in the microenvironment of MSCs might play a crucial regulatory role in the maintenance of stem cell properties.
It has been reported that hypoxia requires notch signaling to maintain undifferentiated cell state. Notch signaling controls cell fate choices [7]. Notch signaling normally inhibits differentiation and maintains cells in progenitor state. The effects of hypoxia are directly regulated by a transcription factor, hypoxia-inducible factor (HIF) [8, 9]. Notch intracellular domain interacts with HIF-1α which is recruited to Notch-responsive promoters upon Notch activation under hypoxic conditions, suggesting cross-talk between HIF and Notch signaling pathways [10].
Presence of hypoxia is also associated with certain pathological conditions such as the formation and growth of tumors [11], wounding [12], arthritic joints [13] and ischemic heart disease [14]. It has been shown that MSCs and progenitor cells, on transplantation, home specifically to hypoxic events in vivo and function as therapeutic agents. MSCs from bone marrow have been reported to induce neovascularization in myocardial infarction and critical limb ischemia [15, 16]. In the ischemic tissues, MSCs experience severe low oxygen conditions. In order for the MSCs to adapt to low oxygen, they must be able to sense and respond accordingly to the change in oxygen level. For therapeutic applications, it is important to generate MSCs which can adapt to the in vivo environment while retaining immunosuppressive properties, stem cell characteristics and multilineage differentiation potential.
Not much has been reported regarding the effect of hypoxia on WJ-MSC characteristics, though hypoxia does prevail in the mammalian reproductive tract and intrauterine oxygen tension is known to be 2% [6]. Hypoxia can influence proliferation and differentiation of various stem/precursor cell populations [17, 18]. There are contradictory reports regarding the effect of hypoxia on self-renewal and differentiation potential of MSCs from other sources [19, 20]. Therefore, in this study we investigated the effect of relatively long-term low oxygen tension on proliferative capacity, multi-lineage differentiation potential and immunophenotypic characteristics of WJ-MSCs. We also investigated the expression levels of some of the notch pathway and its downstream target genes in WJ-MSCs in response to hypoxia. Hypoxia and HIFs are known to play a role in tumor progression and therefore we analyzed the expression of several DNA repair and transformation markers in hypoxic WJ-MSCs. Oxygen works as a signaling molecule and hypoxia can induce differentiation towards certain cell types [21, 22]. Here we analyzed the transcriptional changes with respect to stem cell specific markers and lineage differentiation genes on exposing WJ-MSCs to hypoxia as compared to normoxia. The purpose is to understand if hypoxic environment can influence WJ-MSCs towards adoption of a particular fate or a particular lineage. This information would be especially useful from a clinical point of view, if WJ-MSCs are used as a therapeutic tool to repair tissue injuries where they would encounter severe low oxygen tension.
MATERIALS & METHODS
Isolation and culture of WJ-MSCs
Human umbilical cords (n=10) from both sexes were collected from full-term births after either cesarean section or normal vaginal delivery with informed consent using the guidelines approved by the Institutional Committee for Stem Cell Research and Therapy (ICSCRT) and Institutional Ethics Committee (IEC) at the Manipal Hospital, Bangalore, India. MSCs, from WJ of umbilical cord, were isolated as previously described [23]. After the enzymatic treatment, cells were suspended in 10% FBS (Hyclone, Victoria, Australia) and Knock out Dulbecco's modified Eagle's medium (DMEM-KO) (Invitrogen, CA, USA) and plated on tissue culture plastic plates (Falcon, Becton, Dickinson, and Company, NJ, USA). WJ-MSCs were cultured in DMEM-KO with 4,500 mg/ml glucose and 2mM L-glutamine (Invitrogen), supplemented with 10% FBS (Hyclone, cat.no.SH30084.03, Lot no.GQM0049). All cultures were plated at a density of 5000 cells/cm2 and passaged when they reached 70-80% confluence. For normoxic studies, WJ-MSCs were cultured at 95% air (21% O2) - 5% CO2. Hypoxia (2-3% oxygen) was achieved using a tri-gas incubator (HERA cell 240, Thermo Scientific, MA, USA) that was flushed with humidified gas mixtures of composition 2% O2- 5% CO2- 93% N2..
Two random fields each from three to five biological replicates of hypoxic and normoxic WJ-MSCs were used to measure cell area via Image-Pro AMS version 6.0 software (Media Cybernetics, Inc, Silver Spring, MD, USA) using bright field images.
Growth Kinetics
WJ-MSCs were plated at passage 1 into two sets: one set was cultured under normoxic conditions and the other under hypoxic conditions. Cells in respective culture conditions were maintained till passage 9-11 and cell numbers were determined at the end of each passage. The total number of cells at each passage was calculated as a ratio of total number of cells harvested to total number of cells seeded multiplied by the total number of cells from the previous passage. Population doublings were calculated using the formula: X = [log10(NH)- log10(NI)]/log10(2) where NI is the inoculum cell number and NH the cell harvest number. To yield the cumulated doubling level, the population doubling for each passage was calculated and then added to the population doubling levels of the previous passages.
The population doubling time was obtained by the formula: TD=tplg2/ (lgNH-lgNI). NI:the inoculum cell number; NH is the cell harvest number and t is the time of the culture (in hours). The mean and standard deviation were calculated for three independent experiments. Statistical analysis was carried out using a t test. P values <0.05 were considered significant.
Senescence assay
Senescence assay was performed with WJ-MSC cultures using Senescence β-Galactosidase Staining kit (Cell Signaling Technologies, Danvers, MA, USA,) according to the manufacturer's protocol. Cells were observed for development of blue color under a microscope using 10X objective (Nikon, Tokyo, Japan).
Immunophenotyping
WJ-MSCs cultured in normoxia and hypoxia were taken for flow cytometric analysis at passage 9-11. The following antibodies were used to mark the cell surface epitopes- CD90-PE, CD44-PE, CD73-PE, CD166-PE, CD34-PE and CD45-FITC (all from BD Pharmingen, CA, USA), CD105-PE (R&D Systems Inc, MN, USA,), and HLA-DR-FITC (BD Biosciences). All analyses were standardized against negative control cells incubated with isotype-specific IgG1-PE and IgG1-FITC (BD Pharmingen). The number of cells staining positive for a marker was determined by the percentage of cells present within a gate established such that 3% of the positive events measured represented nonspecific binding by the PE or FITC- conjugated isotype-matched control. At least 10,000 events were acquired on BD LSR II flow cytometer and the results were analyzed using WIN MDI v2.8 software.
Differentiation
Osteogenic and adipogenic differentiation potential of WJ-MSCs, when cultured under hypoxia and normoxia, was examined using standard protocols [23]. Chondrogenic differentiation was induced in confluent monolayer cultures of WJ-MSCs, cultured under normoxia and hypoxia, using STEMPRO Chondrogenesis Differentiation Kit (Invitrogen). Differentiated cells were stained with Alcian blue 8GX between 14-17 days (Sigma, St Louis, MO, USA). Images were captured using Nikon microscope (Nikon, Tokyo, Japan).
Reverse Transcription-Polymerase Chain Reaction Analysis (RT-PCR)
WJ-MSCs were exposed to normoxia and hypoxia for the specified time intervals, RNA was extracted and RT-PCR performed as described [23] using primer sequences listed in table 1.
Karyotype Analysis
Karyotyping of WJ-MSCs cultured under normoxia and hypoxia was performed at passage 10-11 using standard Giemsa staining procedure. Images were acquired using Nikon-Eclipse-90i microscope (Nikon).
Taqman low density arrays
The Human Stem Cell Pluripotency Array (Applied Biosystems), containing a well-defined set of validated gene expression markers to characterize undifferentiated stem cells and early differentiation lineage markers, was used. The 384 wells of each Human stem cell pluripotency array card were preloaded with fluorogenic probes and primers (Applied Biosystems). cDNAs were loaded on the microfluidic cards for thermal cycling on an ABI PRISM 7900HT Sequence Detection System (Applied Biosystems). Expression values for target genes were normalized to the expression of 18s rRNA. Transcriptional analysis was performed with WJ-MSCs at passage 11. For data analysis, the ABI PRISM 7900HT Sequence Detection System software (SDS 2.1 software) calculated the levels of target (WJ-MSC) gene expression in hypoxic samples relative to the level of expression in normoxia. A cut off cycle threshold value of 35.0 was arbitrarily assigned. Samples with a cycle threshold of 35 or less were considered for calculating the fold change in expression.
RT-PCR validation of array data
To confirm the gene expression profile obtained by TLDA, a number of select genes were subjected to quantitative and semi-quantitative RT-PCR analysis. Quantitative amplifications were carried out in duplicate using SYBR green master mix (Applied Biosystems). PCR reactions were run on an ABI Prism 7500HT (Applied Biosystems) and SDS v2.1 software was used to analyze the results. All measurements were normalized by 18s rRNA. Semi-quantitative RT-PCR products were resolved on 1.5-2% agarose gel and the band densities were quantitated using the GeneTools software (Syngene, Cam-bridge, UK). The primer sequences used in RT-PCR analysis and amplicon size are listed in Table 1.
Primer sequence used for semi-quantitative RT-PCR analysis.
| GENE | FORWARD PRIMER | REVERSE PRIMER | PRODUCT SIZE (bp) |
|---|---|---|---|
| 18s | CGGCTACCACATCCAAGGAA | GCTGGAATTACCGCGGCT | 186 |
| CD34 | AATGAGGCCACAACAAACATCACA | CTGTCCTTCTTAACCTCCGCACAGC | 380 |
| c-MYC | AAGACTCCAGCGCCTTCTCTC | GTTTTCCAACTCCGGGATCTG | 526 |
| DESMIN | CCAACAAGAACAACGACG | TGGTATGGACCTCAGAACC | 407 |
| ERCC3 | CCAGGAAGCGGCACTATGAGG | GGTCGTCCTTCAGCGGCATTT | 171 |
| FLT 1 | CGTAGAGATGTACAGTGAAA | GGTGTGCTTATTTGGACATC | 305 |
| GATA 6 | GCCTCACTCCACTCGTGTCT | TCAGATCAGCCACACAATATGA | 546 |
| HES 1 | CCAGTTTGCTTTCCTCATTCC | TCTTCTCTCCCAGTATTCAAGTTCC | 253 |
| HIF-1α | CCTGCACTCAATCAAGAAGTTGC | TTCCTGCTCTGTTTGGTGAGGCT | 618 |
| HIF-2α | AGGGGACGGTCATCTACAACC | ATGGCCTTGCCATAGGCTGAG | 327 |
| IFITM 1 | CCCCAAAGCCAGAAGATGCACAAGGAG | CGTCGCCAACCATCTTCCTGTCCCTAG | 229 |
| JAGGED 1 | AGTCACTGGCACGGTTGTAG | TCGCTGTATCTGTCCACCTG | 226 |
| NOTCH 1 | GACATCACGGATCATATGGA | CTCGCATTGACCATTCAAAC | 665 |
| NOTCH 2 | CCAGAATGGAGGTTCCTGTA | GTACCCAGGCCATCAACACA | 377 |
| p16 | TTATTTGAGCTTTGGTTCTG | CCGGCT TTCGTAGTTTTCAT | 354 |
| p21 | GAGGCCGGGATGAGTTGGGAGGAG | CAGCCGGCGTTTGGAGTGGTAGAA | 220 |
| p53 | TTGGATCCATGTTTTGCCAACTGGCC | TTGAATTCAGGCTCCCCTTTCTTGCG | 488 |
| RAD51 | TTTGGAGAATTCCGAACTGG | AGGAAGACAGGGAGAGTCG | 588 |
| SOX 17 | CGCACGGAATTTGAACAGTA | GGATCAGGGACCTGTCACAC | 181 |
| XRCC4 | AAGATGTCTCATTCAGACTTG | CCGCTTATAAAGATCAGTCTC | 233 |
Statistical data analysis
Data were presented as means ± standard error mean. Statistical comparisons were performed using either the student's two-tailed t-test (unpaired/paired) or the Wilcoxon matched pairs test as appropriate according to data distribution. P values <0.05 were considered significant. Data analysis and graphical representations were performed by using GraphPad Prism 5 software (GraphPad, San Diego, CA, USA).
RESULTS
Proliferative response of WJ-MSCs under hypoxic conditions
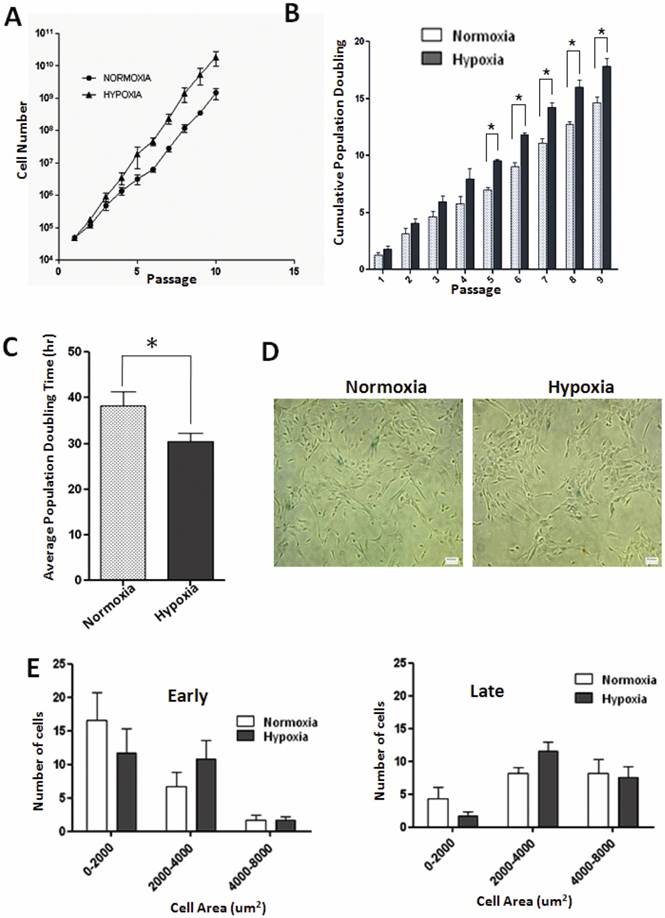
Effect of low oxygen tension on proliferative capacity of WJ-MSCs has not yet been demonstrated. We found that WJ-MSCs cultured under hypoxic conditions grew at faster rates and yielded higher total cell numbers as compared to WJ-MSCs cultured at 21% O2. WJ-MSCs cultured under hypoxia resulted in maximal cell numbers 1.87 x 1010±8.9 x 109 while normoxic cultures resulted in 1.46 x 109±5.49 x 108cells (p=0.17) at the end of ten passages. Again, under hypoxia, WJ-MSCs displayed higher cumulative population doublings as compared to those cultured under normoxia with a peak of 17.8±1.2 and 14.6±0.8 respectively, at passage 10, p value < 0.05 (Fig. 1B). The mean population doubling time of WJ-MSCs cultured under hypoxia was 28.7±1.9 h while that for normoxic cultures was 36.0±3.3 h (p=0.02), as shown in Fig. 1C.
To assess the effect of hypoxia on the morphology of WJ-MSCs, which typically demonstrated a fibroblast-like appearance (Fig. S1 M and N), cell size was measured at early (P2-P5) and late (P10) passages and a size histogram was plotted for the hypoxic and normoxic cell populations. Cells were categorized according to their size and the proportion of large flattened cells, area between 2000-4000 um2, was greater under hypoxic conditions both at early and late passages (Fig. 1E) as compared to normoxic cells though the difference was not significant.
Growth kinetics and senescence in WJ-MSCs cultured under normoxia and hypoxia. (A) Growth curves of WJ-MSCs cultured under normoxia and hypoxia are demonstrated (B) Comparison of cumulative population doubling (PD) at each passage between different culture conditions: Normoxia and Hypoxia. Significant differences were observed between normoxia and hypoxia at later passages (p < 0.05). (C) Analysis of mean PD time in hour±SEM of WJ MSCs cultured under hypoxia and normoxia. (D) Senescence associated β- galactosidase staining of WJ-MSCs at passage 10 under normoxia and hypoxia. (E) Histogram of WJ-MSC area when cultured under hypoxia and normoxia at early (P2-P5) and late passages (P10). Area of individual cells was quantified via automated image analysis from bright-field images. Results represent the average of at least three culture replicates (n=3) with SEM. Scale bar=50µm. Abbreviation: WJ-MSCs, Wharton's jelly-derived mesenchymal stem cells; SEM- standard error mean.
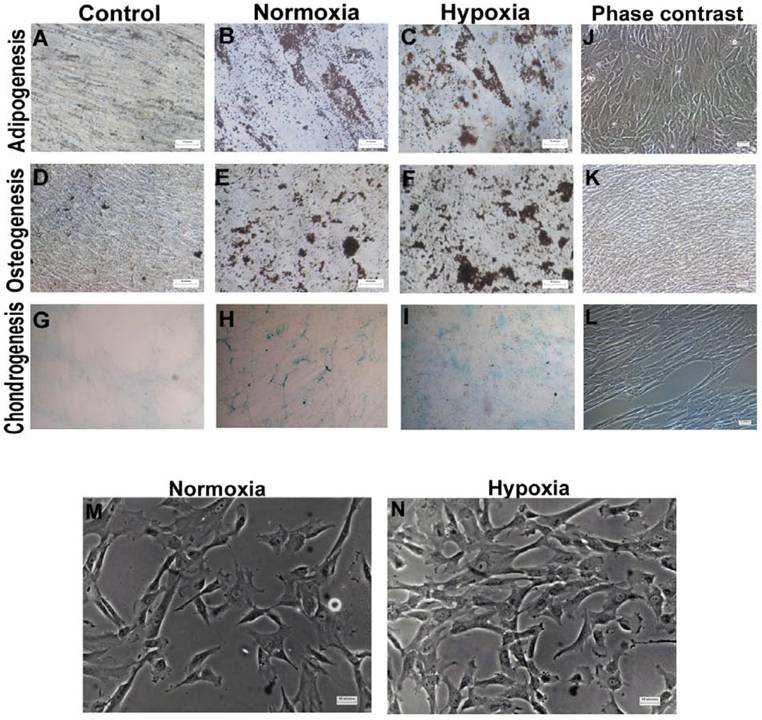
Morphology and multilineage differentiation potential of WJ-MSCs cultured under hypoxic and normoxic condition till late passages. WJ-MSCs (P10-P13) were investigated for in vitro multilineage differentiation capacity when cultured under hypoxia and normoxia. Adipogenesis was confirmed by neutral oil droplet formation stained with Oil Red O at day 18 (B & C). Formation of mineralized matrix was detected by von Kossa staining at day 21 (E & F). Chondrogenic differentiation (n=2) was demonstrated by Alcian blue staining (H & I), 4x objective. Phase contrast images of adipogenesis (J), osteogenesis (K) and chondrogenesis (L). Non-induced control cultures in growth medium without adipogenic (A), osteogenic (D) or chondrogenic differentiation (G) stimuli are shown. Morphology of WJ-MSCs, cultured under normoxia and hypoxia, as observed at passage 9 (M & N). Representative results of three independent experiments are shown. Abbreviation: WJ-MSCs- Wharton's Jelly derived mesenchymal stem cells. (Scale bar for A, B, C =25µm; D-F and J-N=50µm).
Since MSCs possess a limited lifespan during in vitro culture, they finally undergo replicative senescence [24]. Senescence is characterized by cell cycle arrest, telomere shortening and altered morphology. We have employed the enzyme lysosomal pH6 β-galactosidase as a senescence marker but did not detect much of senescence associated β-galactosidase (SA- β-gal) staining in normoxic or hypoxic cultures of WJ-MSCs till passage 10 (Fig. 1D).
Differentiation capacity under hypoxic conditions
To explore the influence of extended period of hypoxia on multi-lineage differentiation potential of WJ-MSCs, adipogenic, osteogenic and chondrogenic differentiation was induced at 21% and 2% oxygen. Oil Red O staining showed that both hypoxic and normoxic WJ-MSCs were positive for staining of neutral lipid vacuoles (Fig. S1 B and C). Similarly, VonKossa assays showed mineralized deposits in osteo-induced cultures of WJ-MSCs under both hypoxia and normoxia (Fig. S1 E and F). In an attempt to quantitate osteogenic differentiation, the percentage of mineral deposit area was calculated and were 10.8%±7.8% and 11.2%±5.7% for hypoxic and normoxic WJ-MSC respectively. No significant difference was found. Hypoxic WJ-MSCs retained the ability to differentiate to chondrocytes as shown by Alcian blue staining with no detectable difference between the two populations (Fig. S1 H and I). Hence, even under hypoxia, WJ-MSCs retained their potential to differentiate in vitro towards osteogenic, chondrogenic and adipogenic lineages.
Surface phenotype characterization
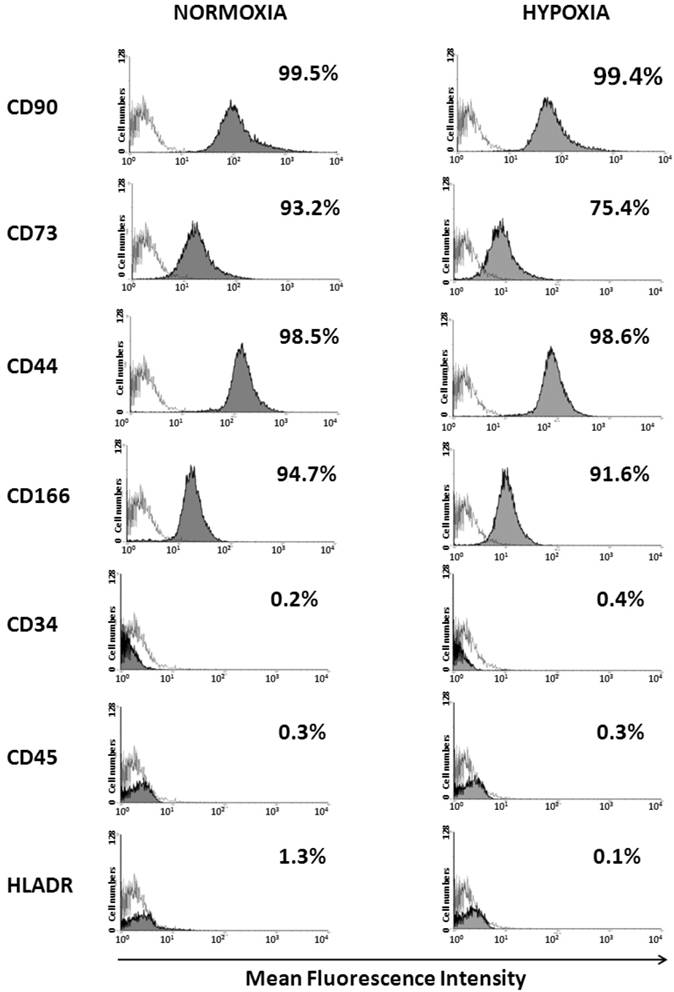
Flow cytometry analysis showed that when cultured under normoxia and hypoxia for ten passages, WJ-MSCs were positive for the MSC markers CD44, CD73, CD90 CD105 and CD166 and negative for CD45, CD34 and HLA-DR and no significant difference was seen in the surface marker expression level between the two populations (Fig. 2).
Upregulation of HIF-1α and Notch signaling pathway genes in WJ-MSCs cultured in hypoxic conditions
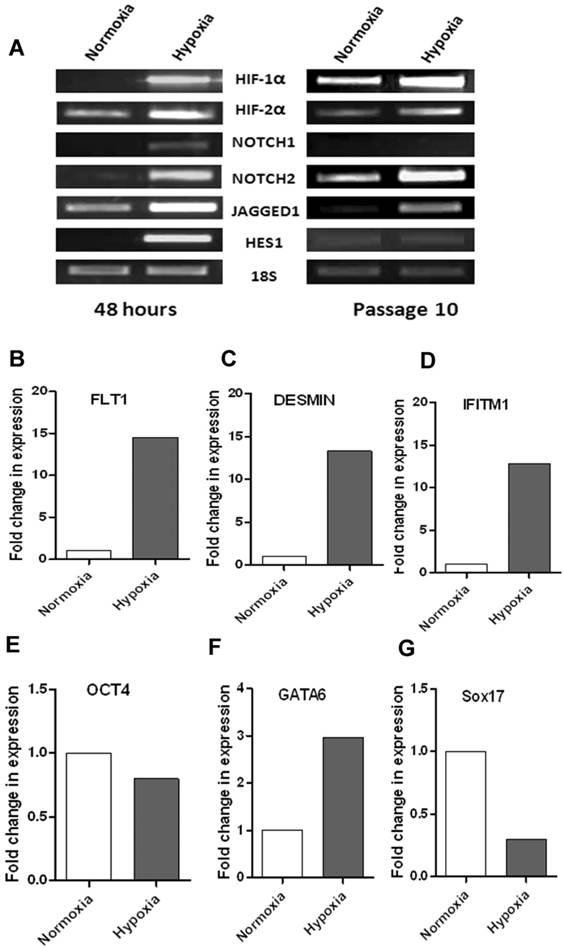
To test if there is any link between hypoxia and notch signaling in WJ-MSCs, changes in expression of HIF-1α and notch pathway genes at early period of hypoxia were investigated (Fig. 4A). Hypoxia led to increased HIF-1α and HIF-2α mRNA levels within 48 hours of exposure of WJ-MSCs to 2-3% oxygen. There was also an increase in the mRNA expression levels of some components of the Notch signaling pathway genes like the Notch receptors, NOTCH1 and NOTCH2 and Notch ligand JAGGED1. Under hypoxia, in four out of six samples, there was an upregulation of these genes as compared to normoxia, while in remaining samples the expression levels were comparable between hypoxia and normoxia. Hypoxia was found to alter the expression of Notch immediate downstream gene, HES1 as there was an increase in mRNA level after 48 hrs of hypoxic treatment as compared to normoxia. Our finding is consistent with previous reports suggesting a link between hypoxia and notch signaling pathway and hypoxia mediated activation of Notch signaling pathway [25]. To study the effect of long term hypoxia on the notch signaling pathway genes, we subjected WJ-MSCs to hypoxia for ten passages with normoxic WJ-MSCs as control (Fig. 4A). Even after culturing for ten passages, hypoxic WJ-MSCs maintained an increased expression of HIF1α, HIF2α, NOTCH2 and JAGGED1 as compared to normoxic WJ-MSCs. Though, the difference in expression level between the two populations was less as compared to that after 48 hr exposure. At passage 10, HES1 expression was faint in both hypoxic and normoxic WJ-MSCs with no difference being detected between the two populations while NOTCH1 was undetectable in both populations.
Karyotype analysis and transformation markers
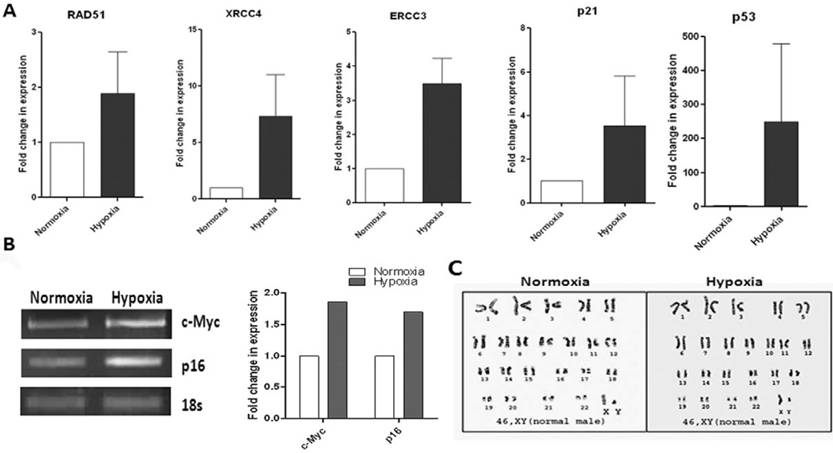
Since adult stem cells are evaluated for various therapeutic approaches, their biosafety criteria need to be addressed. It is reported that MSCs in long term cultures immortalize at high frequency and undergo spontaneous transformation [26]. Again, hypoxia has received considerable attention as an inducer of tumor metastasis. Therefore, we analyzed expression at the mRNA level of some transformation markers such as DNA-repair enzymes, RAD51, ERCC3, XRCC4, oncogene c-Myc and tumour suppressor genes p16, p21 and p53 in our WJ-MSCs, when cultured under hypoxia and normoxia for ten passages. An increase in the expression level was observed for most of these markers under hypoxic conditions when compared to normoxia (Fig. 3A & B). But the difference was not statistically significant. There was variation between the different hypoxic samples with respect to levels of expression of these genes. This suggests that care needs to be taken, from the point of bio safety, for every single MSC preparation cultured under hypoxia before they are planned for clinical applications.
The hypoxic WJ-MSCs maintained a normal karyotype though. To evaluate numerical and structural chromosomal abnormalities in WJ-MSCs cultured under hypoxic conditions, samples were GTG- banded. Both the populations of WJ-MSCx, cultured under hypoxia and normoxia, had normal 2n karyotype up till ten passages (Fig. 3C).
Detection of surface marker expression of WJ-MSCs cultured under hypoxia and normoxia by flow cytometry analysis. WJ-MSCs were cultured for ten passages under hypoxia and normoxia, labeled with the indicated antibodies and analyzed by flow cytometry. An open area represents an antibody isotype control for background fluorescence and a shaded area shows signal from MSC surface marker antibodies. Representative histograms are depicted. Abbreviations: WJ-MSC- Wharton's Jelly derived mesenchymal stem cells.
Karyotype and RT-PCR analysis of transformation markers and notch pathway genes and validation of array data for WJ-MSCs cultured under hypoxia and normoxia. (A) Reverse transcription polymerase chain reaction (RT-PCR) analysis of DNA repair, tumor suppressor and oncogenes of WJ-MSCs cultured under hypoxic and normoxic conditions for 10 passages. Expression of genes RAD51, ERCC3, XRCC4, p21 and p53 were analyzed by real-time RT-PCR using SYBR green reagent and values are normalised to expression of 18s ribosomal RNA. Bars represent the mean ± SE of the ratio of message expressed under hypoxia as compared to that under normoxia for three independent experiments performed in duplicate. (B) Expression p16 and c-Myc using semi-quantitative RT-PCR. Band densities were quantified and plotted. (C) Karyotype analysis of WJ-MSCs using standard Giemsa-banding procedure represents Normal 46, XY karyotype of WJ-MSCs cultured under hypoxic and normoxic conditions. A representative analysis of three independent experiments is shown (E) Abbreviations: WJ-MSCs - Wharton's Jelly derived mesenchymal stem cells; SE- standard error.
Functional classification of stem cell and early lineage genes upregulated in WJ-MSC under hypoxia
Hypoxia is understood to be a potent differentiation inducer of numerous cell types and a stimulus of gene expression. Hypoxia is known to support faster proliferation and maintenance of undifferentiated characteristics in MSCs. Hence, we investigated the gene expression profile of the hypoxia response in WJ-MSCs with respect to stemness markers and early lineage differentiation genes. We used a Human Stem Cell Pluripotency Low Density Array to investigate the difference in gene expression profile between WJ-MSCs cultured in hypoxia and normoxia. The array includes pluripotent/stem cell markers used to characterize undifferentiated stem cells and also some early differentiation lineage markers. Low density arrays allow multiple genes to be studied from a single sample. In order to evaluate the transcriptional changes between the WJ-MSCs cultured under hypoxia and normoxia, we focused on genes which have >1.5 fold change. The results demonstrated that there was an up-regulation in stem cell markers such as CRABP2, DNMT3B, GRB7, IFITM1, KIT, LIN28, IMP2 and IL6ST in WJ-MSCs cultured under hypoxia as compared to WJ-MSCs cultured in normoxia for ten passages. A few other genes, up-regulated under hypoxia as compared to normoxia, such as NOGGIN, RUNX2, DES, COL1A and ACTC belonged to mesoderm lineage. CD34 and FLT1, both endothelial markers, were also up-regulated under hypoxic culture conditions. A few endodermal genes such as FN1, GATA6 and LAMC1 showed increased expression under hypoxia as compared to normoxia while there was a strong down regulation of a definitive endoderm gene SOX17 under hypoxia (Table 2).
Quantitative and semi-quantitative RT-PCR, with pooled cDNA samples used in the array, was carried out to verify the fidelity of the array data where select genes expressed in accordance with their differential expression pattern in the array (Fig. 4B-G). There was good agreement between expression by RT-PCR and the array data. Some variation in the Ct values or fold change in expression could be due to Taqman chemistry and ABI 7900 HT instrument being used for the PCR array while the validation experiments were carried out using SYBR green reagent and ABI 7500HT instrument.
(A) RT-PCR based comparison of HIFs and Notch pathway genes expressed by WJ-MSCs when exposed to hypoxia, with normoxia as control, for 48 hrs and for ten passages. Results are representative of at least three independent experiments. RT-PCR analysis of select differentially expressed genes to validate PCR array data. cDNA was pooled from three different samples each of hypoxic and normoxic WJ-MSCs at passage 11. (B-E) Expression of FLT1, DESMIN, IFITM1 and OCT4 was analyzed by real-time PCR using SYBR green reagent and values were normalized to 18s rRNA. (F & G) Semi-quantitative RT-PCR analysis of GATA6 and SOX17 with intensity of each band being measured by densitometry and plotted. 18S ribosomal RNA was used as an internal control for all semi-quantitative RT-PCRs. Abbreviations: WJ-MSCs - Wharton's Jelly derived mesenchymal stem cells; SE- standard error.
Comparison of transcription profile of stem cell and early lineage differentiation genes of Wharton's Jelly-derived mesenchymal stem cells cultured under hypoxic and normoxic conditions. cDNA from four different samples (n=4) at passage 11 were pooled before being used in the array. Genes with > 1.5 fold change in expression are shown.
| No. | Gene Symbol | Gene Name | Description | Fold change (Normoxia=1) |
|---|---|---|---|---|
| 1. | ACTC | actin, alpha, cardiac muscle 1 | Cardiac/mesoderm | 5.6 |
| 2. | CD34 | CD34 molecule | Endothelial | 8.0 |
| 3. | CGB | chorionic gonadotropin, beta polypeptide | Trophoblast | 2.8 |
| 4. | COL1A | collagen, type I, alpha 1 | Bone/mesoderm | 2.15 |
| 5. | CRABP2 | cellular retinoic acid binding protein 2 | Stem cell | 2.18 |
| 6. | DES | Desmin | Muscle/mesoderm | 9.7 |
| 7. | DNMT3B | DNA (cytosine-5-)-methyltransferase 3 beta | Stem cell | 2.04 |
| 8. | FLT1 | fms-related tyrosine kinase 1 (vascular endothelial growth factor/vascular permeability factor receptor) | Endothelial/endoderm | 2.12 |
| 9. | FN1 | fibronectin 1 | Endoderm | 1.6 |
| 10. | GATA4 | GATA binding protein 4 | Endoderm | 0.57 |
| 10. | GATA6 | GATA binding protein 6 | Endoderm | 2.15 |
| 11. | GRB7 | growth factor receptor-bound protein 7 | Stem cell | 2.8 |
| 12. | IFITM1 | interferon induced transmembrane protein 1 (9-27) | Stem cell | 9.3 |
| 13. | IL6ST | interleukin 6 signal transducer (gp130, oncostatin M receptor) | Stem cell | 1.7 |
| 14. | IMP2 | insulin-like growth factor 2 mRNA binding protein 2 | Stem cell | 1.7 |
| 15. | KIT | v-kit Hardy-Zuckerman 4 feline sarcoma viral oncogene homolog | Stem cell | 2.05 |
| 16. | LAMC1 | laminin, gamma 1 (formerly LAMB2) | Endoderm | 1.6 |
| 17. | LIN28 | lin-28 homolog (C. elegans) | Stem cell | 2.0 |
| 18. | NOG | Noggin | Mesoderm | 2.8 |
| 19. | NR6A1 | nuclear receptor subfamily 6, group A, member 1 | Stem cell | 1.6 |
| 20. | POU5F1 | POU domain, class 5, transcription factor 1 | Stem cell | 0.34 |
| 20. | RUNX2 | runt-related transcription factor 2 | Bone/mesoderm | 1.67 |
| 21. | SERPINA1 | Serpin peptidase inhibitor, clade A (alpha-1 antiproteinase, antitrypsin), member 1 | Endoderm | 1.54 |
| 22. | SOX17 | SRY (sex determining region Y)-box 17 | Endoderm | 0.07 |
DISCUSSION
Potential for self-renewal and multilineage differentiation makes MSCs an attractive therapeutic tool. WJ-MSCs from the umbilical cord are of epiblast origin [27] and possess multipotent properties between embryonic stem cells and adult stem cells. They have higher proliferation rates and lower tendency to differentiate to adipocytes as compared to bone marrow MSCs [3, 28]. Moreover, WJ-MSCs can replicate through many passages without karyotypic changes or senescence and can retain stemness properties for a long time in vitro [29]. They are similar to bone marrow stromal and other MSCs in their surface marker expression since WJ-MSCs too are positive for CD 10, CD 13, CD 29, CD 44, CD 73, CD 90, CD 105 and negative for CD 34, CD 45, CD 14, CD 33 and HLA-DR [3]. Stem cell niche plays a prominent role in stem cell fate determination and oxygen concentration is an important component of the stem cell niche. Low oxygen tension or hypoxia plays an important role in maintaining the plasticity and proliferation of stem cells. We demonstrate here that under hypoxia WJ-MSCs show higher proliferation, evident from the cumulative population doublings, and shorter mean population doubling time as compared to normoxic WJ-MSCs. This finding is consistent with previous reports which demonstrated enhanced proliferation of bone marrow-derived MSCs (BM-MSCs) under hypoxic conditions [30, 31]. Another study reported differential gene expression between freshly isolated MNCs and their cultured MSC subpopulation, in response to hypoxia, with upregulation of several genes involved in cell proliferation and survival in MSCs [32]. Based on senescence associated β-gal staining, very few senescent cells could be found even after culturing WJ-MSCs for ten passages under hypoxia, though morphology changes were noted. WJ-MSC cultures under hypoxia exhibited a higher proportion of large, flattened cells both at early and late passages as compared to normoxic cultures. The enlargement in cell size under hypoxia could be due to a natural response to low oxygen, where an increased surface area would allow for an increase in oxygen diffusion rate. Flow cytometry analysis of MSC-specific surface marker expression showed that when cultured under both experimental conditions of hypoxia and normoxia for ten passages, WJ-MSCs were positive for CD44, CD73, CD90, CD105 and CD166 and negative for CD34, CD45 and HLA-DR and no significant difference was detected between the two populations. Also, under both experimental conditions of hypoxia and normoxia, WJ-MSCs maintained their multilineage differentiation potential in vitro.
Transcriptional responses to hypoxia are mediated by HIFs [33], and there was an increase in the mRNA expression levels of HIF-1α and HIF-2α in hypoxic WJ-MSCs. The expression levels of Notch receptors NOTCH1 and NOTCH2, Notch ligand JAGGED1 and Notch downstream gene HES1 also were elevated in WJ-MSCs cultured under hypoxia as compared to normoxic WJ-MSCs. Our finding is consistent with previous reports suggesting a link between hypoxia and notch signaling pathway and hypoxia mediated activation of Notch signaling pathway [10, 25]. Notch is known to influence proliferation and hence this could also explain the increased proliferation that we observed in the hypoxic WJ-MSCs.
The presence of hypoxic condition in human tumors is well known. HIF levels are found to be elevated in a variety of cancers [34, 35]. Again, MSCs after prolonged in vitro culture or under stressful conditions can undergo spontaneous transformation [26]. After culturing the WJ-MSCs for ten passages under conditions of hypoxia and normoxia, we analyzed them by RT-PCR for certain transformation markers such as DNA repair, tumor suppressors and oncogenes. In a few of the hypoxic WJ-MSC samples, we found an upregulation for some of these markers though we did not detect statistically significant changes for any of these genes between normoxia and hypoxia. The hypoxic WJ-MSCs maintained a normal karyotype though. This suggests that care needs to be taken, from the point of bio safety, for every single MSC preparation cultured under hypoxia before they are planned for clinical applications.
MSCs have been reported to home specifically to hypoxic events in vivo following transplantations. As hypoxia mimicks the ischemic environment in vitro, from a clinical point of view, we wanted to investigate the changes in the expression profile of stem cell markers and early development lineage genes in WJ-MSCs when exposed to low oxygen environment. Using a low density PCR array for stem cell and early lineage markers, we found a higher expression of several stem cell markers such as CRABP2, DNMT3B, GRB7, IFITM1, KIT, LIN28, IMP2 and IL6ST under hypoxia as compared to normoxia. This is in agreement with reports from other groups suggesting that oxygen concentration plays a role in maintaining the plasticity of stem cells [6]. Notch maintains cells in a progenitor state and thus, there could be a possible link between the activation of notch pathway and the upregulation of these stem cell markers that we observe in WJ-MSCs when exposed to hypoxia.
Mesodermal/endothelial genes such as NOGGIN, RUNX2, DESMIN, COL1A, FLT1, CD34 and ACTC were also up-regulated in WJ-MSCs in response to hypoxia. In support for our finding, there are a number of reports in literature confirming hypoxia-driven neo-vascularisation and angiogenesis of MSCs [36, 37]. Recently, an up regulation in FLT1 expression in umbilical cord blood derived-MSCs under hypoxia has been demonstrated [38]. Another set of up-regulated genes in hypoxic WJ-MSCs were the endodermal markers FN1, GATA6 and LAMC1. GATA6, again, has been shown to play a role in vascular smooth muscle differentiation [39] while reports suggest that hypoxic stress up regulates fibronectin mRNA in early placenta [40].
Thus, to conclude, different cells of the human body are exposed to and function in different microenvironments. All mammalian cells do not respond to hypoxia in an identical manner and also they differ in their sensitivity towards hypoxia. We found that in response to chronic hypoxia for up to ten passages, WJ-MSCs from umbilical cord exhibited increased proliferative potential while maintaining immunophenotypic characteristics and in vitro differentiation capacity. Transcriptional profiling identified several mesodermal/endothelial genes upregulated under hypoxia, as opposed to normoxia, in WJ-MSCs suggesting that hypoxic culture condition leads to different developmental outcomes or differently committed cells. To our knowledge, this is the first report of the effect of hypoxia on growth characteristics and gene expression of WJ-derived MSCs.
Earlier we reported higher expression levels of many early endodermal markers, including SOX17, in WJ-MSCs as compared to bone marrow-derived MSCs [23]. Here we noticed a sharp downregulation of SOX17 along with upregulation of mesoderm lineage markers in WJ-MSCs under hypoxia. This indicates that modulation of culture conditions can be used to generate tailor-made MSCs, suitable for different therapeutic applications. Thus, WJ-MSCs when exposed to hypoxia in vitro or in vivo, could find a therapeutic role in ischemic tissues or muscle degenerative disorders. Additional in vitro differentiation experiments or in vivo studies are needed to support the above.
Acknowledgements
This work was fully funded by internal funding of Stempeutics Research Pvt Ltd, Bangalore, India. We are grateful to Dr. Praveena Shenoi, Consultant Obstetrician & Gynaecologist, Manipal Hospital, for generously providing the umbilical cord samples. We thank Ms Majahar Jan for technical assistance with karyotyping and Mr. Vinay B. Rao, Mr. Kartikeya V. and Ms Lipsa Mohanty for assistance with umbilical cord processing and osteo quantification. Special thanks to Dr. Ramesh Bhonde, Dr. Anoop C H. and Dr. Swathi Sundar Raj for reading of the manuscript and providing valuable suggestions and to Dr. Sudha Balasubramanian for manuscript revision.
Conflict of Interests
The authors declare no competing financial interests.
References
1. Pittenger MF, Mackay AM, Beck SM. et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-147
2. Le Blanc K. Immunomodulatory effects of fetal and adult mesenchymal stem cells. Cytotherapy. 2003;5:485-489
3. Troyer DL, Weiss ML. Wharton's jelly-derived cells are a primitive stromal cell population. Stem cells. 2008;26:591-599
4. Ezashi T, Das P, Roberts RM. Low O2 tensions and the prevention of differentiation of hES cells. Proc Natl Acad Sci U S A. 2005;102:4783-4788
5. Gassmann M, Fandrey J, Bichet S. et al. Oxygen supply and oxygen-dependent gene expression in differentiating embryonic stem cells. Proc Natl Acad Sci U S A. 1996;93:2867-2872
6. Ma T, Grayson WL, Fröhlich M. et al. Hypoxia and stem cell-based engineering of mesenchymal tissues. Biotechnol Prog. 2009;25(1):32-42
7. Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770-776
8. Wang GL, Semenza GL. Purification and characterization of hypoxia-inducible factor 1. J Biol Chem. 1995;270:1230-1237
9. Kallio PJ, Pongratz I, Gradin K. et al. Activation of hypoxia-inducible factor 1alpha: posttranscriptional regulation and conformational change by recruitment of the Arnt transcription factor. Proc Natl Acad Sci U S A. 1997;94:5667-5672
10. Gustafsson MV, Zheng X, Pereira T. et al. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev Cell. 2005;9:617-628
11. Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38-47
12. Kivisaari J. Oxygen and carbon dioxide tensions in healing tissue. Acta Chir Scand. 1975;141:693-696
13. Stevens CR, Williams RB, Farrell AJ. et al. Hypoxia and inflammatory synovitis: observations and speculation. Ann Rheum Dis. 1991;50:124-132
14. Jürgensen JS, Rosenberger C, Wiesener MS. et al. Persistent induction of HIF-1alpha and -2alpha in cardiomyocytes and stromal cells of ischemic myocardium. Faseb J. 2004;18:1415-1417
15. Tse HF, Kwong YL, Chan JK. et al. Angiogenesis in ischaemic myocardium by intramyocardial autologous bone marrow mononuclear cell implantation. Lancet. 2003;361:47-49
16. Tateishi-Yuyama E, Matsubara E, Murohara T. et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet. 2002;360:427-435
17. Studer L, Csete M, Lee SH. et al. Enhanced proliferation, survival, and dopaminergic differentiation of CNS precursors in lowered oxygen. J Neurosci. 2000;20:7377-7383
18. Morrison SJ, Csete M, Groves AK. et al. Culture in reduced levels of oxygen promotes clonogenic sympathoadrenal differentiation by isolated neural crest stem cells. J Neurosci. 2000;20:7370-7376
19. Malladi P, Xu Y, Chiou M. et al. Effect of reduced oxygen tension on chondrogenesis and osteogenesis in adipose-derived mesenchymal cells. Am J Physiol Cell Physiol. 2006;290:C1139-1146
20. Lennon DP, Edmison JM, Caplan AI. Cultivation of rat marrow-derived mesenchymal stem cells in reduced oxygen tension: effects on in vitro and in vivo osteochondrogenesis. J Cell Physiol. 2001;187:345-355
21. Dachs GU, Patterson AV, Firth JD. et al. Targeting gene expression to hypoxic tumor cells. Nat Med. 1997;3:515-520
22. Fischer S, Wiesnet M, Marti HH. et al. Simultaneous activation of several second messengers in hypoxia-induced hyperpermeability of brain derived endothelial cells. J Cell Physiol. 2004;198:359-369
23. Nekanti U, Rao VB, Bahirvani AG. et al. Long-term Expansion and Pluripotent Marker Array Analysis of Wharton's Jelly-Derived Mesenchymal Stem Cells. Stem Cells Dev. 2010;19:117-130
24. Wagner W, Horn P, Castoldi M. et al. Replicative senescence of mesenchymal stem, cells: a continuous and organized process. PLoS One. 2008;3:2213
25. Prasad SM, Czepiel M, Cetinkaya C. et al. Continuous hypoxic culturing maintains activation of Notch and allows long-term propagation of human embryonic stem cells without spontaneous differentiation. Cell Prolif. 2009;42:63-74
26. Rubio D, Garcia-Castro J, Martín MC. et al. Spontaneous human adult stem cell transformation. Cancer Res. 2005;65:3035-3039
27. Bongso A, Fong CY, Gauthaman K. Taking stem cells to the clinic: Major challenges. J Cell Biochem. 2008;105:1352-1360
28. Karahuseyinoglu S, Cinar O, Kilic E. et al. Biology of stem cells in human umbilical cord stroma: in situ and in vitro surveys. Stem Cells. 2007;25:319-331
29. Lund RD, Wang S, Lu B. et al. Cells isolated from umbilical cord tissue rescue photoreceptors and visual functions in a rodent model of retinal disease. Stem Cells. 2007;25:602-611
30. Ren H, Cao Y, Zhao Q. et al. Proliferation and differentiation of bone marrow stromal cells under hypoxic conditions. Biochem Biophys Res Commun. 2006;347:12-21
31. Grayson WL, Zhao F, Bunnell B. et al. Hypoxia enhances proliferation and tissue formation of human mesenchymal stem cells. Biochem Biophys Res Commun. 2007;358:948-953
32. Ohnishi S, Yasuda T, Kitamura S. et al. Effect of hypoxia on gene expression of bone marrow-derived mesenchymal stem cells and mononuclear cells. Stem Cells. 2007;25:1166-1177
33. Ratcliffe PJ. HIF-1 and HIF-2: Working alone or together in hypoxia? J Clin Invest. 2007;117:862-865
34. Rankin EB, Giaccia AJ. The role of hypoxia-inducible factors in tumorigenesis. Cell Death Differ. 2008;15:678-685
35. Zhong H, De Marzo AM, Laughner E. et al. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999;59:5830-5835
36. Potier E, Ferreira E, Andriamanalijaona R. et al. Hypoxia affects mesenchymal stromal cell osteogenic differentiation and angiogenic factor expression. Bone. 2007;40:1078-1087
37. Rosová I, Dao M, Capoccia B. et al. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells. 2008;26:2173-2182
38. Nagano M, Kimura K, Yamashita T. et al. Hypoxia responsive mesenchymal stem cells derived from human umbilical cord blood are effective for bone repair. Stem Cells Dev. 2010 [Epub ahead of print]
39. Yin F, Herring BP. GATA-6 can act as a positive or negative regulator of smooth muscle-specific gene expression. J Biol Chem. 2004;280:4745-4752
40. Iwaki T, Yamamoto K, Matsuura T. et al. Alteration of integrins under hypoxic stress in early placenta and choriocarcinoma cell line BeWo. Gynecol Obstet Invest. 2004;57:196-203
Author contact
![]() Corresponding author: Malancha Ta, PhD, Manipal Institute of Regenerative Medicine, Manipal University, 10, Service Road, Domlur Layout, Airport Road, Bangalore-560 071, India. Phone: +91-80-25356663; Fax: +91-80-25356662; Email: malancha.taedu
Corresponding author: Malancha Ta, PhD, Manipal Institute of Regenerative Medicine, Manipal University, 10, Service Road, Domlur Layout, Airport Road, Bangalore-560 071, India. Phone: +91-80-25356663; Fax: +91-80-25356662; Email: malancha.taedu

 Global reach, higher impact
Global reach, higher impact