10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2011; 7(9):1334-1344. doi:10.7150/ijbs.7.1334 This issue Cite
Review
Paratransgenic Control of Vector Borne Diseases
Center for Global Health, Department of Internal Medicine, University of New Mexico and New Mexico VA Health Care System, Albuquerque, New Mexico, USA.
1. Institute of Dentistry, Queen Mary, University of London, England.
* Present address: Department of Internal Medicine, University of Washington Medical Center, Seattle, Washington, USA.
Received 2011-9-1; Accepted 2011-10-1; Published 2011-11-1
Abstract
Conventional methodologies to control vector borne diseases with chemical pesticides are often associated with environmental toxicity, adverse effects on human health and the emergence of insect resistance. In the paratransgenic strategy, symbiotic or commensal microbes of host insects are transformed to express gene products that interfere with pathogen transmission. These genetically altered microbes are re-introduced back to the insect where expression of the engineered molecules decreases the host's ability to transmit the pathogen. We have successfully utilized this strategy to reduce carriage rates of Trypanosoma cruzi, the causative agent of Chagas disease, in the triatomine bug, Rhodnius prolixus, and are currently developing this methodology to control the transmission of Leishmania donovani by the sand fly Phlebotomus argentipes. Several effector molecules, including antimicrobial peptides and highly specific single chain antibodies, are currently being explored for their anti-parasite activities in these two systems. In preparation for eventual field use, we are actively engaged in risk assessment studies addressing the issue of horizontal gene transfer from the modified bacteria to environmental microbes.
Keywords: Paratransgenesis, Chagas disease, triatomine bugs, visceral leishmaniasis, sand flies, microbiology, risk assessment, horizontal gene transfer
Introduction
Paratransgenesis is a “Trojan Horse” approach to control of disease transmission. In this strategy, bacterial flora native to disease-transmitting vectors are isolated and genetically transformed in vitro to export molecules that interfere with pathogen transmission. The genetically altered symbionts are then re-introduced into the host vector where expression of engineered molecules affects the host's ability to transmit the pathogen, i.e. its vector competence. This approach attempts to decrease pathogen transmission without adverse effects on vectors themselves. Further, it employs, as a gene delivery mechanism, bacterial flora native to the host vector.
There are several requirements for such an approach to work[1]: (a) a population of symbiotic bacteria must exist within a given disease-transmitting vector (b) symbiotic bacteria should be specific to a given vector (c) bacterial symbionts should be amenable to culture and genetic manipulation (d) genetically altered symbionts should remain stable (e) fitness of the genetically altered symbionts to re-infect host vectors should not be compromised, nor should their normal symbiotic functions be altered (f) transgene products released from the genetically altered symbionts should interact with the target pathogen(s) and (g) a method must exist for dispersal of the genetically altered symbionts amongst naturally occurring populations of vectors with minimal non-target spread of foreign genes to environmental bacteria and other arthropods.
Our laboratory had pioneered the paratransgenic approach to control Trypanosoma cruzi transmission by triatomine vectors of Chagas disease[2, 3]. We have since applied this technique to a number of other arthropod systems, including sand fly-transmitted leishmaniasis[4] and sharpshooter-mediated Pierce's disease of grapes[5]. We are further developing a modification of this procedure to control diseases in shrimp mariculture[6]. In this review, we will describe how this approach is utilized to impart passive immunity to arthropod vectors - triatomine bugs and sand flies - to render them incompetent to parasitic infections.
Chagas disease
Chagas disease is caused by the protozoan Trypanosoma cruzi and is transmitted to humans by blood-sucking triatomine bugs. These bugs infest thatch and adobe of poorly constructed homes and transmit the parasite to humans via fecal droplets deposited following a bloodmeal. This human parasitic disease is endemic throughout much of rural Mexico as well as Central and South America. In recent years, human migration from these regions of the world has significantly changed the epidemiology of this disease. Today, there are a significant number of people infected with T. cruzi in the United States (>300,000), Canada (>5,500), Europe and the Western Pacific (>80,000), Japan (>3,000), and Australia (>1,500)[7-9]. Of the 8 -11 million people infected with T. cruzi globally, approximately 50,000 will die each year[10]. Chagas disease accounts for the loss of 500,000 disability-adjusted life-years annually, second only to malaria and leishmaniasis in the global calculus of vector borne diseases.
There are three successive stages of Chagas disease. The acute stage - characterized by generalized malaise, fever, lymphadenopathy, hepatosplenomegaly - occurs shortly after infection and is minimally symptomatic for 4 to 8 weeks. The indeterminate phase can last between 10 to 20 years and is characterized by paucity of clinical manifestations but active replication of T. cruzi and periodic release of bloodstream forms of the parasite into the circulation. Decades following the primary infection, between 10-30% of infected individuals progress to chronic Chagas disease. Patients develop irreversible lesions of the autonomous nervous system in the heart, esophagus, colon and the peripheral nervous system[11, 12]. The chronic phase of Chagas disease is incurable and is associated with, on average, a ten year shortening of life span. Gastrointestinal manifestations of chronic Chagas disease are geographically restricted[13] and play a lesser role in the overall disease burden of Chagas disease. Progressive heart disease, a hallmark of chronic Chagas disease, is a leading public health concern throughout much of Central and South America[14].
In the absence of vaccines and drug therapies, control of Chagas disease relies largely on measures aimed at vector eradication. Though screening of blood banks to reduce transfusion-associated Chagas disease is ongoing, public health measures aimed at interrupting vector transmission to humans in endemic regions have the potential to eliminate this devastating disease. Several large-scale insecticide-based efforts have been undertaken with considerable success to date. The Southern Cone Initiative, a joint agreement between the governments of Argentina, Bolivia, Brazil, Chile, Paraguay, Uruguay and Peru, was launched in 1991 to control Chagas disease by the elimination of the main vector, Triatoma infestans. In some Southern Cone nations, such as Brazil, Uruguay and Chile, results have been dramatic and new cases of Chagas disease have been virtually eliminated[15, 16]. However, several factors raise concern about the re-emergence of Chagas disease in the Southern Cone. Reduced effectiveness of pyrethroids in peridomestic habitats had led to the incomplete elimination of T. infestans in several Southern Cone countries[17-19]. Further, several Triatomine populations have been documented to develop resistance to a variety of insecticides in Chagas disease endemic area[20]. For these reasons, alternate methods aimed at controlling Chagas disease transmission must be explored.
Paratransgenic control of transmission of T. cruzi
The symbiotic associations between triatomine vectors, specifically T. infestans and Rhodnius prolixus, and nocardiform actinomycetes is well characterized[21]. Descriptions of nocardia-like bacteria in colony populations of T. infestans date back to the mid-1900's[22]. These bacteria are thought to aid in the processing of B complex vitamins in the restricted blood diets of the host and are essential to the survival of the triatomine. The widespread carriage of nocardiform actinomycetes by several triatomine species and the amenability of these bacteria to genetic manipulation are the cornerstones of the paratransgenic strategies aimed at interrupting vectorial transmission of Trypanosoma cruzi.
The soil-associated nocardiform actinomycete Rhodococcus rhodnii resides extracellularly in the gut lumen of R. prolixus in close proximity to T. cruzi. This microbe is transmitted effectively from adult triatomine bugs to their progeny through coprophagy, the ingestion of fecal material from other bugs. The vital role of R. rhodnii in the growth and development of R. prolixus has been demonstrated repeatedly under laboratory conditions. R. prolixus nymphs that lack gut-associated symbionts (aposymbiotic) do not reach the sexually mature adult stage, and most will die after the second developmental molt. Introduction of the bacteria to first or second instar nymphs permits normal growth and maturation. In 1992, we transformed R. rhodnii with pRr1.1, a shuttle plasmid containing a gene encoding resistance to the antibiotic thiostrepton, to support the hypothesis that a transgene-carrying symbiont could be introduced into R. prolixus. We showed that the modified symbiont is maintained through the insect's development without adverse effects on insect survival and fitness[23]. We have since developed a number of anti-trypanosomal molecules - antimicrobial peptides (AMP's), recombinant endoglucanases that disrupt the surface of the parasite and functional transmission-blocking single chain antibodies - for expression in triatomine vectors.
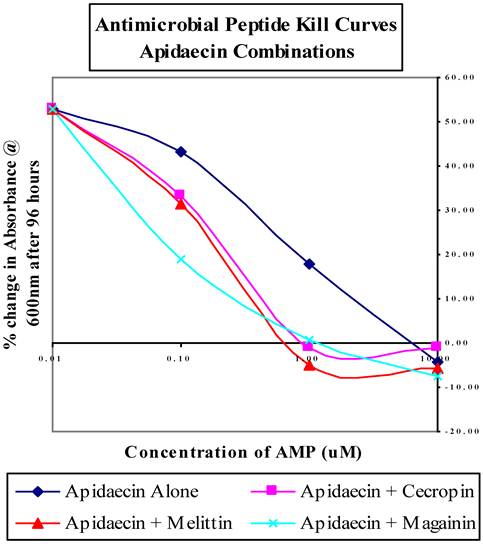
In a seminal study, we re-populated aposymbiotic R. prolixus nymphs with R. rhodnii transformed to express the AMP cecropin A[3]. These paratransgenic insects were then allowed to engorge on T. cruzi-laden human blood. At the end of the trial, 65% of the insects examined had complete clearance of viable T. cruzi. The remaining 35% of the bugs had substantially reduced numbers of parasites, demonstrating that the in vivo expression of an AMP from a genetically modified symbiont can significantly reduce carriage of the infectious parasite from the host vector. AMP's are a class of peptides that are recognized as important components of innate cell defense mechanisms[24, 25]. They are produced in insects, plants, and animals, are present on a variety of cellular surfaces, and vary considerably in primary and secondary structure[26]. AMP's are amphipathic, highly basic molecules that can discriminate between host and bacterial membranes by charge and composition[26]. While most AMP's disrupt membranes of non-host cells, other modes of actions, including interfering with host metabolism and targeting cytoplasmic components have been reported[25, 27, 28]. We recently reported on the in vitro activities of several other AMP's against T. cruzi. We showed that apidaecin, magainin and mellittin had toxicity profiles that may be applicable to the Chagas paratransgenic system, killing T. cruzi at concentrations lower than 10 uM and requiring greater than 100 uM to show any effect on R. rhodnii[29]. Further, when used in pair-wise combinations, these anti-microbial peptides resulted in much improved T. cruzi killing efficiency. For example, when used alone, apidaecin kills T. cruzi at 10 uM, but when used in combination with cecropin, melittin or magainin, the concentration required for killing is decrease by 10-fold (Figure 1). We have now generated strains of R. rhodnii that produce four different AMP's; cecropin, apidaecin, melittin and magainin. Lysates from these cells are biologically active against T. cruzi (Fieck et al, in prep). Work is currently in progress to introduce these transformants either individually, or in combination, into aposymbiotic triatomine nymphs. Based on our in vitro data (Fieck et al, in prep), we would expect close to 100% clearance of T. cruzi following challenge.
A number of other anti-trypanosomal molecules are currently being assessed for expression in R. rhodnii. One such molecule is β1,3-glucanase. The surface of T. cruzi is covered by a thick coat of glycoconjugates, the major component of which is a family of mucin-like glycoproteins. These glycoconjugates are required by the parasites for attachment, and subsequent infection, in the hindgut of the triatomine vector[30]. In unpublished work, we had shown that Arthrobacter luteus lyticase, an endoglucanases complex consisting of β1,3-glucanase and alkaline proteases, is very efficient in lysing T. cruzi in vitro, and is non-toxic to R. prolixus. We recently inserted the cDNA encoding Arthrobacter luteus β-1,3-glucanase into pRrExpA, our E. coli/R. rhodnii shuttle vector. Recombinant β-1,3-glucanse is biologically active, and clears T. cruzi at low concentrations (Jose et al, in prep), suggesting that it could potentially be used as another effector molecule for the paratransgenic control of Chagas disease.
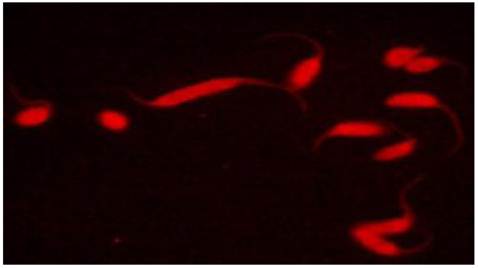
Competence of the triatomine vector to transmit T. cruzi depends in part on parasite maturation in the insect gut. This maturation process, also known as metacyclogenesis or the transformation from noninfective epimastigotes into metacyclic trypomastigotes, is dependent in part on interactions between surface epitopes of T. cruzi and the gut lumen of the triatomine bug. These interactions could therefore be a potential target for paratransgenic expression of antibody fragments. Recombinant single chain antibodies (scFv) are fusion proteins containing the variable regions of the heavy and light chains of immunoglobulins. These regions are connected to one another with a short linker of about 10 amino acid residues. Despite the absence of the constant regions, these proteins retain specificity to target antigens comparable to that of parent immunoglobulins. Because of their small size, scFv's are readily produced in bacterial cultures. In previous work, we genetically modified R. rhodnii to produce a functional murine three-domain antibody fragment (rDB3) that binds progesterone[31]. The scFv-producing cells were subsequently fed to aposymbiotic R. prolixus. We showed that the rDB3 antibody fragment was synthesized and secreted into the insect gut lumen by the engineered symbiont in a stable fashion over the 6-month period of the study. We later repeated these studies via the successful transformation of a Corynebacterium symbiont of T. infestans, with the same shuttle plasmid[32]. Recently, we reported on the design, assembly, production and characterization of two scFV's that are tagged with intrinsic red fluorescent properties to the sialyl-Tn and sialyl-(le)a surface glycans of T. cruzi[33]. The monoclonal antibodies B72.3 and CA19.9 bind to the above glycans, respectively. Using these monoclonals as a template, synthetic DNA sequences of B72.3 and CA19.9 antibody variable domains in VH-VL orientation were generated. Instead of a standard linker, a monomeric red fluorescent protein (mRFP) from Discosoma was inserted as a rigid linker between the VH and VL domains, generating a novel and highly stable REDantibody. The resulting recombinant fluorescent molecules were demonstrated to specifically bind to fixed T. cruzi epimastigotes by confocal microscopy (Figure 2). The ability of these molecules to bind and inactivate live parasites is currently being investigated. We are also in the process of subcloning genes encoding these novel antibodies into our shuttle vector system for expression in R. rhodnii.
Antimicrobial peptide kill curves for dual-combination treatments of T. cruzi liquid cultures. Results averaged from triplicate samples in three separate experiments and displayed as the percent change in absorbance at 600 nm compared to untreated controls. Figure is adapted from Fieck et al, Trypanosoma cruzi: synergistic cytotoxicity of multiple amphipathic anti-microial peptides to T. cruzi and potential bacterial hosts, Exp Parasitol, 2010, 125:342-347
Confocal image of anti-sialy-Tn REDantibody targeting glycan structures on the surface of T. cruzi epimastigotes. Figure adapted from Markiv et al. Module based antibody engineering: a novel synthetic REDantibody. J Immunol Methods, 2011, 364:40-49
Though many triatomine vectors of T. cruzi exist, we have focused primarily on genetically modifying R. rhodnii, the symbiont of R. prolixus, for paratransgenic purposes. As described earlier, the paratransgenic approach derives from the close symbiotic association between bacteria and arthropod vector. In light of this, we had examined the specificity of symbionts in several triatomine vectors. A wide variety of bacterial genera, including Nocardia, Corynebacteria and Streptococci, were isolated from the midgut of T. infestans[34]. In previous studies, we had identified a novel Corynebacterium symbiont from laboratory colonies of T. infestans isolated from the Gran Chaco region of Argentina[32], and studied the role of nocardiform actinomycetes such as Rhodococci, Nocardia spp, Gordonia spp, Corynebacterium spp and Tsukumurella [1-3, 31, 32] in a number of triatomine bugs, including T. dimidiata and T. sordida (Pennington and Durvasula, unpublished data). In all cases, we noted that absence of matched actinomycetes in the arthropod resulted in growth arrest and death of nymphs. However, when introduced into the correct vector, the actinomycetes supported growth and sexual maturation of these bugs. These results suggest that symbionts are indeed specific to their respective hosts, and would need to be first identified for each arthropod vector of T. cruzi prior to initialization of the paratransgenic strategy. Once modified, the genetically altered symbiont should not impair vector survival and fecundity. In previous studies, we had shown that transformed R. rhodnii expressing either cecropin A or rDB3 resulted in no adverse effects on R. prolixus[3, 31]. The effects of apidaecin, mellitin, magainin and β1,3-glucanase-expressing R. rhodnii on R. prolixus are currently being evaluated. Finally, since scFv's bind with high specificity to sites on the surface of T. cruzi, we expect that they will exert minimal toxicity to the vector.
In preparation for field trials, we tested the efficacy of a simulated triatomine-fecal preparation, CRUZIGARD, consisting of an inert guar gum matrix dyed with India ink, as a method for delivery of engineered R. rhodnii to colonies of R. prolixus. The CRUZIGARD preparation was mixed with 108 CFU/ml of transformed R. rhodnii, and used to impregnate cages constructed of thatch and adobe-building materials from the Chagas-endemic regions of Guatemala (Olopa)[35]. Adult R. prolixus from the Olopa district were placed in the cages and removed after eggs were laid. The experiments were conducted over a 9-month period. Genetically altered R. rhodnii were detected in approximately 50% of F1 adults and comprised nearly 95% of the colony forming units (CFU) in these bugs, providing evidence that CRUZIGARD may be useful as a gene dispersal strategy under conditions of microbial competition. To increase the likelihood of CRUZIGARD probing, we have on-going collaborations to develop triatomine attractants and semiochemicals to supplement the current CRUZIGARD formulation. The addition of these semiochemicals is further anticipated to increase the volume and duration of CRUZIGARD ingestion, and consequently, increase rates of vector inoculation with transformed symbiont.
Demonstrating vector uptake of genetically altered symbionts and, ultimately, refractoriness to T. cruzi infection via CRUZIGARD does not suggest or substantiate a strategy for field delivery. A practical strategy in an endemic region should specify the minimum amount of transformed symbionts necessary to prevent T. cruzi transmission to humans, and a method of CRUZIGARD application suitable for at-risk domiciles in the endemic region. In other on-going studies, we are working with collaborators to develop a theoretical framework to assess parameters such as minimum effective release of CRUZIGARD and inoculation of target triatomine population.
We further realize that the deployment of genetically altered lines of bacteria to target field populations of triatomine bugs may have profound environmental consequences. Horizontal gene transfer, uptake of foreign genes by non-target arthropods and microbial biodiversity may all be adversely affected by such a strategy. We have developed a mathematical model predicting horizontal gene transfer (HGT) between genetically modified R. rhodnii and G. rubropertinctus.[36] The model treats HGT as a composite event whose probability is determined by the joint probability of three independent events: gene transfer through the modalities of transformation, transduction, and conjugation. Genes are represented in matrices, with Monte Carlo method and Markov chain analysis used to simulate and evaluate environmental conditions. The model is intended as a risk assessment instrument and predicts an HGT frequency of less than 1.14 x 10-16 per 100,000 generations at the 99% certainty level. This predicted transfer frequency is less than the estimated average mutation frequency in bacteria, 10-1 per gene per 1,000 generations. This suggests even if HGT were to occur between R. rhodnii and G. rubropertinctus, the transgene would likely not persist in the recipient organism, and that the likelihood of these unwanted events are low.
The field release of engineered bacteria cannot occur until a rigorous risk assessment framework and regulatory apparatus are in place. To inform the regulatory process, evaluation of the risks and benefits of the paratransgenic strategy are required. Since such information will not readily be available through field release trials, we are working with several collaborators to develop a framework involving rigorous mathematical modeling and simulations. Outputs of these models will be integral to informing risk assessment and regulatory oversight of the paratransgenic program, and ultimately, to permit field trials of the paratransgenic strategy.
Visceral leishmaniasis
Leishmaniasis is a devastating neglected tropical disease caused by the obligate intracellular protozoa Leishmania donovani in India, L. infantum in Europe, and L. chagasi in South America. Natural transmission occurs through the bite of an infected sand fly of the genus Phlebotomus (Old World) or Lutzomyia (New World). This disease is present in 88 countries, affecting approximately two million people per year with a global prevalence of 12 million with 350 million people at risk for infection[37].
Visceral leishmaniasis (VL) is among the most neglected diseases based on dearth of research and notable lack of innovations in disease treatment and control. It is by far the most devastating form of leishmaniasis, and is nearly 100% fatal if untreated. There are 500,000 annual new cases of VL in the world, one-half of which occur in India. Bihar, the most affected state in India, is home to nearly 90% of the new cases each year[38] followed by West Bengal and Uttar Pradesh. Bihar is also the region where approximately 95% of the mortality occurs among the reported VL cases in India[38]. The disease often presents as an indolent fever, malaise and wasting with co-existent splenomegaly, bone marrow involvement and hepatomegaly.
Treatment of Visceral Leishmaniasis
Treatment of leishmaniasis varies greatly with the type of disease (cutaneous, mucosal, or visceral), Leishmania species, and geographic location. Old World cutaneous disease often self-heals and as a result requires no treatment. New World cutaneous disease is less likely to self-resolve and because of the risk of development of mucosal leishmaniasis with Leishmania braziliensis, systemic treatment is more commonly undertaken. Treatment courses are often lengthy and cure is difficult to achieve with many cutaneous and mucocutaneous forms. Treatment modalities for cutaneous leishmaniasis range from local therapy including topical treatments and intralesional injections to systemic therapy with pentavalent antimonials, amphotericin B or miltefosine being used most commonly.
The treatment of VL has changed substantially over the last decade. Pentavalent antimonial drugs were the standard drug of choice until the emergence of drug non-responsiveness in highly endemic regions such as Bihar, India[39]. Newer treatment modalities, while effective, pose practical limitations as the majority of patients suffering from VL are unable to pay for costly medications and hospitalization. Newer therapies include multiple formulations of amphotericin B, paramomycin, and miltefosine. Dose finding studies have suggested significant efficacy with even a single dose of liposomal amphotericin B[40], however, it is unlikely that this single-dose regimen will be adopted into practice because of the risk of emergence of drug resistance. Paramomycin, an injectable aminoglycoside medication, has also been studied and found effective in clinical trials[41]. Miltefosine, an oral agent with excellent skin penetration has proven successful in treatment of VL and has the advantage of not requiring hospitalization for administration[42]. Most recently, there has been evaluation of combinations of drugs for the treatment of VL. Combination therapy has the distinct advantage of decreasing the risk of drug resistance, decreasing the side effects and improving cost-effectiveness. Regimens found to be effective include combination therapy with amphotericin B and miltefosine, amphotericin B and paramomycin and miltefosine in combination with paramomycin[43].
In the absence of a vaccine, control of VL has focused largely on vector eradication through pesticide use. Several large-scale control efforts have targeted the elimination of domestic and peri-domestic populations of sand flies including Plebotomus argentipes. Initially, DDT spraying in India associated with the National Malaria Control Program in the 1960's was very successful in reducing sand fly populations and cases of VL were nearly eliminated[44]. However, as with triatomine vectors, recent studies show certain populations of sand flies gaining insecticide resistance[45-47], resulting in growing concern about insecticide use that is cost prohibitive and responsible for considerable environmental toxicity. Furthermore, in many parts of Bihar and West Bengal, return of peri-domestic populations of sand flies has been observed within one year of insecticide spraying[48].
Paratransgenic control of leishmania transmission
We are developing a paratransgenic strategy to control transmission of L. donovani by P. argentipes. As in the case of Chagas disease control, we intend to prevent disease transmission by effectively providing the sand flies with passive immunity against the invading parasite. We chose to focus our paratransgenic efforts in Bihar, India where P. argentipes is the main vector for VL for several reasons. Though multiple studies have reported the difficulties in identifying breeding sites of New World sandflies[49-52], there is substantial data attesting to identification of breeding sites of P. argentipes in eastern India[53-57]. Ghosh and Bhattacharya[58] described P. argentipes breeding sites in West Bengal and further described the population biology of this vector[59]. A study in Pondicherry, India delineated breeding sites of both P. argentipes and P. papatasi[60]. In VL-endemic regions of Bihar State, Kesari et al.[61] were highly successful in isolating sand fly larvae from soil samples of mixed dwellings and isolated cowsheds. The accuracy in identifying these sites and retrieving larvae from the soil suggest that breeding sites of P. argentipes in the endemic states of Bihar are characterized to some degree.
To date, there are no known reports of any symbiotic bacteria associated with P. argentipes. However, the gut microbiology of adult sandflies has been described in the past[62]. In 2008, we detailed an extensive analysis of the gut flora of P. argentipes trapped from four VL-endemic sites in Bihar, India, and concluded that sand fly-microbial associations reflect the environment in which the sand flies reside[4]. In this survey, we identified 28 distinct gut microorganisms through 16S rDNA sequencing. A number of these microbes, including Staphylococcus spp., Escherichia coli, and Enterobacter, are all human pathogens, thereby precluding their use in a paratransgenic system. However, we also identified an abundance of several nonpathogenic soil bacteria that are used in industry including Bacillus megaterium, Brevibacterium linens, Bacillus subtilis, and Bacillus pumilis. Our goal is to use these “generally regarded as safe” microbes as delivery vehicles for anti-leishmanial compounds to sandflies.
How would the paratransgenic strategy work in this vector? The life cycle of P. argentipes is short with a brief window of parasite development. Female sand flies acquire the parasite when they ingest macrophages infected with amastigotes during a blood meal. The parasite matures within the alimentary tract of the sand fly as a promastigote form. Four to five days after feeding, the promastigotes move forward to the esophagus and the salivary glands of the insect. When the sand fly subsequently takes a blood meal from the mammalian host, leishmania promastigotes are transferred to the host. For the paratransgenic strategy to be successful in this system, the recombinant commensal bacteria should preferably persist within the sand fly gut until the first blood meal. However, an anti-leishmania recombinant molecule may remain active in the sand fly gut in the absence of recombinant carrier (Figure 3). In our experience with paratransgenic triatomine bugs, recombinant cecropin A persisted for over 6 months in the gut lumen with biological activity [3]. Such an outcome would actually be viewed as highly desirable as the intended biological effect would occur with reduced risk of unwanted spread of transgenic bacteria via activities of the adult flies[63].
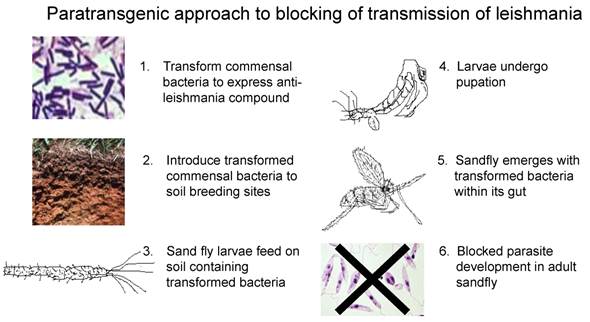
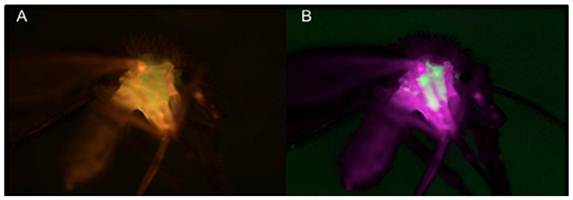
The acquisition of bacteria in P. argentipes has not been studied. However, the large number of soil and environmental bacteria isolated in our study suggests that sand flies are typically colonized by microbes encountered at breeding sites or during sugar meals[4]. Our strategy is to deploy large concentrations of transformed commensal bacteria at sand fly breeding sites to selectively colonize the emerging flies. We recently demonstrated the transstadial passage of GFP-B. subtilis transformants from 4th instar larvae to the emergent sand fly stage, thereby generating the first paratransgenic sand fly (Figure 4)[63]. These results suggest that this deployment strategy can be a viable option for field delivery of transformed, non-pathogenic commensal bacteria to sand fly breeding sites. We are currently evaluating several molecules, including AMP's and scFv's, for anti-leishmania activity for ultimate field use.
Paratransgenic strategy for control of leishmaniasis. In this scheme, we propose to transform sand fly commensal bacteria to express anti-leishmania molecules (1), these genetically altered microbes will be introduced to sand fly breeding sites (2) where they will be consumed by sand fly larvae (3). We have demonstrated that the genetically altered bacteria will be retained by the sand fly as it undergoes metamorphosis (4). Expression of the anti-leishmania molecule in the gut of the emergent paratransgenic sand fly (5) would kill invading leishmania species, therefore rendering the sand fly refractory to infection (6), and thus transmission of the parasite.
Whole mount of paratransgenic sand fly. A: shows the auto-fluorescence associated with the outer carapace and specific GFP fluorescence within the sand fly. B: shows GFP-specific fluorescence signal uncoupled from the background. These 4x-images were captures using a Nuance multispectral imaging system. GFP-specific fluorescence is contained to the midgut chamber of the adult sand fly with no evidence of transfer to other regions of the insect. Figure is adapted from Hurwitz et al, The paratransgenic sand fly: a platform for control of Leishmania transmission. Parasit Vectors, 2011, 4:e82
Conclusions
Despite great advances in public health worldwide, insect-transmitted infectious diseases remain a leading cause of morbidity and mortality. Currently, the best methods for control of many insect-borne diseases involve the use of chemical pesticides. Though such campaigns may, in the short term, yield spectacular results, environmental toxicity and adverse effects on human health limit the use of many chemical pesticides. Further, the emergence of insect resistance to a wide variety of insecticides has greatly undermined their efficacy. Finally, the cost of repeated applications of pesticides is often prohibitive. We describe a novel method, termed paratransgenesis, for transmission control of two specific vector borne diseases, Chagas disease and visceral leishmaniasis, via expression of gene products in the insect vectors via transformed symbiotic or commensal microbes. We show that the expression of AMP's can significantly reduce carriage of T. cruzi in the hindgut of triatomine bugs. We have recently added other molecules, such as endoglucanases and highly specific scFv's, to our armamentarium of effector molecules for the paratransgenic control of T. cruzi transmission. Effector molecules that may be effective against leishmania are currently being studied.
Unlike current arthropod eradication strategies with insecticides, the paratransgenic approach does not aim to eliminate triatomine bugs or sand flies. Rather, the overarching goal is to modulate the insect's ability to transmit a parasite. Such an approach could provide valuable tools for control of Chagas disease and VL in highly endemic regions of the world.
Acknowledgements
This work is supported by NIH/NIAID RO1AI66045-4 (RD). Nuance multispectral images of sand flies were generated in the University of New Mexico & Cancer Center Fluorescence Microscopy Shared Resource, funded as detailed on: http://hsc.unm.edu/crtc/microscopy/Facility.html.
Conflict of Interests
The authors have declared that no conflict of interest exists.
References
1. Beard CB, Cordon-Rosales C, Durvasula RV. Bacterial symbionts of the triatominae and their potential use in control of Chagas disease transmission. Annu Rev Entomol. 2002;47:123-141
2. Beard CB, Durvasula RV, Richards FF. Bacterial symbiosis in arthropods and the control of disease transmission. Emerg Infect Dis. 1998;4:581-591
3. Durvasula RV, Gumbs A, Panackal A, Kruglov O, Aksoy S, Merrifield RB, Richards FF, Beard CB. Prevention of insect-borne disease: an approach using transgenic symbiotic bacteria. Proc Natl Acad Sci USA. 1997;94:3274-3278
4. Hillesland H, Read A, Subhadra B, Hurwitz I, McKelvey R, Ghosh K, Das P, Durvasula R. Identification of aerobic gut bacteria from the kala azar vector, Phlebotomus argentipes: a platform for potential paratransgenic manipulation of sand flies. Am J Trop Med Hyg. 2008;79:881-886
5. Miller T, Lauzon C, Lampe D, Durvasula R, Matthews S. Paratransgenesis applied to insect-transmitted disease: The Pierce's Disease Case. In: (ed.) Miller T, Bourtzis K. Insect Symbiosis 2. London: Taylor and Francis. 2006
6. Subhadra B, Hurwitz I, Fieck A, Subba Rao G, Subba Rao DV, Durvasula RV. Development of paratransgenic artemia as a platform for control of infectious diseases in shrimp aariculture. J Appl Microbiol. 2009;108:831-840
7. Schmunis GA. Epidemiology of Chagas disease in non-endemic countries: the role of international migration. Mem Inst Oswaldo Cruz. 2007;102(Suppl 1):75-85
8. Schmunis GA, Yadon ZE. Chagas disease: a Latin American health problem becoming a world health problem. Acta Trop. 2010;115:14-21
9. Tanowitz HB, Weiss LM, Montgomery SP. Chagas disease has now gone global. PLoS Negl Trop Dis. 2011;5:e1136
10. WHO. Chagas disease: control and elimination. Report by the Secretariat. Sixty-second World Health Assembly; A62/17. WHO. 2009
11. Maguire JH, Hoff R, Sherlock I, Guimaraes AC, Sleigh AC, Ramos NB, Mott KE, Weller TH. Cardiac morbidity and mortality due to Chagas' disease: prospective electrocardiographic study of a Brazilian community. Circulation. 1987;75:1140-1145
12. Rassi AJr, Rassi A, Marin-Neto JA. Chagas disease. Lancet. 2010;375:1388-1402
13. Kirchhoff LV. American trypanosomiasis (Chagas' disease). In: (ed.) Guerrant R, Walker DH, Weller PF. Tropical infectious diseases: Principles, pathogens & practice. Philadelphia: Churchill Livingstone. 2006:1082
14. Bestetti RB, Muccillo G. Clinical course of Chagas' heart disease: a comparison with dilated cardiomyopathy. Int J Cardiol. 1997;60:187-193
15. Moncayo A. Chagas disease: current epidemiological trends after the interruption of vectorial and transfusional transmission in the Southern Cone countries. Mem Inst Oswaldo Cruz. 2003;98:577-591
16. Moncayo A, Ortiz Yanine MI. An update on Chagas disease (human American trypanosomiasis). Ann Trop Med Parasitol. 2006;100:663-677
17. Cecere MC, Vazquez-Prokopec GM, Gurtler RE, Kitron U. Spatio-temporal analysis of reinfestation by Triatoma infestans (Hemiptera: Reduviidae) following insecticide spraying in a rural community in northwestern Argentina. Am J Trop Med Hyg. 2004;71:803-810
18. Gurtler RE, Canale DM, Spillmann C, Stariolo R, Salomon OD, Blanco S, Segura EL. Effectiveness of residual spraying of peridomestic ecotopes with deltamethrin and permethrin on Triatoma infestans in rural western Argentina: a district-wide randomized trial. Bull WHO. 2004;82:196-205
19. Gurtler RE, Petersen RM, Cecere MC, Schweigmann NJ, Chuit R, Gualtieri JM, Wisnivesky-Colli C. Chagas disease in north-west Argentina: risk of domestic reinfestation by Triatoma infestans after a single community-wide application of deltamethrin. Trans R Soc Trop Med Hyg. 1994;88:27-30
20. Picollo MI, Vassena C, Santo Orihuela P, Barrios S, Zaidemberg M, Zerba E. High resistance to pyrethroid insecticides associated with ineffective field treatments in Triatoma infestans (Hemiptera: Reduviidae) from Northern Argentina. J Med Entomol. 2005;42:637-642
21. Baines S. The role of the symbiotic bacteria in the nutrition of Rhodnius prolixus. J Exp Biol. 1956;33:533-541
22. Dasch GA, Weiss E, Chang S. Endosymbionts in Insects. Baltimore, MD: Williams & Wilkins. 1984
23. Beard CB, Mason PW, Aksoy S, Tesh RB, Richards FF. Transformation of an insect symbiont and expression of a foreign gene in the Chagas' disease vector Rhodnius prolixus. Am J Trop Med Hyg. 1992;46:195-200
24. Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3:710-720
25. Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389-395
26. Hale JD, Hancock RE. Alternative mechanisms of action of cationic antimicrobial peptides on bacteria. Expert Rev Anti Infect Ther. 2007;5:951-959
27. Hancock RE, Scott MG. The role of antimicrobial peptides in animal defenses. Proc Natl Acad Sci USA. 2000;97:8856-8861
28. Yang D, Biragyn A, Kwak LW, Oppenheim JJ. Mammalian defensins in immunity: more than just microbicidal. Trends Immunol. 2002;23:291-296
29. Fieck A, Hurwitz I, Kang AS, Durvasula R. Trypanosoma cruzi: synergistic cytotoxicity of multiple amphipathic anti-microbial peptides to T. cruzi and potential bacterial hosts. Exp Parasitol. 2010;125:342-347
30. Cooper R, de Jesus AR, Cross GA. Deletion of an immunodominant Trypanosoma cruzi surface glycoprotein disrupts flagellum-cell adhesion. J Cell Biol. 1993;122:149-156
31. Durvasula RV, Gumbs A, Panackal A, Kruglov O, Taneja J, Kang AS, Cordon-Rosales C, Richards FF, Whitham RG, Beard CB. Expression of a functional antibody fragment in the gut of Rhodnius prolixus via transgenic bacterial symbiont Rhodococcus rhodnii. Med Vet Entomol. 1999;13:115-119
32. Durvasula RV, Sundaram RK, Kirsch P, Hurwitz I, Crawford CV, Dotson E, Beard CB. Genetic transformation of a Corynebacterial symbiont from the Chagas disease vector Triatoma infestans. Exp Parasitol. 2008;119:94-98
33. Markiv A, Anani B, Durvasula RV, Kang AS. Module based antibody engineering: a novel synthetic REDantibody. J Immunol Methods. 2011;364:40-49
34. Figueiro AR, Nunes ZG, Silvia AAL, Giordano-Dias CMG, Conra JR, Hofer E. Isolation of microorganisms of triatomines maintained in artificial and sylvatic conditions. Mem Inst Oswaldo Cruz. 1995;90:228
35. Durvasula RV, Kroger A, Goodwin M, Panackal A, Kruglov O, Taneja J, Gumbs A, Richards FF, Beard CB, Cordon-Rosales C. Strategy for introduction of foreign genes into field populations of Chagas disease vectors. Annnals Entomol Soc Am. 1999;92:937-943
36. Matthews S, Sreehari Rao V, Durvasula RV. Modeling horizontal gene transfer (HGT) in the gut of the Chagas disease vector Rhodnius prolixus. Parasit Vectors. 2011;4:e77
37. WHO. Control of the Leishmaniases. WHO Technical Report Series. 2010;949:22-26
38. Planning Commission, Government of India. Kala azar; In 9th Five Year Plan; Sec3.4.89. 1996.
39. Guerin PJ, Olliaro P, Sundar S, Boelaert M, Croft SL, Desjeux P, Wasunna MK, Bryceson AD. Visceral leishmaniasis: current status of control, diagnosis, and treatment, and a proposed research and development agenda. Lancet. 2002;2:494-501
40. Sundar S, Chakravarty J, Agarwal D, Rai M, Murray HW. Single-dose liposomal amphotericin B for visceral leishmaniasis in India. N Engl J Med. 2010;362:504-512
41. Sundar S, Agrawal N, Arora R, Agarwal D, Rai M, Chakravarty J. Short-course paromomycin treatment of visceral leishmaniasis in India: 14-day vs 21-day treatment. Clin Infect Dis. 2009;49:914-918
42. Sundar S, Jha TK, Thakur CP, Bhattacharya SK, Rai M. Oral miltefosine for the treatment of Indian visceral leishmaniasis. Trans R SocTrop Med Hyg. 2006;100:S26-33
43. Sundar S, Sinha PK, Rai M, Verma DK, Nawin K, Alam S, Chakravarty J, Vaillant M, Verma N, Pandey K. et al. Comparison of short-course multidrug treatment with standard therapy for visceral leishmaniasis in India: an open-label, non-inferiority, randomised controlled trial. Lancet. 2011;377:477-486
44. Thakur CP, Kumar K. Post kala-azar dermal leishmaniasis: a neglected aspect of kala-azar control programmes. Ann Trop Med Parasitol. 1992;86:355-359
45. Dhiman RC, Raghavendra K, Kumar V, Kesari S, Kishore K. Susceptibility status of Phlebotomus argentipes to insecticides in districts Vaishaii and Patna (Bihar). The J Commun Dis. 2003;35:49-51
46. Kishore K, Kumar V, Kesari S, Bhattacharya SK, Das P. Susceptibility of Phlebotomus argentipes against DDT in endemic Districts of North Bihar, India. J Commun Dis. 2004;36:41-44
47. Singh R, Das RK, Sharma SK. Resistance of sandflies to DDT in Kala-azar endemic districts of Bihar, India. Bull WHO. 2001;79:793
48. Mukhopadhyay AK, Hati AK, Chakraborty S, Saxena NB. Effect of DDT on Phlebotomus sandflies in Kala-Azar endemic foci in West Bengal. J Commun Dis. 1996;28:171-175
49. Feliciangeli MD, Rodriguez N, De Guglielmo Z, Rodriguez A. The re-emergence of American visceral leishmaniasis in an old focus in Venezuela. II. Vectors and parasites. Parasite. 1999;6:113-120
50. Ferro C, Pardo R, Torres M, Morrison AC. Larval microhabitats of Lutzomyia longipalpis (Diptera: Psychodidae) in an endemic focus of visceral leishmaniasis in Colombia. J Med Entomol. 1997;34:719-728
51. Hanson WJ. The breeding places of Phlebotomus in Panama (Diptera: Psychodidae). Ann Entomol Soc Am. 1961;54:317-322
52. Travi BL, Velez ID, Brutus L, Segura I, Jaramillo C, Montoya J. Lutzomyia evansi, an alternate vector of Leishmania chagasi in a Colombian focus of visceral leishmaniasis. Trans R Soc Trop Med Hyg. 1990;84:676-677
53. Dhiman RC, Shetty PS, Dhanda V. Breeding habitats of phlebotomine sandflies in Bihar, India. Indian J Med Res. 1983;77:29-32
54. Hati AK. Current status of leishmaniasis - vector biology. In Proceedings of Indo-UK Workshop on Leishmaniasis. Edited by Mahajan RC. New Delhi, India: Indian Council of Medical Research. 1983:84-91
55. Pandya AP, Niyogi AK. Ecological study on immature stages of phlebotomid sandflies in Gujarat. Indian J Med Res. 1980;72:355-358
56. Shortt HE, Smith ROA, Swaminath CS. The breeding nature of Phlebotomus argentipes Ann & Brun. Bull Entomol Res. 1930;21:269-271
57. Smith ROA, Mukherjee S, Lal D. Bionomics of P. argentipes Part III. The breeding sites of P. argentipes and an attempt to control these insects by antilarval measures. Indian J Med Res. 1936;24:557-562
58. Ghosh KN, Bhattacharya A. Breeding places of Phlebotomus argentipes Annandale and Brunetti (Diptera: Psychodidae) in West Bengal, India. Parassitologia. 1991;33:267-272
59. Ghosh KN, Mukhopadhyay JM, Guzman H, Tesh RB, Munstermann LE. Interspecific hybridization and genetic variability of Phlebotomus sandflies. Med Vet Entomol. 1999;13:78-88
60. Ilango K, Dhanda V, Srinivasan R, Sadanand AV, Lane RP. Phlebotomine sandflies (Diptera: Psychodidae) of Tamil Nadu and Pondicherry, southern India, in relation to visceral leishmaniasis. Ann Trop Med Parasitol. 1994;88:413-431
61. Kesari S, Kishore K, Palit A, Kumar V, Roy MS, Sivakumar S, Kar SK. An entomological field evaluation of larval biology of sandfly in Kala-azar endemic focus of Bihar--exploration of larval control tool. J Commun Dis. 2000;32:284-288
62. Volf P, Kiewegova A, Nemec A. Bacterial colonisation in the gut of Phlebotomus duboseqi (Diptera: Psychodidae): transtadial passage and the role of female diet. Folia Parasitol (Praha). 2002;49:73-77
63. Hurwitz I, Hillesland H, Fieck A, Das P, Durvasula R. The paratransgenic sand fly: a platform for control of Leishmania transmission. Parasit Vectors. 2011;4:e82
Author contact
![]() Corresponding author: Ravi Durvasula (505)991 3812, ravi.durvasulagov.
Corresponding author: Ravi Durvasula (505)991 3812, ravi.durvasulagov.

 Global reach, higher impact
Global reach, higher impact