10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2010; 6(6):525-536. doi:10.7150/ijbs.6.525 This issue Cite
Research Paper
Alpha-Calcitonin Gene-Related Peptide Can Reverse The Catabolic Influence Of UHMWPE Particles On RANKL Expression In Primary Human Osteoblasts
1. Department of Trauma Surgery, University of Duisburg-Essen, Hufelandstraße 55, 45147 Essen, Germany
2. Department of Orthopaedics, University of Duisburg-Essen, Pattbergstrasse 1-3, 45239 Essen, Germany
3. Department of Orthopaedics, The second affiliated hospital of Sun Yat-sen University Guangzhou, China
* Both authors contributed equally to this work and share the first authorship.
Received 2010-4-1; Accepted 2010-9-13; Published 2010-9-15
Abstract
Background and purpose: A linkage between the neurotransmitter alpha-calcitonin gene-related peptide (alpha-CGRP) and particle-induced osteolysis has been shown previously. The suggested osteoprotective influence of alpha-CGRP on the catabolic effects of ultra-high molecular weight polyethylene (UHMWPE) particles is analyzed in this study in primary human osteoblasts. Methods: Primary human osteoblasts were stimulated by UHMWPE particles (cell/particle ratios 1:100 and 1:500) and different doses of alpha-CGRP (10-7 M, 10-9 M, 10-11 M). Receptor activator of nuclear factor-κB ligand (RANKL) and osteoprotegerin (OPG) mRNA expression and protein levels were measured by RT-PCR and Western blot. Results: Particle stimulation leads to a significant dose-dependent increase of RANKL mRNA in both cell-particle ratios and a significant down-regulation of OPG mRNA in cell-particle concentrations of 1:500. A significant depression of alkaline phosphatase was found due to particle stimulation. Alpha-CGRP in all tested concentrations showed a significant depressive effect on the expression of RANKL mRNA in primary human osteoblasts under particle stimulation. Comparable reactions of RANKL protein levels due to particles and alpha-CGRP were found by Western blot analysis. In cell-particle ratios of 1:100 after 24 hours the osteoprotective influence of alpha-CGRP reversed the catabolic effects of particles on the RANKL expression. Interpretation: The in-vivo use of alpha-CGRP, which leads to down-regulated RANKL in-vitro, might inhibit the catabolic effect of particles in conditions of particle induced osteolysis.
Keywords: Aseptic loosening, polyethylene, alpha-CGRP, hOB, RANKL.
Introduction
Mechanical wear in the joint of a total hip replacement induces a severe inflammatory reaction leading to aseptic prosthetic loosening [1]. A linkage between the nervous system and aseptic loosening has been discussed since the discovery of calcitonin gene-related peptide (CGRP)-immunoreactive nerve fibres in the interface membrane of loosened arthroplasty [2]. In our previous in-vivo study of alpha-CGRP knock-out mice we found a down-regulation of osteogenesis and osteolysis that were partly mediated via the osteoprotegerin/receptor activator of nuclear factor-κB ligand/receptor activator of nuclear factor-κB (OPG/RANKL/RANK) system [3]. Alpha-CGRP is a neurotransmitter and transmembrane protein with multiple physiological roles. It affects the metabolism of the skeletal muscle, the liver and the kidneys, and inhibits glycogen synthesis [4, 5]. It acts as a potent vasodilatator, as a neurotrophic effector and as a mediator in the neurogenic inflammatory response [6, 7]. The OPG/RANKL/RANK system plays a key role in the cross-talk between osteoblasts and osteoclasts [8, 9]. RANKL and OPG are members of a ligand-receptor system that directly regulates osteoclast differentiation and bone resorption, and both are produced and secreted by osteoblastic lineage cells [9, 10]. RANKL binds to its receptor RANK, which is expressed on osteoclast progenitors, and leads to osteoclast activation. OPG binds to RANKL and thereby inhibits osteoclast activation. Recent in-vitro studies of primary human osteoblasts (hOBs) showed an increase of RANKL expression and induction of a catabolic phenotype by ultra-high molecular weight polyethylene (UHMWPE) particles [11, 12].
To closer study the direct influence of alpha-CGRP on bone metabolism, we analyzed the expression of RANKL and OPG in hOBs hypothesizing an osteoprotective influence of alpha-CGRP in the presence of wear particles in-vitro.
Patients and Methods
Peptide
Alpha-CGRP (Sigma Aldrich, Cat. No. C0167, Saint Louis, Missouri, USA) was dissolved in 1% acetic acid or water and stored at -20° Celsius before use. During cell seeding, alpha-CGRP was added daily to the experimental wells to form different concentrations (10-7 M, 10-9 M or 10-11 M), as introduced by Villa et al. while an alpha-CGRP-free medium was added to the control group [13].
Preparation of wear particles
The commercially pure UHMWPE particles (Ceridust VP 3610, Clariant, Gersthofen, Germany) with a mean particle size (given as equivalent circle diameter) of 1.74 ± 1.43 μm (range 0.05-11.06) were used in this study [14]. For endotoxin removal, the particles were treated for 24 hours with 99% ethanol at room temperature and dried in a desiccator. The efficiency of the method was checked using Limulus Amebocyte Lysate (LAL) Assay (Charles River, Kent, United Kingdom) with a sensitivity of 0.25 EU/ml according to the manufacturer's directions. The test was found to be negative. Subsequently, particles were re-suspended in 10% endotoxin-free fetal calf serum (FCS), vortexed and treated in a sonicating water bath. Flow cytometry was used to measure the number of particles per unit volume of solution.
Primary human osteoblasts
Explants from the upper femur were obtained from 5 patients undergoing arthroplasty for fractured neck of femur analog to comparable publications [13, 15-18]. The donors of this study aged 57 to 64 years. They did not suffer from known metabolic or systemic diseases and did not take any drugs affecting bone metabolism. All donors gave their informed consent prior to their inclusion in the study. Osteoblastic cells from cancellous bone fragments were obtained by enzymatic digestion using type II collagenase (Gibco BRL Life Technologies, Eggenstein, Germany) as described by Beloti et al. [19]. These cells were cultured in α-minimum essential medium (Gibco), supplemented with 10% fetal bovine serum (Gibco), 50ug/mL vancomycin (Acros Organics, Gell, Belgium), 20ug/mL ampicillin (USB Corp., Cleveland, USA), 0.3 mg/mL fungizone (Gibco), 10-7M dexamethazone (Sigma, St. Louis, USA), 50 µg/mL ascorbic acid (Gibco), and 7 mmol/L β-glycerophosphate (Sigma). Subconfluent cells in primary culture were harvested after treatment with 1 mmol/L ethylenediamine tetracetic acid (Gibco) and 0.25% trypsin (Gibco) and subcultured in 24-well culture plates (Falcon, Franklin Lakes, NJ) at a cell density of 2x104 cells/well in culture medium. During the culture period, cells were incubated at 37°C in a humidified atmosphere of 5% CO2 and the medium was changed every 3 days. We identified the hOBs by alkaline phosphatase staining, von Kossa staining and alizarin red staining.
For the experiment, hOB cells were seeded into 6-well flat bottomed culture plates at the quantity of approximately 1.5×105 cells per well. After 24 hours, an 80% confluence of the cells was reached. The supernatant was removed and a fresh medium containing UHMWPE particles was added. The hydrophobicity of polyethylene was addressed by inverted cell-culturing procedures analog to Fang et al. to allow contact of hOBs and UHMWPE particles [20]. During this procedure, different quantities of particles were added to form two different cell-particle ratios (1:100 and 1:500).
Isolation of RNA and quantitative Real Time RT-PCR analysis
Total RNA was isolated using Qiashredder (Qiagen, Hilden, Germany) and purified using the RNeasy Mini Kit (Qiagen, Hilden, Germany). Both procedures were performed according to the manufacturer's specification. The purification included a DNase treatment using the RNase free DNase Set (Qiagen, Hilden, Germany). The yield and purity of the RNA was measured photometrically. RNA was analyzed by quantitative real time polymerase chain reaction (RT-PCR) in a Rotorgene Cycler (Corbett Research, Mortlake, Australia) using the QuantiFast SYBR Green RT-PCR kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. A conventional PCR was performed to obtain a product of amplification suitable for the construction of standard curves with the real-time PCR procedures. The incorporation of Sybr Green into the PCR products was monitored in real time after each PCR cycle, resulting in the calculation of the threshold cycle or Ct value that defines the PCR cycle number at which an exponential growth of PCR products begins. PCR cycle conditions were as follows: 10 minutes at 50°C, 5 minutes at 95°C, 35 to 40 cycles of 10 seconds at 95°C and 30 seconds at 60°C. Each PCR procedure included a negative control reaction without a template. To exclude residual DNA contamination of the RNA samples, RT-PCR was also performed without reverse transcriptase. For mRNA amplification, the validated primers were obtained from Qiagen (Qiagen, Hilden, Germany): ß-actin (Cat. No. QT00095431), RANKL (Cat. No. QT00215614) and OPG (Cat. No. QT00014294). The PCR products were sequenced and found to be identical to the published sequences. The ß-actin housekeeping gene was used as reference for the relative quantification of the gene of interest, which was expressed as the ratio of 'concentration of the target' to 'concentration of ß-actin'.
Western Blot
HOBs were stimulated with and without particles (the cell-particle ratios were 1:100 and 1:500 respectively) for 24, 48 and 72 hours. The cells were washed twice with ice-cold phosphate-buffered saline (PBS) and directly lysed in Laemmli buffer. The lysate was sonicated, boiled for 5 minutes and centrifuged at 16,000 g for 10 minutes at 4°C. The supernatant was recovered as total cell lysate, sub-packaged and stored at -80°C. Equal amounts of protein (10 μg) were separated by 8% SDS-PAGE and electro-transferred to 0.45 μm polyvinylidene difluoride membranes (Millipore, Bedford, USA). Following transfer, membranes were blocked with a solution of 0.1% Tween 20/TBS (TBS/T) containing 5% non-fat milk for one hour at room temperature and then incubated with monoclonal mouse anti-human OPG antibody (GTX11994, GeneTex, USA, final dilution 1:300) or rabbit polyclonal human RANKL (AB1862, Chemicon, Temecula, California, USA, final dilution 1:3500) overnight at 4°C. Specifically bounded primary antibodies were detected with peroxidase-coupled secondary antibody and enhanced chemiluminescence (Cell Signaling Technologies, Beverly, MA). The bands were visualized by nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl-phosphate. Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) was used as house-keeping gene.
For densitometric analyzes, blots were scanned and quantified using Quantity One analysis software (Bio-Rad, Hercules, CA, USA). The results were expressed as the percentage of GAPDH immunoreactivity.
Alkaline phosphatase specific activity
Upon termination of culture, the medium was carefully aspirated from each well. The QuantiChromeTM Alkaline Phosphatase Assay Kit (Cat. No. DALP-250; BioAssay Systems, Hayward, CA) was used to measure alkaline phosphatase (AP) activity levels in lysate samples of 104 cells, following the manufacturer's instructions.
Statistical analysis
Results from representative experiments are shown. They were expressed as mean ± standard deviation. A repeated measurement ANOVA for all continuous dependent variables determined if there was (a) a time-by-group interaction effect, (b) a time effect and (c) inter-group effect. When F-values corresponding to a time-by-group interaction effect for a given variable were found to be significant, simple effects testing was performed to determine a time effect within each experimental group. Subsequently, one-way ANOVA tests were used to determine the detectable change between the groups at each time point. One-way ANOVA tests, at each time point relative to the previous time point, determined if there were significant changes from each time-point. A p-value<0.05 was considered to indicate statistical significance.
Ethics
The study was performed in accordance with the ethical standards laid down in the Helsinki Declaration of 1975, as revised in 2008.
Results
HOBs without co-incubation of particles and alpha-CGRP did not show time-dependent differences in RANKL mRNA expression.
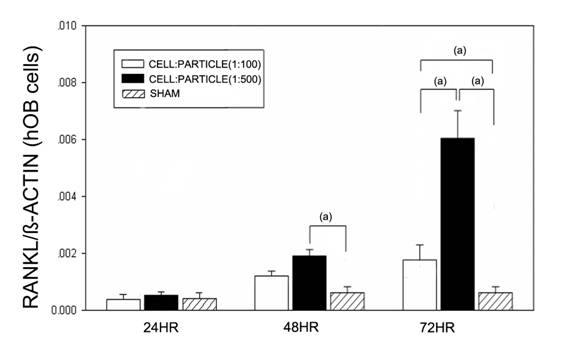
After 24 hours no effects of particles were found (Figure 1). After 48 hours high (1:500) cell-particle concentrations lead to a significant increase of RANKL mRNA compared to the sham group (p<0.05). After 72 hours, the RANKL mRNA expression was significantly (p<0.05) increased at cell-particle concentrations of 1:100 and 1:500 compared to the sham group. Furthermore, high cell-particle concentrations (1:500) lead to a significantly (p<0.05) higher RANKL mRNA expression compared to cell-particle concentrations of 1:100.
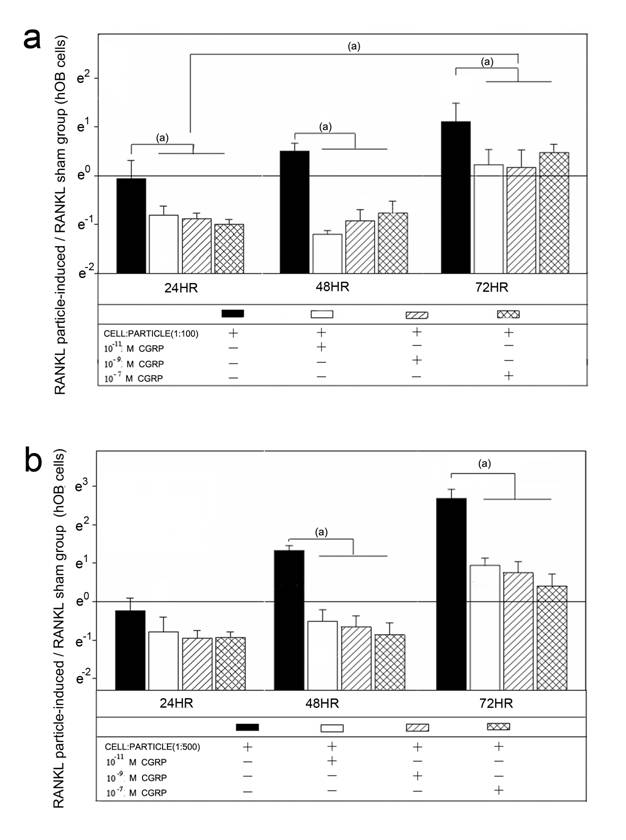
Alpha-CGRP treatment of the particle treated hOBs lead to a down-regulation of RANKL mRNA (Figure 2). In cell-particle ratios of 1:100, RANKL mRNA expression was significantly decreased by all concentrations of alpha-CGRP at all time points (p<0.05) (Figure 2a). In particle concentrations of 1:500 no effect of alpha-CGRP on RANKL mRNA was detected at 24 hours, whereas at 48 hours and 72 hours RANKL mRNA levels were significantly decreased (p<0.05) (Figure 2b). The down-regulation of RANKL mRNA expression due to alpha-CGRP was not influenced by the tested concentrations of 10-7 M, 10-9 M or 10-11 M (Figure 2).
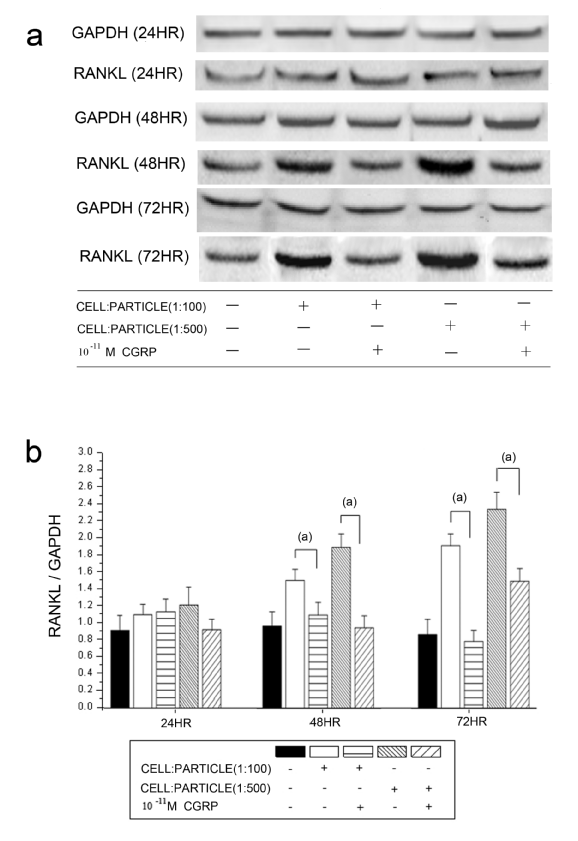
The detected RANKL protein levels corresponded to the mRNA expression. A significantly decreasing RANKL/GAPDH ratio was found after alpha-CGRP incubation in both cell-particle ratios at 48 hours and 72 hours (Figure 3). The tested concentrations of alpha-CGRP (10-7 M, 10-9 M and 10-11 M) showed no influence on the decrease of RANKL protein levels (results not shown).
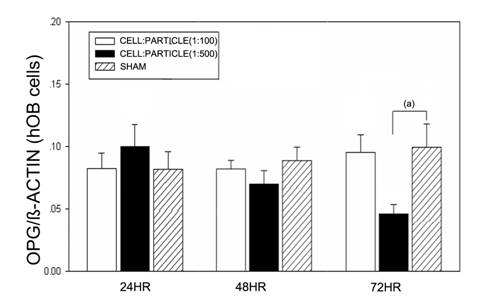
Particle treatment of hOBs did not lead to significant changes of OPG mRNA expression after 24 and 48 hours. In cell-particle concentrations of 1:500 a significant decrease of OPG mRNA was found (Figure 4).
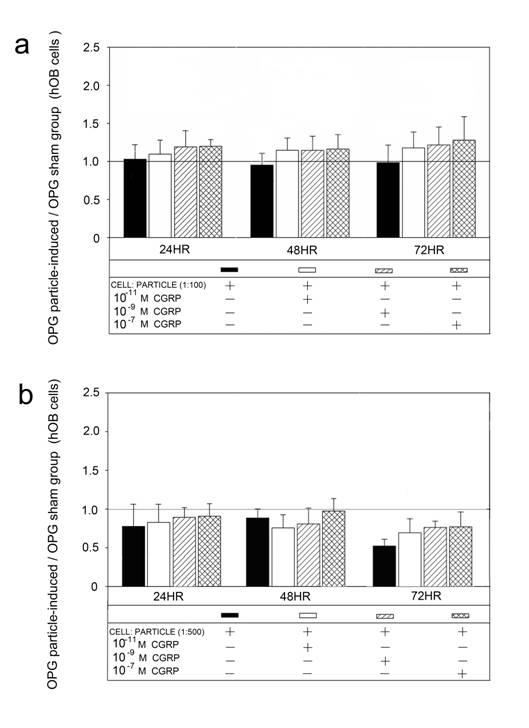
Alpha-CGRP treatment of the particle incubated hOBs did not show an effect on OPG mRNA compared to the sham groups (Figure 5).
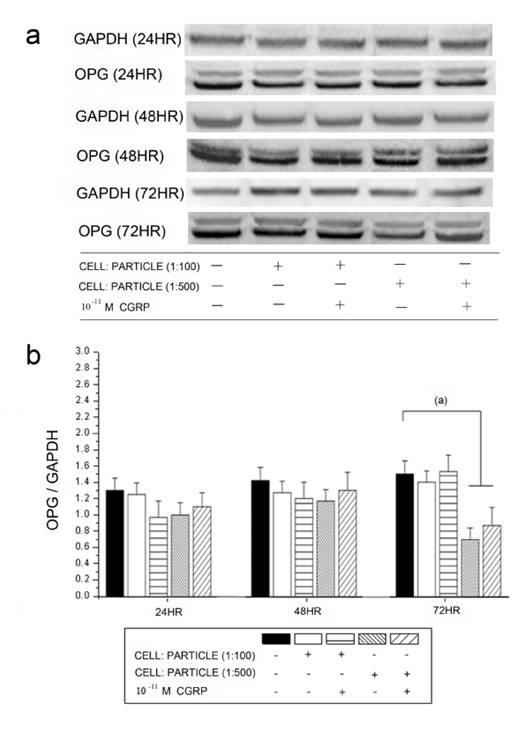
The OPG protein levels detected by Western blot analysis corresponded to the mRNA expression (Figure 6). A significant down-regulation of OPG was found after particle incubation with cell-particle ratios of 1:500. Alpha-CGRP substitution did not lead to significant changes in any groups.
AP activity was measured to further show the influence of particle dose and alpha-CGRP concentration on the activity of hOBs. The untreated hOBs had an AP activity of 22.4 IU/L ± 4.67 IU/L. AP activity in cells treated with cell-particle ratios of 1:100 and 1:500 was significantly (p<0.05) decreased compared to the control at all time points.
AP activity after alpha-CGRP treatment did not differ from the corresponding group without alpha-CGRP treatment (Table 1).
RANKL mRNA expression of hOBs after stimulation with different UHMWPE particle concentrations. Data are reported as mean ± standard deviation. Significant differences are marked. ((a) p<0.05).
Time course of RANKL mRNA expression in hOBs after treatment with different alpha-CGRP doses. The ratios of particle-stimulated hOBs and the corresponding incubation without particle treatment are shown. Data are reported as mean ± standard deviation. Significant differences are marked. ((a) p<0.05). a Cell-particle ratio of 1:100 b Cell-particle ratio of 1:500
Time courses of RANKL protein levels in hOBs stimulated by UHMWPE particles and alpha-CGRP using Western blot analysis. a Representative Western blot for RANKL in the untreated group and the alpha-CGRP-incubated groups b Densitometric quantification of RANKL in particle-treated (particle concentrations of 1:100 and 1:500) group with and without alpha-CGRP incubation. RANKL protein levels are expressed relatively to GAPDH. Data are reported as mean ± standard deviation (n=5) ((a) p<0.05).
OPG mRNA expression of hOBs after stimulation with different UHMWPE particle concentrations. Data are reported as mean ± standard deviation. Significant differences are marked. ((a) p<0.05).
Time course of UHMWPE particle-induced OPG mRNA expression after treatment with different alpha-CGRP doses. The ratios of particle-stimulated hOBs and the corresponding incubation without particle treatment are shown. Data are reported as mean ± standard deviation. Significant differences are marked. ((a) p<0.05). a Cell-particle ratio of 1:100 b Cell-particle ratio of 1:500.
Time courses of OPG protein levels in hOBs stimulated by UHMWPE particles and alpha-CGRP using Western blot analysis. GAPDH protein levels serve as control. OPG protein levels are expressed relatively to GAPDH. Data are reported as mean ± standard deviation. ((a) p<0.05). a Representative Western blots for OPG in untreated group and the alpha-CGRP-incubated groups. b Densitometric quantification of OPG in particle-treated (particle concentrations of 1:100 and 1:500) group with and without alpha-CGRP incubation. OPG protein levels are expressed relatively to GAPDH. Data are reported as mean ± standard deviation. (n=5) ((a) p<0.05).
Alkaline phosphatase specific activity of hOBs incubated with different cell-particle ratios (1:100, 1:500) and different doses of alpha-CGRP (10-7 M, 10-9 M, 10-11 M). Treatment/control ratios (T/C) are shown. Data are reported as mean ± standard deviation. Significant differences between treated and control group are shown. ((a) p<0.05).
| 1:100 (a) | 1:100 10-11 M CGRP (a) | 1:100 10-9 M CGRP (a) | 1:100 10-7 M CGRP (a) | 1:500 (a) | 1:500 10-11 M CGRP (a) | 1:500 10-9 M CGRP (a) | 1:500 10-7 M CGRP (a) | |
|---|---|---|---|---|---|---|---|---|
| 24HR | 0.80±0.03 | 0.78±0.04 | 0.77±0.03 | 0.82±0.03 | 0.73±0.02 | 0.72±0.03 | 0.71±0.04 | 0.73±0.04 |
| 48HR | 0.78±0.03 | 0.77±0.03 | 0.77±0.04 | 0.80±0.04 | 0.71±0.03 | 0.70±0.05 | 0.71±0.03 | 0.71±0.04 |
| 72HR | 0.78±0.03 | 0.77±0.04 | 0.75±0.04 | 0.79±0.04 | 0.66±0.03 | 0.69±0.04 | 0.69±0.02 | 0.66±0.02 |
Discussion
Until now the effects of alpha-CGRP in polyethylene particle-induced osteolysis have not been described in hOBs. For in-vitro study of osteoblast model systems, hOBs are widely regarded as standard procedure for analyzing osteoblasts as they are grown from biopted bone fragments [13, 21-23]. We believe that this is the first time that the effect of alpha-CGRP on RANKL and OPG expression has been examined in hOBs under particle incubation.
This in vitro study of hOBs showed a possible osteoprotective influence of alpha-CGRP on hOBs in conditions of UHMWPE particle stimulation. UHMWPE particles caused a significant dose-dependent increase in RANKL mRNA expression and RANKL protein levels, as well as a decrease of AP activity. High RANKL levels and low OPG levels lead to a stimulation of osteoclasts via the OPG/RANKL/RANK system [8, 24]. Cell culture and animal studies of UHMWPE particle induced osteolysis suggested that the OPG/RANKL/RANK system plays an essential role in UHMWPE particle-induced bone resorption [3, 25]. Analog to our results, Atkins et al. recently found that polyethylene particles lead to increased RANKL and decreased OPG [12]. They suggested that PE particles switch mature osteoblastic cells from an anabolic to a more catabolic phenotype. We were surprised by the mostly non-significant influence of particles on the OPG mRNA expression. In our study, OPG expression was only decreased in cell-particle concentrations of 1:500 after 72 hours. Atkins et al. found comparable results with an up-regulation of RANKL mRNA and a down-regulation of OPG mRNA 7 to 21 days after particle treatment [12]. The results of our study correspond to the findings that macrophage-osteoclast differentiation occurs in the presence of soluble RANKL and that this process is inhibited by OPG [26]. Up-regulation of RANKL and down-regulation of OPG are dual enhancers for osteoclast activity, particularly in patients with a large accumulation and high concentration of wear particles. Down-regulated RANKL and up-regulated OPG mRNA expression could theoretically inhibit the differentiation and activity of osteoclasts. Consequently, we suggest that the discrepancy between RANKL and OPG mRNA expression of osteoblasts affected by UHMWPE particles is one of the reasons for periprosthetic osteolysis.
The results of the AP activity after particle treatment correspond to the induction of a catabolic phenotype due to UHMWPE particles as described by Atkins et al. [12]. The non-significant AP activity reaction to different alpha-CGRP levels might be due to the analyzed time course. Chen et al. found the maximal AP activity after 25 days in hOB cells [27].
The results after alpha-CGRP treatment underline the influence of the neurotransmitter on particle-induced osteolysis and a linkage of the nervous system and particle-induced osteolysis as described previously [2, 3]. Our study showed a significant inhibition of RANKL production after alpha-CGRP co-incubation in nearly all particle and alpha-CGRP concentrations. These results are comparable to Wang et al. who recently described an inhibitory effect of alpha-CGRP on osteoclastogenesis and bone resorption with decreased RANKL expression [28] whereas Villa et al. reported on a down-regulation of OPG following alpha-CGRP treatment in hOBs [13]. Unfortunately, Villa did not analyze the RANKL expression in his study, as this dominated in our study. Nevertheless, the role of the neurotransmitter alpha-CGRP on bone metabolism has been controversial in the past decade. Alpha-CGRP receptors are expressed in brain tissue, adrenal and pituitary glands, the exocrine pancreas, peripheral tissue and on osteoblasts [29-32]. On the one hand, Qian et al. found elevated CGRP levels in the synovial fluids from loosened total hip arthorplasty [33]. On the other hand, alpha-CGRP has shown to be a physiological activator of bone formation. Cornish et al. found that osteoblasts respond to alpha-CGRP by increased growth [34]. Transgenic mice with an over-expression of alpha-CGRP present a phenotype of increased trabecular bone volume caused by an increased bone formation rate due to osteoblast activity [35]. Alpha-CGRP knock-out mice showed a phenotype of decreased bone formation and osteopenia [36]. Moreover, alpha-CGRP inhibits the differentiation and recruitment of osteoclast precursors [37, 38]. The finding that alpha-CGRP favors osteoclast formation via its pro-osteoclastogenic effect on the OPG/RANK/RANKL triad apparently contradicts its direct inhibitory action on bone resorption [37, 39].
Contrary to the above described anabolic effects of alpha-CGRP that are in line with the results of this in-vitro study, we observed a decrease in the number of osteoclasts and osteolysis in the alpha-CGRP-deficient mice after UHMWPE implantation in a previous study [3]. In 2007 we had difficulties to explain the seemingly osteoprotective influence of alpha-CGRP. We found a misbalance in the OPG/RANKL/RANK system and concluded that the alpha-CGRP deficiency has a complex influence on bone metabolism in these animals. A disadvantage of the experiment was that the direct influence of alpha-CGRP was not studied in 2007 because we did not substitute animals with alpha-CGRP. To further analyze the in-vivo results we started with cell-culture experiments as these better show the direct influence of a single factor. We re-evaluated our previous study and believe that a down-regulation of osteoclasts and osteoblasts takes place. On the one hand, there were significantly less (p<0.001) activated osteoclasts in alpha-CGRP mice compared to wild type mice. On the other hand, RANKL staining of alpha-CGRP knock-out mice showed significantly less (p<0.001) RANKL indicating either less osteoblast activation or less osteoblasts. A decreased bone formation rate of these animals has already been described [36]. These cell-culture experiments describe an osteoprotective influence of alpha-CGRP on osteoblasts comparable the in-vitro study of Wang et al. [28]. Therefore, the conclusion of our in-vivo study must be discussed after these in-vitro experiments. For a better understanding of our results in alpha-CGRP knock-out mice, further in-vivo studies of particle-induced osteolysis with alpha-CGRP substitution or alpha-CGRP over-expressing animals should be undertaken to prove or reject the possible osteoprotective effect of alpha-CGRP substitution on particle-induced osteolysis.
However, the cellular interactions involved in the bone resorption phases, i.e. formation, activation and survival of osteoblasts/osteoclasts, are subjected to a complex of other factors besides OPG/RANK/RANKL. Bone remodeling is a tightly coupled process consisting of repetitive cycles of bone resorption and formation. Both processes are governed by mechanical signals, which operate in conjunction with local and systemic factors in a discrete anatomic structure designated by basic multicellular units (BMU). Several pathways regulating the function of BMU have been reported in the past, including TNF-alpha/TNFR/TRAF1 and IL-6/CD126/JAK/STAT [40, 41]. Furthermore, osteoblast activity is strongly regulated by surrounding pH and growth factors released from resorbed bone matrix that stimulate osteoblasts to promote or inhibit bone formation [42, 43]. This may have an impact on the bone mass outcome at each remodeling cycle [44]. Meanwhile, the direct effect of alpha-CGRP to induce osteoblast proliferation cannot be ignored. It has been confirmed that alpha-CGRP was able to stimulate [3H]thymidine incorporation in human osteoblast-like cells and increase cyclic adenosine monomphsopate (cAMP) production [45, 46]. Protein kinase C (PKC) activity, which is important for cell proliferation, was also stimulated by this peptide [45].
Furthermore, wear debris has a direct influence on macrophage-osteoclast differentiation. Human macrophages isolated directly from periprosthetic tissues surrounding loosened implants can differentiate into multinucleated cells and show all the functional and cytochemical characteristics of osteoclasts [47]. Therefore, it can be suggested that alpha-CGRP, by activating bone remodeling, may contribute to the precise adjustment of this process favoring the gain or loss of bone mass depending on the local environment. Finally, a discrepancy between bone formation and resorption due to alpha-CGRP might appear with the increase in the concentration of wear particles.
It remains uncertain whether our in vitro findings in hOBs can be directly transferred to an up-regulation or down-regulation of bone formation in aseptic loosening of joint replacements. A limiting factor of this study is the partial focus on the hOBs, as the OPG/RANKL/RANK system in vivo interacts between both osteoblasts and osteoclasts. The interaction of osteoclasts and osteoblasts might have a major impact on the later osteoprotective or catabolic result. Furthermore, this cell culture experiment has a limited perspective of time as we analyzed the hOBs for only 72 hours. As aseptic loosening is a process which can take years, the reactions by osteoblasts might change in the course of time. Nevertheless, we can show a significant up-regulation of RANKL expression and an AP activity depression due to UHMWPE particles.
In conclusion, the present study provides data describing the activation of signaling pathways in hOBs under incubation with UHMWPE particles. Our data supports the concept of a linkage between the peripheral nervous system and aseptic loosening. Our results improve the understanding of alpha-CGRP having an osteoprotective influence on particle-induced osteolysis via the OPG/RANKL/RANK-system. Further studies of the interaction of osteoclasts, osteoblasts, neurotransmitters and the OPG/RANKL/RANK system have to be undertaken to gain a better understanding of the multi-factorial process of aseptic loosening and possible therapeutic options.
Acknowledgements
The study was supported by IFORES/ University of Duisburg-Essen. The authors would like to thank Kaye Schreyer for editorial assistance with the manuscript.
Conflict of Interests
The authors have declared that no conflict of interest exists.
References
1. Bauer T.W. Particles and periimplant bone resorption. Clin Orthop Relat Res. 2002;405:138-43
2. Ahmed M, Bergstrom J, Lundblad H. et al. Sensory nerves in the interface membrane of aseptic loose hip prostheses. J Bone Joint Surg Br. 1998;80:151-5
3. Wedemeyer C, Neuerburg C, Pfeiffer A. et al. Polyethylene particle-induced bone resorption in alpha-calcitonin gene-related peptide-deficient mice. J Bone Miner Res. 2007;22:1011-9
4. Beaumont K, Pittner R.A, Moore C.X. et al. Regulation of muscle glycogen metabolism by CGRP and amylin: CGRP receptors not involved. Br J Pharmacol. 1995;115:713-5
5. Leighton B. and Foot E.A. The role of the sensory peptide calcitonin-gene-related peptide(s) in skeletal muscle carbohydrate metabolism: effects of capsaicin and resiniferatoxin. Biochem J. 1995;307( Pt 3):707-12
6. Wimalawansa S.J. Calcitonin gene-related peptide and its receptors: molecular genetics, physiology, pathophysiology, and therapeutic potentials. Endocr Rev. 1996;17:533-85
7. Wimalawansa S.J. Amylin, calcitonin gene-related peptide, calcitonin, and adrenomedullin: a peptide superfamily. Crit Rev Neurobiol. 1997;11:167-239
8. Khosla S. Minireview: the OPG/RANKL/RANK system. Endocrinology. 2001;142:5050-5
9. Yasuda H, Shima N, Nakagawa N. et al. Identity of osteoclastogenesis inhibitory factor (OCIF) and osteoprotegerin (OPG): a mechanism by which OPG/OCIF inhibits osteoclastogenesis in vitro. Endocrinology. 1998;139:1329-37
10. Hofbauer L.C, Khosla S, Dunstan C.R. et al. The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. J Bone Miner Res. 2000;15:2-12
11. Granchi D, Ciapetti G, Amato I. et al. The influence of alumina and ultra-high molecular weight polyethylene particles on osteoblast-osteoclast cooperation. Biomaterials. 2004;25:4037-45
12. Atkins G.J, Welldon K.J, Holding C.A. et al. The induction of a catabolic phenotype in human primary osteoblasts and osteocytes by polyethylene particles. Biomaterials. 2009;30:3672-81
13. Villa I, Mrak E, Rubinacci A. et al. CGRP inhibits osteoprotegerin production in human osteoblast-like cells via cAMP/PKA-dependent pathway. Am J Physiol Cell Physiol. 2006;291:C529-37
14. von Knoch M, Sprecher C, Barden B. et al. [Size and shape of commercially available polyethylene particles for in-vitro and in-vivo-experiments]. Z Orthop Ihre Grenzgeb. 2004;142:366-70
15. Torricelli P, Fini M, Giavaresi G. et al. Comparative interspecies investigation on osteoblast cultures: data on cell viability and synthetic activity. Biomedicine & Pharmacotherapy. 2003;57:57-62
16. Rattner A, Sabido O, Le J. et al. Mineralization and alkaline phosphatase activity in collagen lattices populated by human osteoblasts. Calcified Tissue International. 2000;66:35-42
17. Di Palma F, Douet M, Boachon C. et al. Physiological strains induce differentiation in human osteoblasts cultured on orthopaedic biomaterial. Biomaterials. 2003;24:3139-3151
18. Guillen C, de Gortazar A.R, Esbrit P. The interleukin-6/soluble interleukin-6 receptor system induces parathyroid hormone-related protein in human osteoblastic cells. Calcified Tissue International. 2004;75:153-159
19. Beloti M.M, Martins WJr, Xavier S.P. et al. In vitro osteogenesis induced by cells derived from sites submitted to sinus grafting with anorganic bovine bone. Clin Oral Implants Res. 2008;19:48-54
20. Fang H.W, Ho Y.C, Yang C.B. et al. Preparation of UHMWPE particles and establishment of inverted macrophage cell model to investigate wear particles induced bioactivites. Journal of Biochemical and Biophysical Methods. 2006;68:175-187
21. Welldon K.J, Atkins G.J, Howie D.W. et al. Primary human osteoblasts grow into porous tantalum and maintain an osteoblastic phenotype. J Biomed Mater Res A. 2008;84:691-701
22. Gundle R, Beresford JN. The isolation and culture of cells from explants of human trabecular bone. Calcif Tissue Int. 1995;56(Suppl 1):S8-10
23. El-Amin S.F, Botchwey E, Tuli R. et al. Human osteoblast cells: isolation, characterization, and growth on polymers for musculoskeletal tissue engineering. J Biomed Mater Res A. 2006;76:439-49
24. Jacobs J.J, Roebuck K.A, Archibeck M. et al. Osteolysis: basic science. Clin Orthop Relat Res. 2001;393:71-7
25. Ren W, Wu B, Peng X. et al. Implant wear induces inflammation, but not osteoclastic bone resorption, in RANK(-/-) mice. J Orthop Res. 2006;24:1575-86
26. Itonaga I, Sabokbar A, Murray D.W. et al. Effect of osteoprotegerin and osteoprotegerin ligand on osteoclast formation by arthroplasty membrane derived macrophages. Ann Rheum Dis. 2000;59:26-31
27. Chen F.P, Hsu T, Hu C.H. et al. Expression of estrogen receptors alfa and beta mRNA and alkaline phosphatase in the differentiation of osteoblasts from elderly postmenopausal women: comparison with osteoblasts from osteosarcoma cell lines. Taiwan J Obstet Gynecol. 2006;45:307-12
28. Wang L, Shi X, Zhao R. et al. Calcitonin-gene-related peptide stimulates stromal cell osteogenic differentiation and inhibits RANKL induced NF-kappaB activation, osteoclastogenesis and bone resorption. Bone. 2010;46:1369-79
29. Bjurholm A, Kreicbergs A, Brodin E. et al. Substance P- and CGRP-immunoreactive nerves in bone. Peptides. 1988;9:165-71
30. Bjurholm A, Kreicbergs A, Terenius L. et al. Neuropeptide Y-, tyrosine hydroxylase- and vasoactive intestinal polypeptide-immunoreactive nerves in bone and surrounding tissues. J Auton Nerv Syst. 1988;25:119-25
31. Brain S.D. and Williams T.J. Inflammatory oedema induced by synergism between calcitonin gene-related peptide (CGRP) and mediators of increased vascular permeability. Br J Pharmacol. 1985;86:855-60
32. Yamamoto I, Kitamura N, Aoki J. et al. Human calcitonin gene-related peptide possesses weak inhibitory potency of bone resorption in vitro. Calcif Tissue Int. 1986;38:339-41
33. Qian Y, Zeng B.F, Zhang X.L. et al. High levels of substance P and CGRP in pseudosynovial fluid from patients with aseptic loosening of their hip prosthesis. Acta Orthop. 2008;79:342-5
34. Cornish J, Callon K.E, Lin C.Q. et al. Comparison of the effects of calcitonin gene-related peptide and amylin on osteoblasts. J Bone Miner Res. 1999;14:1302-9
35. Ballica R, Valentijn K, Khachatryan A. et al. Targeted expression of calcitonin gene-related peptide to osteoblasts increases bone density in mice. J Bone Miner Res. 1999;14:1067-74
36. Schinke T, Liese S, Priemel M. et al. Decreased bone formation and osteopenia in mice lacking alpha-calcitonin gene-related peptide. J Bone Miner Res. 2004;19:2049-56
37. Akopian A, Demulder A, Ouriaghli F. et al. Effects of CGRP on human osteoclast-like cell formation: a possible connection with the bone loss in neurological disorders? Peptides. 2000;21:559-64
38. Zaidi M, Fuller K, Bevis P.J. et al. Calcitonin gene-related peptide inhibits osteoclastic bone resorption: a comparative study. Calcif Tissue Int. 1987;40:149-54
39. Ishizuka K, Hirukawa K, Nakamura H. et al. Inhibitory effect of CGRP on osteoclast formation by mouse bone marrow cells treated with isoproterenol. Neurosci Lett. 2005;379:47-51
40. Dempsey P.W, Doyle S.E, He J.Q. et al. The signaling adaptors and pathways activated by TNF superfamily. Cytokine Growth Factor Rev. 2003;14:193-209
41. Rakshit D.S, Ly K, Sengupta T.K. et al. Wear debris inhibition of anti-osteoclastogenic signaling by interleukin-6 and interferon-gamma. Mechanistic insights and implications for periprosthetic osteolysis. J Bone Joint Surg Am. 2006;88:788-99
42. Arnett T.R. Extracellular pH regulates bone cell function. J Nutr. 2008;138:415S-418S
43. Sato S, Futakuchi M, Ogawa K. et al. Transforming growth factor beta derived from bone matrix promotes cell proliferation of prostate cancer and osteoclast activation-associated osteolysis in the bone microenvironment. Cancer Sci. 2008;99:316-23
44. Theoleyre S, Wittrant Y, Tat S.K. et al. The molecular triad OPG/RANK/RANKL: involvement in the orchestration of pathophysiological bone remodeling. Cytokine Growth Factor Rev. 2004;15:457-75
45. Villa I, Dal Fiume C, Maestroni A. et al. Human osteoblast-like cell proliferation induced by calcitonin-related peptides involves PKC activity. Am J Physiol Endocrinol Metab. 2003;284:E627-33
46. Villa I, Melzi R, Pagani F. et al. Effects of calcitonin gene-related peptide and amylin on human osteoblast-like cells proliferation. Eur J Pharmacol. 2000;409:273-8
47. Sabokbar A, Fujikawa Y, Neale S. et al. Human arthroplasty derived macrophages differentiate into osteoclastic bone resorbing cells. Ann Rheum Dis. 1997;56:414-20
Author contact
![]() Corresponding author: Dr. med. Max Daniel Kauther, Department of Trauma Surgery, University of Duisburg-Essen, Hufelandstraße 55, 45147 Essen, Germany. Phone: 0049-201-7231341; Fax: 0049-201-7235629; E-Mail: maxdaniel.kautherde
Corresponding author: Dr. med. Max Daniel Kauther, Department of Trauma Surgery, University of Duisburg-Essen, Hufelandstraße 55, 45147 Essen, Germany. Phone: 0049-201-7231341; Fax: 0049-201-7235629; E-Mail: maxdaniel.kautherde

 Global reach, higher impact
Global reach, higher impact