10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2010; 6(7):691-699. doi:10.7150/ijbs.6.691 This issue Cite
Review
Lipid metabolism, adipocyte depot physiology and utilization of meat animals as experimental models for metabolic research
1. Department of Animal Sciences, Washington State University, Pullman, WA 99164, USA
2. USDA-ARS, Richard B. Russell Agricultural Research Station, Athens, GA 30604, USA
3. Department of Agricultural, Food and Nutritional Science, University of Alberta, Edmonton, Alberta T6G 2P5, USA
4. Department of Animal Science, University of Wyoming, Laramie, WY 82071, USA
5. Department of Pharmaceutical Sciences, University of Connecticut, Storrs, CT 06269, USA
6. The Coca-Cola Company, Research and Technology, Atlanta, GA 30313, USA
7. Agriculture and Agri-Food Canada Research Centre, Lethbridge T1J 4B1, CA, , USA
8. Program in Cellular and Molecular Biosciences and Animal Sciences, Auburn University, AL 36849, USA
9. The Hartz Mountain Corporation, Secaucus, NJ 07094, USA
10. Department of Animal and Range Sciences, South Dakota State University, Brookings, SD 57007, USA
11. Department of Animal Sciences, University of Arizona, Tucson, AZ 85721, USA
12. Universidad Publica de Navarra, Campus Arrosadia, Pamplona 31006, Spain
13. Animal Sciences, Iowa State University, Ames, IA 50011, USA
14. Department of Animal Sciences, The Ohio State University/OARDC, Wooster, OH 44691, USA
Received 2010-10-7; Accepted 2010-11-22; Published 2010-11-22
Abstract
Meat animals are unique as experimental models for both lipid metabolism and adipocyte studies because of their direct economic value for animal production. This paper discusses the principles that regulate adipogenesis in major meat animals (beef cattle, dairy cattle, and pigs), the definition of adipose depot-specific regulation of lipid metabolism or adipogenesis, and introduces the potential value of these animals as models for metabolic research including mammary biology and the ontogeny of fatty livers.
Keywords: Meat animals, lipid metabolism, adipose depots, adipocytes, adipogenesis
1. Introduction
Selective lipid deposition in meat animals is a relatively new strategy for improving production efficiency while improving meat quality. Efforts of reducing lipid deposition include genetic/breeding selection, feeding strategies, housing and environmental strategies, and hormone supplementation [1]. While these efforts have improved production efficiency and reduced carcass lipid deposition, one negative impact is thought to be reduced meat quality because of reduced lipid deposition in muscle, also known as marbling fat [1]. Although there is growing interest in developing alternative technologies to alter lipid deposition to selectively enhance marbling fat in meat animals, the mechanisms leading to differential lipid accumulation in visceral, subcutaneous, intermuscular and intramuscular fat depots remain unclear [1, 2]. Selection of ruminant sires [3] and breeds of cattle (Wagyu and Angus) that have the potential to partition energy to muscle adipocytes early in the growth period in their offspring has shown promise in promoting marbling [4]. Studying the cellular development, metabolism, and regulatory mechanisms of various cell types in different anatomical adipose depots [1, 5] has also provided insight for selective lipid deposition. Research with meat animals may very well lead to a new understanding of the regulation of lipid metabolism and adipocyte physiology. In addition, increased understanding of ectopic lipid storage observed in humans will help our overall understanding of the complex process of lipid storage. Collectively, methods developed to modulate selective lipid deposition may serve the dual purpose of improving meat quality and animal husbandry and metabolic regulation in humans, with potential to impact human health, particularly in individuals with lipodystrophy and obesity with concomitant ectopic lipid storage.
2. Lipid metabolism in meat animals
Meat animals are either non-ruminants (pigs and poultry; poultry will not be considered here) and ruminants (cattle, sheep, goats). Non-ruminants possess a gastrointestinal tract anatomically and functionally similar to that of humans and rodents. Alternatively, ruminants possess a forestomach anaerobic fermentation system (rumen-reticulum), anterior to the gastric organ and intestines. In ruminants, the adipose tissue is the principal site of de novo fatty acid (FA) synthesis [6, 7], which is markedly different from de novo lipogenesis in non-ruminants which occurs in the liver. Smith and Crouse [8] found that in ruminants glucose is the favoured substrate for lipogenic adipocytes in muscle, unlike subcutaneous adipocytes where acetate is favoured. In corroboration, Pethick et al. [9] found that visual marbling fat was elevated in ruminants that were fed carbohydrate that had escaped rumen fermentation and was later digested into glucose units in the intestine. The extent of carbohydrate escape from rumen fermentation is dependent on feed processing and other factors, but beef cattle fed high starch-containing diets will have more glucose directly absorbed from the small intestine than forage-fed animals [10]. Lipid deposition is increased in skeletal muscle in humans when there is insulin resistance in other adipose tissue depots, and the energy-bearing carbon is redirected to skeletal muscle [11]. Thus, major meat animals like both pigs and ruminants exhibit patterns of lipid metabolism distinctly different from humans and rodents (Table 1).
Major difference in lipid metabolism between ruminant and non-ruminant animals.
| Non-ruminants | Ruminants | |
|---|---|---|
| Fatty acid absorption | Fatty acids are absorbed directly in small intestine and proceed into the blood | Fatty acids are fermented into volatile fatty acids in rumen, which are absorbed. |
| Principal site of fatty acid de novo synthesis | Liver (humans; rodents) Adipose (pigs) | Adipose tissue, as well as mammary gland for lactating ruminants |
| Precursor for fatty acid de novo synthesis | Glucose | Acetate |
| Fatty acids in circulation | Very low density lipoproteins and chylomicrons | Volatile fatty acids and low density lipoproteins |
| Fatty acid composition | Long chain fatty acids with a sizeable proportion of unsaturated fatty acids | High proportion of short chain saturated fatty acids |
Lipid metabolism in non-ruminant animals
Non-ruminant farm animals such as pigs eat diets containing both carbohydrates and dietary fats. Typically, pigs are fed cereal grains (starch) and low amounts of fat. Monosaccharides and FAs are absorbed directly from the small intestine and monosaccharides arrive at the liver via the portal vein, while dietary FAs are incorporated into chylomicrons and transported to tissues via the lymphatic system and then into the general (blood) circulation. Any glucose not immediately metabolized (or in excess of energy needs) will be used for de novo FA synthesis and then triacylglycerol (TAG) storage in adipose tissue depots [6, 7]. Thus unlike in humans and rodents, this excess energy will not be immediately converted to FA, TAG and then very low density lipoproteins (VLDL) in the porcine liver [7]. Further in pigs fed low fat diets, hepatic VLDL synthesis will depend on FA available from adipose TAG lipolysis. Unlike in humans and rodents, this excess energy will thus not be immediately converted to FA but into TAG and very low density lipoproteins (VLDL) in the liver [7]. Fatty acids may be released from chylomicrons by lipoprotein lipase (LPL) into adipose tissues, cardiac and skeletal muscle and liver. The incorporation of dietary lipids into various tissues is well-established and can be observed as a change in the relative fatty acid composition of tissues. High dietary lipid content may contribute to increased hepatic fat content and VLDL synthesis. For specific VLDL production, FAs are released from storage adipose depots by lipolysis and may be used for hepatic VLDL synthesis. Thus, de novo lipid synthesis and lipoprotein synthesis are functionally and anatomically separated in pigs. Moreover, during citric acid overflow, when there is excess acetyl-CoA, upon rapid mobilization of lipid stores, pig liver mitochondria release acetate instead of ketones [12]. The lipid metabolism of pigs is regulated by similar hormones and relevant transcription factors as in rats and humans [13, 14], but in pigs the liver has much less of a central role than in rodents, particularly when high carbohydrate diets are fed.
Lipid metabolism in ruminant animals
Ruminants consume forages and cereal grains. In the forestomach fermentation system, the cell wall and soluble carbohydrates of feedstuffs are degraded and fermented into volatile fatty acids (VFA).
These fatty acids are shorter than 6 carbons long and are the principal energy source of these animals. Intake of diets containing more than 5 % fat will inhibit forestomach fermentation and are not recommended for ruminants. Consequently, exogenous FAs are less evident in ruminant tissues as compared to non-ruminants [15]. However, application of technology for dietary lipids exists that permits these molecules to pass the rumen and to be absorbed in the small intestine. As such, feed components escape rumen fermentation. Major sites of FA use are the mammary gland, subcutaneous adipose tissue and intramuscular lipid deposits in skeletal muscle adipocytes located in the perimysium and epimysium. The principal precursor for de novo FA synthesis is acetate and not glucose [7]; irrespective of the carbon source, almost all fatty acids are produced (except for a few resulting from lower gut digestion of microorganisms) via endogenous synthesis (DNL) in adipose and mammary glands during lactation only. The molecular regulation of DNL, lipid deposition and oxidation is affected by the same transcription factors and molecular mechanisms in both mammary tissue and subcutaneous fat stores [16, 17]. For example, during periods of rapid depot fat mobilization as often typical during the first month of lactation, hepatic accumulation of NEFA results first in ketosis followed by TAG synthesis [18], but the TAG is not immediately derived from lipoproteins, and TAG accumulates in the liver resulting in a adipose tissue-lipid mobilization/NEFA release-dependent fatty liver. This problem is accentuated as bovine liver has a limited capacity for NEFA oxidation. While rates of liver TAG synthesis in ruminants are similar to those of non-ruminants, hepatic VLDL secretion is very slow compared to non-ruminants [19]. Indeed, mechanistically hepatic steatosis in early postpartum dairy cows may be related to a lesser hepatic apoB availability [15, 20]. Ruminant species not associated with high milk production are much less likely to develop fatty livers [18].
Dairy cattle. Work on milk fat synthesis in dairy cattle has primarily centered on quantifying the relative contribution of endogenously synthesized FA (arising from lipid depots) as compared to fatty acids synthesized in the mammary. Typically, about one half of lipid of secreted milk arises from either endogenous adipose FA or dietary, rumen- protected fats, with the remainder being synthesized in the mammary tissue [16, 21]. Mammary lipid synthesis results in the production of a softer secreted fat via esterification of a mixture of short, medium and long chain FA to glycerol phosphate. More recently the role of conjugated fatty acids (CLA), arising from rumen partial hydrogenation of poly-unsaturated FA (PUFA) and subsequent animal metabolism, on total mammary fat synthesis as well as the roles of dietary and hormonal factors that influence the composition of milk fat have been widely explored [22].
Beef cattle. With beef cattle the emphasis in lipid metabolism has been on the extent of fat deposition and intramuscular lipid synthesis. Presently work is proceeding on the role of transcription factors on adipogenesis and fat synthesis in preadipocytes in muscle tissues, with both tissue culture and in vivo studies [23]. It appears that molecular regulation of preadipocyte differentiation in bovine muscle may be somewhat similar to the mechanisms described for 3T3-L1 adipocytes [24] in culture with key roles by CCAAT enhancer binding protein (C/EBP α,β,δ) and peroxisome proliferator-activated receptor (PPARγ) [1, 25, 26]. Comparative differences of beef cattle present a unique resource to study various aspects of lipid metabolism. Thus, major meat animals, both pigs and ruminants exhibit patterns of lipid metabolism distinctly different from humans and rodents.
3. Adipose depot physiology
Differences at a cellular, metabolic and genetic level among adipose depots have been reported in meat animals [1, 2, 28]. For example, subcutaneous porcine preadipocytes proliferated more actively and showed more rapid accumulation of TAG than visceral-derived adipocytes [29]. During differentiation, subcutaneous and visceral preadipocytes showed different, and depot-specific, effects on the expression of C/EBP, carnitine palmitoyl transferase 1B (CPT1B) and fatty acid binding protein 4 (FABP4) [24]. Using sheep as a model, it was determined that visceral-derived preadipocytes were less able to differentiate than subcutaneous-derived preadipocytes [30]. A recent study performed by Yamada et al. [25] in cattle demonstrated differences in the expression of the adipogenic transcription factors of the C/EPB family in subcutaneous and intramuscular adipose tissue, depending on the dietary roughage level of the feeds. Regional differences also exist in developmental morphology, cellularity, lipid content, responsiveness to metabolic regulators, and the ability to mobilize lipids in animal and tissue-specific adipose depots [31-33]. Moreover, adipogenesis [1, 5] inside skeletal muscle during the fetal and early postnatal stages has a dominant effect on the number of postnatal intramuscular adipocytes [1, 34], because the total number of adipocytes appears to be set when reaching adolescence in some (but not all) meat animals [35]. However, the mechanisms that control adipogenesis in the fetal and postnatal stages in all of the adipose depots of meat animals remain poorly defined [1].
Dynamics of depot adipogenesis
Adipose tissue is a dynamic tissue [1, 36], and from a cellular perspective adipose depot-specific hyperplasia may occur into adulthood [37]. A key question is where do these new adipocytes come from [28]? The classic view is that a majority of these cells are derived from the precursor cells in the connective tissue fraction of adipose tissue [38]. However, the dedifferentiation of mature adipocytes and daughter cell proliferation constitutes another significant source of new adipocytes. Adipocytes from both ruminant animals like beef cattle [5, 39-41] and monogastric animals like pigs [42, 43] possess ability to dedifferentiate [44, 39-41] and form proliferative competent progeny cells in vitro [39, 41-43]. These cells are capable of undergoing population expansion [41], and may re-differentiate to form lipid-filled adipocytes [41-43]. Classic research showed that specific numbers may be assigned to different adipose depots in beef cattle throughout postnatal aging [37]. Moreover, the total amount of adipose tissue (in kg) has been determined on an animal and adipose tissue depot basis [37]. Fernyhough et al. [39] proposed that 1 cell out of every 100 mature adipocytes possessed the ability to dedifferentiate and form proliferative-competent progeny cells in vitro. In the subcutaneous adipose depot of 19 month old beef steers (alone) there exists 43.2 kg of tissue and 14.306 billion cells [37]. Should, in fact, 1/100 mature adipocytes possess the ability to dedifferentiate and form proliferative-competent progeny cells, then approximately 100 million of the cells are potentially capable of forming new adipocytes, or other types of cells, if subjected to the appropriate physiological regulation. So, the possibility that almost every cell type in the adipocyte lineage, including mature adipocytes, are capable of proliferation and differentiation affords a great potential to very adipocyte-filled tissues, and supersedes the traditional idea that new cells added to any adipose depot are only from preadipocytes, adipofibroblasts, or (as yet) undefined stem cells residing in the depot [39]. This research needs to be resolved [45, 46]. Species influences on cell physiology, and depot-specific regulation differences must be included in any research design. However, the potential of outcomes of this research being applied to animal growth and development, human health and dysfunction resolution and alleviating the adverse effects of aging on body composition make the research area ripe for much participation. Indeed, the potential impact of mature adipocyte dedifferentiation in terms of cell numbers may benefit new modalities such as tissue regeneration, may change current ideas regarding postnatal stem cells, and may be useful in a variety of applications of tissue engineering [47].
Genetic control of different adipose depots
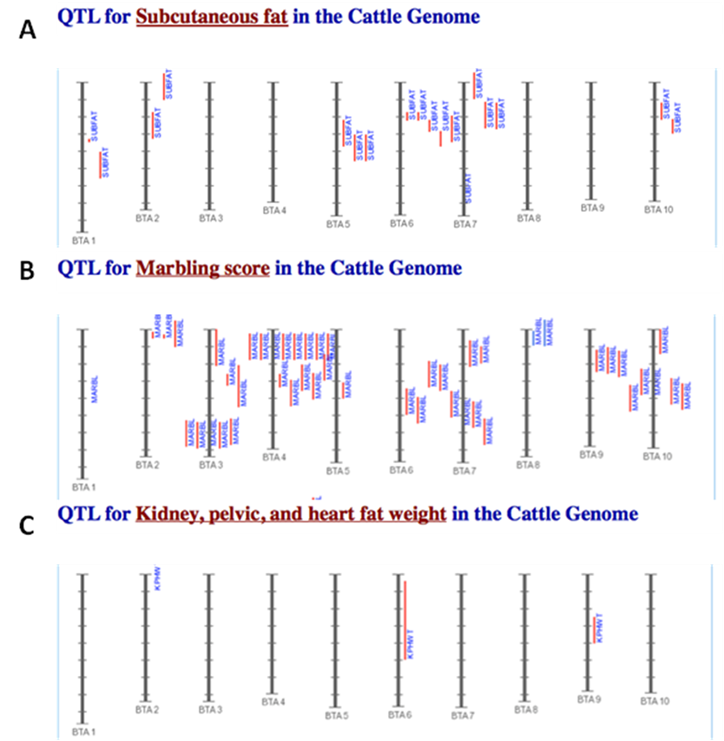
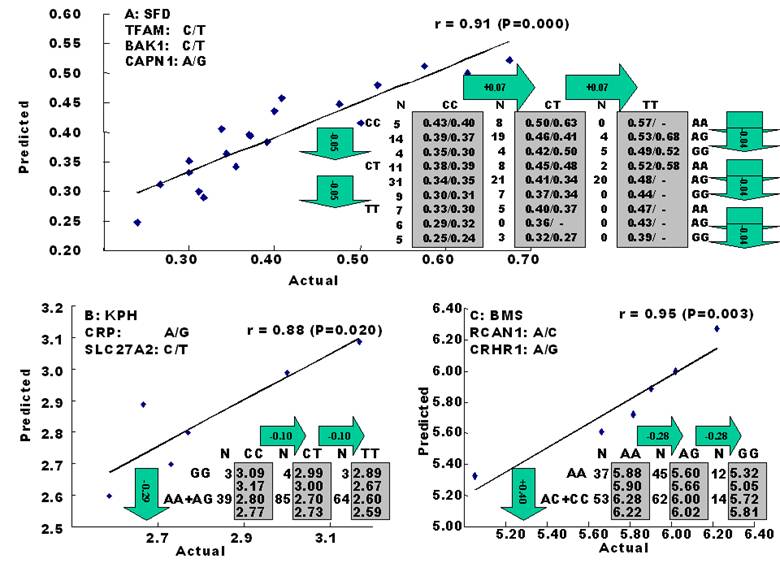
The amounts of body fat stored as subcutaneous, internal, and intramuscular adipose tissue depots are quantitative traits or complex phenotypes in nature, which are generally determined by the combined effects of many loci and are affected by genetic networks or molecular pathways. A quick perusal of the Cattle QTLdb [48] reveals that researchers have identified quantitative trait loci (QTL) for marbling (Marb), subcutaneous adipose thickness (SAT), and kidney, heart and pelvic fat (KPH). As shown in Figure 1, many of these QTL have only been associated with a single adipose trait. However, there are some instances where a given QTL has been associated with two of these adipose traits (for example, compare SAT with Marb QTL on chromosome 7 in Figure 1). Recently, Jiang and colleagues [49] identified possible genetic networks that control variation for three classes of adipose tissue depots using a Wagyu x Limousin reference population (Table 2). Subcutaneous fat depth (SFD) was measured at the 12-13th rib interface perpendicular to the outside surface at a point three-fourths the length of the longissimus muscle from its chine bone end [49]. The internal fat depots were measured as kidney, pelvic and heart (KPH) fat percentage, which is calculated as estimated weight of KPH fat divided by hot carcass weight. The amount of fat within the muscle is known as intramuscular fat or beef marbling score (BMS), which is scored in the ribeye muscle at the 12th rib based on USDA standards (http://www.ams.usda.gov/). The authors genotyped a total of 157 mutations, mainly single nucleotide polymorphisms derived from 82 functional genes on the animals and found 7 significant single gene-trait associations for SFD, 6 for KPH and 6 for BMS (Table 2), but involving 16 genes. Only three genes, C-reactive protein (CRP), a regulator of calcineurin 1(RCAN1) and solute carrier family 27 (fatty acid transporter) member 2 (SLC27A2) are associated with two traits. Incorporating these single gene-trait associations for each depot into a regression model revealed a three gene TFAM-BAK1-CAPN1 network for SFD, a three gene CRP-SLC27A2-PON1 network for KPH and a two gene RCAN1-CRHR1 network for BMS (Figure 2 A, B and C), respectively [49]. These results indicate that subcutaneous, internal, and intramuscular adipose tissue depots may rarely share common genetic networks or genetic backgrounds (the genetic background is the same but the epigenic regulation is different among different depots).
Comparison of identified QTL associated with SAT (A), Marb (B), and KPH (C). The red vertical lines next to each chromosome denote the location of each QTL. The results shown here are intended as a demonstration and are not intended to be comprehensive for the bovine genome.
Genetic networks for SFD (A), KPH (B) and BMS (C) by the linear regression analysis for all significant single gene-trait associations. The numbers in arrows represent substitution effects of one type of genotypes or allele for another one. Each combined genotype(s) among different genes has two means of performance: predicted (top or left side) and actual (bottom or right side). “-“ indicates that no animals were identified with the combined genotype(s) in the population [49].
Functional genes associated with subcutaneous (SAT), internal (KPH) or intramuscular adipose tissue (Marb) depots discovered in a Wagyu x Limousin reference population [49]*.
| Symbol | Description | SFD | KPH | BMS |
|---|---|---|---|---|
| ABCA1 APOE BAK1 | ATP binding cassette A1 Apolipoprotein E BCL2-antagonist/killer 1 | A D A | ||
| CAPN1 | Calpain 1 | A | ||
| CRHR1 | Corticotropin releasing hormone receptor 1 | A | ||
| CRP | C-reactive protein, pentraxin-related | D | D | |
| DHCR7 | 7-dehydrocholesterol reductase | O | ||
| FABP4 | Fatty acid binding protein 4 | O | ||
| PAPD1 | Poly (A) polymerase associated domain containing 1 | O | ||
| RCAN1 | Regulator of calcineurin 1 | D | A | |
| PON1 SKIV2L | Paraoxidase I Superkiller viralicidic activity 2-like (S. cerevisiae) | A | D | |
| SLC27A2 | Solute carrier family 27, member 2 | A | D | |
| TFAM | Transcription factor A, mitochondrial | A | ||
| TFBM1 | Transcription factor B1, mitochondrial | A | ||
| UCN3 | Urocortin 3 | O |
*Only significant associations (P<0.05) were presented for each gene with additive (A), dominant (D) and overdominant (O) effects.
Specific proteins produced by adipose depots
As described above, specific genes can control and regulate adipocyte differentiation and metabolism. Through the life span of an individual, such differences may be observed, since age, nutritional input and environment factors can impact changes of gene expression. Only if there is a synthesis of functional/viable proteins for fat metabolism and development might something actually occur. To this end, it has been suggested that transcriptome expression patterns are not always correlated with the protein expression profiles, since biologically active proteins can be modified by the efficiency of translation, by post-translational modification(s), and by the rate and extent of proteolysis. Therefore, it is essential and critical to obtain and evaluate both transcriptome and proteome data sets, and (then) to use bioinformatics tools to identify protein functions and to predict the molecular mechanisms by which the proteins regulate adipogenesis and lipid metabolism. It is also increasingly recognized that post-translational modification of proteins plays an essential role in metabolism. Zhao et al. [50] summarized the proteome analysis on bovine subcutaneous adipose tissue from different crossbreed animals. In this study, annexin 1 was determined to be a novel protein that was expressed differentially, depending on animal breed. The annexins have functions as calcium-dependent phospholipid-binding protein that is involved in inhibition of phospholipase activity, exocytosis, endoctyosis, signal transduction, organization of the extracellular matrix, resistance to reactive oxygen species and DNA replication [50]. The altered protein expression of annexin 1 in animals with a higher backfat thickness suggests that the cell membrane-binding proteins may also play a role in fat depot formation in beef animals. Furthermore, in studies performed in vitro [51], a conventional proteomics approach coupled with monoclonal antibody cytokine arrays demonstrated 12 cytokines including several pro-inflamatory interleukins were secreted by neonatal preadipocytes during early adipogenesis. A number of other proteins secreted early in adipogenesis were also detected or identified with several protein assay approaches including apolipoprotein-A1, apolipoprotein-E, relaxin, brain-derived neurotrophic factor, and IGF binding protein-5 [51]. Several of these factors have also been identified and studied in vivo studies of neonatal and older pigs [52]. Therefore, a multifaceted approach in animal studies, clearly, can be more insightful than can in vitro studies of preadipocytes/cell lines alone.
4. Utilization of meat animals as models for metabolic research
The use of ruminants as models for biomedical research with adipocytes, or lipid metabolism, has been modest. Cattle are large and expensive animals to maintain. Even so, applicable research might be in mammary biology, the ontogeny of fatty livers, or the definition of adipose depot-specific regulation of lipid metabolism or adipogenesis. In addition, molecular regulation of excessive (visible) fat stores in muscle during obesity and associated maladies is difficult to study with tissue culture and cell lines. However, by using muscle biopsies sampled at various intervals during intramuscular adipose tissue development, coupled with laser dissection microscopy, adipocytes can be isolated from meat animals for transcriptomic and proteomic analyses. A potential concern to the use of farm animals as models for metabolic studies would be species-specific differences. An example of this would be the results obtained with CLAs, widely studied for their anticarcinogenic actions and potential to modify lipid deposition, but which might have different effects depending on the species. For instance, the 9-11 CLA isomer causes an inhibition of differentiation in 3T3 L1 cells [53], but the contrary was found in pig primary preadipocytes [14], showing that preadipocyte differentiation varies depending on the species of origin of the cell line or donor. In spite of these, the use of meat animals as models to test hypotheses on the accumulation of lipids in the myo- or peri-muscular cells and its association to insulin resistance can lead to a better understanding of the intramuscular fat deposition.
5. Conclusions
Meat animals have been highly studied in order to determine ways to make them grow more efficiently (thereby saving producers money), achieve optimum consumer appeal (nutrition and health benefits), and as potential models for metabolic research (with application towards other animals and humans). Lipid metabolism (lipogenesis and lipolysis) and adipogenesis (formation of adipose tissue from precursor cells) in meat animals may/may not be similar to other animal species. As such, study of these physiological mechanisms in meat animals may produce benefits for both health and animal production disciplines.
Conflict of Interests
The authors have declared that no conflict of interest exists.
References
1. Hausman GJ, Dodson MV, Ajuwon K. et al. Board Invited Review: The biology and regulation of preadipocytes and adipocytes in meat animals. J Anim Sci. 2009;87:1218-1246
2. Dodson MV, Jiang Z, Chen J. et al. Allied industry approaches to alter intramuscular fat content and composition in beef animals. J Food Sci. 2010;75:R1-8
3. Francis SM, Bickerstaffe R, Clarke JN. et al. Effect of selection for glucose tolerance in sheep on carcass fat and plasma glucose, urea and insulin. J Agric Sci Camb. 1994;123:279-286
4. Mir PS, Bailey DRC, Mir Z. et al. Growth, carcass and meat quality characteristics of beef cattle with 0, 50 and 75 percent Wagyu genetic influence. Can J Anim Sci. 1999;79:129-137
5. Fernyhough ME, Helterline DL, Vierck JL. et al. Dedifferentiation of mature adipocytes to form adipofibroblasts: More than just a possibility. Adipocytes. 2005;1:17-24
6. Hanson RW, Ballard FJ. The relative significance of acetate and glucose as precursors for lipid synthesis in liver and adipose tissue from ruminants. Biochem J. 1967;105:529-536
7. Bergen WG, Mersmann HJ. Comparative aspects of lipid metabolism: impact on contemporary research and use of animal models. J Nutr. 2005;135:2499-2502
8. Smith SB, Crouse JD. Relative contributions of acetate, lactate and glucose to lipogenesis in bovine intramuscular and subcutaneous adipose tissue. J Nutr. 1984;114:792-800
9. Pethick DW, McIntyre ML, Tudor G. et al. The partitioning of fat in ruminants-can nutrition be used as a tool to regulate marbling? Recent advances in Animal Nutrition in Australia. Armidale Australia: University of New England. 1997:151-158
10. Mir PS, McAllister TA, Scott S. et al. Conjugated linoleic acid-enriched beef production. Am J Clin Nutr. 2004;79:1207S-1211S
11. Frayn KN. Adipose tissue and the insulin resistance syndrome. Proc Nutr Soc. 2001;60:375-380
12. Lin X, Shim K, Odle J. Carnitine palmitoyltransferase I control of acetogenesis, the major pathway of fatty acid β-oxidation in liver of neonatal swine. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1435-R1443
13. Reiter SS, Halsey CHC, Stronach BM. et al. Lipid metabolism related gene-expression profiling in liver, skeletal muscle and adipose tissue in crossbred Duroc and Pietrain pigs. Comp Biochem Physiol Part D. 2007;2:200-206
14. Ding ST, Schinckel AP, Weber TE. et al. Expression of porcine transcription factors and genes related to fatty acid metabolism in different tissues and genetic populations. J Anim Sci. 2000;78:2127-2134
15. Boggs DL, Bergen WG, Hawkins DR. Effects of tallow supplementation and protein withdrawal on ruminal fermentation, microbial synthesis and site of digestion. J Anim Sci. 1987;64:907-914
16. Bauman DE, II JWP, Harvatine KJ. et al. Regulation of fat synthesis by conjugated linoleic acid: Lactation and the ruminant model. J Nutr. 2008;138:403-409
17. Harvatine KJ, Perfield JWn, Bauman DE. Expression of enzymes and key regulators of lipid synthesis is upregulated in adipose tissue during CLA-induced milk fat depression in dairy cows. J Nutr. 2009;139:849-854
18. Pullen DL, Liesman JS, Emery RS. A species comparison of liver slice synthesis and secretion of triacylglycerol from nonesterified fatty acids in media. J Anim Sci. 1990;68:1395-1399
19. Bremmer DR, Bertics SJ, Grummer RR. Differences in activity of hepatic microsomal triglyceride transfer protein among species. Comp Biochem Physiol A Mol Integr Physiol. 1999;124:123-131
20. Bernabucci U, Ronchi B, Basirico L. et al. Abundance of mRNA of apolipoprotein b 100, apolipoprotein e, and microsomal triglycerisde transfer protein in liver from periparturient dairy cows. J Dairy Sci. 2004;87:2881-2888
21. Emery RS, Herdt TH. Lipid nutrition. Vet Clin North Am Food Anim Pract. 1991;7:341-352
22. Nafikov RA, Beitz DC. Carbohydrate and lipid metabolism in farm animals. J Nutr. 2007;137:702-705
23. Lengi AJ, Corl BA. Factors influencing the differentiation of bovine preadipocytes in vitro. J Anim Sci. 2010;88:1999-2008
24. Kokta TA, Dodson MV, Gertler A. et al. Intercellular signaling between adipose tissue and muscle tissue. Domest Anim Endocrinol. 2004;27:303-331
25. Yamada T, Kawakami SI, Nakanishi N. Effects of dietary roughage levels on the expresión of adipogenic transcription factors in Wagyu stress. Meat Sci. 2009;83:775-781
26. Mandrup S, Lane MD. Regulating adipogenesis. J Biol Chem. 1997;272:5367-5370
27. Fernyhough ME, Okine E, Hausman G. et al. PPARγ and GLUT-4 expression as developmental regulators/markers for preadipocyte differentiation into an adipocyte. Domest Anim Endocrinol. 2007;33:367-378
28. Dodson MV, Hausman GJ, Guan LL, Du M, Rasmussen TP, Poulos SP, Mir P, Bergen WG, Fernyhough ME, McFarland DC, Rhoads RP, Soret B, Reecy JM, Velleman SG, Jiang Z. Skeletal muscle stem cells from animals I. Basic cell biology. Intern J Biol Sci. 2010;6(5):465-474
29. Samulin J, Berget I, Lien S. et al. Differential gene expression of fatty acid binding proteins during porcine adipogenesis. Comparitive Biochem Physiol B Biochem Mol Biol. 2008;151:147-152
30. Soret B, Lee HJ, Finley E. et al. Regulation of differentiation of sheep subcutaneous and abdominal preadipocytes in culture. J Endocrinol. 1999;161:517-524
31. Coppack SW. Adipose tissue changes in obesity. Biochem Soc Trans. 2005;33:1049-1052
32. Jensen MD. Role of body fat distribution and the metabolic complications of obesity. J Clin Endocrinol Metab. 2008;93:S57-S63
33. Santosa S, Jensen MD. Why are we shaped differently, and why does it matter? Am J Physiol Endocrinol Metab. 2008;295:E532-E535
34. Du M, Tong J, Zhao J. et al. Fetal programming of skeletal muscle development in ruminant animals. J Anim Sci. 2010;88:E51-60
35. Spalding KL, Arner E, Westermark PO. et al. Dynamics of fat cell turnover in humans. Nature. 2008;453:783-787
36. Hausman GJ, Kauffman R. The histology of developing porcine adipose tissue. J Anim Sci. 1986;63:642-658
37. Cianzio DS, Topel DG, Whitehurst GB. et al. Adipose tissue growth and cellularity: Changes in bovine adipocyte size and number. J Anim Sci. 1985;60:970-976
38. Hausman GJ, Poulos SP. Recruitment and differentiation of intramuscular preadipocytes in stromal-vascular cell cultures derived from neonatal pig semitendinosus muscles. J Anim Sci. 2004;82:429-437
39. Fernyhough ME, Vierck JL, Hausman GJ. et al. Primary adipocyte culture: adipocyte purification methods may lead to a new understanding of adipose tissue growth and development. Cytotechnol. 2004;46:163-172
40. Fernyhough ME, Bucci LR. et al. Gaining a solid grip on adipogenesis. Tissue Cell. 2005;37:335-338
41. Fernyhough ME, Hausman GJ, Dodson MV. Progeny from dedifferentiated bovine adipocytes display protracted adipogenesis. Cells Tissues Organs. 2008;188:359-372
42. Chen J, Guridi M, Fernyhough ME. et al. Initial differences in lipid processing leading to pig- and beef-derived mature adipocyte dedifferentiation. Basic and Appl Myol. 2009;19:243-246
43. Chen J, Dodson MV, Jiang Z. Cellular and molecular comparison of redifferentiation of intramuscular- and subcutaneous- adipocyte derived progeny cells. Internat J Biol Sci. 2010;6:80-88
44. Dodson MV, Fernyhough ME, Vierck JL, Hausman GJ. Adipocytes may not be a terminally differentiated cell type: Implications for animal production. Anim Sci. 2005;80:239-240
45. Dodson MV, Fernyhough ME. Mature adipocytes: Are there still novel things that we can learn from them? Tissue Cell. 2008;40:307-308
46. Fernyhough ME, Vierck JL, Dodson MV. Assessing a nontraditional view of adipogenesis: adipocyte dedifferentiation--mountains or molehills? Cells Tissues Organs. 2006;182:226-228
47. Fernyhough ME, Hausman GJ. et al. Mature adipocytes may be a source of stem cells for tissue engineering. Biochem Biophys Res Commun. 2008;368:455-457
48. Hu ZL, Fritz ER, Reecy JM. AnimalQTLdb: a livestock QTL database tool set for positional QTL information mining and beyond. Nucleic Acids Res. 2007;35:D604-609
49. Jiang Z, Michal JJ, Chen J. et al. Discovery of novel genetic networks associated with 19 economically important traits in beef cattle. Int J Biol Sci. 2009;5:528-542
50. Zhao YM, Basu U, Dodson MV. et al. Proteome difference associated with fat accumulation in bovine subcutaneous adipose tissues. Proteome Sci. 2010;8:14-24
51. Hausman GJ, Poulos SP, Richardson RL. et al. Secreted proteins and genes in fetal and neonatal pig adipose tissue and stromal-vascular cells. J Anim Sci. 2006;84:1666-1681
52. Poulos SP, Dodson MV, Hausman GJ. Cell line models for differentiation: Preadipocytes and adipocytes. Submitted.
53. Brodie AE, Manning VA, Ferguson KR. et al. Conjugated linoleic acid inhibits differentiation of pre- and post- confluent 3T3-L1 preadipocytes but inhibits cell proliferation only in preconfluent cells. J Nutr. 1999;129:602-606
Author contact
![]() Corresponding author: FAX +1 509 335 1082; E-mail address: dodsonedu (M.V. Dodson)
Corresponding author: FAX +1 509 335 1082; E-mail address: dodsonedu (M.V. Dodson)

 Global reach, higher impact
Global reach, higher impact