Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2010; 6(6):556-568. doi:10.7150/ijbs.6.556 This issue Cite
Research Paper
Activity and interactions of antibiotic and phytochemical combinations against Pseudomonas aeruginosa in vitro
1. Biomedical Engineering Research Centre, Nanyang Technological University, Singapore;
2. Advanced Design and Modeling Lab, Nanyang Technological University, Singapore;
3. School of Life and Environmental Sciences, University of Tsukuba, Ibaraki, Japan;
4. Infectious Disease Cluster, Advanced Medical & Dental Institute, Universiti Sains Malaysia, Penang, Malaysia.
Received 2010-7-4; Accepted 2010-9-16; Published 2010-9-21
Abstract
In this study the in vitro activities of seven antibiotics (ciprofloxacin, ceftazidime, tetracycline, trimethoprim, sulfamethoxazole, polymyxin B and piperacillin) and six phytochemicals (protocatechuic acid, gallic acid, ellagic acid, rutin, berberine and myricetin) against five P. aeruginosa isolates, alone and in combination are evaluated. All the phytochemicals under investigation demonstrate potential inhibitory activity against P. aeruginosa. The combinations of sulfamethoxazole plus protocatechuic acid, sulfamethoxazole plus ellagic acid, sulfamethoxazole plus gallic acid and tetracycline plus gallic acid show synergistic mode of interaction. However, the combinations of sulfamethoxazole plus myricetin shows synergism for three strains (PA01, DB5218 and DR3062). The synergistic combinations are further evaluated for their bactericidal activity against P. aeruginosa ATCC strain using time-kill method. Sub-inhibitory dose responses of antibiotics and phytochemicals individually and in combination are presented along with their interaction network to suggest on the mechanism of action and potential targets for the phytochemicals under investigation. The identified synergistic combinations can be of potent therapeutic value against P. aeruginosa infections. These findings have potential implications in delaying the development of resistance as the antibacterial effect is achieved with lower concentrations of both drugs (antibiotics and phytochemicals).
Keywords: Synergy, combination therapy, phytochemicals, drug resistance.
Introduction
Pseudomonas aeruginosa is a major nosocomial pathogen, particularly dangerous to cystic fibrosis patients and populations having weak immune system. Moreover, this organism is resistant to many antibacterial drugs [1]. Several mechanisms for resistance against antibiotics have been proposed - which include antibiotic inactivation by enzymatic action, altering the efflux pump mechanisms, target mutation, and decreased uptake of antibiotics [2]. Due to the frequent development of resistance during monotherapy treatment of infected patients, multiple combinations of antibacterial agents are proposed [3-4]. Synergy is reported for β-lactams in combination with aminoglycoside antibiotics. However, the increasing resistance of Pseudomonas sp. to β-lactams and the toxicity concerns with the aminoglycosides limit the use of these combinations [5]. It is also reported that polymyxin B can be used in combination with other antimicrobials to treat multidrug resistant (MDR) gram-negative respiratory tract infections caused by several clinical strains of P. aeruginosa [6]. Also, fluoroquinolones in combination with potent anti-pseudomonal β-lactam agents are reported to prevent the development of resistance in P. aeruginosa [7-8]. Earlier, Marie et al. reported that cystic fibrosis patients infected with P. aeruginosa can be effectively treated with clarithromycin/tobramycin combination [9]. However, alarmingly numbers of P. aeruginosa strains are reported to show resistance to multiple antibiotics. Concomitantly, multidrug resistance and dual resistance are major issues in developing a definitive therapy for P. aeruginosa infections [10].
Since long, natural products are in use for the development of novel drugs to treat various bacterial infections. Several studies have proposed that natural compounds in combination with antibiotics are a new strategy for developing therapies for infections caused by bacterial species and that natural plant products can potentiate the activity of antibiotics in combination [10-13]. For example, secondary metabolites like essential oils, flavonols and alkaloids have been reported to have antimicrobial properties [14-18]. Also, Michael and colleagues reported that the use of bacterial resistance modifiers such as efflux pump inhibitors, derived from natural sources mainly from plants can suppress the emergence of MDR strains [19].
Phenolic compounds from medicinal herbs and dietary plants possess a range of bioactivities like - antibacterial, antifungal, antiviral, antimutagenic and anti-inflammatory activities. Moreover, extensive clinical evidence has shown that chemoprevention by phenolic phytochemicals is an inexpensive, readily applicable approach in the chemotherapy and management [20]. Here, we investigate the in vitro antimicrobial activities of 7 different antibiotics (ciprofloxacin, ceftazidime, tetracycline, trimethoprim, sulfamethoxazole, polymyxin B and piperacillin) in combination with 6 phytochemicals (protocatechuic acid, gallic acid, ellagic acid, rutin, berberine and myricetin) against 5 strains of P. aeruginosa using minimum inhibitory concentrations (MIC), checkerboard test, and time-kill assay. All antibiotics under investigation have earlier been reported as anti-pseudomonal drugs and have been reported to have different mechanisms of action (have different targets). The results of our analysis with data on antibiotic-phytochemical interaction network are presented.
Materials and Methods
Antimicrobial agents
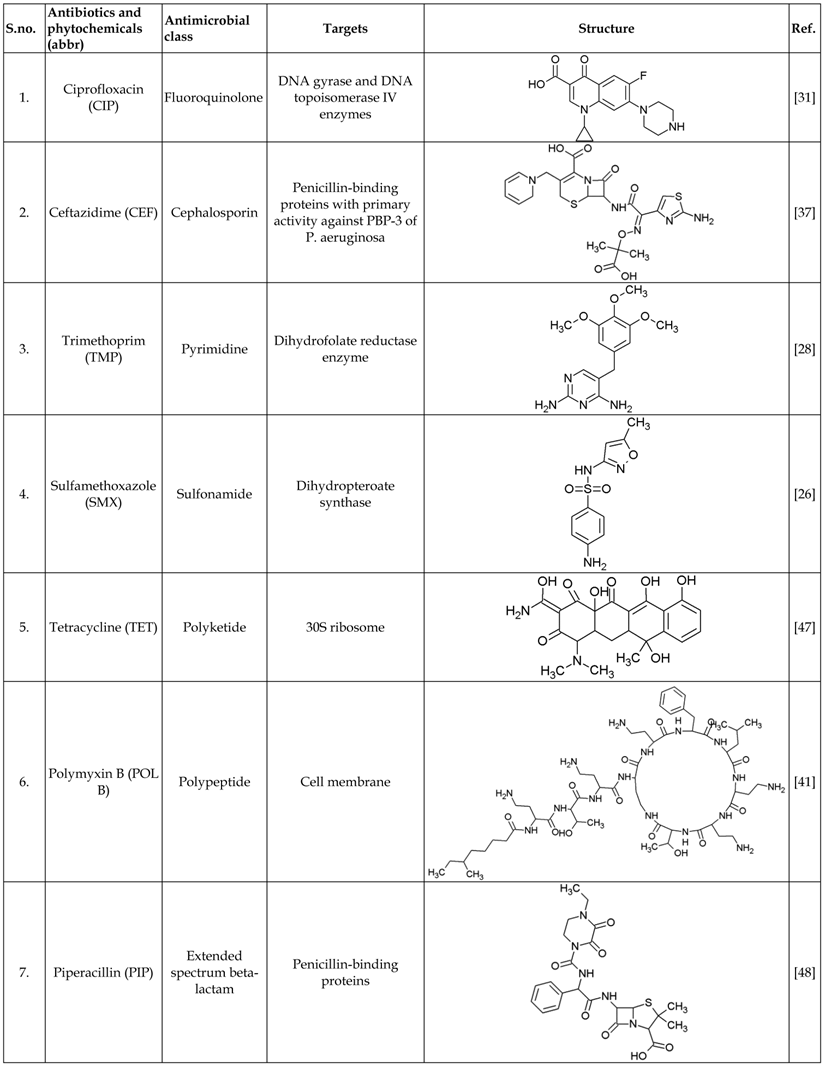
Standard powders of antibiotics and phytochemicals listed in Table 1 were obtained from Sigma - Aldrich, Singapore. Stock solutions were prepared and diluted according to Clinical and Laboratory Standards Institute (CLSI) standards and manufacturer's recommendations and stored at -20°C.
Bacterial isolates
Five P. aeruginosa strains were used in this study including two clinical isolates (DB5218, DR3062) which was obtained from Singapore General Hospital, Singapore. The other three strains were P. aeruginosa ATCC 15692 (American Type Culture Collection, Manassas, USA), P. aeruginosa PA01 (University of Geneva, Switzerland), P. aeruginosa PT121 (University of Geneva, Switzerland). Before use, bacterial suspension was made from fresh culture and stored at -80⁰C using 30% glycerol.
Antimicrobial susceptibility testing
MIC is defined as the lowest concentration of the antimicrobial agent that inhibits the bacterial growth as detected by lack of visual turbidity. Broth microdilution method was used to determine the MICs for 7 antibiotics and 6 phytochemicals according to Clinical and Laboratory Standards Institute guidelines [21]. The medium used for the broth microdilution test was Iso-sensitest Broth (ISB). For each strain of P. aeruginosa, three to five colonies were transferred from overnight growth plate into 5 ml of ISB to approximate the density of 0.5 McFarland standard and incubated at 35⁰C for 2-3 h. This suspension with the inoculum concentration of 108 colony forming units (CFU/ml) was then diluted to 106 CFU/ml with the Iso-sensitest broth. Serial two-fold dilutions of all the antimicrobial agents with the following concentrations (µg/ml): ciprofloxacin (0.06 - 32), ceftazidime (0.25 - 32), trimethoprim (4 - 128), sulfamethoxazole (8 - 512), tetracycline (4 - 128), polymyxinpolymyxin B (0.25 - 32), piperacillin (0.25 - 32), protocatechuic acid (250 - 8000), ellagic acid (1000 - 16000), gallic acid (250- 8000), rutin (1000 - 16000), berberine (1000-16000), myricetin (100 - 1000) were prepared with ISB and placed in 96-well microtiter plates and the lowest concentration inhibiting visible growth after 18-20 h at 35⁰C was recorded as MIC.
List of antibiotics and phytochemicals used in this study
Checkerboard assay
The activity of phytochemicals and antibiotics in combination was investigated using the checkerboard broth microdilution method. Two fold serial dilutions of the antibiotic and two fold serial dilutions of the phytochemicals were prepared for every combination tested and 50 µl aliquots of each component was placed into the wells of the sterile 96-well microtiter plate. The inoculum was prepared using the above described MIC determination method. The microtiter plates were then incubated at 35⁰C and MIC was determined after 18-20 h of incubation.
The Fractional inhibitory concentration (FIC index) for all the combinations was determined using the following formula,
FIC index = FICA + FICB = [A]/MICA + [B]/MICB
FICA, FICB - Fractional inhibitory concentration of drug A & B respectively. MICA, MICB - Minimum inhibitory concentration of drug A & B respectively.
[A], [B] - Concentration of drug A & B respectively.
FIC index by checkerboard method is interpreted as follows: ≤ 0.5- synergy; > 0.5 and ≤ 4- additivity; and > 4 - antagonism.
The effects of sub-inhibitory concentrations of test compounds individually and in combination on PA01 growth were also evaluated by measuring the growth rates after 20 h of incubation at 35⁰C in microtitre plates and OD600 was measured.
Time-kill assay
Time-kill assays were performed using the identified synergistic combinations (SMX plus PA, SMX plus EA, SMX plus GA and TET plus GA) against a single bacterial isolate (ATCC 15692). Individual colonies were isolated from the overnight growth plate and suspended in sterile ISB to approximate the density of 0.5 McFarland standard. The suspension was diluted 1:10 in ISB to obtain a standard inoculum of 1 x 106 CFU/ml. 100 µl of the diluted suspension was added to 0.9 ml of ISB. Double dilutions for each antibiotic and phytochemicals were prepared. Tubes containing individual drugs and the combination were incubated at 35°C for 24 h. From each tube 100 µl of sample was removed at 0, 4, 8 and 24 h, respectively and plated to count the viable cells. Growth control and sterility control were included for each assay. The killing rate was determined by plotting viable colony counts (CFU/ml) against time. Synergy was defined as a ≥2 log10 CFU/ml decrease of viable count by the combination compared with the most active single agent.
Statistical analysis
The dose response values and time-kill data are presented as mean ± SD (Standard deviation). The analysis was performed in triplicate.
Results
Antimicrobial susceptibility testing
This study explored the antimicrobial activities of the phytochemicals alone and in combination with 7 anti-pseudomonal drugs (antibiotics) against 5 strains of P. aeruginosa (including 2 clinical strains). All the 5 strains of P. aeruginosa were susceptible to the anti-pseudomonal drugs chosen for investigation i.e. ciprofloxacin, ceftazidime, trimethoprim, sulfamethoxazole, tetracycline, polymyxin B and piperacillin. MIC value was found to be highest for sulfamethoxazole (128µg/ml) and lowest for ciprofloxacin (0.125µg/ml) among the antibiotics (Table 2).
The phytochemicals used in this study significantly inhibited the growth of all the bacterial strains under investigation. Among the phytochemicals tested, myricetin (500µg/ml), protocatechuic acid (2000µg/ml) and gallic acid (2000µg/ml) showed lower MIC than ellagic acid (4000µg/ml), rutin (4000µg/ml) and berberine (4000µg/ml). The minimum inhibitory concentrations of the phytochemicals are listed in Table 2.
Antimicrobial activity (MIC values) of all the antibiotics and phytochemicals against five strains of P. aeruginosa
| Antimicrobials | Minimum inhibitory concentration (µg/ml) | ||||
|---|---|---|---|---|---|
| P. aeruginosa strains | |||||
| ATCC | PA01 | PT121 | DB5218 | DR3062 | |
| Ciprofloxacin | 0.125 | 0.125 | 0.125 | 0.125 | 0.25 |
| Ceftazidime | 2 | 2 | 2 | 4 | 8 |
| Trimethoprim | 32 | 32 | 32 | 32 | 32 |
| Sulfamethoxazole | 128 | 128 | 128 | 128 | 128 |
| Tetracycline | 32 | 32 | 32 | 32 | 32 |
| Polymyxin B | 2 | 2 | 2 | 2 | 2 |
| Piperacillin | 2 | 2 | 2 | 2 | 16 |
| Protocatechuic acid | 2000 | 2000 | 2000 | 2000 | 2000 |
| Ellagic acid | 4000 | 4000 | 4000 | 4000 | 4000 |
| Gallic acid | 2000 | 2000 | 2000 | 2000 | 2000 |
| Rutin | 4000 | 4000 | 4000 | 4000 | 4000 |
| Berberine | 4000 | 4000 | 4000 | 4000 | 4000 |
| Myricetin | 500 | 500 | 500 | 500 | 500 |
Combination of phytochemicals and antibiotics against P. aeruginosa
The combination effects of anti-pseudomonal drugs and phytochemicals are summarized in Table 3.
Our results show that the combinations of sulfamethoxazole plus protocatechuic acid, sulfamethoxazole plus ellagic acid and sulfamethoxazole plus gallic acid have synergistic mode of interactions for all the strains of P. aeruginosa under investigation. It was also observed that the antifolate drug, sulfamethoxazole is synergistic with myricetin in three strains (PA01, DB5218 and DR3062) and additive for two strains (ATCC and PT121) of P. aeruginosa. Also, tetracycline in combination with gallic acid demonstrates synergy for all the isolates under investigation. Other antimicrobials - ciprofloxacin, ceftazidime, trimethoprim, polymyxin B and piperacillin show additivity in combination with phytochemicals, for all the strains under investigation. It is noteworthy, that none of the antibiotic -phytochemical combinations show antagonism.
The FIC indices of the combinations sulfamethoxazole plus protocatechuic acid and sulfamethoxazole plus ellagic acid range from 0.25 - 0.5, showing significant synergistic activity for all the strains under investigation (Table 3).
In vitro activity of all antimicrobial combinations against five strains of P. aeruginosa. The values show the FIC index.
| Antibiotics + Phytochemicals Combination | P. aeruginosa strains | ||||
|---|---|---|---|---|---|
| ATCC | PA01 | PT121 | DB5218 | DR3062 | |
| Checkerboard FIC index (Interpretation) | |||||
| CIP+PA | 2(I) | 2(I) | 2(I) | 2(I) | 1(I) |
| CIP+EA | 2(I) | 2(I) | 2(I) | 2(I) | 1(I) |
| CIP+GA | 2(I) | 2(I) | 2(I) | 2(I) | 1(I) |
| CIP+RUT | 1(I) | 1(I) | 1(I) | 1(I) | 1(I) |
| CIP+BER | 2(I) | 2(I) | 2(I) | 2(I) | 1(I) |
| CIP+MYR | 2(I) | 2(I) | 2(I) | 2(I) | 2(I) |
| CF+PA | 1(I) | 1(I) | 1(I) | 1(I) | 1(I) |
| CF+EA | 1(I) | 1(I) | 1(I) | 2(I) | 1(I) |
| CF+GA | 1(I) | 1(I) | 1(I) | 2(I) | 1(I) |
| CF+RUT | 1(I) | 1(I) | 1(I) | 1(I) | 2(I) |
| CF+BER | 1(I) | 1(I) | 1(I) | 1(I) | 2(I) |
| CF+MYR | 1(I) | 1(I) | 1(I) | 1(I) | 2(I) |
| TMP+PA | 1(I) | 1(I) | 1(I) | 1(I) | 1(I) |
| TMP +EA | 1(I) | 1(I) | 1(I) | 1(I) | 1(I) |
| TMP +GA | 1(I) | 1(I) | 1(I) | 1(I) | 1(I) |
| TMP +RUT | 2(I) | 2(I) | 2(I) | 2(I) | 2(I) |
| TMP +BER | 2(I) | 2(I) | 2(I) | 2(I) | 2(I) |
| TMP +MYR | 2(I) | 2(I) | 2(I) | 2(I) | 2(I) |
| SMX+PA | 0.5(S) | 0.25(S) | 0.25(S) | 0.25(S) | 0.25(S) |
| SMX +EA | 0.5(S) | 0.25(S) | 0.25(S) | 0.25(S) | 0.25(S) |
| SMX +GA | 0.5(S) | 0.5(S) | 0.5(S) | 0.5(S) | 0.5(S) |
| SMX +RUT | 1(I) | 1(I) | 1(I) | 1(I) | 1(I) |
| SMX +BER | 1(I) | 1(I) | 1(I) | 1(I) | 1(I) |
| SMX +MYR | 1(I) | 0.5(S) | 1(I) | 0.5(S) | 0.5(S) |
| TET+PA | 1(I) | 1(I) | 1(I) | 1(I) | 1(I) |
| TET +EA | 1(I) | 1(I) | 1(I) | 1(I) | 1(I) |
| TET +GA | 0.5(S) | 0.5(S) | 0.5(S) | 0.5(S) | 0.5(S) |
| TET +RUT | 1(I) | 1(I) | 1(I) | 1(I) | 1(I) |
| TET +BER | 1(I) | 1(I) | 1(I) | 1(I) | 1(I) |
| TET +MYR | 1(I) | 1(I) | 1(I) | 1(I) | 1(I) |
| POL B+PA | 2(I) | 2(I) | 2(I) | 2(I) | 2(I) |
| POL B +EA | 2(I) | 2(I) | 2(I) | 2(I) | 2(I) |
| POL B +GA | 2(I) | 2(I) | 2(I) | 2(I) | 2(I) |
| POL B +RUT | 2(I) | 2(I) | 2(I) | 2(I) | 2(I) |
| POL B +BER | 2(I) | 2(I) | 2(I) | 2(I) | 2(I) |
| POL B +MYR | 4(I) | 4(I) | 4(I) | 4(I) | 4(I) |
| PIP+PA | 1(I) | 1(I) | 1(I) | 1(I) | 1(I) |
| PIP+EA | 2(I) | 2(I) | 2(I) | 2(I) | 1(I) |
| PIP+GA | 1(I) | 1(I) | 1(I) | 1(I) | 1(I) |
| PIP+RUT | 2(I) | 2(I) | 2(I) | 2(I) | 2(I) |
| PIP+BER | 1(I) | 1(I) | 1(I) | 1(I) | 2(I) |
| PIP+MYR | 2(I) | 2(I) | 2(I) | 2(I) | 4(I) |
S = Synergistic; I = Additivity/Indifferent.
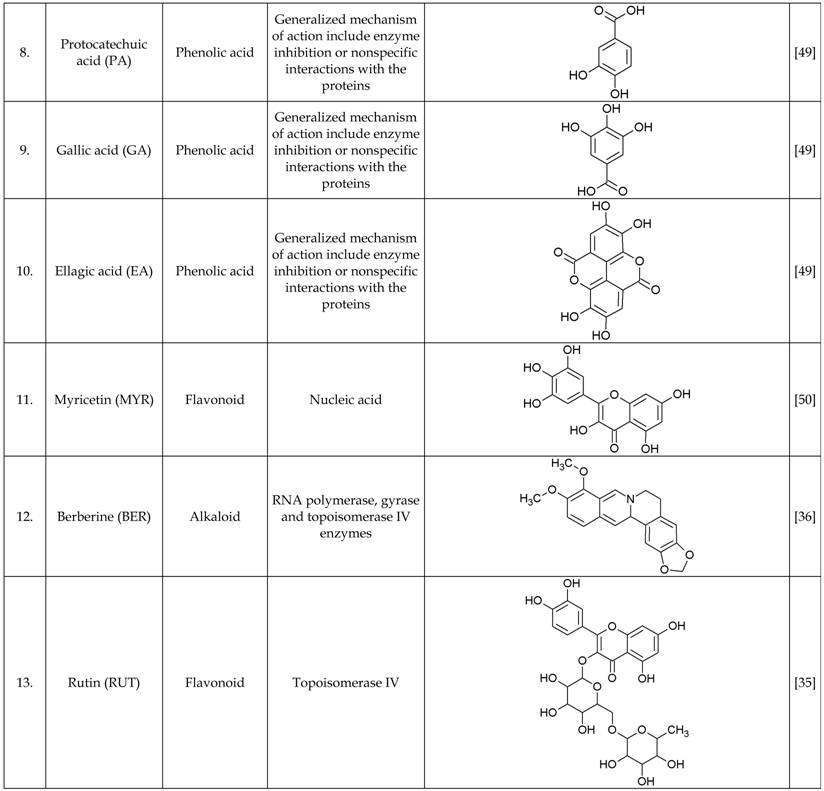
The growth inhibitory properties of all the test compounds and additive combinations assayed over 20 h period in microtiter wells at sub-inhibitory concentration was similar to the growth control well (no antimicrobials). Moreover, the synergistic combinations were found to inhibit the test strain (PA01) growth at similar concentrations (Figure 1). The drug interaction data between various classes of antibiotics and phytochemicals is represented as a figure to provide some insights on their mechanism of action (Figure 2).
PA01 growth in subminimum inhibitory concentrations of individual antibiotics and phytochemicals and their combination. Dose response and classification of interactions for antibiotics and phytochemicals used in this study. Each panel consists of bars representing measured growth rates (OD600 nm) after 24 hr incubation with sub-inhibitory concentration of the antimicrobials, from left to right: growth control (no antibiotics), antibiotics alone (1/4 x MIC), phytochemicals alone (1/4 x MIC), antibiotics + phytochemicals (1/4 + 1/4 x MIC). The background colour represents the type of interactions, red - additive/indifferent, green - synergistic.
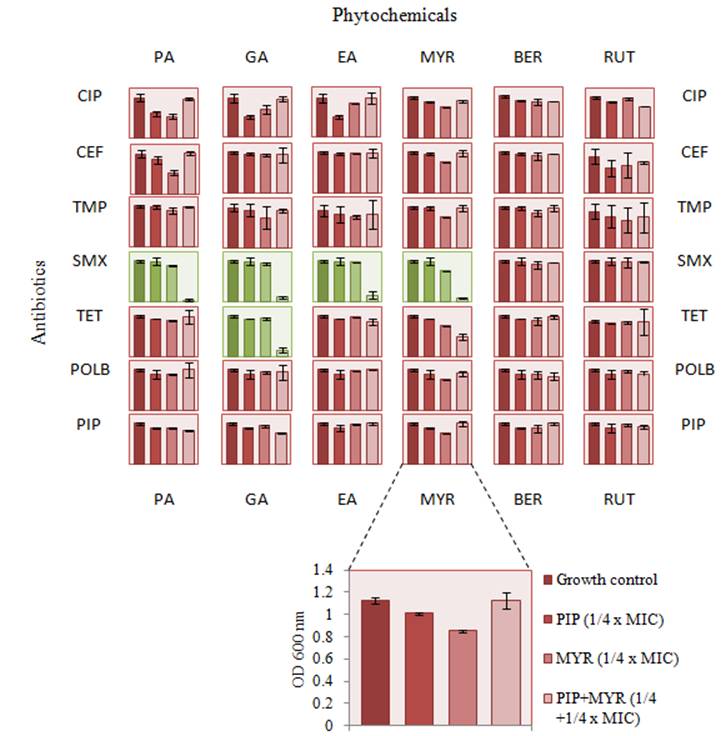
Network interactions between antibiotics and phytochemicals. Antibiotics are classified based on their respective targets and mechanism of action. CE, ceftazidime; PI, piperacillin - cell wall inhibitors; T, trimethoprim; S, sulfamethoxazole - folic acid biosynthesis inhibitors; POL B, polymyxin B - cell membrane inhibitors; TET, tetracycline - 30S ribosome inhibitors; CIP, ciprofloxacin - DNA gyrase and toposiomerase IV inhibitors. Phytochemicals are classified based on their classes. PA, protocatechuic acid; GA, gallic acid; EA, ellagic acid -polyphenol antioxidants (phenolic acids); BER, berberine - alkaloid; RUT, rutin; MYR, myricetin - flavonoids.
Time-kill curves
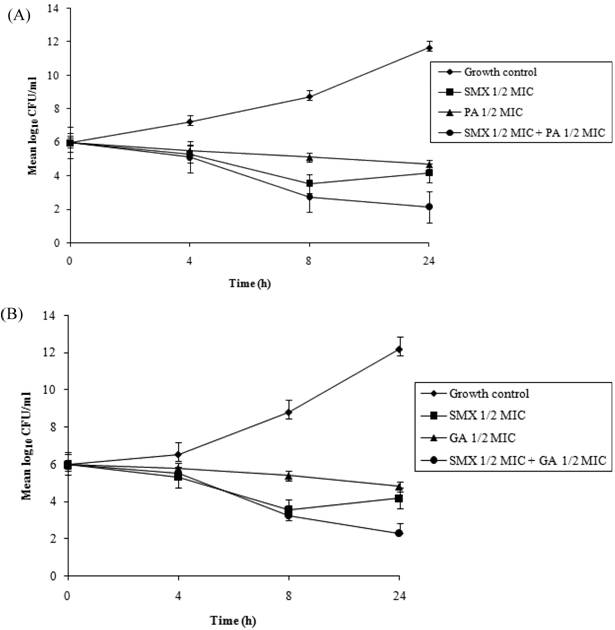
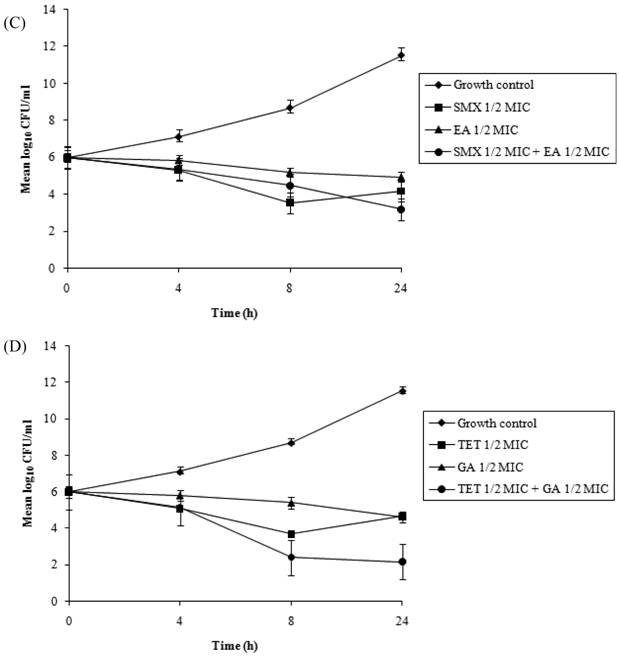
Time-kill assays for the most potent synergistic combinations (sulfamethoxazole plus protocatechuic acid, sulfamethoxazole plus ellagic acid, sulfamethoxazole plus gallic acid and tetracycline plus gallic acid) on ATCC strain are shown (Figures 3: A-D). The net reduction in colony count was seen consistently (throughout 24 h) at (1/2th + 1/2th) x MIC for the identified synergistic drug-herb combinations. Any single active agent alone at 1/2th x MIC did not show significant inhibition of the isolates tested. The rate of killing was higher at 8 h with maximum reduction in the colony count at 24 h. When 1/2th x MIC sulfamethoxazole was combined with 1/2th x MIC protocatechuic acid, 1/2th x MIC sulfamethoxazole was combined with 1/2th x MIC gallic acid, 1/2th x MIC tetracycline was combined with 1/2th x MIC gallic acid, significant decrease in bacterial count was observed between 8 - 24 h with maximum reduction in the growth baseline by 4 - 5 log10 CFU/ml. However, when 1/2th x MIC sulfamethoxazole was combined with 1/2th x MIC ellagic acid a reduction of 1 - 2 log10 decrease in viable count occurred at 24 h.
Discussion
P. aeruginosa is a tenacious pathogen because of its ability to develop resistance to multiple antibiotics. Though, there are a few anti-pseudomonal drugs in the pipeline [22], there is an urgent need for novel and effective infection control strategy to face this highly critical therapeutic challenge of drug resistance [23-24].
Since long, bioactive compounds from natural sources have shown the potential to inhibit resistance mechanisms [25]. Here, we report on the synergistic and additive interactions between selected phytochemicals and antibiotics which are folate biosynthesis inhibitors, DNA/protein synthesis inhibitors, and cell permeability/cell wall inhibitors.
Antibiotics and phytochemicals - interaction analysis
Folate Biosynthesis inhibitors
Sulfamethoxazole (a sulfonamide), competitively inhibits the binding of para aminobenzoic acid (pABA) to the enzyme dihydropteroate synthase (DHPS) that catalyses the formation of dihydrofolate [26]. Trimethoprim inhibits dihydrofolate reductase (DHFR) enzyme which catalyses the NADPH-dependent reduction of dihydrofolate to tetrahydrofolate, an important cofactor involved in supplying one carbon for the synthesis of purines, pyrimidines, methionine and many other amino acids. It must be mentioned that nearly all prokaryotes must synthesize folate compounds de novo, starting with GTP and utilizing several different enzymes in a multi-step pathway, whereas eukaryotes (including human) are able to utilize the dietary folates by uptake through a carrier-mediated transport system [27]. Hence, DHFR and DHPS are good targets for antifolates [28].
Recently, we proposed that phytochemicals (protocatechuic acid, gallic acid, quercetin and myricetin) possibly bind to P. aeruginosa DHFR and in combination with sulfamethoxazole may inhibit different steps in the same pathway, thereby resulting in synergistic mode of action. On the other hand indifferent/additive interaction of trimethoprim and phytochemicals may be due to the binding of these phytochemicals into the active site cavity of DHFR, thereby resulting in interactions with common target residues similar to that of trimethoprim, leading to competitive inhibition and no inhibition of cell growth [29]. Here, we reconfirm the above findings using 5 strains - including two clinical strains- of P. aerugoinosa and also provide data on MICs, and time-kill. We further report on synergism between the combination of ellagic acid and sulfamethoxazole and propose similar mode of action, as ellagic acid may also potentially bind in P. aeruginosa DHFR active site.However, combinations of sulfamethoxazole plus rutin, sulfamethoxazole plus berberine, trimethoprim plus rutin and trimethoprim plus berberine resulted in indifferent/additive mode of interaction.
Time-kill data for synergistic combinations at ½ x MIC against P. aeruginosa ATCC strain 15692. (A) Combinations of sulfamethoxazole (SMX) and protocatechuic acid (PA); (B) Combinations of sulfamethoxazole (SMX) and gallic acid (GA); (C) Combinations of sulfamethoxazole (SMX) and ellagic acid (EA); (D) Combinations of tetracycline (TET) and gallic acid (GA). Filled diamond, control; Filled square, antibiotics; Filled triangle, phytochemicals; Filled circle, antibiotic and phytochemical combination.
Protein synthesis (30S ribosome) inhibitor
Synergistic interactions were observed for combination of tetracycline and gallic acid for all the P. aeruginosa strains under investigation. Sudano et al., reported that epigallocatechin gallate enhances the tetracycline activity for resistant staphylococcal isolates by impairment of tetracycline efflux pump activity and increased intracellular retention of the drug leading to synergistic drug combination [30]. Since, gallic acid is a structural component of epigallocatechin galate, there is a possibility that it may behave similarly in combination with tetracycline. Additive mode of interactions was observed for combinations of tetracycline and protocatechuic acid, ellagic acid, rutin, berberine and myricetin for all the P. aeruginosa strains under investigation.
DNA replication inhibitor
The combinations of ciprofloxacin/phytochemicals were found to be additive. Ciprofloxacin has been earlier reported to have two targets (DNA gyrase and DNA topoisomerase IV) [31]. It is suggested that quercetin, inhibits supercoiling activity of E. coli bacterial gyrase by binding to the ATP binding site of gyrase B [32]. It is also reported that quercetin and myricetin bind to DNA and induce enzymic DNA breakage [33]. Recently, epigallocatechin gallate is reported to inhibit E. coli bacterial DNA gyrase [34]. Bernard et al. found that rutin induces topoisomerase IV-mediated DNA cleavage and growth inhibition in E. coli [35]. The mechanism of action of berberine was found to be similar to that of rifampicin and norfloxacin possibly targeting RNA polymerase, DNA gyrase and topoisomerase IV enzymes [36]. The present study interaction data from (Figure 2) shows similar mechanism of action of the above phytochemicals as DNA synthesis inhibitors by having indifferent activity in combination with ciprofloxacin.
Cell wall / cell membrane permeability inhibitors
Ceftazidime, causes cell lysis in P. aeruginosa due to its primary activity against membrane proteins to which penicillin is reported to bind [37]. Also, quercetin is reported to cause increase in permeability of the inner bacterial membrane and loss of membrane potential [38]. On the other hand, galangin, a structural analogue of quercetin, induces cytoplasmic membrane interference damage and potassium leakage leading to cell wall damage [39]. The correlation between indifferent activities of phytochemicals in combination with ceftazidime from the experimental data may possibly suggest that these compounds may act at same target either at different sites in the cytoplasmic membrane with direct contact with agonist site or at the same site.
Polymyxin B is reported to have a detergent like mechanism of action and interacts with lipoplysaccharide (LPS) of the outer membrane of P. aeruginosa [40-41]. Polymyxin B in combination with the tested phytochemicals results in additive/indifferent mode of interaction. The LPS molecules in the outer leaflet form specific contacts with integral outer membrane proteins and are required for LPS transport to the bacterial cell surface [42]. It is earlier suggested that in the presence of lipopolysaccharide as a barrier in gram-negative bacteria, the susceptibility of catechins may be low [43]. The combinations of polymyxin B plus phytochemicals may help overcome this barrier due to the detergent like activity of polymyxin B and cytoplasmic membrane damage inducing ability of phytochemicals. However, it is possible that there may be a direct competition for binding to LPS or by indirectly affecting the same site of the same target by overlapping actions subsequently resulting in additive mode of action. Similarly, piperacillin, an extened spectrum β-lactam antibiotic shows additive effect in combination with the phytochemicals under investigation.
This study highlights the various systemic interaction patterns and may help identify new drug combinations with novel mechanism of action. It must be noted that no antagonistic interaction was observed for any antibiotic- phytochemical combination studied. These results suggest that the identified combinations could be potential and effective strategy, without the concern of antagonistic interactions. One of the major concerns for the dietary phytochemicals under investigation is the higher MIC values in comparison to antibiotics, which are generally in the range of < 10 µg/ml range [44]. However, it is noteworthy that, the main polyphenol dietary sources are fruits and beverages with an average total intake of 1g/day and after the consumption of 10-100 mg of a single phenolic compound, the maximum concentration in plasma rarely exceeds 1 µM [45]. Additionally the toxicity of these phytochemicals are very low and as of date, very few adverse side effects are reported [46]. Nonetheless, detailed analysis needs to be carried out to characterize the interactions of antibacterial phytochemicals and antibiotics with the use of different approaches and methods facilitated by targets profile and toxicity data.
Conclusion
The present study clearly highlights the low toxic potential of phytochemicals as antibacterial compounds and suggest on the possibility of use of the above shown synergistic drug-herb combinations for combating infections caused by this pathogen. The drug-herb network presented in this study shows the level of interactions between various classes of antibiotics and phytochemicals and provides a baseline to identify the potential mechanism of action of these potential phytochemicals. Moreover, phytochemicals are reported to have the capability of increasing the susceptibility of the pathogen to various drugs and also reduce the toxicity of the drugs when used in combination. Finally, the experimental findings encourage further studies with these agents and other antimicrobial classes and in vivo animal experiments to validate these interesting observations before clinical test can move forward. The investigations are underway to further characterize the interaction of the above phytochemicals and antibiotics.
Acknowledgements
This work was funded by a seed grant from Biopharmaceutical Engineering Cluster, Nanyang Technological University, Singapore and AcRF grant from NTU to MKS, KRS and CSL. The authors would like to thank Dr. Tan Ai Ling, Senior consultant and Head of the Diagnostic Bacteriology section at the Department of pathology, Singapore General Hospital, for providing the clinical strains of Pseudomonas aeruginosa.
Conflict of Interests
The authors have declared that no conflict of interest exists.
References
1. Mesaros N, Nordmann P, Plésiat P. et al. Pseudomonas aeruginosa: Resistance and therapeutic options at the turn of the new millennium. Clinical Microbiology and Infection. 2007;13:560-578
2. Singh SB, Barrett JF. Empirical antibacterial drug discovery - Foundation in natural products. Biochemical Pharmacology. 2006;71:1006-1015
3. Campbell Jr GD, Niederman MS, Broughton WA. et al. Hospital-acquired pneumonia in adults: Diagnosis, assessment of severity, initial antimicrobial therapy, and preventative strategies: A consensus statement. American Journal of Respiratory and Critical Care Medicine. 1996;153:1711-1725
4. El Solh AA, Alhajhusain A. Update on the treatment of Pseudomonas aeruginosa pneumonia. Journal of Antimicrobial Chemotherapy. 2009;64:229-238
5. Gould IM, Milne K. In-vitro pharmacodynamic studies of piperacillin/tazobactam with gentamicin and ciprofloxacin. Journal of Antimicrobial Chemotherapy. 1997;39:53-61
6. Sobieszczyk ME, Furuya EY, Hay CM. et al. Combination therapy with polymyxin B for the treatment of multidrug-resistant Gram-negative respiratory tract infections. Journal of Antimicrobial Chemotherapy. 2004;54:566-569
7. Fish DN, Choi MK, Jung R. Synergic activity of cephalosporins plus fluoroquinolones against Pseudomonas aeruginosa with resistance to one or both drugs. Journal of Antimicrobial Chemotherapy. 2002;50:1045-1049
8. Kanellakopoulou K, Sarafis P, Galani I. et al. In vitro synergism of β-lactams with ciprofloxacin and moxifloxacin against genetically distinct multidrug-resistant isolates of Pseudomonas aeruginosa. International Journal of Antimicrobial Agents. 2008;32:33-39
9. Tré-Hardy M, Vanderbist F, Traore H. et al. In vitro activity of antibiotic combinations against Pseudomonas aeruginosa biofilm and planktonic cultures. International Journal of Antimicrobial Agents. 2008;31:329-336
10. Obritsch MD, Fish DN, MacLaren R. et al. National surveillance of antimicrobial resistance in Pseudomonas aeruginosa isolates obtained from intensive care unit patients from 1993 to 2002. Antimicrobial Agents and Chemotherapy. 2004;48:4606-4610
11. Coutinho HDM, Costa JGM, Lima EO. et al. Herbal therapy associated with antibiotic therapy: Potentiation of the antibiotic activity against methicillin - Resistant Staphylococcus aureus by Turnera ulmifolia L. BMC Complementary and Alternative Medicine. 2009;9:35
12. Coutinho HDM, Costa JGM, Lima EO. et al. Enhancement of the antibiotic activity against a multiresistant Escherichia coli by Mentha arvensis L. and chlorpromazine. Chemotherapy. 2008;54:328-330
13. Garo E, Eldridge GR, Goering MG. et al. Asiatic acid and corosolic acid enhance the susceptibility of Pseudomonas aeruginosa biofilms to tobramycin. Antimicrobial Agents and Chemotherapy. 2007;51:1813-1817
14. Cowan MM. Plant Products as Antimicrobial Agents. Clin Microbiol Rev. 1999;12:564-582
15. Gurib-Fakim A. Medicinal plants: Traditions of yesterday and drugs of tomorrow. Molecular Aspects of Medicine. 2006;27:1-93
16. Harvey AL. Natural products in drug discovery. Drug Discovery Today. 2008;13:894-901
17. Lewis K, Ausubel FM. Prospects for plant-derived antibacterials. Nature Biotechnology. 2006;24:1504-1507
18. Nascimento GGF, Locatelli J, Freitas PC. et al. Antibacterial activity of plant extracts and phytochemicals on antibiotic-resistant bacteria. Brazilian Journal of Microbiology. 2000;31:247-256
19. Stavri M, Piddock LJV, Gibbons S. Bacterial efflux pump inhibitors from natural sources. Journal of Antimicrobial Chemotherapy. 2007;59:1247-1260
20. Huang WY, Cai YZ, Zhang Y. Natural phenolic compounds from medicinal herbs and dietary plants: Potential use for cancer prevention. Nutrition and Cancer. 2010;62:1-20
21. National Committee for Clinical Laboratory Standards. Methods for determining bactericidal activity of antimicrobial agents; Tentative guideline; NCCLS document M26-T. Villanova, Pa: National Committee for Clinical Laboratory Standards. 1992
22. Page MG, Heim J. Prospects for the next anti-Pseudomonas drug. Current Opinion in Pharmacology. 2009;9:558-565
23. Brown PD, Izundu A. Antibiotic resistance in clinical isolates of Pseudomonas aeruginosa in Jamaica. Revista Panamericana de Salud Publica/Pan American Journal of Public Health. 2004;16:125-130
24. Kipnis E, Sawa T, Wiener-Kronish J. Targeting mechanisms of Pseudomonas aeruginosa pathogenesis. Medecine et Maladies Infectieuses. 2006;36:78-91
25. Gibbons S. Phytochemicals for bacterial resistance - Strengths, weaknesses and opportunities. Planta Medica. 2008;74:594-602
26. Huovinen P. Resistance to trimethoprim-sulfamethoxazole. Clinical Infectious Diseases. 2001;32:1608-1614
27. Achari A, Somers DO, Champness JN. et al. Crystal structure of the anti-bacterial sulfonamide drug target dihydropteroate synthase. Nature Structural Biology. 1997;4:490-497
28. Hawser S, Lociuro S, Islam K. Dihydrofolate reductase inhibitors as antibacterial agents. Biochemical Pharmacology. 2006;71:941-948
29. Jayaraman P, Sakharkar M, Sing L. et al. Insights into antifolate activity of phytochemicals against Pseudomonas aeruginosa. J Drug Target. 2010 [Epub ahead of print]
30. Roccaro AS, Blanco AR, Giuliano F. et al. Epigallocatechin-gallate enhances the activity of tetracycline in staphylococci by inhibiting its efflux from bacterial cells. Antimicrobial Agents and Chemotherapy. 2004;48:1968-1973
31. Hirai K. History of mode of action and resistance mechanisms of quinolones. Japanese Journal of Chemotherapy. 2005;53:349-356
32. Plaper A, Golob M, Hafner I. et al. Characterization of quercetin binding site on DNA gyrase. Biochemical and Biophysical Research Communications. 2003;306:530-536
33. Austin CA, Patel S, Ono K. et al. Site-specific DNA cleavage by mammalian DNA topoisomerase II induced by novel flavone and catechin derivatives. Biochemical Journal. 1992;282:883-889
34. Gradišar H, Pristovšek P, Plaper A. et al. Green tea catechins inhibit bacterial DNA gyrase by interaction with its ATP binding site. Journal of Medicinal Chemistry. 2007;50:264-271
35. Bernard FX, Sablé S, Cameron B. et al. Glycosylated flavones as selective inhibitors of topoisomerase IV. Antimicrobial Agents and Chemotherapy. 1997;41:992-998
36. Yi ZB, Yan Y, Liang YZ. et al. Evaluation of the antimicrobial mode of berberine by LC/ESI-MS combined with principal component analysis. Journal of Pharmaceutical and Biomedical Analysis. 2007;44:301-304
37. Hayes MV, Orr DC. Mode of action of ceftazidime: Affinity for the penicillin-binding proteins of Escherichia coli K12, Pseudomonas aeruginosa and Staphylococcus aureus. Journal of Antimicrobial Chemotherapy. 1983;12:119-126
38. Mirzoeva OK, Grishanin RN, Calder PC. Antimicrobial action of propolis and some of its components: The effects on growth, membrane potential and motility of bacteria. Microbiological Research. 1997;152:239-246
39. Cushnie TPT, Lamb AJ. Detection of galangin-induced cytoplasmic membrane damage in Staphylococcus aureus by measuring potassium loss. Journal of Ethnopharmacology. 2005;101:243-248
40. Evans ME, Feola DJ, Rapp RP. Polymyxin B sulfate and colistin: Old antibiotics for emerging multiresistant gram-negative bacteria. Annals of Pharmacotherapy. 1999;33:960-967
41. Hancock REW. Peptide antibiotics. Lancet. 1997;349:418-422
42. Bos MP, Tommassen J. Biogenesis of the Gram-negative bacterial outer membrane. Current Opinion in Microbiology. 2004;7:610-616
43. Ikigai H, Nakae T, Hara Y. et al. Bactericidal catechins damage the lipid bilayer. Biochimica et Biophysica Acta - Biomembranes. 1993;1147:132-136
44. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Twelfth Informational Supplement M100-S12. Wayne, PA: Clinical and Laboratory Standards Institute. 2002
45. Scalbert A, Williamson G. Dietary intake and bioavailability of polyphenols. Journal of Nutrition. 2000;130:2073S-2085S
46. Bode AM, Dong Z. Molecular and cellular targets. Mol Carcinog. 2006;45:422-430
47. Chopra I, Roberts M. Tetracycline antibiotics: Mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiology and Molecular Biology Reviews. 2001;65:232-260
48. Iida K, Hirata S, Nakamuta S. et al. Inhibition of cell division of Escherichia coli by a new synthetic penicillin, piperacillin. Antimicrobial Agents and Chemotherapy. 1978;14:257-266
49. Mason TL, Bruce P W. Inactivation of red beet β-glucan synthase by native and oxidized phenolic compounds. Phytochemistry. 1987;26:2197-2202
50. Mori A, Nishino C, Enoki N. et al. Antibacterial activity and mode of action of plant flavonoids against Proteus vulgaris and Staphylococcus aureus. Phytochemistry. 1987;26:2231-2234
Author contact
![]() Corresponding author: Meena K. Sakharkar (Ph.D.), Professor, Graduate School of Life and Environmental Sciences, Tsukuba University, Japan. Tel: +81-29-853-8834; Email: meena.sak.gntsukuba.ac.jp. Kishore R. Sakharkar (Ph.D.), Professor, USM, Malaysia. Tel: +60-4562 2387; Email: ksakharkarcom.
Corresponding author: Meena K. Sakharkar (Ph.D.), Professor, Graduate School of Life and Environmental Sciences, Tsukuba University, Japan. Tel: +81-29-853-8834; Email: meena.sak.gntsukuba.ac.jp. Kishore R. Sakharkar (Ph.D.), Professor, USM, Malaysia. Tel: +60-4562 2387; Email: ksakharkarcom.

 Global reach, higher impact
Global reach, higher impact