Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2012; 8(3):363-374. doi:10.7150/ijbs.3796 This issue Cite
Research Paper
Protective Effects of Garlic Oil on Hepatocarcinoma Induced by N-Nitrosodiethylamine in Rats
Institute of Toxicology, School of Public Health, Shandong University, 44 West Wenhua Road, Jinan 250012, P.R. China
Received 2011-11-14; Accepted 2012-2-16; Published 2012-2-22
Abstract
To investigate the protective effects and the possible mechanisms of garlic oil (GO) against N-nitrosodiethylamine (NDEA)-induced hepatocarcinoma in rats, Wistar rats were gavaged with GO (20 or 40 mg/kg) for 1 week, and then were gavaged with GO and NDEA (10 mg/kg) for the next 20 weeks. The changes of morphology, histology, the biochemical indices of serum, and DNA oxidative damage of liver were examined to assess the protective effects. Lipid peroxidation (LPO), antioxidant defense system, and apoptosis-related proteins were measured to investigate potential mechanisms. At the end of the study (21 weeks), GO administration significantly inhibited the increase of the nodule incidence and average nodule number per nodule-bearing liver induced by NDEA, improved hepatocellular architecture, and dramatically inhibited NDEA-induced elevation of serum biochemical indices (alanine aminotransferase , aspartate aminotransferase, alkaline phosphatase and gamma-glutamyl transpeptidase) and hepatic 8-hydroxy-2'-deoxyguanosine (8-OHdG) levels in a dose-dependent manner. The mechanistic studies demonstrated that GO counteracted NDEA-induced oxidative stress in rats illustrated by the restoration of glutathione (GSH), superoxide dismutase (SOD), catalase (CAT), glutathione reductase (GR), glutathione peroxidase (GPx), glutathione-S-transferase (GST) levels, and the reduction of the malondialdehyde (MDA) levels in liver. Furthermore, the mRNA and protein levels of Bcl-2, Bcl-xl, andβ-arrestin-2 were significantly decreased whereas those of Bax and caspase-3 were significantly increased. These data suggest that GO exhibited significant protection against NDEA-induced hepatocarcinogenesis, which might be related with the enhancement of the antioxidant activity and the induction of apoptosis.
Keywords: Garlic oil, Nitrosodiethylamine, Hepatocarcinoma, Antioxidant, Apoptosis
Introduction
Liver cancer is the second most frequent cause of death in 2010 throughout the world [1]. Exposure to environmental carcinogens is an important factor leading to the formation of hepatocarcinoma. N-nitrosodiethylamine (NDEA), which exists widespreadly in nature such as in cheese, soybean, processed meats, alcoholic beverages, tobacco products, cosmetics and agricultural chemicals, is one of the most important environmental carcinogens [2-4]. NDEA can induce carcinoma in all animal species as well as humans [5], and has been used as an experimental carcinogen in many studies.
The conventional therapy of hepatocarcinoma including chemotherapy, radiation, surgical resection and ablation gives little hope for restoration of health because of poor diagnosis and serious side effects. Liver transplantation is considered to be the most effective treatment for patients with hepatocarcinoma. However, low availability of organs limits the offer of this option to all candidates, and the high risk of tumor recurrence after transplantation further compromises its efficiency [6]. Therefore, developing more effective and less toxic anti-cancer agents, including natural products, is necessary to prevent or retard the process of hepatocarcinogenesis.
Garlic (Allium sativum) is cultivated and consumed worldwide. The application of garlic for medicinal purposes can be dated back to antiquity. Previous studies have shown that garlic possesses many biological activities, such as antimicrobial, antiatherosclerotic, antihypertensive, immunomodulation, radioprotection and anticancer [7-10]. Multiple lines of experimental and clinical evidence suggested the organosulfur compounds (OSCs) originating from garlic, accounting for only 1% of garlic weight, are the main anti-cancer active constituents [9, 11-13], and the variability in composition of OSCs influences the biological properties of garlic [14-15]. Currently, the steam distillation method is widely used to extract and condense volatile OSCs from garlic and the final oily product is called garlic oil (GO), which contains more than 30 OSCs [16]. It has been reported that diallyl sulfide (DAS), diallyl disulfide (DADS) and diallyl trisulfide (DATS) are the three major components involved in GO [17]. In contrast with the numerous studies on the pharmacological investigations about GO, fewer studies have been conducted with regard to its bioavailability. However, several studies have determined the metabolites of the above three diallyl sulfides in the breath of the volunteers as well as in different organs of rats [18-19]. It could be speculated that GO may have good bioavailability in humans.
Previous studies also indicated that GO could reduce cancer risk including gastric carcinoma, kidney cancer, skin tumor [20-22], however, the role of GO against NDEA-induced hepatocarcinoma remains unclear.
Accumulating evidence has demonstrated that overproduction of reactive oxygen species (ROS) plays a key role in the etiology of hepatocarcinoma. ROS could result in oxidative damage of DNA, which facilitating the formation of hepatocellular carcinomas [23]. Besides, dysregulated apoptosis was related to hepatocarcinogenesis induced by many chemicals including NDEA [24]. As GO has excellent antioxidant capacity [23, 25] and the constituents of GO have proapoptotic activities in many cancer cell lines[26-28], it is interesting to examine whether or not GO could inhibit NDEA-induced hepatocarcinogenesis by antioxidant capacity and proapoptosis.
Materials and Methods
Materials
GO (drug grade) was purchased from Xuchang Yuanhua Biotechnology, Inc. (Xuchang, CN). NDEA was obtained from Sigma Chemical Co. (St. Louis, MO, USA). DNeasy tissue kit was obtained from Promega Corporation (Madison, WI, USA). 8-hydroxy-2'-deoxyguanosine (8-OHdG) ELISA kit was provided by Cell Biolabs, Inc. (San Diego, CA, USA). BCATM protein assay kits were purchased from Pierce Biotechnology, Inc. (Rockford, IL, USA). Commercial antioxidantion assay kits for malondialdehyde (MDA), glutathione (GSH), superoxide dismutase (SOD), glutathione peroxidase (GPx), catalase (CAT), and glutathione reductase (GR) were bought from Nanjing Jiancheng Bioengineering Insititute (Nanjing, CN). All antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Animals and treatments
60 Male Wistar rats, weighing 120 - 140 g, were provided by Laboratory Animal Center of Shandong University. All animals received professional humane care in compliance with the guidelines of the Ethical Committee of School of Public Health, Shandong University, China. Rats were housed individually and a standard laboratory diet and water were available ad libitum. The animal room was maintained at constant temperature of 23 ± 1 ℃ and 50% relative humidity with a 12 h (7:00-19:00) light/dark cycle. After 1 week on basal diet, the animals were randomly divided into 4 groups (n=15). Rats in GO groups were pretreated with GO (20 or 40 mg/kg bw) by gavage for 7 days, while the rats in the control and NDEA groups received equal volume of corn oil. Then, all the animals except those in control group orally received NDEA (10 mg/kg bw, 5 time/week) for consecutive 20 weeks, and the rats in GO groups were continuously administered with GO until the end of the experiment. The body weight was measured weekly, and the dosage was varied accordingly. After 20 weeks of NDEA administration, all rats were anesthetized at 24 hours after the last treatment. Blood was collected by cervical decapitation and centrifuged at 1 500 g for 20 minutes at 4 ℃ to obtain serum. Liver tissue was excised, weighed. The amount of nodules on the liver surface was counted. A portion of the liver was fixed in paraformaldehyde (4%) for histopathological analysis. The other portion of liver tissue was quickly frozen in liquid nitrogen before storing at -80 ℃.
Liver morphology and histology
The amount of nodules in liver was counted by 3 mm cross-sections, and the nodule incidence and average nodule number per nodule-bearing liver in all groups were calculated. For histological examination, about 5 μm paraffin-embedded specimens were prepared, deparaffinized, rehydrated, stained with hematoxylin and eosin (H&E), and viewed using an Olympus AX70 microscope (Olympus, Tokyo, Japan)
Serum biochemical assays
The levels of serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transpeptidase (GGT), alkaline phosphatase (ALP), albumin (ALB), globulin (GLB) and total protein (TP) were determined using an automatic biochemical analyser (BTS-370, BioSystems S.A., Barcelona, Spain) according to the instructions supplied with the commercial assay kits (Roche, Switzerland).
Quantification of 8-OHdG levels in liver DNA by competitive ELISA
Genomic DNA in liver was extracted from frozen tissues using a DNeasy tissue kit. The 8-OHdG levels in liver DNA were determined using an ELISA kit. Briefly, the 8-OHdG antibody and the sample were added to ELISA plate which had been precoated with 8-OHdG. The 8-OHdG in the sample competed with the 8-OHdG bond on the plate for the 8-OHdG antibody binding sites. The average concentration of 8-OHdG per microgram of DNA for each group was calculated for each sample. Sample DNA assays were performed in duplicate.
Antioxidant status in liver
The liver tissue was homogenized in 9 volumes of ice-cold buffer (pH 7.4) containing 0.01 M Tris-HCl, 0.1 mM EDTA-2Na, 0.01 M saccharose, and 0.8% saline. The homogenates were centrifuged at 1000×g for 20 min at 4℃. The supernatant was collected, aliquoted, and stored at -80℃ for antioxidant assay.
LPO was determined by measuring the accumulation of thiobarbituric acid-reactive substance and expressed as MDA content. The levels of MDA, GSH and the activities of antioxidant enzymes including SOD, CAT, GR, GPx, and GST, were assayed using commercial assay kits according to the manufacturer's instructions. The MDA and GSH level was expressed as nmol/mg protein and mg/g protein, respectively. The activities of antioxidant enzymes were expressed as U/mg protein or U/g protein.
Western blotting analysis
The protein samples were extracted and western blotting was performed as we previously reported[29]. Briefly, the liver tissue was homogenized in lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 1% Triton X-100, 1% sodium deoxychlolate, 0.1% sodium dodecylsulphate, 50 mM β-glycerophosphate, 1 mM Na3VO4, 5 mM NaF, 1 % cocktail protein inhibitors and 1 mM phenylmethylsulfphonylfloride), then were centrifuged at 14 000g for 15 min. The protein extracts (20-50 μg) were separated on 12% or 15% SDS (sodium dodecyl sulfate, SDS)-polyacrylamide gels, transferred to polyvinylidene fluoride (PVDF) membranes, incubated with primary antibodies (Bcl-2, Bcl-xl, β-arrestin-2, Bax, cleaved Caspase-3 and β-actin) and the corresponding horseradish peroxidase conjugated secondary antibodies. The signals were detected by enhanced chemiluminescence kit (Pierce, Rockford, IL, USA) and the relative optical densities of the bands were quantified using Kodak Imaging Program and Image-Pro Plus software.
RNA extraction and cDNA synthesis
Total RNA was isolated from the rat livers using Trizol reagent (Invitrogen Corp., Carlsbad, CA, USA) according to the manufacture's instructions. The RNA pellet was dissolved in DEPC water. The concentration and integrity of total RNA was measured using a Nanodrop spectrophotometer (Nanodrop 2000c, Thermo Scientific, Wilmington, DE, USA) and Agilent 2100 Bioanalyzer (Agilent Technologies, Waldbronn, Germany). Complementary DNA was synthesized using the RevertAid TM First Strand cDNA Synthesis Kit (Fermentas) according to the manufacture's protocol.
Real-time PCR analysis
The levels of gene expression in liver tissue were quantified by real-time PCR. The primers were synthesized by Sangon Biotech Co., Ltd (Shanghai, CN) (Table 1). All PCR reactions were performed using Maxima SYBR Green qPCR Master Mix (Fermentas) and were carried out under the following conditions using MasterCycler TM eppendorf realplex 4 (Eppendorf, Westbury, NY, USA): initial denaturation at 95 ℃ for 10 min followed by 40 cycles of 15 s at 95 ℃, 30 s at 60 ℃ and 30 s at 72 ℃. Each sample was analyzed in triplicate. Differences in gene expression between groups were calculated using the △△Ct (cycle time, Ct) method [30], which were normalized against glyceraldehydes-3-phosphate dehydrogenase (GAPDH) and expressed as relative mRNA levels compared with controls.
Statistical analysis
SPSS13.0 was used for the statistical analysis. The Chi-Square test was used for nodule incidence analysis. Other data were expressed as mean ± SD, and were analyzed by one-way ANOVA, followed with Tukey for the multiple comparisons. Differences were considered significantly at P < 0.05 level.
Results
Changes of body weight and relative liver weight
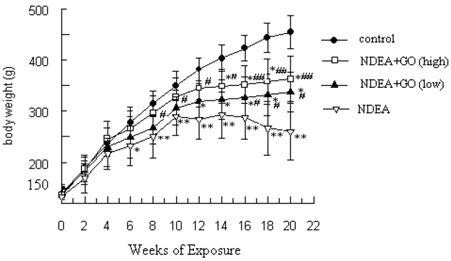
Figure 1 summarizes the effect of GO on body weight changes induced by NDEA. On first week following NDEA treatment, the rats began to show a slow growth. Along with the NDEA exposure, the mean weight gain decreased gradually. After 14 weeks of exposure, the body weight in NDEA group showed negative growth. At the end of 21 weeks, the body weight of rats in control group was increased to about 2.62-fold of the initial value, while that of rats in NDEA group was only about 1.46-fold. In contrast, GO treatment significantly recovered the body weight gain of rats (1.86-fold and 2.06-fold in 20 and 40 mg/kg group, respectively), compared to that in NDEA group (Figure 1).
Table 2 shows the final body weight and liver weight of different groups of rats. The relative liver weight in NDEA group was increased compared with that in control group (P<0.01). Administration of 20 and 40 mg/kg GO significantly reduced the relative liver weight as compared with NDEA group (P < 0.01).
Changes of rat body weight. The data of body weight were presented as mean ± S.D. Statistical difference was shown as: *P < 0.05, **P < 0.01, with respect to control; # P < 0.05, ##P < 0.01, with respect to NDEA group.
Sequences of primers used for the Real-time PCR analysis.
| Gene symbol | Forward primer | Reverse primer |
|---|---|---|
| Bcl-2 | CCCCAGAAGAAACTGAACC | GCATCTCCTTGTCTACGC |
| Bcl-XL | GCCACAAAGAAACCAATTCTG | CCGGTTGCTCTGAGACATTT |
| β-arrestin-2 | CCACGTCACCAACAATTCTG | TTGGTGTCTTCGTGCTTGAG |
| Bax | GTTGCCCTCT TCTACTTTGC | ATGGTCACTG TCTGCCATG |
| Caspase-3 | CTGGACTGCGGTATTGAGAC | CCGGGTGCGGTAGAGTAAGC |
| GAPDH | TCAAGAAGGTGGTGAAGCAG | AGGTGGAAGAATGGGAGTTG |
Body and liver weights of different groups of rats at the end of the study (mean±S.D.).
| Groups | Final body weights (g) | Liver weights (g) | Relative liver weight (g liver/100g body weight) |
|---|---|---|---|
| control | 500±32 | 14.98±1.33 | 3.006±0.334 |
| NDEA+GO(low ) | 398±39*# | 16.59±5.46## | 4.242±1.673## |
| NDEA+GO(high) | 413±44*## | 15.45±4.50## | 3.709±1.204## |
| NDEA | 327±49** | 25.38±7.52** | 7.861±2.360** |
Compared with control, *P < 0.05, **P < 0.01; compared with NDEA group, #P < 0.05, ##P < 0.01.
Morphological and histological changes of the liver
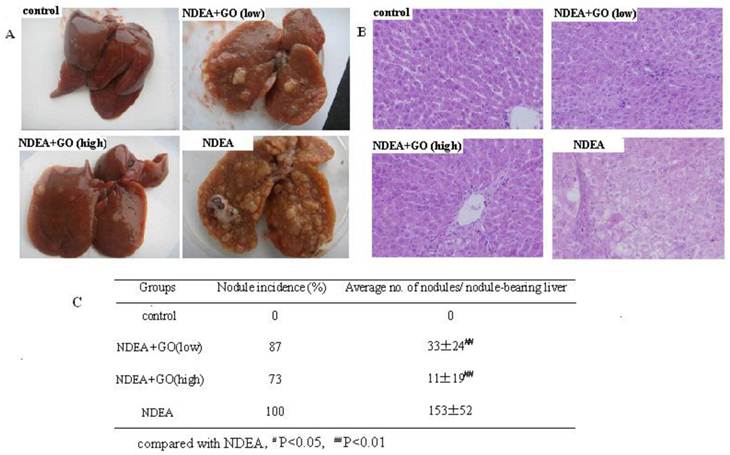
The appearance of livers in control group was normal and no macroscopically detectable nodules. The rats in NDEA group revealed enlarged liver, and the nodule incidence of NDEA group was 100% and the maximum diameter of nodules was about 10mm. At the same time, there were clear necrosis regions in livers of NDEA-treated rats. Interestingly, a significant reduction in liver enlargement, nodule incidence and average nodule numbers per nodule-bearing liver was observed in GO-treated rats compared with that of NDEA group (Fig. 2A and Fig. 2C).
Representative histology photos of the liver in all groups were depicted in Fig. 2B. The livers of control group showed normal liver and hepatocyte architecture. The hepatocellular architecture in NDEA group was completely destroyed, with many hyperplastic nodules and obvious heteromorphism. The nuclei were prominent and occupied most of the cells. These histopathological features were greatly reduced in the liver of rats treated with GO. Again, the histology analysis in GO group (40 mg/kg) showed significant improvement of hepatocellular architecture as compared with NDEA group.
Serum biochemical assays
The serum ALT, AST, ALP, and GGT levels were elevated in NDEA group compared with those in control group (P < 0.01), which indicating liver damage and preneoplastic lesion. The serum albumin was decreased, while globulin was increased in NDEA group compared with those in control group. Therefore, the A/G ratio was declined by NDEA treatment. All the above adverse effects of NDEA were suppressed by GO (Table 3).
GO inhibited the formation of 8-OHdG in liver DNA induced by NDEA
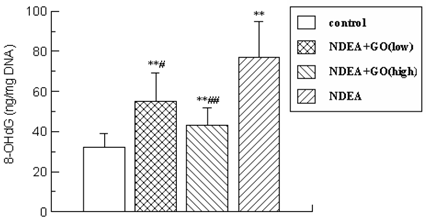
As shown in Figure 3, after 21 weeks of NDEA treatment, the content of 8-OHdG was augmented to 2.41-fold (P < 0.01) compared with that in control group.
However, this elevation of 8-OHdG induced by NDEA was significantly reduced in GO-treated groups and decreased by 29% (P<0.05) and 44% (P<0.01) in 20 mg/kg and 40 mg/kg groups, respectively.
Effect of GO on hepatic morphology and histology in NDEA-intoxicated rats. A: The gross appearance of liver. B: Representative photomicrographs of hepatic histology changes in 4 groups (HE staining, ×200). C: Changes of nodule incidence and average number of nodules per nodule-bearing liver in rats.
Changes of biochemical parameters in serum (mean±S.D.).
| Groups | ALT(U/L) | AST(U/L) | ALP(U/L) | GGT(U/L) | TP(g/L) | ALB(g/L) | GLB(g/L) | A/G |
|---|---|---|---|---|---|---|---|---|
| Control | 66±11 | 188±27 | 138±25 | 1.1±0.7 | 77.9±4.1 | 40.3±3.7 | 37.6±4.9 | 1.08±0.10 |
| NDEA+GO(low) | 118±33**## | 232±39**## | 248±36 | 24.4±11.5 | 83.4±3.0 | 39.2±1.5 | 44.2±5.9* | 0.91±0.08*# |
| NDEA+GO(high) | 109±18**## | 226±31## | 227±63## | 20.3±12.3## | 84.0±3.8* | 41.2±3.9 | 42.8±4.3# | 0.98±0.11## |
| NDEA | 186±39** | 388±58** | 362±61** | 120.2±37.7** | 84.8±3.5* | 37.8±2.7* | 47.1±4.7** | 0.82±0.12** |
*P<0.05, **P<0.01, compared with control group; #P<0.05, ##P<0.01, compared with NDEA group.
The content of 8-OHdG in liver DNA. The data was presented as mean ± S.D. *P<0.05, **P<0.01, compared with control group; #P<0.05, ##P<0.01, compared with NDEA group.
Changes of lipid peroxidation and antioxidative status
Considering the key roles of ROS in the development of NDEA-induced hepatocarcinoma, it was necessary and important to investigate the changes of the hepatic antioxidant system, which were displayed in Table 4.
NDEA significantly increased the MDA level, which was markedly suppressed by GO treatment. Compared with that of NDEA group, the MDA level was decreased by 34% and 44% (P<0.01) in 20 mg/kg and 40 mg/kg GO groups, respectively. No significant difference of MDA level was observed between GO and control groups (P > 0.05). Furthermore, NDEA caused significant decreases in the level of GSH, and impaired the activities of SOD, GR, GST, CAT and GPx. Compared with NDEA group, 20 and 40 mg/kg GO significantly increased the hepatic level of GSH and the activities of SOD, GR, GST, CAT and GPx by 143%, 17%, 47%, 43%, 27%, 75% and 636%, 24%, 79%,54%, 34%, 94% (P < 0.01), respectively. The high dose of GO inhibited NDEA-induced oxidative stress more effectively than the low dose of GO.
GO accelerated apoptosis in liver of NDEA-induced rats
Because dysregulation of apoptosis is an important cause for the hepatocarcinoma formation [31], we further investigated the mRNA and protein expression levels of the apoptosis-related proteins.
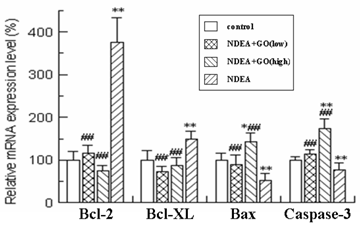
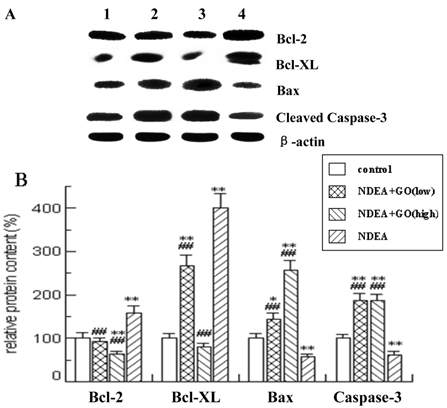
As shown in Figure 4 and Figure 5, quantitative real-time PCR and Western blotting results showed significant increases in mRNA and protein expression levels of anti-apoptotic Bcl-2 and Bcl-xl in NDEA group, as compared with the control group (P<0.05). Similarly significant decreases in Bax and Caspase-3 mRNA and protein expression levels were noticed in NDEA group. Therefore, the Bcl-2/Bax ratio remained significantly higher in NDEA group than that in control group. In contrast, GO administration markedly suppressed the expressions of Bcl-2 and Bcl-xl and increased the expressions of Bax and Caspase-3. Interestingly, the Bcl-2/Bax ratio was significantly decreased in GO groups compared with that in NDEA group.
Changes in β-arrestin-2 expression following treatment with GO in NDEA-induced hepatocarcinoma in rats
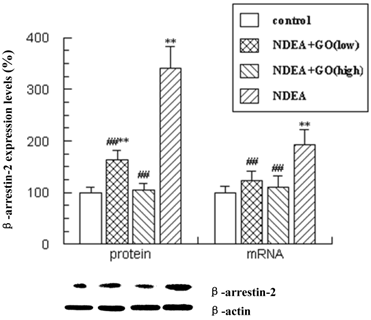
Recently, β-arrestin-2 was shown to mediate anti-apoptotic signaling, so we detected the expression levels of β-arrestin-2 in liver tissues. β-arrestin-2 mRNA and protein levels showed significant increases in NDEA-treatment rats compared with those in control rats (P<0.01). Treatment with GO inhibited the increases of β-arrestin-2 mRNA and protein levels induced by NDEA (P<0.01).
Changes of lipid peroxidation and antioxidative status in liver homogenates (mean±S.D.).
| Groups | MDA (nmol/mg prot) | GSH (mg/g prot) | SOD (U/mg prot) | GR (U/g prot) | GST (U/mg prot) | CAT (U/mg prot) | GPx (U/mg prot) |
|---|---|---|---|---|---|---|---|
| control | 0.84±0.13 | 16.31±3.00 | 111.54±4.15 | 11.52±1.98 | 22.88±2.13 | 43.08±4.79 | 651.65±59.20 |
| NDEA+ GO(low) | 0.99±0.26## | 2.43±0.88** ## | 104.04±15.86## | 7.43±1.25* # | 18.12±2.72** ## | 22.80±3.91**# | 550.95±93.26*## |
| NDEA+ GO(high) | 0.94±0.11## | 7.36±1.97* ## | 109.96±13.80## | 9.05±1.76## | 19.54±3.63** ## | 24.19±3.58**## | 610.82±97.77## |
| NDEA | 1.51±0.36** | 1.00±0.43** | 88.69±10.51** | 5.07±1.03** | 12.69±2.67** | 18.01±3.47** | 315.33±73.75** |
*P<0.05, **P<0.01, compared with control group; #P<0.05, ##P<0.01, compared with NDEA group.
Effect of GO and NDEA on the mRNA level of Bcl-2, Bcl-XL, Bax and Caspase-3. The mRNA levels were quantified with GAPDH as an internal control. The control group was defined as 100%. *P<0.05, **P<0.01, compared with control group; #P<0.05, ##P<0.01, compared with NDEA group.
Effect of GO and NDEA on the protein level of Bcl-2, Bcl-XL, Bax and Caspase-3. A: A representative immunoblot. B: Data presented the expression of Bcl-2, Bcl-XL, Bax and Caspase-3 as percentage of control group (mean ± SD) in triplicate. The protein levels were quantified with β-actin as an internal control. *P<0.05, **P<0.01, compared with control group; #P<0.05, ##P<0.01, compared with NDEA group. (lane 1) control; (lane 2) NDEA+GO (low); (lane 3) NDEA+GO (high); (lane 4) NDEA.
Effect of GO and NDEA on the mRNA and protein levels of β-arrestin-2. The protein levels were quantified with β-actin as an internal control. The mRNA levels were quantified with GAPDH as an internal control. *P<0.05, **P<0.01, compared with control group; #P<0.05, ##P<0.01, compared with NDEA group.
Discussion
In this study, GO (20 or 40 mg/kg) was demonstrated to significantly reduce the numbers and sizes of the tumor nodules, improve the histopathological damages and serum biochemical indices, and inhibit the DNA damage induced by NDEA. We further found that GO dose-dependently activated the hepatic antioxidant system and regulated the apoptotic-related proteins. These results provided strong evidence that GO significantly prevented the development of NDEA-induced hepatocarcinoma.
GO administration (20 or 40 mg/kg) resulted in fewer rats developing visible nodules and smaller nodule number per nodule-bearing rat liver than those observed in NDEA group rats. GO also significantly inhibited the NDEA-induced liver weight increases. The hepatic histopathological damage induced by NDEA was improved in GO-treated rats. Because the correlation of the number of hyperplastic nodules and hepatocarcinoma in experimental and human disease [32], the inhibition of nodule growth by GO in our experiment was important for hepatocarcinoma prevention.
The activities of ALT, AST and ALP in serum are generally accepted as indices of liver injury and this tendency is also known to be distinct in rodents[33]. The serum GGT activity is considered to biomarker of preneoplastic lesion. These enzymatic activities elevation may potentially be attributed to the release of these enzymes from the cytoplasm into the blood circulation after liver cellular damage [34]. Therefore, we first detected the changes of these marker enzymes. The results of our study showed a sharply inhibitory effect of GO on the enzymatic activities increase initiated by NDEA, which indicated that GO could suppress NDEA-induced liver damage. As there is a close connection between GGT activation and carcinogenesis, the increase of GGT activity could be correlated with a high nodule incidence and nodule number.
Liver is the main site of NDEA metabolism, the generation of ROS in the liver is recognized as an important contributor in NDEA-induced carcinogenic effects [34]. ROS are continuously generated in vivo as a result of NDEA administration causing oxidative stress that seriously damaged the biological systems by injuring tissues, altering biochemical compounds, causing chromosomal instability, eroding cell membranes and mutation, which are involved in all steps of carcinogenesis, i.e. initiation, promotion and progression [35]. LPO is a useful marker of oxidative stress because it is correlated with increased production of ROS [36], and plays an important role in carcinogenesis induced by NDEA [37]. MDA has long been used as a particular biomarker of oxidative damage and LPO, and the increase of MDA reflects the enhancement of the LPO [38]. In the present study, we found that the level of MDA was significantly elevated by NDEA. However, the rats pretreated with GO displayed a manifest reduction in the level of MDA compared with that in NDEA group. The observed reduction in the level of LPO in GO-treated animals was presumably due to the increase of antioxidative capabilities.
For the purpose of preventing cellular damage induced by ROS, the organism has a lot of antioxidative defense system, including the non-enzymatic (mainly GSH) and enzymatic antioxidant defenses (including SOD, GR, GST, CAT and GPx). GSH plays an important role in maintaining the normal reduced state of cells and counteracting the harmful effects of oxidative stress [39]. GSH can effectively scavenge free radicals and other oxygen species through nonenzymatic and enzymatic process by conjugation with GPx and GST [40]. GPx ubiquitously exists both in cytosol and mitochondria of the hepatocytes. GST locates in cytosol and plays an important role in detoxification and excretion of xenobiotics [41]. GST catalyzes the conjugation of the thiol functional groups of GSH to electrophilic xenobiotics, leading to elimination or conversion of xenobiotic-GSH conjugate [42]. In such reaction, the GSH is oxidized into GSSG, which can be reduced to GSH by GR with the consumption of NADPH [43]. In addition, GSH can also react with various electrophiles, physiological metabolites and xenobiotics to form mercapturates, which are catalyzed by glutathione S-transferase (GST) [44-45]. In addition to GSH and GSH-related antioxidant enzymes, other antioxidant enzymes including SOD and CAT also take important role in the antioxidant defense system. SOD can catalyze the dismutation of two superoxide radicals to H2O2 and O2. CAT acts as supporting antioxidant enzymes by transforming H2O2 to H2O, thereby providing protection against ROS [36].
Banerjee et al. (2002) reported that garlic had a powerful antioxidative defense system and minimized intracellular oxidative stress. Among various garlic components, the OSCs are considered as main bioactive components. Wu et al. reported GO, DADS and DATS but not DAS significantly increased the activities of GST and GSH reductase [16]. Our previous study also showed that administration of DATS (one of the components of GO) significantly decreased LPO and increased the activities of endogenous antioxidants, such as SOD, CAT, GPx [44]. In the present study, NDEA resulted in significant decreases in the level of GSH and the activities of antioxidation enzymes including GR, GPx and GST. The reduction in the activities of these enzymes may be resulted from excessive LPO during NDEA metabolism. However, the effects of NDEA were partially counteracted by GO, which suggests that the elevation of GSH might be one of the important mechanisms for GO against NDEA-induced hepatocarcinoma
Oxidative DNA damage maybe an important underlying event that leads to cancer [46]. Cherng etal found that DAS, one of the principal components of GO, protected against ultraviolet B-induced skin cancers in SKH-1 hairless mouse by preventing DNA damage and facilitating DNA repair [47]. To further evaluate the roles of GO against NDEA-induced devolvement of hepatocarcinoma, we examined the levels of 8-OHdG, a DNA base-modified product generated by reactive oxygen species, which is a good marker for DNA oxidative damage due to aging, cancer and other degenerative diseases [48]. The data clearly showed that GO administration significantly decreased the levels of 8-OHdG in liver DNA compared with that in NDEA group, which suggested that GO could significantly prevent NDEA-induced DNA damage.
Tumor initiation, progression, and maintenance commonly involve alterations in apoptosis. Studies have shown that dysregulation of apoptosis is an important cause for the hepatocarcinoma formation [31]. Accumulating data indicate that induction of apoptosis is a crucial event for chemoprevention of cancer by naturally occurring dietary agents [49]. Thus, we evaluated the changes of apoptotic-related factors in mRNA and protein expression levels.
The antiapoptotic Bcl-2 family proteins act by neutralizing proapoptotic proteins and play a pivotal role in determining the ability of a cancer cell to undergo apoptosis [50]. Bax, normally residing in the cytosol, translocates to mitochondria to promote apoptosis, but Bax activity is counteracted by antiapoptotic proteins, such as Bcl-2 and Bcl-xL [51]. Many cancer cells evade apoptosis by up-regulation of the expressions of Bcl-2 and Bcl-xL [52-53]. In the present study, GO up-regulated the expression of Bax and down-regulated the expression of Bcl-2 and Bcl-xL, when compared with NDEA group. DATS, one of the important components of GO, have been proven to induce apoptosis in MCF7 human breast cancer cells by decreasing the expression of Bcl-2 and enhancing the expression of Bax [54], which is consistent with our experimental results.
The ratio of Bcl-2 to Bax, rather than the levels of the individual proteins, is considered to be critical in determining the survival/death of cells [55]. Decreased Bcl-2/Bax ratio will induce apoptosis [56]. Our results showed that the ratio of Bcl-2 to Bax was prominently decreased by GO which may lead to apoptotic response in hepatocarcinogenesis induced by NDEA. Many researches also demonstrated that one of important mechanisms of preventing hepatocarcinoma is reducing the ratio of Bcl-2/Bax for capsaicin, zerumbone, matrine and leptin [57-60].
Caspase activation plays a central role in the execution of apoptosis. The activation of Caspase-3 is a common event in two major pathways, death receptor and mitochondrial pathways [61-63]. In the present study, NDEA administration inhibited the expression of Caspase-3, but GO activated the expression of Caspase-3. The apoptosis induced by PTX-2 in human hepatocellular carcinoma cells was associated with the down-regulation of Bcl-2 and Bcl-xL, the up-regulation of Bax and the activation of Caspase-3 [64]. Therefore, GO induced-apoptosis by down-regulation of Bcl-2, Bcl-xL and up-regulation of Bax and Caspase-3 could be one of important mechanisms of preventing hepatocarcinoma.
β-arrestin-2, originally discovered as terminators of G protein-coupled receptor signaling, has been revealed novel functions as signal transducers in various signaling pathways [65-66]. Among these processes, β-arrestin-2 has been reported to mediate anti-apoptotic signaling by activating the intrinsic apoptotic pathway [67-68]. Khan et al. first reported the changes of β-arrestin-2 expression in neoplastic nodules in 2011 [68]. Our results showed that β-arrestin-2 expression increase in NDEA treatment rats may lead to antiapoptotic effect, which correlated well with the upregulation of Bcl-2, Bcl-xL and downregulation of Bax and Caspase-3. Reversely, GO treatment led toβ-arrestin-2 expression decrease in mRNA and protein levels, thus promoting apoptotic signaling as evidenced by the upregulation of Bax and Caspase-3. Furthermore, β-arrestin-2 participated in the Wnt signaling pathway [69], which is frequently imbalanced in hepatocarcinoma.
Increasing ROS generation is one of the mechanisms of some exogenous chemicals inducing apoptosis, and thus antioxidation is theoretically to produce anti-apoptotic effects. However, some chemicals prevented cancer by enhancing antioxidant activity and inducing apoptosis. For example, saffron and polyphenols exert a significant chemopreventive effect against cancer through the antioxidant activity and induction of apoptosis [4, 70]. Therefore, it is not paradoxical that GO exhibited potent antioxidant capacity as well as pro-apoptotic effects. Pre-treatment with GO reduced the generation of ROS, but it did not inhibit the pro-apoptotic activities of GO, suggesting that multiple events overlap to explain the mechanisms of hepatocarcinoma prevention induced by GO.
Taken together, the present study is the first to demonstrate the in vivo chemoprotective effect of GO on NDEA induced hepatocarcinoma. This chemoprotective effect of GO may be associated with enhancing antioxidant activity and inducing apoptosis. Given that there is no effective treatment measure for hepatocarcinoma, our findings may suggest a potential use of GO for hepatocellular carcinoma chemoprevention.
Acknowledgements
This work has been supported by Scientific and Technological Project of Shandong Province (2008GG2NS02012) and Innovation Fund for Young Scholar of Public Health School, Shandong University.
Conflict of Interests
The authors have declared that no conflict of interest exists.
References
1. Jemal A, Bray F, Center M.M. et al. Global cancer statistics. CA Cancer Clin J. 2011;61:69-90
2. Subramanian P, Mirunalini S, Dakshayani K.B. et al. Prevention by melatonin of hepatocarcinogenesis in rats injected with N-nitrosodiethylamine. J Pineal Res. 2007;43:305-12
3. Park D.H, Shin J.W, Park S.K. et al. Diethylnitrosamine (DEN) induces irreversible hepatocellular carcinogenesis through overexpression of G1/S-phase regulatory proteins in rat. Toxicol Lett. 2009;191:321-6
4. Amin A, Hamza A.A, Bajbouj K. et al. Saffron: A potential candidate for a novel anticancer drug against hepatocellular carcinoma. Hepatology. 2011Sep2;54(3):857-67
5. Loeppky R.N. The mechanism of bioactivation of N-nitrosodiethanolamine. Drug Metab Rev. 1999;31:175-93
6. Tabone M, Pellicano R. Prevention of intrahepatic hepatocarcinoma recurrence in patients with viral cirrhosis: two potential options. Minerva Gastroenterol Dietol. 2006;52:47-52
7. Agarwal K.C. Therapeutic actions of garlic constituents. Med Res Rev. 1996;16:111-24
8. Amagase H, Petesch B.L, Matsuura H. et al. Intake of garlic and its bioactive components. Nutr J. 2001;131:955S-62S
9. Amagase H. Clarifying the real bioactive constituents of garlic. Nutr J. 2006;136:716S-725S
10. Banerjee S.K, Mukherjee P.K, Maulik S.K. Garlic as an antioxidant: the good, the bad and the ugly. Phytother Res. 2003;17:97-106
11. Nagini S. Cancer chemoprevention by garlic and its organosulfur compounds-panacea or promise? Anticancer Agents Med Chem. 2008;8:313-21
12. Iciek M, Kwiecien I, Wlodek L. Biological properties of garlic and garlic-derived organosulfur compounds. Environ Mol Mutagen. 2009;50:247-65
13. Jones M.G, Hughes J, Tregova A. et al. Biosynthesis of the flavour precursors of onion and garlic. J Exp Bot. 2004;55:1903-18
14. Gonzalez R.E, Soto V.C, Sance M.M. et al. Variability of solids, organosulfur compounds, pungency and health-enhancing traits in garlic (Allium sativum L.) cultivars belonging to different ecophysiological groups. J Agric Food Chem. 2009;57:10282-8
15. Cerella C, Dicato M, Jacob C. et al. Chemical properties and mechanisms determining the anti-cancer action of garlic-derived organic sulfur compounds. Anticancer Agents Med Chem. 2011;11:267-71
16. Wu C.C, Sheen L.Y, Chen H.W. et al. Effects of organosulfur compounds from garlic oil on the antioxidation system in rat liver and red blood cells. Food Chem Toxicol. 2001;39:563-9
17. Zeng T, Zhang C.L, Zhao X.L. et al. The roles of garlic on the lipid parameters: a systematic review of the literature. Critical Reviews in Food Science and Nutrition. 2011 DOI:10.1080/10408398.2010.523148
18. Germain E, Auger J, Ginies C. et al. In vivo metabolism of diallyl disulphide in the rat: identification of two new metabolites. Xenobiotica. 2002;32:1127-38
19. Rosen R.T, Hiserodt R.D, Fukuda E.K. et al. The determination of metabolites of garlic preparations in breath and human plasma. Biofactors. 2000;13:241-9
20. Perchellet J.P, Perchellet E.M, Belman S. Inhibition of DMBA-induced mouse skin tumorigenesis by garlic oil and inhibition of two tumor-promotion stages by garlic and onion oils. Nutr Cancer. 1990;14:183-93
21. Li X, Xie J, Li W. et al. Effects of garlic oil on tumoragenecity and intercellular communication in human gastric cancer cell line. Sci China C Life Sci. 2000;43:82-7
22. Agarwal M.K, Iqbal M, Athar M. Garlic oil ameliorates ferric nitrilotriacetate (Fe-NTA)-induced damage and tumor promotion: implications for cancer prevention. Food Chem Toxicol. 2007;45:1634-40
23. Lin P.H, Lin C.H, Huang C.C. et al. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) induces oxidative stress, DNA strand breaks, and poly(ADP-ribose) polymerase-1 activation in human breast carcinoma cell lines. Toxicol Lett. 2007;172:146-58
24. Finnberg N, Stenius U, Hogberg J. Xenobiotics modulate the p53 response to DNA damage in preneoplastic enzyme-altered foci in rat liver; effects of diethylnitrosamine and phenobarbital. Toxicol Sci. 2000;54:95-103
25. Zeng T, Zhang C.L, Pang G.B, Zhao S, Dou D.D, Xin X, Xie K.Q. The protective effects of garlic oil on acute ethanol-induced oxidative stress in the liver of mice. J. Sci. Food Agric. 2008;88:2238-2243
26. Wang H.C, Yang J.H, Hsieh S.C. et al. Allyl sulfides inhibit cell growth of skin cancer cells through induction of DNA damage mediated G2/ arrest M and apoptosis. J Agric Food Chem. 2010;58:7096-103
27. Gayathri R, Gunadharini D.N, Arunkumar A. et al. Effects of diallyl disulfide (DADS) on expression of apoptosis associated proteins in androgen independent human prostate cancer cells (PC-3). Mol Cell Biochem. 2009;320:197-203
28. Hui C, Jun W, Ya L.N. et al. Effect of Allium sativum (garlic) diallyl disulfide (DADS) on human non-small cell lung carcinoma H1299 cells. Trop Biomed. 2008;25:37-45
29. Zeng T, Zhang C.L, Song F.Y. et al. The modulatory effects of garlic oil on hepatic cytochrome P450s in mice. Hum Exp Toxicol. 2009;28:777-83
30. Livak K.J, Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402-8
31. Fabregat I. Dysregulation of apoptosis in hepatocellular carcinoma cells. World Gastroenterol J. 2009;15:513-20
32. Bishayee A, Chatterjee M. Inhibitory effect of vanadium on rat liver carcinogenesis initiated with diethylnitrosamine and promoted by phenobarbital. Br Cancer J. 1995;71:1214-20
33. Ha W.S, Kim C.K, Song S.H. et al. Study on mechanism of multistep hepatotumorigenesis in rat: development of hepatotumorigenesis. J Vet Sci. 2001;2:53-8
34. Shaarawy S.M, Tohamy A.A, Elgendy S.M. et al. Protective effects of garlic and silymarin on NDEA-induced rats hepatotoxicity. Int J Biol Sci. 2009;5:549-57
35. Karbownik M, Lewinski A, Reiter R.J. Anticarcinogenic actions of melatonin which involve antioxidative processes: comparison with other antioxidants. Int J Biochem Cell Biol. 2001;33:735-53
36. Vasquez-Garzon V.R, Arellanes-Robledo J, Garcia-Roman R. et al. Inhibition of reactive oxygen species and pre-neoplastic lesions by quercetin through an antioxidant defense mechanism. Free Radic Res. 2009;43:128-37
37. Banakar M.C, Paramasivan S.K, Chattopadhyay M.B. et al. 1alpha, 25-dihydroxyvitamin D3 prevents damage DNA and restores antioxidant enzymes in rat hepatocarcinogenesis induced by diethylnitrosamine and promoted by phenobarbital. World Gastroenterol J. 2004;10:1268-75
38. Lykkesfeldt J. Malondialdehyde as biomarker of oxidative damage to lipids caused by smoking. Clin Chim Acta. 2007;380:50-8
39. Ramakrishnan G, Raghavendran H.R, Vinodhkumar R. et al. Suppression of N-nitrosodiethylamine induced hepatocarcinogenesis by silymarin in rats. Chem Biol Interact. 2006;161:104-14
40. Saydam N, Kirb A, Demir O. et al. Determination of glutathione, glutathione reductase, glutathione peroxidase and glutathione S-transferase levels in human lung cancer tissues. Cancer Lett. 1997;119:13-9
41. Bansal A.K, Bansal M, Soni G. et al. Protective role of Vitamin E pre-treatment on N-nitrosodiethylamine induced oxidative stress in rat liver. Chem Biol Interact. 2005;156:101-11
42. Rao G.M, Rao C.V, Pushpangadan P. et al. Hepatoprotective effects of rubiadin, a major constituent of Rubia cordifolia Linn. Ethnopharmacol J. 2006;103:484-90
43. Fang Y.Z, Yang S, Wu G. Free radicals, antioxidants, and nutrition. Nutrition. 2002;18:872-9
44. Zeng T, Zhang C.L, Zhu Z.P. et al. Diallyl trisulfide (DATS) effectively attenuated oxidative stress-mediated liver injury and hepatic mitochondrial dysfunction in acute ethanol-exposed mice. Toxicology. 2008;252:86-91
45. Lei X.G. In vivo antioxidant role of glutathione peroxidase: evidence from knockout mice. Methods Enzymol. 2002;347:213-25
46. Hagen T.M, Huang S, Curnutte J. et al. Extensive oxidative DNA damage in hepatocytes of transgenic mice with chronic active hepatitis destined to develop hepatocellular carcinoma. Proc Natl Acad Sci U S A. 1994;91:12808-12
47. Cherng J.M, Tsai K.D, Perng D.S. et al. Diallyl sulfide protects against ultraviolet B-induced skin cancers in SKH-1 hairless mouse: analysis of early molecular events in carcinogenesis. Photodermatol Photoimmunol Photomed. 2011;27:138-46
48. Tsubota A, Yoshikawa T, Nariai K. et al. Bovine lactoferrin potently inhibits liver mitochondrial 8-OHdG levels and retrieves hepatic OGG1 activities in Long-Evans Cinnamon rats. Hepatol J. 2008;48:486-93
49. Khan N, Adhami V.M, Mukhtar H. Apoptosis by dietary agents for prevention and treatment of prostate cancer. Endocr Relat Cancer. 2010;17:R39-52
50. Tse C, Shoemaker A.R, Adickes J. et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68:3421-8
51. Edlich F, Banerjee S, Suzuki M. et al. Bcl-x(L) retrotranslocates Bax from the mitochondria into the cytosol. Cell. 2011;145:104-16
52. Hanahan D, Weinberg R.A. The hallmarks of cancer. Cell. 2000;100:57-70
53. Danial N.N, Korsmeyer S.J. Cell death: critical control points. Cell. 2004;116:205-19
54. Malki A, El-Saadani M, Sultan A.S. Garlic constituent diallyl trisulfide induced apoptosis in MCF7 human breast cancer cells. Cancer Biol Ther. 2009;8:2175-85
55. Fukamachi Y, Karasaki Y, Sugiura T. et al. Zinc suppresses apoptosis of U937 cells induced by hydrogen peroxide through an increase of the Bcl-2/Bax ratio. Biochem Biophys Res Commun. 1998;246:364-9
56. Gardner C.R. Anticancer drug development based on modulation of the Bcl-2 family core apoptosis mechanism. Expert Rev Anticancer Ther. 2004;4:1157-77
57. Jung M.Y, Kang H.J, Moon A. Capsaicin-induced apoptosis in SK-Hep-1 hepatocarcinoma cells involves Bcl-2 downregulation and caspase-3 activation. Cancer Lett. 2001;165:139-45
58. Sakinah S.A, Handayani S.T, Hawariah L.P. Zerumbone induced apoptosis in liver cancer cells via modulation of Bax/Bcl-2 ratio. Cancer Cell Int. 2007;7:4
59. Ma L, Wen S, Zhan Y. et al. Anticancer effects of the Chinese medicine matrine on murine hepatocellular carcinoma cells. Planta Med. 2008;74:245-51
60. Chen C, Chang Y.C, Liu C.L. et al. Leptin induces proliferation and anti-apoptosis in human hepatocarcinoma cells by up-regulating cyclin D1 and down-regulating Bax via a Janus kinase 2-linked pathway. Endocr Relat Cancer. 2007;14:513-29
61. Sarada S.K, Himadri P, Ruma D. et al. Selenium protects the hypoxia induced apoptosis in neuroblastoma cells through upregulation of Bcl-2. Brain Res. 2008;1209:29-39
62. Hengartner M.O. The biochemistry of apoptosis. Nature. 2000;407:770-6
63. Paris C, Bertoglio J, Breard J. Lysosomal and mitochondrial pathways in miltefosine-induced apoptosis in U937 cells. Apoptosis. 2007;12:1257-67
64. Qi F, Li A, Inagaki Y. et al. Induction of apoptosis by cinobufacini preparation through mitochondria- and Fas-mediated caspase-dependent pathways in human hepatocellular carcinoma cells. Food Chem Toxicol. 2012Feb;50(2):295-302
65. Hall R.A, Premont R.T, Lefkowitz R.J. Heptahelical receptor signaling: beyond the G protein paradigm. J Cell Biol. 1999;145:927-32
66. DeWire S.M, Ahn S, Lefkowitz R.J. et al. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483-510
67. Ahn S, Kim J, Hara M.R. et al. {beta}-Arrestin-2 Mediates Anti-apoptotic Signaling through Regulation of BAD Phosphorylation. J Biol Chem. 2009;284:8855-65
68. Khan M.S, Devaraj H, Devaraj N. Chrysin abrogates early hepatocarcinogenesis and induces apoptosis in N-nitrosodiethylamine-induced preneoplastic nodules in rats. Toxicol Appl Pharmacol. 2011;251:85-94
69. Bryja V, Gradl D, Schambony A. et al. Beta-arrestin is a necessary component of Wnt/beta-catenin signaling in vitro and in vivo. Proc Natl Acad Sci U S A. 2007;104:6690-5
70. D'Archivio M, Santangelo C, Scazzocchio B. et al. Modulatory effects of polyphenols on apoptosis induction: relevance for cancer prevention. Int J Mol Sci. 2008;9:213-28
Author contact
![]() Corresponding author: Tel: +86-531-8838-2132; Fax: +86-531-8838-2129; E-mail: keqinxedu.cn
Corresponding author: Tel: +86-531-8838-2132; Fax: +86-531-8838-2129; E-mail: keqinxedu.cn

 Global reach, higher impact
Global reach, higher impact