Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2010; 6(7):639-648. doi:10.7150/ijbs.6.639 This issue Cite
Research Paper
Comparative Analysis of Cytotoxic T Lymphocyte Response Induced by Dendritic Cells Loaded with Hepatocellular Carcinoma -Derived RNA or Cell Lysate
State Key Laboratory of Oncology in Southern China and Department of Experimental Research, Cancer Center, Sun Yat-sen University, Guangzhou 510060, P.R. China.
Received 2010-7-25; Accepted 2010-8-29; Published 2010-10-13
Abstract
The choice of the tumor antigen preparation used for dendritic cell (DC) loading is important for optimizing DC vaccines. In the present study, we compared DCs pulsed with hepatocellular carcinoma (HCC) total RNA or cell lysates for their capacity to activate T cells. We showed here that HCC total RNA pulsed-DCs induced effector T lymphocyte responses which showed higher killing ability to HCC cell lines, as well as higher frequency of IFN-γ producing of CD4+ and CD8+ T cells when compared with lysate pulsed-DCs. Both of RNA and lysate loading did not influence the changes of mature DC phenotype and the capacity of inducing T cell proliferation. However, HCC lysate loading significantly inhibited the production of inflammatory cytokines IL-12p70, IFN-γ and enhanced the secretion of anti-inflammatory cytokines IL-10 of mature DCs. Our results indicated that DCs loaded with HCC RNA are superior to that loaded with lysate in priming anti-HCC CTL response, suggesting that total RNA may be a better choice for DCs-based HCC immunotherapy.
Keywords: Dendrtic cells, tumor total RNA, tumor lysate, hepatocellular carcinoma, cytotoxic T cells
Introduction
Hepatocellular carcinoma (HCC) is one of the most common cancers that typically has poor prognosis. It is the third leading cause of cancer deaths worldwide, especially in Asia (1-3). Traditional therapeutic modalities such as hepatic resection did not significantly increase the 5-year survival rate. Therefore, it is urgently required to develop novel treatment strategies for HCC. Dendritic cells (DC) are highly specialized antigen-presenting cells (APC) that control a spectrum of innate and adaptive immune responses, and could effectively induce cytotoxic T lymphocyte (CTL) response against cancer (4, 5). The objective regressions after DC-based treatments are observed in some patients, even in those with metastatic disease (6, 7). Recently, increasing reports demonstrated that HCC antigen-loaded DC could promote the priming of specific CTL responses, including in vivo and in vitro model (8, 9). In clinical trial, some of HCC patients showed stable disease after vaccinated with HCC lysate-pulsed DCs (10-12). These studies clearly demonstrated that DC-based immunotherapy could be a promising approach for HCC treatment.
A critical issue in optimizing DC vaccines is the choice of tumor antigen for DC loading. Loading MHC class I and class II molecules at the cell surface of DCs with peptides derived from defined antigens is the most commonly used strategy (13). The limitations of this method are that the tumor antigen must have been identified, and only a restricted repertoire of T-cell clones could be induced, thereby limiting the ability of the immune system to control tumor antigen variation (5). An alternative strategy is loading DC with total tumor antigen such as tumor lysate, which could generate a diverse immune response that involves many CD4+ T-cell and CTL clones against tumor antigens, including both identified and unidentified antigens. Tumor cell lysate has been widely used as total antigen for DC-based cancer immunotherapy, including HCC (11, 12). However, several studies recently reported that tumor lysate would impair the function of DCs and result in limited immune responses, indicated the potential disadvantage of tumor lysate (14-16). Another commonly used total antigen is tumor-derived RNA, which is an attractive method because its isolation and use in clinical settings is more straightforward than the use of exogenously provided peptides and proteins (17). Loading DC with RNA has been turned out to efficiently stimulate robust CTL responses and antitumor immunity in human, including in vitro study and clinical trial (18-24), suggesting that RNA-loaded DC might be a promise strategy for cancer immunotherapy. So far, there are few studies on the relative efficacy of lysate or RNA loading methods to be performed in HCC.
Thus, in the present study we compared DC either loaded with tumor lysates or RNA from the same HCC cell line for their capacity to stimulate specific anti-HCC immune responses. In a separate in vitro model of whole HCC antigen loading, our studies showed that pulsing DCs with HCC total RNA induced a higher frequency of activated CTLs and T-helper cells, as well as stronger tumor cell lysis compared with lysate. These results provide new information for optimization of DCs-based immunotherapy in HCC.
Material and Methods
Cell Lines and Culture
Different human HCC cell lines, Hep-G2, Hep-3B, were obtained from the American Type Culture Collection (ATCC). Huh-7, SMMC-7721 cell lines were obtained from the Committee of Type Culture Collection of Chinese Academy of Sciences (Shanghai, China). Cells were grown in RPMI 1640 supplemented with 10% (v/v) FBS (fetal bovine serum) and antibiotics (50 μg/ml each of penicillin, streptomycin and gentamicin) at 37 °C in a humidified 5% CO2 atmosphere.
Culture of Human DCs
PBMC were isolated from peripheral blood of healthy donors by Ficoll-Hypaque gradient centrifugation. And then, monocytes were allowed to adhere in six-well culture plates for 1 hour at 37ºC. Nonadherent cells were removed and cultured in 20 U/ml IL-2 medium for further use. Adherent cells were cultured in AIM-V complete medium supplemented with 1,000 U/ml GM-CSF and 400 U/ml IL-4 (BioSource, USA). Half of the old medium was replaced every 2 days with fresh medium. After culture for 6 days, immature dendritic cells (iDCs) were generated.
Tumor cell RNA or Lysates Loading of Cultured DCs
Total RNA was extracted from HCC cell lines using Trizol reagent (Invitrogen, USA) according to the manufacturer's instructions. Tumor cell lysates were generated by four rapid freeze-thaw cycles as described (25). Both RNA and protein loading of DCs was performed by simple coincubation of DCs without any transfection reagent. In brief, immature DCs generated on day 6 were washed twice in PBS, counted, and resuspended in AIM-V medium supplemented with GM-CSF and IL-4. Then tumor cells RNA or lysates were added (20 μg RNA or 100 μg lysates/5×105 DCs in 1 ml medium) and incubated with DCs for 3 hour in a humidified incubator at 37°C/5% CO2. After 3 hours, antigen-loaded DCs were treated with 0.1 KE/ml (10 μg/ml) OK432 (Shandong Lukang Pharmaceutical Co., China) for 48 hour.
In Vitro Generation of CTLs and Cytotoxicity Assays
Antigen loading-DCs were cocultured with autologeous nonadherent PBMCs from the same healthy donor at a ratio of 1:10 in AIM-V medium containing IL-2 (25 units/ml) in 24 well plates. Half of the old medium was replaced every 2 days with fresh medium. After 5 days of coculture, the cells were used for cytotoxicity assays using the lactat dehydrogenase-release assay (CytoTox 96; Promega, USA) according to the protocol.
Phenotypic Analysis of DC by Flow Cytometry
DCs or T cells were re-suspended at 2.5 × 105 cells in 100 μl of PBS and incubated for 30 min at 4ºC with different fluorescein isothiocyanate (FITC)-conjugated monoclone antibodies (mAbs) or appropriate isotype controls. After three washes in cold phosphate-buffered saline (PBS) supplemented with 0.5% of bovine serum albumin (BSA), cells were fixed with 2% paraformaldehyde in PBS. The following mAbs were used: FITC-anti-CD80, FITC-anti-CD83, FITC-anti-CD86, FITC-anti-HLA-DR (BD Biosciences, USA). Cells were collected and analyzed using a CytomicsTM FC500 Flow Cytometry (Beckman Coulter). Data analysis was performed by CXP Analysis software (Beckman Coulter).
Mixed Lymphocyte Reaction (MLR)
For proliferation assays, non-adherent cells collected from freshly isolated PBMC were resuspended in PBS (1 x 107 cells/ml) containing carboxyfluorescein diacetate succinimidyl ester (CFSE, Molecular Probes) at a final concentration of 1 µM, incubated at 37°C for 10 min, and washed three times. And then the labeled cells were coincubated with antigen-loading DC pretreated with OK-432 for 5 days at 1:10 ratios on a 48-well plate in AIM-V medium. Cells were harvested and analyzed by FACS. Data analysis was performed using CXP Analysis software (Beckman Coulter).
Analysis of Cytokines
Cytokines TNF-α, IL-12p70, IFN-γ, IP-10, IL-10 and TGF-β in the supernatants of differently treated DCs cultures were measured by ELISA kits (BioSource Systems) according to the manufacturer's protocols.
Flow Cytometric Analysis of Intracellular Cytokine Expression
To determine the stimulation of T cells by HCC antigen-loading DCs, IFN-γ and IL-4 expression was assessed by intracellular staining. Briefly, T cells were collected and incubated at 37°C for 6 h in AIM-V medium containing 1 μg/ml anti-CD3 mAb and 1 μg/ml anti-CD28 mAb (BD Biosciences, USA). Brefeldin A, 10 ng/ ml, was added for the final 5 h of incubation to block cytokine secretion. The cells were harvested, washed and stained with PE-conjugated anti-CD4 and FITC-conjugated anti-CD8 mAb for 30 min on ice. Then the cells were fixed with 2% paraformaldehyde in phosphate buffered saline (PBS) for 15 min at room temperature and permeabilized with PBS/0.5% saponin (Sigma, St Louis, MO) for another 10 min at room temperature. Finally, cells were further labeled with APC-conjugated anti-IFN-γ or anti-IL-4 mAb and analyzed by flow cytometer.
Statistical Analysis
Data were compared using the Student's t test, and differences of p<0.05 were considered to be significant. Each experiment was repeated at least three times.
Results
Cytolytic Activity of the T cells Against HCC Cell Lines in vitro
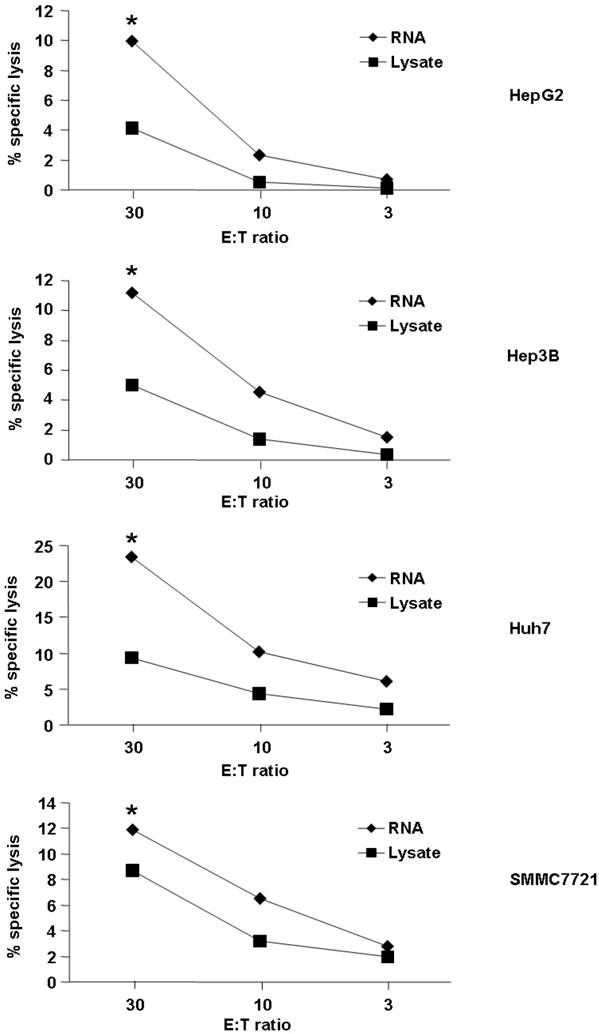
First, we detected cytotoxic activity of T cells induced by DC loaded with RNA or lysate from the HCC cell line. As demonstrated in Fig. 1, both HCC RNA and lysate-pulsed DCs were capable of stimulating CTLs that recognized and lysed HCC cell lines. However, loading DC with RNA could induce the better lytic activity than lysate (the percentages of cytotoxicity were between approximately 10% to 23% for RNA loading group vs 4% to 9% for lysate-pulsed group at an effector : target ratio of 30 : 1, p<0.05). We used four different HCC cell lines for detection, and the same trend was observed (Fig. 1), indicated that loading DC with RNA might stimulate higher cytolytic activity of the T cells against HCC.
Cytokine Production of CD4+ and CD8+ T Cells
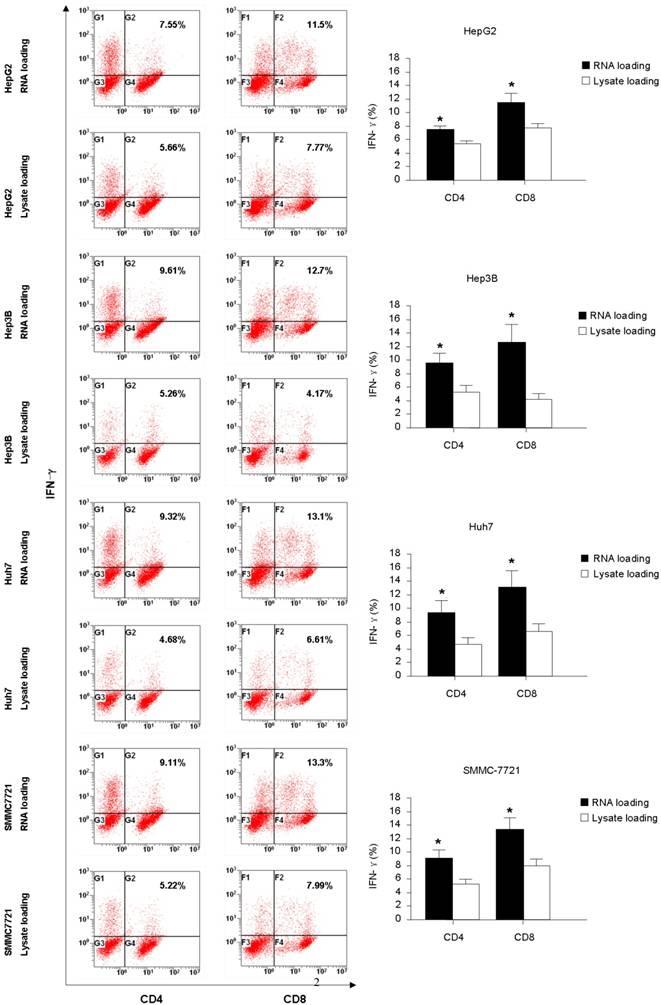
The intracellular staining for IFN-γ and IL-4 production were carried out to further assess the function of the T cells generated from co-culture with HCC RNA or lysate pulsed-DCs. From the Fig. 2, we found that RNA pulsed-DCs induced significant higher numbers of IFN-γ+CD4+ and IFN-γ+CD8+ T cells compared to lysate pulsed-DCs. Low levels of IL-4 producing were detected in T cells stimulated by both type antigen pulsed-DCs (data not show). These results indicate that HCC RNA pulsed-DCs activate Th1 and CTL functional T cells more efficiently than HCC lysate pulsed-DCs.
Effect of HCC Total RNA or Lysate Pulsing on DCs Phenotype Changes and Cytokines Production
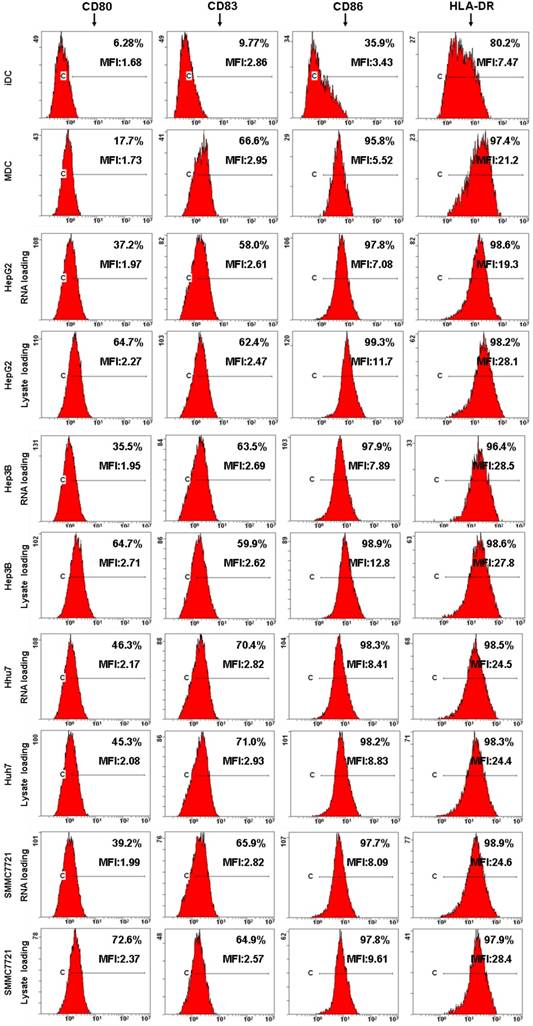
Next, DCs immunophenotype changes and cytokine production were detected by flow cytometre and ELISA to analyze the effects of HCC total RNA or lysate on DCs maturation. As shown in Fig. 3, untreated DCs exhibited low-level expression of costimulatory molecules CD80, CD86 as well as the maturation marker CD83, HLA-DR. OK-432 stimulation significantly increased CD80, CD83, CD86 and HLA-DR expression. Both of HCC total RNA and lysate pulsing enhanced CD80 expression but did not significantly alter CD83, CD86 and HLA-DR expression of mature DCs. These results indicated that HCC total RNA or lysate pulsing did not inhibit costimulatory molecules and maturation marker expression of mature DCs.
Tumor cell killing by T cells stimulated with tumor antigen-pulsed DCs. Autologeous nonadherent PBMCs were cocultured with DCs pulsed with HCC total RNA or lysate. After 5 days of incubation, cytotoxic activity of T cells against Hep-G2, Hep-3B, Huh-7 and SMMC-7721 was evaluated using a LDH release assay at different E:T ratios. *p<0.05.
Intracellular IFN-γ production of CD4+ and CD8+ T-cells stimulated with DC pulsed with HCC total RNA or lysate. Autologeous nonadherent PBMCs were cocultured with RNA-DCs or lysate-DCs for 5 days. And then, T cells were collected and accessed for intracellular expression of IFN-γ in CD4+ and CD8+ T cells using flow cytometry. Original histograms are shown with percentage of dual-positive cells. *p<0.05.
Analysis of DC phenotype by flow cytometry. Different group of DCs were harvested and processed for staining with a panel of mAb as indicated. The staining of the indicated surface marker is shown and the percentage of positive cells is stated. The MFI value are also indicated.
Effects of HCC total RNA or lysate loading on the cytokines release of mature DCs. Immature DCs were incubated with RNA (black bar) or lysate (white bar) for 3 hour and then stimulated with OK-432 (0.1 KE/ml) for 2 days. The supernatant were collected for TNF-α, IL-12p70, IFN-γ, IP-10, IL-10 and TGF-β production determine by using ELISA method. The supernatant come from immature DC and OK-432-stimulated DC without loading any antigen are as control (stripe bar). Data are means ± SD of triplicates from one experiment. *p<0.05 (RNA loading compared with lysate loading).
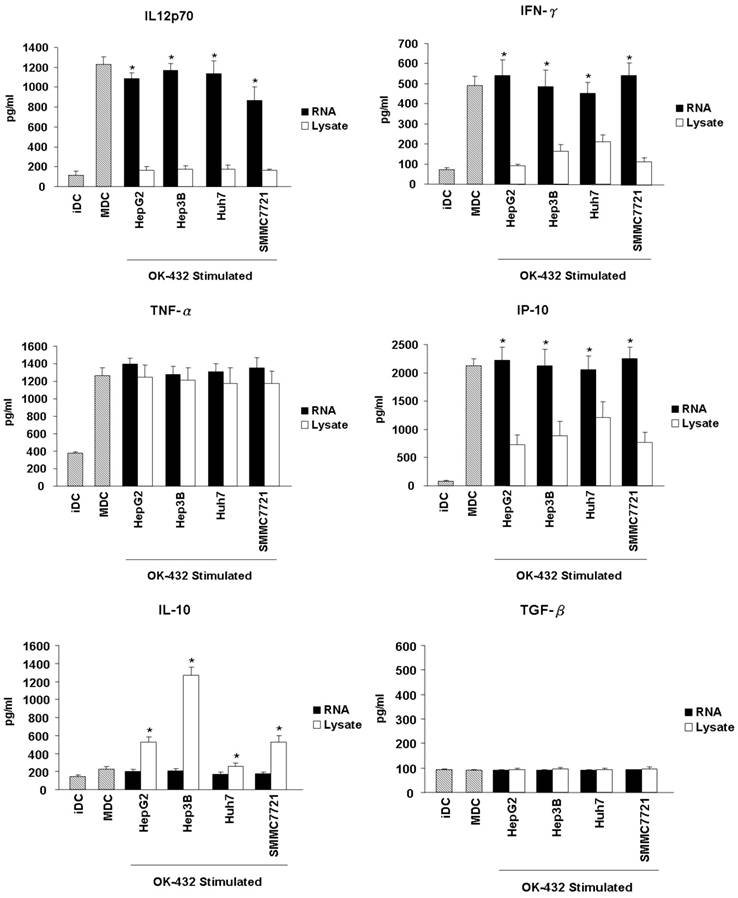
Another important feature of functional DCs is the production of inflammatory cytokines. Thus, the secretion of cytokines from DCs, including IL-12p70, IFN-γ, TNF-α, IP-10, IL-10 and TGF-β were further detected. The level of IL-12p70, IFN-γ, TNF-α and IP-10 increased significantly in the culture supernatants when immature DCs were stimulated with OK-432 (Fig. 4). However, OK-432 treatment did not induce IL-10 and TGF-β production of DCs. HCC RNA loading slightly decreased the production of IL-12p70, and did not exert the production of IFN-γ, TNF-α and IP-10. However, tumor lysate loading markedly decreased the production of IL-12p70, IFN-γ and IP-10, meanwhile, increased the production of IL-10 especially when the Hep3b cell line is used. Both HCC RNA and lysate loading did not enhance the production of TGF-β. These results demonstrated that HCC lysate pulsing will impair Th1-type cytokines secretion and promote anti-inflammatory cytokines production of DCs.
Effect of HCC RNA or Lysate Pulsing on the Stimulatory Capacity of DCs
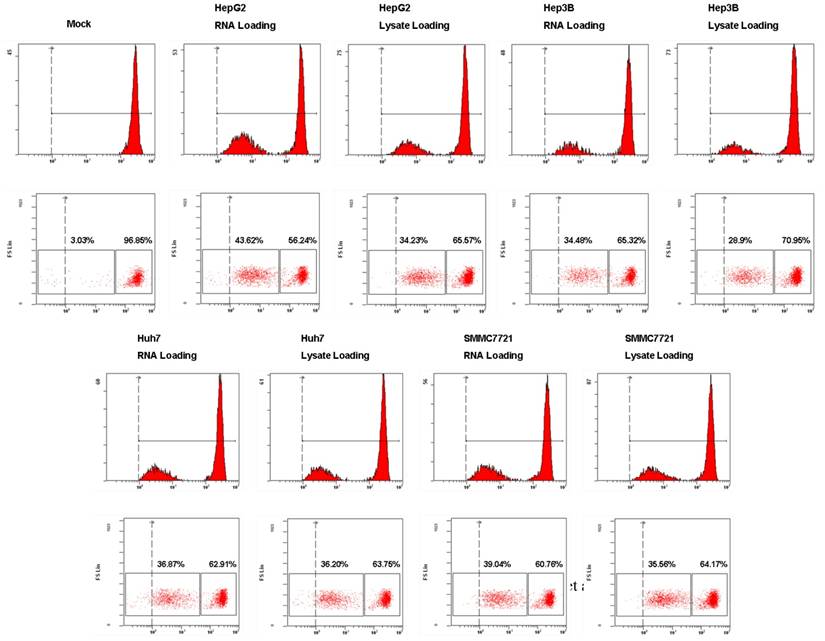
To assess the functional consequences of HCC tumor whole antigen-pulsed DCs, we examined the T cell stimulatory activity of tumor RNA or lysate-pulsed DCs in MLR by CFSE dilution experiments. As shown in Fig. 5, both tumor RNA and lysate-pulsed DCs have potent stimulatory capacity. T cell proliferation was 43.62% (RNA loading) vs. 34.23% (lysate loading) for HepG2; 34.48% (RNA loading) vs. 28.9% (lysate loading) for Hep3B; 36.87% (RNA loading) vs. 36.20% (lysate loading) for Huh7, and 39.04% (RNA loading) vs. 35.56% (lysate loading) for SMMC7721 respectively. The amount of proliferation of T cells was comparable for two types of antigen-loaded DCs.
The stimulatory capacity of DCs pulsed with HCC total RNA or lysate. Analysis of T cells proliferation. Autologous T lymphocytes labeled with CFSE were mixed with RNA-DCs or lysate-DCs at a ratio of 10:1 (T cells to DCs) and cocultured for 5 days. T cell proliferation was performed using flow cytometry and the percentage of proliferated T cells is stated.
Discussion
Dendritic cell (DC)-based therapy has proved to be effective in patients with a variety of malignancies, including HCC. However, an optimal preparation protocol using DCs and the best means for enhancing antigens has not yet been described completely. In the present study, we have demonstrated that DCs loaded with HCC total RNA are more potent antigen than that loaded with lysates in activating effctor T cells such as Th1 and CTL against HCC cell lines, which was evidenced by a higher percentage of tumor cell killing, as well as higher IFN-γ secretion of CD4+ and CD8+ T cells in DCs loaded with HCC total RNA. As well known, activation and polarization of naive T cells requires three signals from DC. Signal 1is the interaction between major histocompatibility complex (MHC) molecules containing peptide fragments on the DC and the T-cell receptor (TCR) on the T cell. Signal 2 is the interaction between costimulatory molecules (e.g. CD80/86) on the surface of the DC with ligands (e.g. CD28) on the T-cell surface. Signal 3 is the secretion of proinflammatory cytokines (e.g. interleukin 12 (IL-12)) by the DC (26-28). Signal 1 and signal 2 allow naive T cells to develop into protective effector cells, while signal 3 promotes the activated CD4+ and CD8+ to differentiate into Th1 and CTL (29-31). In our study, we found that loading DC with HCC total RNA and lysate did not result in the decreased expression of MHC class II and costimulatory molecules, indicating that the two type of antigen did not impair the signal 1 and signal 2. However, unlike RNA, HCC lysate pulsing significantly suppressed the inflammatory cytokines such as IL-12 and IFN-γ production, indicating that HCC lysate would disorder signal 3. Thus, loading DC with HCC lysate induced limited T cells responses might be due to decreased inflammatory cytokines environment.
The cytokine microenvironment plays a key role in T helper cell differentiation toward Th1 or Th2 cell type during immune responses (32). The IL-12 produced by DC polarizes the T-cell response towards a Th1 phenotype, which is believed to be preferential for cancer immunotherapy (33, 34). The absence of IL-12p70 leads to depressed Th1-responses and hence a paucity of IFN-γ-support for CD8+ CTL. It has been reported that tumor lysate, such as melanoma, would suppress IL-12p70 secretion, resulting in less efficiently generating Th1-responses from naïve T-cells, and restoring IL-12p70 production of DC permitted effective generation of Th1-responses (14-16). These results are in agreement with our studies. Thus, tumor RNA loading may be a better choice to induce Th1 and CTL response compared with tumor lysate.
IL-12 production is positively regulated by IFN-γ, which IL-12 itself induces (35, 36). Multiple studies have shown that DCs also produce IFN-γ (37, 38), suggesting the presence of an autocrine-positive feedback pathway between IL-12 and IFN-γ for DC activation (39). In our study, we found OK-432 stimulation could also promote human DCs to secrete high level of IFN-γ, and HCC lysate loading significantly suppress IFN-γ production as well as IL-12, indicating HCC lysate could destroy the positive feedback loop of IL-12 and IFN-γ, and then influence the Th1 response induction.
In summary, our data showed that pulsing DCs with total RNA as antigen seems to be a promising alternative to lysate for priming better Th1 and CTL response against hepatoma cells. Our study might provide a reasonable strategy for DC-based immunotherapy in HCC. Further investigation should be done to evaluate the efficacy of this strategy in clinical application.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (30571717, u0772002, 30700985). We thank Dr. Hong-wei Liu for critical reading of this manuscript.
Conflict of Interest
The authors declare that they do not have any conflict of interest with respect to this manuscript.
References
1. Okuda K. Hepatocellular carcinoma. J Hepatol. 2000;32(1 Suppl):225-237
2. Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153-156
3. Thomas MB, Zhu AX. Hepatocellular carcinoma: the need for progress. J Clin Oncol. 2005;23:2892-2899
4. Palucka AK, Ueno H, Fay JW, Banchereau J. Taming cancer by inducing immunity via dendritic cells. Immunol Rev. 2007;220:129-150
5. Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol. 2005;5:296-306
6. Lesimple T, Neidhard EM, Vignard V, Lefeuvre C, Adamski H, Labarrière N. et al. Immunologic and clinical effects of injecting mature peptide-loaded dendritic cells by intralymphatic and intranodal routes in metastatic melanoma patients. Clin Cancer Res. 2006;12:7380-7388
7. Wierecky J, Müller MR, Wirths S, Halder-Oehler E, Dörfel D, Schmidt SM. et al. Immunologic and clinical responses after vaccinations with peptide-pulsed dendritic cells in metastatic renal cancer patients. Cancer Res. 2006;66:5910-5918
8. Lee WC, Wang HC, Jeng LB, Chiang YJ, Lia CR, Huang PF. et al. Effective treatment of small murine hepatocellular carcinoma by dendritic cells. Hepatology. 2001;34:896-905
9. Wang XH, Qin Y, Hu MH, Xie Y. Dendritic cells pulsed with gp96-peptide complexes derived from human hepatocellular carcinoma (HCC) induce specific cytotoxic T lymphocytes. Cancer Immunol Immunother. 2005;54:971-980
10. Iwashita Y, Tahara K, Goto S, Sasaki A, Kai S, Seike M. et al. A phase I study of autologous dendritic cell-based immunotherapy for patients with unresectable primary liver cancer. Cancer Immunol Immunother. 2003;52:155-161
11. Lee WC, Wang HC, Hung CF, Huang PF, Lia CR, Chen MF. Vaccination of advanced hepatocellular carcinoma patients with tumor lysate-pulsed dendritic cells: a clinical trial. J Immunother. 2005;28:496-504
12. Palmer DH, Midgley RS, Mirza N, Torr EE, Ahmed F, Steele JC. et al. A phase II study of adoptive immunotherapy using dendritic cells pulsed with tumor lysate in patients with hepatocellular carcinoma. Hepatology. 2009;49:124-132
13. Figdor CG, de Vries IJ, Lesterhuis WJ, Melief CJ. Dendritic cell immunotherapy: mapping the way. Nat Med. 2004;10:475-480
14. Jackson AM, Mulcahy LA, Zhu XW, O'Donnell D, Patel PM. Tumour-mediated disruption of dendritic cell function: inhibiting the MEK1/2-p44/42 axis restores IL-12 production and Th1-generation. Int J Cancer. 2008;123:623-632
15. Yang DH, Park JS, Jin CJ, Kang HK, Nam JH, Rhee JH. et al. The dysfunction and abnormal signaling pathway of dendritic cells loaded by tumor antigen can be overcome by neutralizing VEGF in multiple myeloma. Leuk Res. 2009;33:665-670
16. Hatfield P, Merrick AE, West E, O'Donnell D, Selby P, Vile R. et al. Optimization of Dendritic Cell Loading With Tumor Cell Lysates for Cancer. Immunotherapy. J Immunother. 2008;31:620-632
17. Gilboa E. DC-based cancer vaccines. J Clin Invest. 2007;117:1195-1203
18. Tyagi RK, Mangal S, Garg N, Sharma PK. RNA-based immunotherapy of cancer: role and therapeutic implications of dendritic cells. Expert Rev Anticancer Ther. 2009;9:97-114
19. Kyte JA, Gaudernack G. Immuno-gene therapy of cancer with tumour-mRNA transfected dendritic cells. Cancer Immunol Immunother. 2006;55:1432-1442
20. Grünebach F, Müller MR, Brossart P. New developments in dendritic cell-based vaccinations: RNA translated into clinics. Cancer Immunol Immunother. 2005;54:517-525
21. Gilboa E, Vieweg J. Cancer immunotherapy with mRNA-transfected dendritic cells. Immunol Rev. 2004;199:251-263
22. Nair S, Boczkowski D. RNA-transfected dendritic cells. Expert Rev Vaccines. 2002;1:507-513
23. Mitchell DA, Nair SK. RNA transfected dendritic cells as cancer vaccines. Curr Opin Mol Ther. 2000;2:176-181
24. Mitchell DA, Nair SK. RNA-transfected dendritic cells in cancer immunotherapy. J Clin Invest. 2000;106:1065-1069
25. Schnurr M, Galambos P, Scholz C, Then F, Dauer M, Endres S. et al. Tumor cell lysate-pulsed human dendritic cells induce a T-cell response against pancreatic carcinoma cells: an in vitro model for the assessment of tumor vaccines. Cancer Res. 2001;61:6445-6450
26. Clark R, Kupper T. Old meets new: the interaction between innate and adaptive immunity. J Invest Dermatol. 2005;125:629-637
27. Reis e Sousa C. Dendritic cells in a mature age. Nat Rev Immunol. 2006;6:476-483
28. Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol. 2003;3:984-993
29. Baxter AG, Hodgkin PD. Activation rules: The two-signal theories of immune activation. Nat Rev Immunol. 2002;2:439-446
30. Curtsinger JM, Schmidt CS, Mondino A, Lins DC, Kedl RM, Jenkins MK. et al. Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T cells. J Immunol. 1999;162:3256-3262
31. Kalinski P, Hilkens CM, Wierenga EA, Kapsenberg ML. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol Today. 1999;20:561-567
32. Kaiko GE, Horvat JC, Beagley KW, Hansbro PM. Immunological decision-making: how does the immune system decide to mount a helper T-cell response? Immunology. 2008;123:326-338
33. Giorgio Trinchieri. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nature Reviews Immunology. 2003;3:133-146
34. Langrish CL, McKenzie BS, Wilson NJ, de Waal Malefyt R, Kastelein RA, Cua DJ. IL-12 and IL-23: master regulators of innate and adaptive immunity. Immunol Rev. 2004;202:96-105
35. Ma X, Chow JM, Gri G, Carra G, Gerosa F, Wolf SF. et al. The interleukin-12 p40 gene promoter is primed by interferon-γ in monocytic cells. J Exp Med. 1996;183:147-157
36. Yoshida A, Koide Y, Uchijima M, Yoshida TO. IFN-γ induces IL-12 mRNA expression by a murine macrophage cell line, J774. Biochem Biophys Res Commun. 1994;198:857-861
37. Ohteki T, Fukao T, Suzue K, Maki C, Ito M, Nakamura M. et al. Interleukin-12-dependent interferon γ production by CD8α+ lymphoid dendritic cells. J Exp Med. 1999;189:1981-1986
38. Pulendran B, Lingappa J, Kennedy MK, Smith J, Teepe M, Rudensky A. et al. Developmental pathways of dendritic cells in vivo: distinct function, phenotype, and localization of dendritic cell subsets in FLT3 ligand- treated mice. J Immunol. 1997;159:2222-2231
39. Frucht DM, Fukao T, Bogdan C, Schindler H, O'Shea JJ, Koyasu S. IFN-γ production by antigen-presenting cells: mechanisms emerge. Trends Immunol. 2001;22:556-560
Author contact
![]() Corresponding author: Jian-Chuan Xia, PhD, MD, State Key Laboratory of Oncology in Southern China and Department of Experimental Research, Cancer Center, Sun Yat-sen University, 651 Dongfeng Road East, Guangzhou, 510060, P. R. China. Tel: +86-20-87343173; Fax: +86-20-87343392; Email: xiajchsysu.edu.cn
Corresponding author: Jian-Chuan Xia, PhD, MD, State Key Laboratory of Oncology in Southern China and Department of Experimental Research, Cancer Center, Sun Yat-sen University, 651 Dongfeng Road East, Guangzhou, 510060, P. R. China. Tel: +86-20-87343173; Fax: +86-20-87343392; Email: xiajchsysu.edu.cn

 Global reach, higher impact
Global reach, higher impact