Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2011; 7(9):1412-1426. doi:10.7150/ijbs.7.1412 This issue Cite
Review
Mechanisms Underlying the Induction of Regulatory T cells and Its Relevance in the Adaptive Immune Response in Parasitic Infections
1. Instituto Nacional de Neurología y Neurocirugía Manuel Velasco Suárez
2. Unidad Periférica para el estudio de neuroinflamación del Instituto de Investigaciones Biomédicas de la UNAM en el INNN, Insurgentes Sur 3877, Col. La Fama, México, D.F. 14269, México
3. Departamento de Inmunología, Instituto de Investigaciones Biomédicas, Universidad Nacional Autónoma de México, México, D.F, 04510, México
Received 2011-9-1; Accepted 2011-10-1; Published 2011-11-1
Abstract
To fulfill its function, the immune system must detect and interpret a wide variety of signals and adjust the magnitude, duration, and specific traits of each response during the complex host-parasite relationships in parasitic infections. Inflammation must be tightly regulated since uncontrolled inflammation may be as destructive as the triggering stimulus and leads to immune-mediated tissue injury.
During recent years, increasing evidence points to regulatory T cells (Tregs) as key anti-inflammatory cells, critically involved in limiting the inflammatory response. Herein, we review the published information on the induction of Tregs and summarize the most recent findings on Treg generation in parasitic diseases.
Keywords: regulatory T cells, parasitic infections, inflammation, dendritic cell, regulation, immune response, cytokines, malaria, Leishmania, Trypanosoma, Schistosoma, nematodes.
Regulatory T cells: phenotypes and induction
Regulatory T cells (Tregs) play an important role in the control of the immune response. Different types of Treg cells have been described, and may be classified into two main groups: thymic and inducible. Thymic or natural Treg cells (nTregs), are produced in the thymus and are present in the host's bloodstream before pathogen or damage exposure. Inducible Treg cells (iTregs) are those cells that acquire a regulatory function in the context of a given infection or a neoplastic process. Inducible Treg cell populations include: T regulatory 1 (Tr1) cells, which secrete IL‑10; T helper 3 (Th3) cells, which secrete TGF-β, and converted Foxp3+ Treg cells [1-3]. This review is focused on the induction of CD4+ Tregs, mostly by Foxp3+ expression, and on their suppressor activities during parasite infections.
Inducible Treg cells are generated in the periphery and exert their suppressor activity mainly by producing IL-10, IL-35, and TGF-β [4, 5]. Initially, these iTreg cells are conventional T cells expressing low or null levels of CD25 (CD25low/-) [6]. CD25 expression could be up-regulated according to environmental conditions [7]. On the other side, Foxp3 expression is up-regulated by signaling pathways initiated by T cell receptor (TCR), co-stimulatory molecules, IL-2R, programmed death ligand 1 (PDL1), transforming growth factor-β (TGF-β) receptor, and Notch [8-10]. In fact, the TCR density on the cell surface and its affinity to the antigen play a key role in Treg induction. It has been demonstrated that TCR stimulation by a strong agonist in low dose resulted in maximal induction of Foxp3 in vivo. Also, the density and duration of TCR interactions define a cumulative TCR stimulation that determines initial Foxp3 induction [11]. In contrast, high doses of TCR engagement in presence of CD28 co-stimulation induce NF-κB signaling, which prevents Foxp3 induction in mice [12]. On the other hand, recently it has been demonstrated that CD28 signals alone, independently of TCR-mediated stimulatory pathways, are sufficient to induce Foxp3 transcription in CD4(+)CD25(-) T cells. Additionally, the stimulation of CD28 associated with TCR can regulate Foxp3 expression; the authors propose that, under these conditions, CD28 signals mediate Foxp3 trans-activation by nuclear translocation of RelA/NF-κB [13, 14]. Furthermore, the presence of regulatory cytokines like IL-10, TGF-β, and IL-35 during the priming of T lymphocytes favors regulatory T cell generation, function and maintenance (this point is discussed later). Most probably, the accumulation of signals that yield a regulatory T cell phenotype depend on the molecules expressed on the cell surface, their interaction with environmental molecules, and the prevalent intracellular signaling. All of these factors may in turn be modified by infective agents as a way to evade immunity.
In that sense, several mechanisms have been reported as involved in Treg-mediated immune suppression, offering a wide range of possibilities to exhibit their function under different environmental and requirement conditions. Some of these mechanisms are reviewed elsewhere [15].
Role of dendritic cells and cytokines in Treg induction during the adaptive immune response
Tregs can be generated by conversion from naïve T cells or by expanding the population of pre-existing Treg cells. The nTregs antigen-specificity issue remains controversial since they are expanded from pre-existing Tregs. In contrast, Tregs induced as a consequence of adaptive immune response are generally antigen-specific [16]; however, Treg cells can inhibit responder T cells of unrelated antigen specificity [17]. On the other hand, the Treg induction pathway depends on the interaction of T cells with tolerogenic dendritic cells, anti-inflammatory cytokines, glucocorticoids, vitamins, or other suppressive molecules produced by pathogens [18].
Role of dendritic cells
The immunological paradigm places fully mature dendritic cells (DCs) as immunity inducers. It is currently believed that DCs integrate a variety of incoming signals and decide whether protective immunity or tolerance develops. Mature DCs might be crucially involved in the expansion and induction of Treg cells. In fact, conventional CD4+ cells become either specific T helper or T regulatory cell subsets depending upon the affinity of their TCR to the antigen, the strength of the co-stimulatory signals provided by antigen-presenting cells (APCs), and the cytokine milieu (Fig. 1).
Different pathways of regulatory T cell induction. A) Semimature dendritic cells promote the conversion of naïve T cells into Treg cells. B) Fully mature dendritic cells generate new antigen-specific regulatory T cells during immune response priming. C) Fully mature DCs may be crucially involved in the expansion of preformed Treg cells. D) Plasmacytoid dendritic cells also promote Treg cell induction by ICOS-L, TLR9, TGF-β and IDO-dependent pathways.
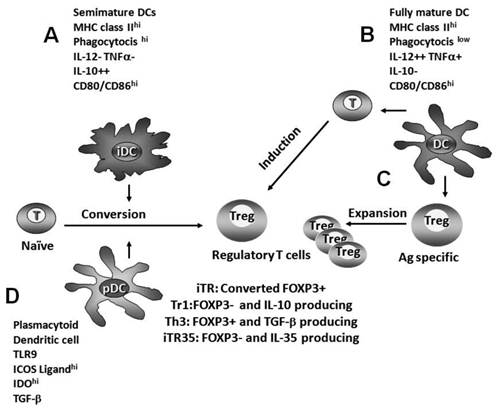
In contrast, semimature DCs promote T cell tolerance by the conversion of naïve T cells into Treg cells. The absence of inflammation arrests dendritic cells into a semimature state (iDC). iDCs are characterized by the expression of MHC II, a high phagocytosis capacity, and low CD80/CD86 expression; they produce IL-10, but neither IL-12 nor TNFα. The inability of producing IL-12p70 in bioactive amounts together with IL-10 generates a unique phenotype of semimature DC that induces Treg cells (Fig. 1) [19]. In turn, these newly formed Treg cells induce a suppressive environment.
Role of cytokines
IL-10
IL-10 plays a role in Treg increase and maintenance [20]. The role of IL-10 in regulatory T cell induction probably involves several events: in APC, IL-10 reduces antigen presentation by trapping peptide-loaded major histocompatibility complex type II (MHCII) molecules, reducing the co-stimulatory molecules CD80/CD86 expression, and destabilizes cytokine mRNAs. Additionally, it has been reported that high levels of CD40, CD86, and PD-L1 in APC favor de novo induction of Tr1 cells [21].
In turn, IL-10 conditions CD4+ T cells to become unresponsive to antigens, and these cells lose the capacity to produce cytokines, probably by induction of suppressors of cytokine signaling (SOCS-1 and -3) [22]. Other IL-10 effects on peripheral generation of Treg cells may be mediated by activation of the Notch-dependent signaling pathway. Transgenic mice carrying the constitutively active intracellular domain of Notch3 in thymocytes and T cells fail to develop experimental autoimmune diabetes. The inability to develop the disease is associated with an increase of CD4+CD25+ Treg cells. In fact, an accumulation of Tregs in lymphoid organs and pancreas infiltrates is observed. This accumulation is paralleled with an increased expression of IL-4 and IL-10 [23]. Furthermore, the stimulation of purified murine CD4+ T cells with antigen, anti-CD3 and anti-CD28 antibodies has proved to induce a transient increase in Notch ligand and receptor expression [24]. Probably, Notch has a key role in boosting the differentiation and possibly the function of Treg cells [23]. Likewise, in naïve mouse CD4+ T cells, Notch induces IL-10 production via a signal transducer and activator of transcription 4 (STAT4). IL-10 could act as a positive autocrine factor in the development of IL-10-producing Tregs [25].
An indirect mechanism of action of IL-10 in the generation of suppressor cells occurs through CD4+ anergic induction. In one pathway, IL-10 induces anergy in CD4+ T cells [26]; in another pathway, treatment of dendritic cells with IL-10 yields anergic T lymphocytes [27, 28]. These anergic T cells act as suppressor cells by competing with other antigen-stimulated T cells for the membrane of APCs and for locally produced IL-2 [20].
TGF-β
Evidence of the in vivo effect of TGF-β in a Treg cell pool expansion was described in diabetic mice stimulated with TGF-β. In these mice, TGF-β inhibited autoimmune type I diabetes development, in addition to an increase in the Treg frequency in pancreatic intraislets. These cells showed high levels of intracellular CTLA-4 and Foxp3 expression [29]. Early studies in human T cells demonstrated that TGF-β was necessary to induce Tregs. Stimulation of human CD4+ cells with TGF-β increases the number of Tregs and intracellular expression of CD25 and CTLA-4. This expansion was due to both an increased proliferation and the protection of these cells from activation-induced apoptosis [30].
TGF-β promotes the induction of Treg cells accompanied by an increase in Foxp3 expression. In mice, it has been demonstrated that TGF-β is able to convert CD4+CD25-Foxp3- non-Tregs into CD4+CD25+Foxp3+ Tregs. Evidence for the role of TGF-β was collected in Foxp3-mRFP mice, in which Foxp3-expressing cells were marked by messenger red fluorescent protein (mRFP) expression. Upon TCR engagement, TGF-β induced de novo Foxp3 expression. Furthermore, only Foxp3+CD4+ cells but not their Foxp3-CD4+ counterparts showed regulatory activity [31]. Also, in an independent study, Zhang et al. reported that engagement to TCR and CTLA-4 delivers a specific signal which cooperates with TGF-β signaling molecules to initiate Smad signaling and recruits co-activators to activate Foxp3 expression [8, 32].
Foxp3 regulation and induction mechanisms are currently being elucidated. Fantini et al. suggested that TGF-β may induce Foxp3 directly by binding to the inhibitory Smad7 promoter region to turn off its expression. Thus, TGF-β might produce a feedback regulation of TGF-β signaling that may result in a cumulative Foxp3 expression, facilitating the conversion of T cells into Tregs [33].
Foxp3 transcription is a key factor in Treg induction, differentiation, function and survival. foxp3 gene expression is controlled by a core promoter and at least three distal enhancers. During T cell activation, a number of transcription factors (NFAT, AP1, CREB, SP1, c-Rel, RUNX, TIEG1, RAR Stat5, ATF, and Smad 3) bind to conserved noncoding sequences (CNS) in the promoter and in intron 2 [34]. In fact, the 5' and 3' CNS in intron 2 serve as enhancer sites. The formation of a Foxp3-specific “enhanceosome” containing nuclear factor-kB, c-Rel, NFATc2, p65, Smad3, and CREB as FOXP3 expression inducers [35-38] has been identified. Recent studies indicate that murine TGF-β drives Foxp3 expression, probably in two ways: 1) through the induction of activated Smad3, as it initially binds to an enhancer site in intron 2 of foxp3 gene and then interacts with nuclear factors to form an “enhanceosome”, which binds to the foxp3 promoter, resulting in Foxp3 transcription; 2) TGF-β induces increased H4 histone acetylation in the region of NFAT/Smad binding; this increases the accessibility of various transcription factors and facilitates the FOXP3 promoter activity [38].
Notably, the relationship between TGF-β and CTLA-4 is essential for Treg induction. Zheng et al. have demonstrated that TGF-β is required to induce Foxp3 expression, but in CTLA-4-/- deficient mice TGF-β stimulation is not able to convert CD4+CD25- cells into Tregs. This group has also demonstrated that CTLA-4 ligation to CD80 shortly after T cell activation enables TGF-β to induce CD4+CD25- cells to expressing FoxP3 and developing suppressor activity. Additionally, Foxp3 has been reported to up-regulate CTLA-4 expression; thus, a TGF-β/CTLA-4/FoxP3/CTLA-4 positive loop may be vital for the induction and maintenance of regulatory T cells [32].
It was suggested that TGF-β alone is unable to mediate Treg cell induction. In fact, the combination of IL-2 and TGF-β play different but complementary roles in Treg induction. In IL-2 KO mice, Foxp3 expression cannot be sustained in the periphery; also, IL-2 KO T cells stimulated with TGF-β are not able to convert CD4+ T cells into Treg cells. Thus, during Treg induction it is likely that TGF-β induces Foxp3 expression in newly induced Tregs, and that Foxp3 stability is apparently IL-2-dependent [39]. In agreement with these observations, Foxp3+ Treg induction requires the expression of IL2R and TGF-β receptors [39, 40]. Additionally, the administration of IL-2 results in the stabilization of Foxp3 expression in TGF-β-induced Tregs in vivo [41]. Additionally, an indirect effect might take place during Treg induction: while IL-2 could promote the growth of these cells, TGF-β would protect them from activation-induced apoptosis [20].
Recently, new molecules have been implied in Foxp3 induction by TGF-β. In mucosal tissue, mature tolerogenic DCs producing retinoic acid also induce Foxp3+ Tregs via a TGF-β-dependent mechanism. In fact, retinoic acid enhances TGF-β signaling by increasing the expression and phosphorylation of Smad3, and this results in increased Foxp3 expression, even in the presence of IL-6 or IL-21 [42].
Furthermore, it has been proposed that Activin A may contribute to the expansion of peripheral Treg cells. Activin A is a pleiotropic TGF-β family member and is expressed in response to inflammatory signals. Huber et al. reported that Activin A together with TGF-β1 shows synergistic effects on the Treg conversion rate and seems to be essential for Treg induction [43].
IL-35
IL-35 is a heterodimeric cytokine composed by an Epstein-Barr virus-induced gene 3 (EBI3) subunit plus the p35 subunit of IL-12. IL-35 is a Treg cell-specific cytokine, required for the maximum regulatory activity of mouse Treg cells in vitro and in vivo [4]. Recently, Collison and colleagues described a newly identified population of CD4+ Treg cells, induced by IL-35, called “iTR35 cells”. They have demonstrated that human and mouse CD4+ T cells activated with beads coated with anti-CD3 and anti-CD28 antibodies in the presence of IL-35 substantially up-regulated EBI3 and IL12A mRNA, which encode the two constituents of IL-35. iTR35 did not express Foxp3 or other key suppressive cytokines (IL-10 or TGF-β); thus, the proposed phenotype is CD4+Foxp3−Ebi3+p35+IL-10−TGF-β−. iTR35 mediated suppression exclusively via IL-35 and seemingly independently of IL-10 and TGF-β. iTR35 expresses CTLA-4 in a relatively low percentage (<15%), and its function is not yet clear. Finally, these authors showed the regulatory capacity of iTR35 by restoration of immune homeostasis and preventing autoimmunity in Foxp3-/- mice, preventing EAE, controlling T cell proliferation in a lymphopenic setting, and limiting the pathogenesis during experimental colitis [44].
Role of glucocorticoids in Treg cell induction
Glucocorticoids (GCs) inhibit the ability of APCs to stimulate effector T lymphocytes and favor Treg cell induction. Emilie et al. showed that GCs and IL-10 stimulate the production of an intracellular factor called glucocorticoid-induced leucine zipper (GILZ) in monocytes/macrophages and in human DCs [45]. GILZ prevents the expression of activation molecules such as MHC II, CD80, CD86, and CD83, and inflammatory chemokines. Besides, GILZ stimulates the expression of immunoregulatory molecules like B7-H1/CD274, ILT3/CD85k and IL-10 [46]. Hamdi et al. showed that GILZ-expressing DCs stimulate in T lymphocytes the expression of the Tregs markers CD25hi, Foxp3, and CTLA-4. Induced Tregs are able to produce IL-10 and inhibit T CD4+ cell proliferation in an antigen-specific manner. In fact, IL-10 production by Tregs is essential for suppression. The mechanism probably implies two different ways: 1) IL-10 acts as a growth factor for Tregs, and 2) IL-10 contributes to their suppressive functions [47, 48].
The concomitant administration of vitamins and dexamethasone induces differentiation of regulatory T cells from naïve T CD4+ cells in both humans and mice. These Treg cells are characterized by: (a) IL-10 production, (b) attenuated secretion of IL-2 and IFNγ, (c) lack of induction of the Th1 or Th2 phenotype, and (d) significant antigen-specific and non-specific suppression of T cell proliferation. The mechanism described may imply a direct effect on dendritic cells, as mentioned above [49-53].
Another mechanism of Treg cell induction may involve a direct effect of vitamin D3 and dexamethasone on T cells. Barrat et al. have shown that vitamin D-Dex treatment induces the development of IL-10-producing T cells in absence of APCs. When naïve CD4 T cells were simultaneously stimulated with vitamin D-Dex, anti-CD3 and anti-CD28, higher numbers of IL-10-producing cells were obtained. The use of anti-IL-10R mAbs inhibits Treg induction in these APC-free systems. Thus, IL-10 acts as a positive autocrine factor in the development of IL-10-producing Treg cells [54]. This effect could be associated to the down-regulation of NF-kB p50 and c-Rel protein expression [50, 54, 55].
Plasmacytoid dendritic cells in Tregs induction
Plasmacytoid DCs (pDCs) play distinct roles in regulating T cell-mediated adaptive immunity. Firstly, pDCs express different PAMS and possess the ability to produce large amounts of type-1 IFNs. Also, maturing pDCs exhibit the ability to generate IL-10-producing Treg cells [56]. These mechanisms are possibly mediated by indoleamine 2,3-dioxygenase (IDO) and inducible T-cell co-stimulator ligand (ICOS-L), among other molecules [56, 57]. pDCs do not express functional IDO constitutively; however, they can be readily activated by tolerogenic ligands or by CpG. In fact, binding of CpG to TLR9 and CTLA-4-Ig or GITR-Ig to B7 in pDCs promotes IDO protein expression. IDO catalyzes the conversion of tryptophan into kynurenine, a critical metabolite involved in the generation of Tregs with potent cell suppressor function from CD4+CD25- T cells [57-59]. Recently it has been demonstrated that IDO acts as an intracellular signal transducer, in response to TGF-β, to induce a stable regulatory phenotype in plasmacytoid dendritic cells. These pDCs are able to generate Tregs from naïve T cells [60].
The role of ICOS-L in Treg generation was elucidated by Ito et al., 2004. They found that naïve peripheral CD4 T cells co-cultured with pDC in the presence of neutralizing antibodies against ICOS-L inhibits IL-10 production by Treg cells, but not other cytokines such as IFN-γ or TNFα. On the other side, in the same study it was shown that the stimulation of naïve CD4 T cells with anti-CD3 alone or anti-CD3 in the presence of ICOS-L generates Treg cells capable of producing high levels of IL-10 but not IL-4 [56]. Furthermore, a specific defect in Treg cell-mediated mucosal tolerance has been observed in ICOS-deficient (ICOS−/−) mice [61]. In agreement with this, ICOS-L has been previously implicated in the generation of IL-10-producing Treg cells, both in human studies and mouse disease models [62, 63].
Moreover, pDC are not a main APC subset and probably this would limit their role in regulatory T cell induction. However, the interaction of TLR9 with CpG favors pDC suppressor activity, as stated above. This may promote a suppressor environment accompanied by Treg induction.
The capacity of parasites to activate TLR9 in pDC and thus induce Treg cells as a part of their control strategies on the host's immune response still needs to be elucidated.
Generation of regulatory T cells in parasitic infections
Regulatory T cells help controlling the immune response, but in some cases pathogens may promote an excessive regulatory control, which allows parasite replication without restraint and compromises the host. The consequence of such control is an enhanced pathogen survival and, in some cases, a long-term pathogen persistence. Even though there are clear evidences of Treg cell induction during many parasitic infections, their role in these parasitoses is not well established yet. Herein we discuss some illustrative examples of parasitic infections that involve Tregs in their pathogenesis.
In leishmaniasis
Parasites of the genus Leishmania are the causative agents of cutaneous, mucocutaneous or visceral leishmaniasis [64]. Leishmania sp. triggers multiple effects on the functions of macrophages and dendritic cells that promote immunomodulation of innate immune response and impair their capacity to initiate Th1 cell immunity (as discussed in [65]).
It has been suggested that intracellular forms of Leishmania (promastigotes and amastigotes) produce yet unknown molecules that promote an immunoregulatory environment, probably through IL-10 and TGF-β. This way, the induction of regulatory T cells permit a low-level persistence of the parasite, which in turn maintains the immunological stimulus (Table 1) [66, 67]. In fact, in vivo experiments have suggested that transferred CD25- T cells become CD25+. These Tregs are homed at the parasite inoculation site, where they promote the infection establishment and ensure the long-term survival of the parasite in the host [66]. Actually, CD103 expression has been shown to be essential for Tregs homing. Genetically susceptible BALB/c mice that lack CD103 become resistant to Leishmania major infection, a phenotype associated with a poor capacity of Tregs to be retained in the infection site (skin) [68]. Conversely, Treg cells accumulate in the lesions of patients with acute cutaneous leishmaniasis. These Tregs are functionally suppressive, since they decrease the IFN-γ production. The main suppressive action observed in intralesional Tregs implies IL-10 and IDO [69].
Different authors have proposed that, during infection, the parasite promotes Treg induction, which might also modulate effector T cells or antigen-presenting cell (APC) functions by means of cell contact-dependent mechanisms [66, 67, 70]. Other teams have observed that lower CD40 expression levels in APC are related to the degree of infection of L. donovani. Interestingly, the parasite down-regulates CD40 expression and up-regulates IL-10 and TGF-β expression in APC during infection. All of these conditions are required for efficient Treg induction [71]. Additionally, Leishmania amastigotes can infect dendritic cells and down-regulate early innate signaling events, resulting in impaired DC function that includes lower CD40 and CD83 expression, suppressed IL-12p40, IL-12p70, and IL-6 production, and increased IL-10 production [70].
Recently, it has been reported that Leishmania major cutaneous infection stimulated IDO expression in local lymph nodes [72]. The known effect of IDO on dendritic cells suggests that this molecule could be involved in Treg induction during cutaneous infection.
Regulatory T cells (Tregs) in leishmaniasis
| Parasites | Host | Molecule involved | Cells involved | Role of Tregs in pathogenesis | Ref # |
|---|---|---|---|---|---|
| L. major | Mouse | IL-10 | *Tr1 | Tregs accumulation favors parasite persistence | [66, 134] |
| L. donovani | Mouse | TGF-β | º Th3 | Enhance parasite growth and exacerbate infection | [135] |
| L. donovani | Human | IL-10 | Tr1 | Enhance skin lesion | [136] |
| L. guyanensis | Human | IL-10, IDO | ‡Tregs | Accumulation in lesions from acute cutaneous leishmaniasis; Tregs contribute to infection chronicity | [69, 137] |
| L. infantum | Mouse | IL-10, TGF-β | Tr1, Th3 | Influence local immune response that favors parasite persistence | [138] |
| L. braziliensis | Mouse/Human | IL-10, TGF-β | Tregs | Enhance cutaneous lesions | [139, 140] |
| L. braziliensis | Macaque | IL-10 | Tregs | Regulate local immune response | [141] |
*Tr1: IL-10 producing cells, ºTh3:TGF-β producing cells, ‡Tregs: conventional Foxp3+ cells
In schistosomiasis
Schistosomiasis is one of the most common parasitic infections worldwide. Upon infection, adult parasites migrate to the mesenteric veins, where they lay hundreds of eggs per day. Some of these eggs are trapped in the liver, gut and other organs, causing a vigorous granulomatose response [73]. The promotion of Tregs could be regarded either as beneficial or detrimental for the parasite. This approach to the role of Treg cells in schistosomiasis has not been explored yet, and deserves further research. We propose two hypothetical possibilities about the role of Tregs in schistosomiasis. In one, the immune system itself induces Tregs to control the exacerbated immune response that produce granulomas. Thus, granulomas would be useful to eliminate the parasite, since they would help locating the eggs and triggering the immune response. As a second possibility, parasite antigens mediate Treg induction during granuloma formation. The fibrosis promoted by collagen production induced by IL-13 [73, 74] supports the first possibility. IL-13 could also be directly involved in the induction of Treg cells. The engagement of the IL-4R alpha-chain by binding cytokines IL-4 and IL-13 was identified as an inducer of CD25+ Tregs from peripheral naïve CD25-CD4 T cells [75]. Nevertheless, it is not known if this IL-13 production during schistosomiasis infection is related or not to Treg cell generation. Supporting the second possibility, Treg cells have been associated to egg antigens during Schistosome infections in mice [76, 77]. In fact, IL-10-secreting CD4+CD25+ cells isolated from granuloma from chronically infected mice can suppress the proliferation of CD4+ T cells [78, 79]. An alternative approach to address the role of Treg cells in granulomas was adopted by Singh et al., 2005, using experimentally infected mice with Schistosoma mansoni. They found that granulomatous livers at 8 and 16 weeks after infection showed 10- and 30-fold increases in Foxp3 expression compared with normal liver. Also, the percentage of Treg cells in granuloma rose from 12% at 8 weeks to 88% at 16 weeks after infection. Retroviral transfer of the Foxp3 gene at the onset of granuloma formation enhanced Foxp3 expression fourfold in granuloma CD4(+) CD25(+) T cells, and strongly suppressed full granuloma development [80]. All these data suggest that Schistosoma may be implicated in the evolution of granulomas (Table 2).
In trypanosomiasis
Trypanosomes are zooflagellate protozoans that cause persistent infections accompanied by a profound immunosuppression [81]. Trypanosoma cruzi is an intracellular parasite, primarily located in the tissues, while African trypanosomes are extracellular forms circulating in the blood. The role of Tregs in trypanosomiasis infection is controversial. On one side, African trypanosomiasis is characterized by gradual expansions of the total number of Tregs, starting at parasite establishment and continuing during the chronic stages of the disease, which favors parasite tolerance (Table 3) [82, 83]. On the other side, Tregs have a limited role on Trypanosoma cruzi pathogenesis [84, 85]. In fact, depletion of Treg cells during acute and chronic infection neither improved nor worsened the course of the infection and the immunity involved [86]. However, it is important to note that T. cruzi and African trypanosomes are completely different, and that immune response induced by an extracellular or by an intracellular parasite will trigger different effector mechanisms. Thus, the role of Tregs in trypanosomiasis may depend on factors such as the parasite biology, the environment, and the host immune response.
Regulatory T cells (Tregs) in schistosomiasis
| Parasites | Host | Molecule involved | Cells involved | Role of Tregs in pathogenesis | Ref # |
|---|---|---|---|---|---|
| S. mansoni | Mouse | IL-10 | *Tr1, ‡Tregs | Suppress Th1 and Th2 response and increase the susceptibility to infection | [78, 79] |
| S. mansoni | Mouse | Tregs | Regulate immune response, favoring eggs survival | [133] | |
| S. japonicum | Mouse | IL-10, TGF-β | Tregs | Regulate immune response, favoring parasite survival | [142] |
| S. mansoni | Mouse | CD103, GITR, OX40, CTLA-4 | Tregs | Limit and control granuloma growth | [143] |
| S. japonicum | Mouse | IL-10 | Tregs | Promote worm burden | [144] |
*Tr1: IL-10 producing cells, ‡Tregs: conventional Foxp3+ cells
Regulatory T cells (Tregs) in trypanosomiasis
| Parasites | Host | Molecule involved | Cells involved | Role of Tregs in pathogenesis | Ref # |
|---|---|---|---|---|---|
| T. congolese | Mouse | IL-10 | ‡Tregs, *Tr1, ∞nTregs | Down-regulate the Th1 response and preserve host parasite clearance | [83] |
| T. cruzi | Human | IL-10, CTLA-4 | Tregs,Tr1 | Control early stages and favor the disease progression | [145] |
| T. congolese | Mouse | Tregs | Prevent early protective response | [82] | |
| T. cruzi | Mouse | GITR | Tregs | Control inflammation | [85, 140] |
| T. cruzi | Mouse | Tregs | Neither improve nor worsen the outcome of immune response | [86] |
*Tr1: IL-10 producing cells, ‡Tregs: conventional Foxp3+ cells, ∞nTregs: Natural Tregs
On the other hand, Treg generation during trypanosome infection is supported by multiple evidences. The mechanism may imply a default in DC activation or a specific cytokine balance to an immunosuppressive environment [87]. At early stages of infection, Guilliams et al., 2007, observed that Treg population is expanded in spleen and liver of mice infected by T. congolense. These cells produced IL-10 and mediated suppression of the type-1 inflammatory immune response [83]. Probably, the effect of these Tregs goes beyond the sole local suppressive activity and the delay of the onset of immune response. The IL-10, TGF-β and CTLA-4 suppressive environment probably yields “special” conditions that favor the expansion of antigen-specific Tregs, as seen in some trypanosome (T. congolense and T. cruzi) mouse models [83, 88]. In addition, T. cruzi trypomastigotes seem to be responsible for the generation of regulatory DC in vitro; in turn, these DCs induce Treg cells in vitro [87]. The mechanism may involve DC activation by the parasite, mediated by TLR4 and ERK, and is associated to the yielding of high IL-10 levels [89]. Nevertheless, the relevance of this phenomenon in Treg induction remains to be elucidated.
In malaria
Malaria is caused by infection with protozoan parasites of the genus Plasmodium. After infection, sporozoites move from the dermis to the liver, where they go through an asymptomatic stage of rapid division before the parasite re-enters the bloodstream. In blood, exponential expansion of parasite populations leads to febrile illness. Typically, acute infection is controlled and chronic infection is established at reasonably low parasite density. However, in some cases the disease is lethal [90].
Infection with malaria parasites induces an immune response, either an effector or a suppressive one. Treg cells induction during malaria infection could be either beneficial or detrimental to the progression of the disease. Controversial results on the immunomodulatory properties of parasite components are reported. There is also conflicting information about the role of Tregs in malaria infection. In mice models, Tregs depletion protects experimental subjects from death when infected with a lethal strain of Plasmodium yoelii. Additionally, Tregs depletion protects mice from Plasmodium falciparum in cerebral malaria (CM) [91-93]. In contrast, during CM associated with Plasmodium berghei or during infection with Plasmodium chabaudi, Tregs depletion does not alter the parasitemia in mice [94-96]. These contradictory effects of Tregs in Plasmodium infection could be related to kinetic differences in the modulation of pro- and anti-inflammatory responses. Moreover, particular parameters such as mouse strain, age, sex, early cytokine profile production, and natural susceptibility to infection may influence the resulting role of Tregs.
In spite of the contradictory evidence on the role of Treg cells on malaria infection, some results suggest the Treg cell induction by Plasmodium. In fact, several reports demonstrate the increase of Tregs with suppressive capacity: Human infections with Plasmodium falciparum and P. vivax induced a significant expansion of CD4+CD25+Foxp3+ Treg cells expressing the key immunomodulatory molecule CTLA-4 [97]. Also, a Treg increase in human cord blood was observed in newborns of women with malaria; these Tregs are able to suppress Th1-type recall responses [98]. In addition, an increased proportion of Tregs with suppressive functions has been observed in P. yoelii and in P. berghei infected mice [95, 99-101]. The effect of this Treg increase at the beginning or during malaria progression needs to be clarified.
Moreover, malaria infection seemingly drives Tregs generation/induction. Couper et al., 2007, have compared the depletion and repopulation of spleen CD4+CD25+Foxp3+ Tregs in uninfected and malaria-infected mice. By adoptive transfer, they have shown that repopulation of the spleen by CD25(high)Foxp3+ cells results from the re-expression of CD25 on peripheral populations of CD25-Foxp3+. Repopulation of the spleen by CD25+Foxp3+ cells occurs extremely rapidly in malaria-infected mice when compared to non-infected mice. Thus, malaria infection may drive proliferation and CD25 expression in peripheral CD4+CD25-Foxp3+ cells and/or conversion of CD4+CD25-Foxp3- cells [102].
On the other hand, T cell induction during malaria may be cytokine-dependent (IL-10 and TGF-β). A correlation has been observed between Tregs and elevated IL-10 levels in susceptible mice, but not in resistant mouse strains [101]. P. yoelii triggers Treg cell induction, probably mediated by TGF-β activation. In fact, thrombospondin-like molecules and metalloproteases present in P. yoelii extracts are able to activate latent TGF-β to its bioactive form. During the priming of malaria-specific T cells, TGF-β might up-regulate Foxp3, leading to the development of regulatory T cells [103].
In humans, a P. falciparum-mediated conversion of CD4(+)CD25(-) T cells into CD25+Foxp3+CD4+ T cells has been described. Induction of Foxp3 cells in vitro required TGF-β1 and IL-10, and these cells show the typical suppressor phenotype (CTLA-4(+), CD127(low), CD39(+), ICOS(+), TNFRII(+)) [104]. In accordance, an increase in systemic IL-10 and TGF-β that correlates with up-regulation of CD4+CD25+Foxp3+ has been observed in humans infected with P. falciparum and P. vivax [105, 106].
The induction of Treg cells during malaria may imply dendritic cells. During Plasmodium yoelii infection, regulatory CD11clowCD45RBhigh DCs become prevalent in the spleen, overtaking the conventional CD11chigh DCs. Additionally, regulatory CD11clowCD45RBhigh DCs induce IL-10-expressing CD4 T cell [107]. In vivo experiments demonstrated that during acute infection of P. yoelii, DCs migrate to the spleen and secrete TGF-β, prostaglandin E2 and IL-10. These DCs inhibit Plasmodium response, probably by generation of new Treg cells [108]. On the other hand, the pigment hemozoin (a metabolite from the parasite's intraerythrocytic stage) and P. falciparum merozoites promoted the generation of immunosuppressive dendritic cells. The maturation of human monocyte-derived dendritic cells (DCs) in vitro with hemozoin and merozoites prevented IL-12 production and augmented the release of IL-10 and TNFα. Additionally, merozoites prevented soluble CD40 ligand-induced phenotypic maturation of DCs [109, 110]. This observation suggests that hemozoin and merozoites induce a suppressive DC phenotype, which may play a role in favor of Treg cell induction. However, the immunosuppressive properties of hemozoin and merozoites remain uncertain. There are reports concerning the immunosuppressive properties of hemozoin and merozoites by inhibiting dendritic cell activity (hemozoin reviewed in [111]) [110]. In contrast, other authors suggest DC activation by these parasitic components [112-114]. Careful investigations are required to identify the molecule(s) responsible of modulating the DC function during malaria and their role in Treg induction.
On the other hand, it has been described that Treg cell induction can be a result of indirect interactions during malaria infection. Scholzen et al. reported an induction of human CD25+Foxp3+ CD4 T cells independent of MHCII interaction. Co-culture of the trophozoite-stage malaria-infected red blood cells with PBMC induces an enhancement of Treg levels. This induction seems not to be necessarily mediated by direct TCR stimulation, but could be mediated by soluble factors, including IL-2, IL-10, and TGF-β [104]. Also, Hisaeda observed that P. yoelii induces Tregs independently of MHCII interaction. Malaria parasites interact with TLR9 in DCs to induce Tregs during infection [115]. This author and some others have proposed that merozoites/protein-DNA complexes or genetic material trapped in hemozoin crystals are ligands for TLR9 [112-115]. Thus, TLR9 signaling might be a key factor for Treg induction [115] (Table 4). In contrast, other authors have observed that TLR9-deficient mice are able to trigger immune responses, control the infection and clear the parasite [113, 116]. Although the latter studies did not report Treg levels, this finding indicate the need to further study the role of TLR9 during this parasitosis and its effect on Treg induction.
Regulatory T cells (Tregs) in malaria
| Parasites | Host | Molecule involved | Cells involved | Role of Tregs in pathogenesis | Ref # |
|---|---|---|---|---|---|
| P. yoelii | Mouse | IL-10, TGF-β | ‡Tregs, *Tr1 | Contribute to immune suppression, impede parasite clearance and promotes mice death | [91, 146, 147] |
| P. yoelii | Mouse | IL-10 | Tregs | Suppress Th1 response early in the infection | [101, 147, 148] |
| P. falciparum | Human | TGF-β, | Tregs | Increase in vivo parasite growth and disease severity | [105, 149] |
| P. falciparum | Human | IL-10, CTLA-4 | Tregs | Suppress Th1 response and favor placental infection | [150, 151] |
| P. falciparum | Mouse | IL-10 | Tregs | Modulate the pro- and anti-inflammatory responses affecting pathogenesis | [92] |
| P. berghei | Mouse | ∞nTregs | Suppress the Th1 response | [93, 100] | |
| P. berghei | Mouse | Tregs | No effect on cerebral malaria | [99] | |
| P. chabaudi | Mouse | nTregs | Control the secretion of pro-inflammatory cytokines | [95] | |
| P. vivax | Human | IL-10, TGF-β, GITR, CTLA-4 | Tregs | Increase parasite burden | [106] |
| P. falciparum | Human | IL-10, TGF-β | Tregs | No effect on acute malarial inflammation Facilitate parasite clearance without causing immunopathology | [152] |
| P. yoelii | Mouse | TLR9 | Tregs | Prevent anti-parasite immunity | [115] |
*Tr1: IL-10 producing cells, ‡Tregs: conventional Foxp3+ cells, ∞nTregs: Natural Tregs
In nematode infections
The filarial worm Onchocerca volvulus has been shown that induces immunosuppressive environment by cytokines such as IL-10 and TGF-β [117, 118]. In fact, infected patients has Tregs expressing CTLA-4 in onchocercomas lesion; and this cells are associated to immunosuppression during chronic infection [119]. Concordantly, hyper-reactive patients show reduced TGF-β expression [120] indicating that this cytokine may participate in down-regulating the immune response.
Other human and animal filarial diseases (Wuchereria bancrofti, Brugia malayi, Mansonella perstans and Litomosoides sigmodontis) promote Treg recruitment and increase Treg levels at the infection site (Table 5) [121-123]. In Brugia malayi infection (larvae and adult stages) there is immunomodulation associated to Treg expansion and to an increase in suppressive molecules such as TGF-β, CTLA-4, PD-1, ICOS, IDO [124, 125]. During L. sigmodontis infection, Tregs are increased both in acute and chronic infections [121, 126]. Taylor et al. observed that Treg depletion during L. sigmodontis infection did not affect larval establishment, but reduced the number of adult parasites [121]. Also, in W. bancrofti and M. perstans infection, Treg expansion has been observed, associated to an IL-10-dominated regulatory environment [122]. A variety of events may contribute to activation or control of the immune response. In fact, not only parasite components may control the immune response, but also other factors affecting the parasite itself. As an example, the endosymbiont Wolbachia (a bacterium living in filarial nematodes) is essential for the worm fertility and survival, and modulates the immune response in patients harboring W. bancrofti. In fact, a recombinant Wolbachia surface protein (WSP) induces suppressive T cell response in Wuchereria bancrofti infections. WSP components induce the expression of CTLA-4, IL-10, and TGF-β in PBMCs from filariasis patients [127]. This suggests that WSP may contribute to the suppression of the immune response in filariasis patients, probably by Treg induction.
Heligmosomoides polygyrus, a gastrointestinal nematode, induces de novo Foxp3 expression in T cells and increases IL-10 and TGF-β levels; these induced Tregs express CD103 and CTLA-4 [128]. Additionally, induced Tregs have suppressive activity in vitro [128, 129]. Rausch et al. have examined the role of effector and regulatory T cells during H. polygyrus infection by adoptive transfer. Their results show that while conventional T effector cells conferred protection and led to a significant decrease in worm burdens, Tregs had no effect on worm burdens in the host. However, not long after infection, the number of Foxp3+ Treg cells temporarily increased in the inflamed tissue. On the other hand, the transfer of a heterogeneous T-cell population containing Treg and T effector cells yields a significant decrease in worm number [129]. This study points out the importance of a balance between effector and regulatory immune response to determinate the course of infection.
Regulatory T cells (Tregs) in nematode infections
| Parasites | Host | Molecule involved | Cells involved | Role of Tregs in pathogenesis | Ref # |
|---|---|---|---|---|---|
| O. volvulus | Human | IL-10, TGF-β, CTLA-4 | *Tr1 | Tregs in onchocercomas are associated with immunosuppression during chronic infection. | [119] |
| L. sigmodontis | Mouse | IL-10, CTLA-4, GITR | ‡Tregs | No effect on larva establishment but favors chronicity. Inhibit protective immunity | [121, 126] |
| L. sigmodontis | Mouse | TGF-β | Tregs | Induce unspecific suppression | [153] |
| W. bancrofti and M. perstans | Human | IL-10 | ∞nTregs, Tr1 | Induce a regulatory environment | [122] |
| W. bancrofti | Human | §WSP, IL-10, TGF-β, CTLA-4 | T cell | Induce hypo-responsiveness in antigen-specific T cells | [127] |
| B. malayi | Human | TGF-β, CTLA-4, PD-1, ICOS, IDO | Tregs | Impairment of specific Th1 and Th2 immune response | [124] |
| H. polygyrus | Mouse | IL-10, TGF-β, CTLA-4 | Tregs | No effect on worm burdens | [129] |
| H. polygyrus | Mouse | IL-10, TGF-β | Tregs | In vitro suppression of effector response | [128] |
| H. polygyrus | Mouse | **HES | DC | Impairment of dendritic cell function and modulation of immune responses | [131] |
| H. polygyrus | Mouse | HES | Treg | Promote suppressive immune response | [132] |
*Tr1: IL-10 producing cells, ‡Tregs: conventional Foxp3+ cells, ∞nTregs: Natural Tregs, §WSP Wolbachia surface protein, **HES: Parasite excretory-secretory products.
The mechanisms of Treg induction in nematode infections are not known. However, a mechanism for Treg induction by Heligmosomoides has been described, and it has been proposed to be common among nematodes. On one side, the secreted and constitutive parasite components act on DCs promoting Treg generation. On the other side, secreted components act directly on T lymphocytes to turn them into Tregs [130]. In fact, H. polygyrus produces in vitro an excretory-secretory antigen termed HES. It has been described that HES and an adult worm homogenate (AWH) inhibit DC activation, cytokine (IL-12p70, IL-6, TNF-α, and IL-10) and chemokine (MCP-1, and RANTES) production, as well as co-stimulatory molecule expression (CD40, CD86 and MHC class II). Also, in DC HES induce the generation of CD4+CD25+ IL-10-producing T cells able to inhibit effector T cell proliferation and IFN-γ production [131].
With regard to the effect of HES in T cells, Grainger et al. have described that HES is able to ligate directly to TGF-β receptor in T cells and promote a signaling pathway (Smad2/3 phosphorilation). Thus, HES triggers a TGF-β-like activity that mimics TGF-β functions. More interestingly, HES induces de novo Foxp3 expression in naïve T cells depending on signaling through the TGF-β receptor. To confirm the role of HES in Treg induction, the authors show that Foxp3 Treg cell conversion is enhanced in vivo by H. polygyrus infection [132]. The fact that nematodes secrete a TGF-β-like molecule suggests that parasites have developed strategies to circumvent the host's immune response. These parasites have been learned during millions of years of co-evolution with their hosts to identify molecules that promote Treg induction.
Conclusions
Parasites use all the mechanisms herein described to induce Treg cells during their establishment and to promote their survival in the host. However, the structure and function of the parasite molecules involved in Treg induction is just partially known. Many parasitic components could act as “cytokine-like” or directly stimulate immune receptors; it is also possible that parasite molecules act directly as Treg cell inducers. Some cases have been described in which a parasite molecule can use TLR4, TLR2 and TLR9 as intermediaries to induce Treg cells [89, 115, 133]. In addition, parasites drive the cytokine balance during infection to yield an environment that promotes Tregs generation. The knowledge on the immune components and parasite molecules involved in controlling the immune response would be useful for a better understanding of the molecular mechanisms of Treg induction. Finally, the use of parasite molecules to induce Tregs in vivo may provide a new alternative to control inflammation in other diseases.
Acknowledgements
This study was supported by CONACYT (46953-M, CB-2008-01 100708 and S0008-08-01-86527) and by DGAPA IN213911, Mexico. Juan Francisco Rodríguez proofread the English version of this manuscript.
Abbreviations
Tregs: regulatory T cells; Tr1: T regulatory 1 cells; Th3: T helper 3 cells; TCR: T cell receptor; PDL1: programmed death ligand 1; TGF-β: transforming growth factor-β; DC: dendritic cells; APCs: antigen-presenting cells; SOCS: suppressors of cytokine signaling; STAT: signal transducer and activator of transcription; GCs: glucocorticoids; GILZ: glucocorticoid-induced leucine zipper; pDCs: plasmacytoid DCs; IDO: indoleamine 2,3-dioxygenase; ICOS: inducible T-cell co-stimulator.
Conflict of Interests
The authors have declared that no conflict of interest exists. The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflicts with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
1. Apostolou I, Sarukhan A, Klein L, von Boehmer H. Origin of regulatory T cells with known specificity for antigen. Nat Immunol. 2002;3:756-763
2. Akbar AN, Taams LS, Salmon M, Vukmanovic-Stejic M. The peripheral generation of CD4(+) CD25(+) regulatory T cells. Immunology. 2003;109:319-325
3. Fantini MC, Dominitzki S, Rizzo A, Neurath MF, Becker C. In vitro generation of CD4+CD25+regulatory cells from murine naive T cells. Nat Protoc. 2007;2:1789-1794
4. Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, Vignali DA. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566-569
5. Sakaguchi S, Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T. Regulatory T cells: how do they suppress immune responses? Int Immunol. 2009;21:1105-1111
6. Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875-1886
7. Zheng SG, Wang JH, Gray JD, Soucier H, Horwitz DA. Natural and induced CD4+CD25+ cells educate CD4+CD25- cells to develop suppressive activity: the role of IL-2, TGF-beta, and IL-10. J Immunol. 2004;172:5213-5221
8. Zhang L, Zhao Y. The regulation of Foxp3 expression in regulatory CD4(+)CD25(+)T cells: multiple pathways on the road. J Cell Physiol. 2007;211:590-597
9. Shen Z, Chen L, Hao F, Wu J. Transcriptional regulation of Foxp3 gene: multiple signal pathways on the road. Med Res Rev. 2009;29:742-766
10. Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015-3029
11. Gottschalk RA, Corse E, Allison JP. TCR ligand density and affinity determine peripheral induction of Foxp3 in vivo. J Exp Med. 2010;207:1701-1711
12. Molinero LL, Miller ML, Evaristo C, Alegre ML. High TCR Stimuli Prevent Induced Regulatory T Cell Differentiation in a NF-{kappa}B-Dependent Manner. J Immunol. 2011;186:4609-4617
13. Scotta C, Soligo M, Camperio C, Piccolella E. FOXP3 induced by CD28/B7 interaction regulates CD25 and anergic phenotype in human CD4+CD25- T lymphocytes. J Immunol. 2008;181:1025-1033
14. Soligo M, Camperio C, Caristi S, Scotta C, Del Porto P, Costanzo A, Mantel PY, Schmidt-Weber CB, Piccolella E. CD28 costimulation regulates FOXP3 in a RelA/NF-kappaB-dependent mechanism. Eur J Immunol. 2011;41:503-513
15. Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523-532
16. Chatenoud L. Natural and induced T CD4+CD25+FOXP3+ regulatory T cells. Methods Mol Biol. 2011;677:3-13
17. Thornton AM, Shevach EM. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol. 2000;164:183-190
18. Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235-238
19. Rutella S, Danese S, Leone G. Tolerogenic dendritic cells: cytokine modulation comes of age. Blood. 2006;108:1435-1440
20. Horwitz DA, Zheng SG, Gray JD. The role of the combination of IL-2 and TGF-beta or IL-10 in the generation and function of CD4+ CD25+ and CD8+ regulatory T cell subsets. J Leukoc Biol. 2003;74:471-478
21. Maynard CL, Weaver CT. Diversity in the contribution of interleukin-10 to T-cell-mediated immune regulation. Immunol Rev. 2008;226:219-233
22. Grutz G. New insights into the molecular mechanism of interleukin-10-mediated immunosuppression. J Leukoc Biol. 2005;77:3-15
23. Anastasi E, Campese AF, Bellavia D, Bulotta A, Balestri A, Pascucci M, Checquolo S, Gradini R, Lendahl U, Frati L, Gulino A, Di Mario U, Screpanti I. Expression of activated Notch3 in transgenic mice enhances generation of T regulatory cells and protects against experimental autoimmune diabetes. J Immunol. 2003;171:4504-4511
24. Hoyne GF, Dallman MJ, Champion BR, Lamb JR. Notch signalling in the regulation of peripheral immunity. Immunol Rev. 2001;182:215-227
25. Rutz S, Janke M, Kassner N, Hohnstein T, Krueger M, Scheffold A. Notch regulates IL-10 production by T helper 1 cells. Proc Natl Acad Sci U S A. 2008;105:3497-3502
26. Ye Z, Huang H, Hao S, Xu S, Yu H, Van Den Hurk S, Xiang J. IL-10 has a distinct immunoregulatory effect on naive and active T cell subsets. J Interferon Cytokine Res. 2007;27:1031-1038
27. Steinbrink K, Graulich E, Kubsch S, Knop J, Enk AH. CD4(+) and CD8(+) anergic T cells induced by interleukin-10-treated human dendritic cells display antigen-specific suppressor activity. Blood. 2002;99:2468-2476
28. Zheng Z, Narita M, Takahashi M, Liu A, Furukawa T, Toba K, Aizawa Y. Induction of T cell anergy by the treatment with IL-10-treated dendritic cells. Comp Immunol Microbiol Infect Dis. 2004;27:93-103
29. Peng Y, Laouar Y, Li MO, Green EA, Flavell RA. TGF-beta regulates in vivo expansion of Foxp3-expressing CD4+CD25+ regulatory T cells responsible for protection against diabetes. Proc Natl Acad Sci U S A. 2004;101:4572-4577
30. Zheng SG, Gray JD, Ohtsuka K, Yamagiwa S, Horwitz DA. Generation ex vivo of TGF-beta-producing regulatory T cells from CD4+CD25- precursors. J Immunol. 2002;169:4183-4189
31. Wan YY, Flavell RA. Identifying Foxp3-expressing suppressor T cells with a bicistronic reporter. Proc Natl Acad Sci U S A. 2005;102:5126-5131
32. Zheng SG, Wang JH, Stohl W, Kim KS, Gray JD, Horwitz DA. TGF-beta requires CTLA-4 early after T cell activation to induce FoxP3 and generate adaptive CD4+CD25+ regulatory cells. J Immunol. 2006;176:3321-3329
33. Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25- T cells through Foxp3 induction and down-regulation of Smad7. J Immunol. 2004;172:5149-5153
34. Xu L, Kitani A, Strober W. Molecular mechanisms regulating TGF-beta-induced Foxp3 expression. Mucosal Immunol. 2010;3:230-238
35. Long M, Park SG, Strickland I, Hayden MS, Ghosh S. Nuclear factor-kappaB modulates regulatory T cell development by directly regulating expression of Foxp3 transcription factor. Immunity. 2009;31:921-931
36. Ruan Q, Kameswaran V, Tone Y, Li L, Liou HC, Greene MI, Tone M, Chen YH. Development of Foxp3(+) regulatory t cells is driven by the c-Rel enhanceosome. Immunity. 2009;31:932-940
37. Venuprasad K, Huang H, Harada Y, Elly C, Subramaniam M, Spelsberg T, Su J, Liu YC. The E3 ubiquitin ligase Itch regulates expression of transcription factor Foxp3 and airway inflammation by enhancing the function of transcription factor TIEG1. Nat Immunol. 2008;9:245-253
38. Tone Y, Furuuchi K, Kojima Y, Tykocinski ML, Greene MI, Tone M. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat Immunol. 2008;9:194-202
39. Zheng SG, Wang J, Wang P, Gray JD, Horwitz DA. IL-2 is essential for TGF-beta to convert naive CD4+CD25- cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J Immunol. 2007;178:2018-2027
40. Wan YY, Flavell RA. The roles for cytokines in the generation and maintenance of regulatory T cells. Immunol Rev. 2006;212:114-130
41. Chen Q, Kim YC, Laurence A, Punkosdy GA, Shevach EM. IL-2 Controls the Stability of Foxp3 Expression in TGF-{beta}-Induced Foxp3+ T Cells In Vivo. J Immunol. 2011;186(11):6329-37
42. Xiao S, Jin H, Korn T, Liu SM, Oukka M, Lim B, Kuchroo VK. Retinoic acid increases Foxp3+ regulatory T cells and inhibits development of Th17 cells by enhancing TGF-beta-driven Smad3 signaling and inhibiting IL-6 and IL-23 receptor expression. J Immunol. 2008;181:2277-2284
43. Huber S, Stahl FR, Schrader J, Luth S, Presser K, Carambia A, Flavell RA, Werner S, Blessing M, Herkel J, Schramm C. Activin a promotes the TGF-beta-induced conversion of CD4+CD25- T cells into Foxp3+ induced regulatory T cells. J Immunol. 2009;182:4633-4640
44. Collison LW, Chaturvedi V, Henderson AL, Giacomin PR, Guy C, Bankoti J, Finkelstein D, Forbes K, Workman CJ, Brown SA, Rehg JE, Jones ML, Ni HT, Artis D, Turk MJ, Vignali DA. IL-35-mediated induction of a potent regulatory T cell population. Nat Immunol. 2010;11:1093-1101
45. Berrebi D, Bruscoli S, Cohen N, Foussat A, Migliorati G, Bouchet-Delbos L, Maillot MC, Portier A, Couderc J, Galanaud P, Peuchmaur M, Riccardi C, Emilie D. Synthesis of glucocorticoid-induced leucine zipper (GILZ) by macrophages: an anti-inflammatory and immunosuppressive mechanism shared by glucocorticoids and IL-10. Blood. 2003;101:729-738
46. Cohen N, Mouly E, Hamdi H, Maillot MC, Pallardy M, Godot V, Capel F, Balian A, Naveau S, Galanaud P, Lemoine FM, Emilie D. GILZ expression in human dendritic cells redirects their maturation and prevents antigen-specific T lymphocyte response. Blood. 2006;107:2037-2044
47. Hamdi H, Godot V, Maillot MC, Prejean MV, Cohen N, Krzysiek R, Lemoine FM, Zou W, Emilie D. Induction of antigen-specific regulatory T lymphocytes by human dendritic cells expressing the glucocorticoid-induced leucine zipper. Blood. 2007;110:211-219
48. Mittelstadt PR, Ashwell JD. Inhibition of AP-1 by the glucocorticoid-inducible protein GILZ. J Biol Chem. 2001;276:29603-29610
49. Griffin MD, Lutz W, Phan VA, Bachman LA, McKean DJ, Kumar R. Dendritic cell modulation by 1alpha,25 dihydroxyvitamin D3 and its analogs: a vitamin D receptor-dependent pathway that promotes a persistent state of immaturity in vitro and in vivo. Proc Natl Acad Sci U S A. 2001;98:6800-6805
50. Tan PH, Sagoo P, Chan C, Yates JB, Campbell J, Beutelspacher SC, Foxwell BM, Lombardi G, George AJ. Inhibition of NF-kappa B and oxidative pathways in human dendritic cells by antioxidative vitamins generates regulatory T cells. J Immunol. 2005;174:7633-7644
51. Pedersen AE, Gad M, Walter MR, Claesson MH. Induction of regulatory dendritic cells by dexamethasone and 1alpha,25-Dihydroxyvitamin D(3). Immunol Lett. 2004;91:63-69
52. Dong X, Bachman LA, Kumar R, Griffin MD. Generation of antigen-specific, interleukin-10-producing T-cells using dendritic cell stimulation and steroid hormone conditioning. Transpl Immunol. 2003;11:323-333
53. Unger WW, Laban S, Kleijwegt FS, van der Slik AR, Roep BO. Induction of Treg by monocyte-derived DC modulated by vitamin D3 or dexamethasone: differential role for PD-L1. Eur J Immunol. 2009;39:3147-3159
54. Barrat FJ, Cua DJ, Boonstra A, Richards DF, Crain C, Savelkoul HF, de Waal-Malefyt R, Coffman RL, Hawrylowicz CM, O'Garra A. In vitro generation of interleukin 10-producing regulatory CD4(+) T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J Exp Med. 2002;195:603-616
55. O'Garra A, Barrat FJ. In vitro generation of IL-10-producing regulatory CD4+ T cells is induced by immunosuppressive drugs and inhibited by Th1- and Th2-inducing cytokines. Immunol Lett. 2003;85:135-139
56. Ito T, Yang M, Wang YH, Lande R, Gregorio J, Perng OA, Qin XF, Liu YJ, Gilliet M. Plasmacytoid dendritic cells prime IL-10-producing T regulatory cells by inducible costimulator ligand. J Exp Med. 2007;204:105-115
57. Puccetti P, Fallarino F. Generation of T cell regulatory activity by plasmacytoid dendritic cells and tryptophan catabolism. Blood Cells Mol Dis. 2008;40:101-105
58. Chen W, Liang X, Peterson AJ, Munn DH, Blazar BR. The indoleamine 2,3-dioxygenase pathway is essential for human plasmacytoid dendritic cell-induced adaptive T regulatory cell generation. J Immunol. 2008;181:5396-5404
59. Baban B, Chandler PR, Sharma MD, Pihkala J, Koni PA, Munn DH, Mellor AL. IDO activates regulatory T cells and blocks their conversion into Th17-like T cells. J Immunol. 2009;183:2475-2483
60. Pallotta MT, Orabona C, Volpi C, Vacca C, Belladonna ML, Bianchi R, Servillo G, Brunacci C, Calvitti M, Bicciato S, Mazza EM, Boon L, Grassi F, Fioretti MC, Fallarino F, Puccetti P, Grohmann U. Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat Immunol. 2011;12:870-878
61. Miyamoto K, Kingsley CI, Zhang X, Jabs C, Izikson L, Sobel RA, Weiner HL, Kuchroo VK, Sharpe AH. The ICOS molecule plays a crucial role in the development of mucosal tolerance. J Immunol. 2005;175:7341-7347
62. Herman AE, Freeman GJ, Mathis D, Benoist C. CD4+CD25+ T regulatory cells dependent on ICOS promote regulation of effector cells in the prediabetic lesion. J Exp Med. 2004;199:1479-1489
63. Vermeiren J, Ceuppens JL, Van Ghelue M, Witters P, Bullens D, Mages HW, Kroczek RA, Van Gool SW. Human T cell activation by costimulatory signal-deficient allogeneic cells induces inducible costimulator-expressing anergic T cells with regulatory cell activity. J Immunol. 2004;172:5371-5378
64. Liese J, Schleicher U, Bogdan C. The innate immune response against Leishmania parasites. Immunobiology. 2008;213:377-387
65. Peters N, Sacks D. Immune privilege in sites of chronic infection: Leishmania and regulatory T cells. Immunol Rev. 2006;213:159-179
66. Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502-507
67. Bogdan C. Mechanisms and consequences of persistence of intracellular pathogens: leishmaniasis as an example. Cell Microbiol. 2008;10:1221-1234
68. Suffia I, Reckling SK, Salay G, Belkaid Y. A role for CD103 in the retention of CD4+CD25+ Treg and control of Leishmania major infection. J Immunol. 2005;174:5444-5455
69. Bourreau E, Ronet C, Darcissac E, Lise MC, Sainte Marie D, Clity E, Tacchini-Cottier F, Couppie P, Launois P. Intralesional regulatory T-cell suppressive function during human acute and chronic cutaneous leishmaniasis due to Leishmania guyanensis. Infect Immun. 2009;77:1465-1474
70. Xin L, Li K, Soong L. Down-regulation of dendritic cell signaling pathways by Leishmania amazonensis amastigotes. Mol Immunol. 2008;45:3371-3382
71. Martin S, Agarwal R, Murugaiyan G, Saha B. CD40 expression levels modulate regulatory T cells in Leishmania donovani infection. J Immunol. 2010;185:551-559
72. Makala LH, Baban B, Lemos H, El-Awady AR, Chandler PR, Hou DY, Munn DH, Mellor AL. Leishmania major attenuates host immunity by stimulating local indoleamine 2,3-dioxygenase expression. J Infect Dis. 2011;203:715-725
73. Wilson MS, Mentink-Kane MM, Pesce JT, Ramalingam TR, Thompson R, Wynn TA. Immunopathology of schistosomiasis. Immunol Cell Biol. 2007;85:148-154
74. Wynn TA. IL-13 effector functions. Annu Rev Immunol. 2003;21:425-456
75. Skapenko A, Kalden JR, Lipsky PE, Schulze-Koops H. The IL-4 receptor alpha-chain-binding cytokines, IL-4 and IL-13, induce forkhead box P3-expressing CD25+CD4+ regulatory T cells from CD25-CD4+ precursors. J Immunol. 2005;175:6107-6116
76. Zaccone P, Burton O, Miller N, Jones FM, Dunne DW, Cooke A. Schistosoma mansoni egg antigens induce Treg that participate in diabetes prevention in NOD mice. Eur J Immunol. 2009;39:1098-1107
77. Zaccone P, Burton OT, Gibbs S, Miller N, Jones FM, Dunne DW, Cooke A. Immune modulation by Schistosoma mansoni antigens in NOD mice: effects on both innate and adaptive immune systems. J Biomed Biotechnol. 2010;2010:795210
78. Hesse M, Piccirillo CA, Belkaid Y, Prufer J, Mentink-Kane M, Leusink M, Cheever AW, Shevach EM, Wynn TA. The pathogenesis of schistosomiasis is controlled by cooperating IL-10-producing innate effector and regulatory T cells. J Immunol. 2004;172:3157-3166
79. McKee AS, Pearce EJ. CD25+CD4+ cells contribute to Th2 polarization during helminth infection by suppressing Th1 response development. J Immunol. 2004;173:1224-1231
80. Singh KP, Gerard HC, Hudson AP, Reddy TR, Boros DL. Retroviral Foxp3 gene transfer ameliorates liver granuloma pathology in Schistosoma mansoni infected mice. Immunology. 2005;114:410-417
81. Tabel H, Wei GJ, Shi MQ. T cells and immunopathogenesis of experimental African trypanosomiasis. Immunol Rev. 2008;225:128-139
82. Wei G, Tabel H. Regulatory T cells prevent control of experimental African trypanosomiasis. J Immunol. 2008;180:2514-2521
83. Guilliams M, Oldenhove G, Noel W, Herin M, Brys L, Loi P, Flamand V, Moser M, De Baetselier P, Beschin A. African trypanosomiasis: naturally occurring regulatory T cells favor trypanotolerance by limiting pathology associated with sustained type 1 inflammation. J Immunol. 2007;179:2748-2757
84. Sales PAJr, Golgher D, Oliveira RV, Vieira V, Arantes RM, Lannes-Vieira J, Gazzinelli RT. The regulatory CD4+CD25+ T cells have a limited role on pathogenesis of infection with Trypanosoma cruzi. Microbes Infect. 2008;10:680-688
85. Mariano FS, Gutierrez FR, Pavanelli WR, Milanezi CM, Cavassani KA, Moreira AP, Ferreira BR, Cunha FQ, Cardoso CR, Silva JS. The involvement of CD4+CD25+ T cells in the acute phase of Trypanosoma cruzi infection. Microbes Infect. 2008;10:825-833
86. Kotner J, Tarleton R. Endogenous CD4(+) CD25(+) regulatory T cells have a limited role in the control of Trypanosoma cruzi infection in mice. Infect Immun. 2007;75:861-869
87. Poncini CV, Alba Soto CD, Batalla E, Solana ME, Gonzalez Cappa SM. Trypanosoma cruzi induces regulatory dendritic cells in vitro. Infect Immun. 2008;76:2633-2641
88. Gazzinelli RT, Oswald IP, Hieny S, James SL, Sher A. The microbicidal activity of interferon-gamma-treated macrophages against Trypanosoma cruzi involves an L-arginine-dependent, nitrogen oxide-mediated mechanism inhibitable by interleukin-10 and transforming growth factor-beta. Eur J Immunol. 1992;22:2501-2506
89. Poncini CV, Gimenez G, Pontillo CA, Alba-Soto CD, de Isola EL, Piazzon I, Cappa SM. Central role of extracellular signal-regulated kinase and Toll-like receptor 4 in IL-10 production in regulatory dendritic cells induced by Trypanosoma cruzi. Mol Immunol. 2010;47:1981-1988
90. Aly AS, Vaughan AM, Kappe SH. Malaria parasite development in the mosquito and infection of the mammalian host. Annu Rev Microbiol. 2009;63:195-221
91. Hisaeda H, Maekawa Y, Iwakawa D, Okada H, Himeno K, Kishihara K, Tsukumo S, Yasutomo K. Escape of malaria parasites from host immunity requires CD4(+)CD25(+) regulatory T cells. Nat Med. 2004;10:29-30
92. Wu JJ, Chen G, Liu J, Wang T, Zheng W, Cao YM. Natural regulatory T cells mediate the development of cerebral malaria by modifying the pro-inflammatory response. Parasitol Int. 2010;59:232-241
93. Amante FH, Stanley AC, Randall LM, Zhou Y, Haque A, McSweeney K, Waters AP, Janse CJ, Good MF, Hill GR, Engwerda CR. A role for natural regulatory T cells in the pathogenesis of experimental cerebral malaria. Am J Pathol. 2007;171:548-559
94. Steeg C, Adler G, Sparwasser T, Fleischer B, Jacobs T. Limited role of CD4+Foxp3+ regulatory T cells in the control of experimental cerebral malaria. J Immunol. 2009;183:7014-7022
95. Cambos M, Belanger B, Jacques A, Roulet A, Scorza T. Natural regulatory (CD4(+)CD25(+)FOXP(+)) T cells control the production of pro-inflammatory cytokines during Plasmodium chabaudi adami infection and do not contribute to immune evasion. Int J Parasitol. 2008;38:229-238
96. Haque A, Best SE, Amante FH, Mustafah S, Desbarrieres L, de Labastida F, Sparwasser T, Hill GR, Engwerda CR. CD4+ natural regulatory T cells prevent experimental cerebral malaria via CTLA-4 when expanded in vivo. PLoS Pathog. 2010;6:e1001221
97. Goncalves RM, Salmazi KC, Santos BA, Bastos MS, Rocha SC, Boscardin SB, Silber AM, Kallas EG, Ferreira MU, Scopel KK. CD4+ CD25+ Foxp3+ regulatory T cells, dendritic cells, and circulating cytokines in uncomplicated malaria: do different parasite species elicit similar host responses? Infect Immun. 2010;78:4763-4772
98. Mackroth MS, Malhotra I, Mungai P, Koech D, Muchiri E, King CL. Human cord blood CD4+CD25hi regulatory T cells suppress prenatally acquired T cell responses to Plasmodium falciparum antigens. J Immunol. 2011;186:2780-2791
99. Vigario AM, Gorgette O, Dujardin HC, Cruz T, Cazenave PA, Six A, Bandeira A, Pied S. Regulatory CD4+ CD25+ Foxp3+ T cells expand during experimental Plasmodium infection but do not prevent cerebral malaria. Int J Parasitol. 2007;37:963-973
100. Nie CQ, Bernard NJ, Schofield L, Hansen DS. CD4(+) CD25(+) regulatory T cells suppress CD4(+) T-cell function and inhibit the development of Plasmodium berghei-specific TH1 responses involved in cerebral malaria pathogenesis. Infect Immun. 2007;75:2275-2282
101. Wu Y, Wang QH, Zheng L, Feng H, Liu J, Ma SH, Cao YM. Plasmodium yoelii: distinct CD4(+)CD25(+) regulatory T cell responses during the early stages of infection in susceptible and resistant mice. Exp Parasitol. 2007;115:301-304
102. Couper KN, Blount DG, de Souza JB, Suffia I, Belkaid Y, Riley EM. Incomplete depletion and rapid regeneration of Foxp3(+) regulatory T cells following anti-CD25 treatment in malaria-infected mice. J Immunol. 2007;178:4136-4146
103. Omer FM, de Souza JB, Corran PH, Sultan AA, Riley EM. Activation of transforming growth factor beta by malaria parasite-derived metalloproteinases and a thrombospondin-like molecule. J Exp Med. 2003;198:1817-1827
104. Scholzen A, Mittag D, Rogerson SJ, Cooke BM, Plebanski M. Plasmodium falciparum-mediated induction of human CD25Foxp3 CD4 T cells is independent of direct TCR stimulation and requires IL-2, IL-10 and TGFbeta. PLoS Pathog. 2009;5:e1000543
105. Walther M, Tongren JE, Andrews L, Korbel D, King E, Fletcher H, Andersen RF, Bejon P, Thompson F, Dunachie SJ, Edele F, de Souza JB, Sinden RE, Gilbert SC, Riley EM, Hill AVS. Upregulation of TGF-beta, FOXP3, and CD4(+)CD25(+) regulatory T cells correlates with more rapid parasite growth in human malaria infection. Immunity. 2005;23:287-296
106. Bueno LL, Morais CG, Araujo FF, Gomes JA, Correa-Oliveira R, Soares IS, Lacerda MV, Fujiwara RT, Braga EM. Plasmodium vivax: induction of CD4+CD25+FoxP3+ regulatory T cells during infection are directly associated with level of circulating parasites. PLoS One. 2010;5:e9623
107. Wong KA, Rodriguez A. Plasmodium infection and Endotoxic shock induce the expansion of regulatory dendritic cells. J Immunol. 2008;180:716-726
108. Ocana-Morgner C, Wong KA, Lega F, Dotor J, Borras-Cuesta F, Rodriguez A. Role of TGF-beta and PGE(2) in T cell responses during Plasmodium yoelii infection. Eur J Immunol. 2007;37:1562-1574
109. Keller CC, Yamo O, Ouma C, Ong'echa JM, Clunah D, Hittner JB, Vulule JM, Perkins DJ. Acquisition of hemozoin by monocytes down-regulates interleukin-12 p40 (IL-12p40) transcripts and circulating IL-12p70 through an IL-10-dependent mechanism: In vivo and in vitro findings in severe malarial anemia. Infect Immun. 2006;74:5249-5260
110. Mukherjee P, Chauhan VS. Plasmodium falciparum-free merozoites and infected RBCs distinctly affect soluble CD40 ligand-mediated maturation of immature monocyte-derived dendritic cells. J Leukoc Biol. 2008;84:244-254
111. Urban BC, Todryk S. Malaria pigment paralyzes dendritic cells. J Biol. 2006;5:4
112. Wu X, Gowda NM, Gowda DC. Plasmodium falciparum: differential merozoite dose requirements for maximal production of various inflammatory cytokines. Exp Parasitol. 2011;127:202-207
113. Wu X, Gowda NM, Kumar S, Gowda DC. Protein-DNA complex is the exclusive malaria parasite component that activates dendritic cells and triggers innate immune responses. J Immunol. 2010;184:4338-4348
114. Parroche P, Lauw FN, Goutagny N, Latz E, Monks BG, Visintin A, Halmen KA, Lamphier M, Olivier M, Bartholomeu DC, Gazzinelli RT, Golenbock DT. Malaria hemozoin is immunologically inert but radically enhances innate responses by presenting malaria DNA to Toll-like receptor 9. Proc Natl Acad Sci U S A. 2007;104:1919-1924
115. Hisaeda H, Tetsutani K, Imai T, Moriya C, Tu L, Hamano S, Duan X, Chou B, Ishida H, Aramaki A, Shen J, Ishii KJ, Coban C, Akira S, Takeda K, Yasutomo K, Torii M, Himeno K. Malaria parasites require TLR9 signaling for immune evasion by activating regulatory T cells. J Immunol. 2008;180:2496-2503
116. Franklin BS, Rodrigues SO, Antonelli LR, Oliveira RV, Goncalves AM, Sales-Junior PA, Valente EP, Alvarez-Leite JI, Ropert C, Golenbock DT, Gazzinelli RT. MyD88-dependent activation of dendritic cells and CD4(+) T lymphocytes mediates symptoms, but is not required for the immunological control of parasites during rodent malaria. Microbes Infect. 2007;9:881-890
117. Ouaissi A. Regulatory cells and immunosuppressive cytokines: Parasite-derived factors induce immune polarization. J Biomed Biotechnol. 2007;2007:94971-94981
118. Doetze A, Satoguina J, Burchard G, Rau T, Loliger C, Fleischer B, Hoerauf A. Antigen-specific cellular hyporesponsiveness in a chronic human helminth infection is mediated by T(h)3/T(r)1-type cytokines IL-10 and transforming growth factor-beta but not by a T(h)1 to T(h)2 shift. Int Immunol. 2000;12:623-630
119. Satoguina J, Mempel M, Larbi J, Badusche M, Loliger C, Adjei O, Gachelin G, Fleischer B, Hoerauf A. Antigen-specific T regulatory-1 cells are associated with immunosuppression in a chronic helminth infection (onchocerciasis). Microbes Infect. 2002;4:1291-1300
120. Korten S, Hoerauf A, Kaifi JT, Buttner DW. Low levels of transforming growth factor-beta (TGF-beta) and reduced suppression of Th2-mediated inflammation in hyperreactive human onchocerciasis. Parasitology. 2011;138:35-45
121. Taylor MD, van der Werf N, Harris A, Graham AL, Bain O, Allen JE, Maizels RM. Early recruitment of natural CD4+ Foxp3+ Treg cells by infective larvae determines the outcome of filarial infection. Eur J Immunol. 2009;39:192-206
122. Metenou S, Dembele B, Konate S, Dolo H, Coulibaly SY, Coulibaly YI, Diallo AA, Soumaoro L, Coulibaly ME, Sanogo D, Doumbia SS, Traore SF, Mahanty S, Klion A, Nutman TB. At homeostasis filarial infections have expanded adaptive T regulatory but not classical Th2 cells. J Immunol. 2010;184:5375-5382
123. Hoerauf A, Satoguina J, Saeftel M, Specht S. Immunomodulation by filarial nematodes. Parasite Immunol. 2005;27:417-429
124. Babu S, Blauvelt CP, Kumaraswami V, Nutman TB. Regulatory networks induced by live parasites impair both Th1 and Th2 pathways in patent lymphatic filariasis: implications for parasite persistence. J Immunol. 2006;176:3248-3256
125. McSorley HJ, Harcus YM, Murray J, Taylor MD, Maizels RM. Expansion of Foxp3+ regulatory T cells in mice infected with the filarial parasite Brugia malayi. J Immunol. 2008;181:6456-6466
126. Taylor MD, Harris A, Babayan SA, Bain O, Culshaw A, Allen JE, Maizels RM. CTLA-4 and CD4+ CD25+ regulatory T cells inhibit protective immunity to filarial parasites in vivo. J Immunol. 2007;179:4626-4634
127. Shiny C, Krushna NS, Haripriya K, Babu S, Elango S, Manokaran G, Narayanan RB. Recombinant Wolbachia surface protein (WSP)-induced T cell responses in Wuchereria bancrofti infections. Parasitol Res. 2011 epub
128. Finney CAM, Taylor MD, Wilson MS, Maizels RM. Expansion and activation of CD4(+)CD25(+) regulatory T cells in Heliomosomoides polygyrus infection. Eur J Immunol. 2007;37:1874-1886
129. Rausch S, Huehn J, Mrchhoff D, Rzepecka J, Schnoeller C, Pillai S, Loddenkemper C, Scheffold A, Hamann A, Lucius R, Hartmann S. Functional analysis of effector and regulatory T cells in a parasitic nematode infection. Infect Immun. 2008;76:1908-1919
130. Maizels RM, Hewitson JP, Murray J, Harcus YM, Dayer B, Filbey KJ, Grainger JR, McSorley HJ, Reynolds LA, Smith KA. Immune modulation and modulators in Heligmosomoides polygyrus infection. Exp Parasitol. 2011 epub
131. Segura M, Su Z, Piccirillo C, Stevenson MM. Impairment of dendritic cell function by excretory-secretory products: a potential mechanism for nematode-induced immunosuppression. Eur J Immunol. 2007;37:1887-1904
132. Grainger JR, Smith KA, Hewitson JP, McSorley HJ, Harcus Y, Filbey KJ, Finney CA, Greenwood EJ, Knox DP, Wilson MS, Belkaid Y, Rudensky AY, Maizels RM. Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-{beta} pathway. J Exp Med. 2010;207:2331-2341
133. Layland LE, Rad R, Wagner H, da Costa CU. Immunopathology in schistosomiasis is controlled by antigen-specific regulatory T cells primed in the presence of TLR2. Eur J Immunol. 2007;37:2174-2184
134. Belkaid Y, Hoffmann KF, Mendez S, Kamhawi S, Udey MC, Wynn TA, Sacks DL. The role of interleukin (IL)-10 in the persistence of Leishmania major in the skin after healing and the therapeutic potential of anti-IL-10 receptor antibody for sterile cure. J Exp Med. 2001;194:1497-1506
135. Murray HW, Flanders KC, Donaldson DD, Sypek JP, Gotwals PJ, Liu J, Ma X. Antagonizing deactivating cytokines to enhance host defense and chemotherapy in experimental visceral leishmaniasis. Infect Immun. 2005;73:3903-3911
136. Ganguly S, Mukhopadhyay D, Das NK, Chaduvula M, Sadhu S, Chatterjee U, Rahman M, Goswami RP, Guha SK, Modak D, Mallik S, Gonju D, Pramanik N, Barbhuiya JN, Saha B, Chatterjee M. Enhanced lesional Foxp3 expression and peripheral anergic lymphocytes indicate a role for regulatory T cells in Indian post-kala-azar dermal leishmaniasis. J Invest Dermatol. 2010;130:1013-1022
137. Bourreau E, Ronet C, Darsissac E, Lise MC, Marie DS, Clity E, Tacchini-Cottier F, Couppie P, Launois P. In leishmaniasis due to Leishmania guyanensis infection, distinct intralesional interleukin-10 and Foxp3 mRNA expression are associated with unresponsiveness to treatment. J Infect Dis. 2009;199:576-579
138. Rodrigues OR, Marques C, Soares-Clemente M, Ferronha MH, Santos-Gomes GM. Identification of regulatory T cells during experimental Leishmania infantum infection. Immunobiology. 2009;214:101-111
139. Campanelli AP, Roselino AM, Cavassani KA, Pereira MS, Mortara RA, Brodskyn CI, Goncalves HS, Belkaid Y, Barral-Netto M, Barral A, Silva JS. CD4+CD25+ T cells in skin lesions of patients with cutaneous leishmaniasis exhibit phenotypic and functional characteristics of natural regulatory T cells. J Infect Dis. 2006;193:1313-1322
140. Salhi A, Rodrigues VJr, Santoro F, Dessein H, Romano A, Castellano LR, Sertorio M, Rafati S, Chevillard C, Prata A, Alcais A, Argiro L, Dessein A. Immunological and genetic evidence for a crucial role of IL-10 in cutaneous lesions in humans infected with Leishmania braziliensis. J Immunol. 2008;180:6139-6148
141. de-Campos SN, Souza-Lemos C, Teva A, Porrozzi R, Grimaldi GJr. Systemic and compartmentalised immune responses in a Leishmania braziliensis-macaque model of self-healing cutaneous leishmaniasis. Vet Immunol Immunopathol. 2010;137:149-154
142. Wang X, Zhou S, Chi Y, Wen X, Hoellwarth J, He L, Liu F, Wu C, Dhesi S, Zhao J, Hu W, Su C. CD4+CD25+ Treg induction by an HSP60-derived peptide SJMHE1 from Schistosoma japonicum is TLR2 dependent. Eur J Immunol. 2009;39:3052-3065
143. Layland LE, Mages J, Loddenkemper C, Hoerauf A, Wagner H, Lang R, da Costa CU. Pronounced phenotype in activated regulatory T cells during a chronic helminth infection. J Immunol. 2010;184:713-724
144. Tang CL, Lei JH, Wang T, Lu SJ, Guan F, Liu WQ, Li YL. Effect of CD4+ CD25+ regulatory T cells on the immune evasion of Schistosoma japonicum. Parasitol Res. 2011;108:477-480
145. Araujo FF, Gomes JA, Rocha MO, Williams-Blangero S, Pinheiro VM, Morato MJ, Correa-Oliveira R. Potential role of CD4+CD25HIGH regulatory T cells in morbidity in Chagas disease. Front Biosci. 2007;12:2797-2806
146. Hisaeda H, Hamano S, Mitoma-Obata C, Tetsutani K, Imai T, Waldmann H, Himeno K, Yasutomo K. Resistance of regulatory T cells to glucocorticoid-induced [corrected] TNFR family-related protein (GITR) during Plasmodium yoelii infection. Eur J Immunol. 2005;35:3516-3524
147. Couper KN, Blount DG, Wilson MS, Hafalla JC, Belkaid Y, Kamanaka M, Flavell RA, De Souza JB, Riley EM. IL-10 from CD4(+)CD25(-)Foxp3(-)CD127(-) adaptive regulatory T cells modulates parasite clearance and pathology during malaria infection. Plos Pathogens. 2008;4:e1000004
148. Chen G, Liu J, Wang QH, Wu Y, Feng H, Zheng W, Guo SY, Li DM, Wang JC, Cao YM. Effects of CD4(+)CD25(+)Foxp3(+)regulatory T cells on early Plasmodium yoelii 17XL infection in BALB/c mice. Parasitology. 2009;136:1107-1120
149. Minigo G, Woodberry T, Piera KA, Salwati E, Tjitra E, Kenangalem E, Price RN, Engwerda CR, Anstey NM, Plebanski M. Parasite-dependent expansion of TNF receptor II-positive regulatory T cells with enhanced suppressive activity in adults with severe malaria. PLoS Pathog. 2009;5:e1000402
150. Bisseye C, van der Sande M, Morgan WD, Holder AA, Pinder M, Ismaili J. Plasmodium falciparum infection of the placenta impacts on the T helper type 1 (Th1)/Th2 balance of neonatal T cells through CD4(+)CD25(+) forkhead box P3(+) regulatory T cells and interleukin-10. Clin Exp Immunol. 2009;158:287-293
151. Brustoski K, Moller U, Kramer M, Hartgers FC, Kremsner PG, Krzych U, Luty AJ. Reduced cord blood immune effector-cell responsiveness mediated by CD4+ cells induced in utero as a consequence of placental Plasmodium falciparum infection. J Infect Dis. 2006;193:146-154
152. Walther M, Jeffries D, Finney OC, Njie M, Ebonyi A, Deininger S, Lawrence E, Ngwa-Amambua A, Jayasooriya S, Cheeseman IH, Gomez-Escobar N, Okebe J, Conway DJ, Riley EM. Distinct roles for FOXP3 and FOXP3 CD4 T cells in regulating cellular immunity to uncomplicated and severe Plasmodium falciparum malaria. PLoS Pathog. 2009;5:e1000364
153. Dittrich AM, Erbacher A, Specht S, Diesner F, Krokowski M, Avagyan A, Stock P, Ahrens B, Hoffmann WH, Hoerauf A, Hamelmann E. Helminth infection with Litomosoides sigmodontis induces regulatory T cells and inhibits allergic sensitization, airway inflammation, and Hyperreactivity in a murine asthma model. J Immunol. 2008;180:1792-1799
Author contact
![]() Corresponding author: Edda Sciutto; Instituto de Investigaciones Biomédicas, Universidad Nacional Autónoma de México, UNAM, AP 70228, México, D.F. 04510, México. Tel.: (55)5622 3153, fax: (55) 5622 3369, E-mail address: argentina54com
Corresponding author: Edda Sciutto; Instituto de Investigaciones Biomédicas, Universidad Nacional Autónoma de México, UNAM, AP 70228, México, D.F. 04510, México. Tel.: (55)5622 3153, fax: (55) 5622 3369, E-mail address: argentina54com

 Global reach, higher impact
Global reach, higher impact