10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2012; 8(7):935-944. doi:10.7150/ijbs.4499 This issue Cite
Research Paper
The Role of α-synuclein and Tau Hyperphosphorylation-Mediated Autophagy and Apoptosis in Lead-induced Learning and Memory Injury
Department of Occupational & Environmental Health and the Ministry of Education Key Lab of Hazard Assessment and Control in Special Operational Environment, School of Public Health, Fourth Military Medical University, Xi'an, 710032, China.
* Jianbin Zhang and Tongjian Cai contributed equally to this work.
Received 2012-4-20; Accepted 2012-7-4; Published 2012-7-7
Abstract
Lead (Pb) is a well-known heavy metal in nature. Pb can cause pathophysiological changes in several organ systems including central nervous system. Especially, Pb can affect intelligence development and the ability of learning and memory of children. However, the toxic effects and mechanisms of Pb on learning and memory are still unclear. To clarify the mechanisms of Pb-induced neurotoxicity in hippocampus, and its effect on learning and memory, we chose Sprague-Dawley rats (SD-rats) as experimental subjects. We used Morris water maze to verify the ability of learning and memory after Pb treatment. We used immunohistofluorescence and Western blotting to detect the level of tau phosphorylation, accumulation of α-synuclein, autophagy and related signaling molecules in hippocampus. We demonstrated that Pb can cause abnormally hyperphosphorylation of tau and accumulation of α-synuclein, and these can induce hippocampal injury and the ability of learning and memory damage. To provide the new insight into the underlying mechanisms, we showed that Grp78, ATF4, caspase-3, autophagy-related proteins were induced and highly expressed following Pb-exposure. But mTOR signaling pathway was suppressed in Pb-exposed groups. Our results showed that Pb could cause hyperphosphorylation of tau and accumulation of α-synuclein, which could induce ER stress and suppress mTOR signal pathway. These can enhance type II program death (autophgy) and type I program death (apoptosis) in hippocampus, and impair the ability of learning and memory of rats. This is the first evidence showing the novel role of autophagy in the neurotoxicity of Pb.
Keywords: lead, tau, α-synuclein, autophagy, learning and memory.
Introduction
Lead (Pb) is a well-known and generally used heavy metal. It can interfere with a variety of body processes and is toxic to body systems including cardiovascular, hematopoietic, reproductive, gastrointestinal, renal, and nervous systems [1]. Pb can impair the development of the nervous system, particularly to children, cause learning and behavior disorders that may be permanent [2,3]. In daily life, routes of exposure to Pb include air, water, soil, food and consumer products [4,5,6,7]. Although many studies have shown that Pb has neurotoxicity to the rats and human beings, but the mechanism of Pb neurotoxicity is still unclear. Clarifying the mechanism of Pb-induced neurotoxicity is important for preventing the toxicity of Pb on children.
α-synuclein is a small, highly charged 140-amino acid residue protein. It is a soluble, heat-stable, and natively unfolded protein, expresses in central nervous system (CNS) neurons and is localized around synaptic vesicles in presynaptic terminals [8,9,10,11]. Accumulated evidence suggests that point mutation or genetic alteration that increase the number of copies of the α-synuclein (scna) gene can cause Parkinson's disease or related disorder dementia with Lewy bodies [12,13,14]. Aberrantly α-synuclein polymerize with typical amyloid properties in the fibrils, which are the major component of many types of pathological inclusions, for instance Lewy bodies that are associated with neurodegenerative diseases [15]. Some heavy metals can induce neurotoxicity through the induction of aberrant α-synuclein accumulation in neurons, for example manganese [16]. It is crucial to know whether Pb-induced neurotoxicity is related to accumulation of α-synuclein.
Tau protein (tubulin-associated unit) was identified in 1975 and is encoded by a single gene locus 17q21.3 in human [17]. Tau is a microtubule-associated protein highly conserved and exclusively found in higher eukaryites [18,19]. Pathological hyperphosphorylation and aggregation of tau deposits as neurofibrillary tangles (NFTs) in the brains of those with Alzheimer`s disease (AD) and other related neurodegenerative disorders called tauopathies.
Endoplasmic reticulum (ER) is a highly dynamic organelle with crucial biosynthetic and signaling functions in eukaryotic cells. ER has two major functions, one is the major intracellular calcium (Ca2+) storage, the other is providing the environment for the synthesis, folding, and modification of proteins [20,21]. The ER stress will happen when the intracellular environment contains large amounts of abnormal protein accumulation. Severe ER stress can cause cell death, usually by activating intrinsic apoptosis. Moreover, the ER stress caused by the accumulation of terminally misfolded protein aggregates that cannot be degraded by the proteasome may also activate the autophagy machinery [22,23]. Autophgy is a process by which cytoplasmic components including macromolecules and organelles are degraded by the lysosome [24]. Autophagy is orchestrated by lots of highly conserved autophagy-related genes (ATGs), which were originally identified in yeast and in mammalian cells [25,26]. Autophagy is activated in response to a host of stimuli including nutrient depletion, hypoxia, and activated oncogenes. But excess auphagy can induce cell programmed death called type II cell programmed death. Akt/mTOR/p70S6k is an important signaling pathway that could regulate autophagy. LC3 and Beclin1 are ATGs required for macroautophagy and its related process. In the process of mature of autophagysome, LC3I is phosphorylated to LC3II and the expression of Beclin1 is enhanced, so the proportion of LC3II and the expression of Beclin1 are always used to assess the level of autophagy in cells.
In our experiments, we supposed that low levels of Pb exposure could induce accumulation of α-synuclein and excessive phosphorylation of tau, while promote ER stress, and stimulate apoptosis and autophagy, thereby affect the number and function of hippocampal neurons in rats, and reduce the ability of learning and memory in rats.
Materials and methods
Drugs and reagents
Monodansylcadaverine (MDC), lead acetate, and anti-β-actin antibody were purchased from Sigma (St. Louis, MO, USA). Anti-LC3, anti-Beclin1, anti-α-synuclein, anti-p70S6kinase, anti-p-p70S6Kinase, anti-caspase-3, anti-mTOR, anti-p-mTOR, anti-tau5, anti-tau396, anti-tau404, anti-Grp78, anti-Bcl2, and anti-ATF4 bodies were purchased from Cell Signaling (Beverly, MA, USA). Secondary horseradish peroxidase-conjugated antibodies and ECL Western detection reagents were purchased from Amersham Biosciences (Little Chalfont, Buckinghamshire, UK). Hoechst 33258 was bought from Beyotime (Nantong, China).
Animals and treatments
This study involved 40 weaned male Sprague-Dawley rats (littermate, from the animal centre of the Fourth Military Medical University) with an average weight of 40~60g. All rats were maintained in 12h dark/12h light cycle room with free access to food and water. The temperature and humidity of the animal room were maintained within a stable range (23±1℃, 65~70%). All animals were handled in accordance with the procedures outlined in the “Guide for the care and use of laboratory animals” (National Institutes of Health Guide), and the institutional committee on animal research approved the study. On day 3, the rats were randomly divided into four groups with 10 rats of each group: control, low-dose Pb exposure group, middle-dose Pb exposure group, and high-dose Pb exposure group. Lead acetate was dissolved with deionized water to different concentrations (0, 100ppm, 200ppm, 300ppm). Through the drinking to use narcotics, the control group drank deionized water. The period of exposure was 8 weeks.
Morris water maze
The water maze was a black circular pool (120cm in diameter, 45cm in high) filled with water (30cm depth) heated to 27 oC. Each subject's path in the pool was recorded with Videomex Water Maze Monitoring Software (Taimeng tech, Chengdu, China) and analyzed off-line. All animals were first screened for visual and/or motor impairments using a visual cue version of the water maze. Two days after the cue task, rats received training on a spatial version of the Morris water maze according to methods previously described by Foster [27,28]. The all-black escape platform, located about 1.5 cm below the water's surface, remained in the same quadrant across trials for each group of animals, but varied from group to group. The curtains surrounding the pool were removed, revealing extra-maze cues within the room, including high-contrast, black and white poster boards. Starting locations were randomized across trials. There were four different trials in the maze in total. Four different starting positions were equally spaced around the perimeter of the pool. In the hidden platform test, 10 rats of each group conducted 4 trails one day and continued 8 days. On each day, all four start positions were used once in a random sequence equal for every rat. If the animal failed to escape within 120 s, it was manually guided to the platform. Latency and path length traveled to escape were used to measure performance in each trial.
The measurement of Pb in blood and brain samples
After experiments of Morris water maze, blood samples (0.1 ml) and hippocampus samples (0.2g) of rats were collected from each group. The whole blood samples were used for determination of blood Pb using electrothermal atomization atomic absorption spectroscopy (AAS). Brain tissues were excised and weighed. The hippocampus was quickly dissected on ice and used for determination of Pb using an electrothermal atomization AAS.
Immunohistofluorescence
The rats were sacrificed by terminal anesthesia followed by transcardial perfusion with ice-cold 0.9% NaCl and fixed with 4% paraformaldehyde (PFA) for 24 h. The tissue were gradient dehydrationed with 20% and 30% sucrose for 24~48 h. And then hippocampus tissues were bladed to sections of 10 μm with freezing microtome (Leica). Sections of hippocampus on glass slides were pre-treated with 3% H2O2 and 10% methanol. Rinsed with Tris-buffered saline (TBS) 3 times for 5 min, and incubated with 1% bovine serum albumin (BSA) in TBS and 0.2% Triton X-100 in TBS for 1 h and then with primary antibodies against α-synuclein (1:500) at 4oC for 16 h. Sections were rinsed with TBS 3 times for 5 min, followed by incubation with secondary antibodies and Hoechst 33258 for 1 h at 37oC. Then sections were rinsed with TBS 3 times for 5 min. Glass slides were mounted on coverslips using anti-fluoresence reagent as the mounting medium. Finally, the slides were observed by using a fluorescence microscope (OLYMPUS, Japan).
The detection of autophagic vacuoles by monodansylcadaverine (MDC) staining
Autophagosomes were detected with MDC according to the Biederbick method [29]. Sections of hippocampus on glass slides were pre-treated with 3% H2O2 and 10% methanol. Rinsed with TBS 3 times for 5 min, and incubated with 1% bovine serum albumin (BSA) in TBS and 0.2% Triton X-100 in TBS for 1 h and then with 0.05 mM MDC for 60 min at 37oC. Then sections were rinsed with TBS 3 times for 5 min. Glass slides were mounted on coverslips using anti-fluoresence reagent as the mounting medium and observed under a fluorescence microscope (OLYMPUS, Japan).
Western blotting
The rats were sacrificed by terminal anesthesia followed by transcardial perfusion with ice-cold 0.9% NaCl and decapitated. Total proteins were isolated from the hippocampus of the rats with mammalian tissue lysis/extraction reagent (Sigma-Aldrich, St. Louis, MO, USA) according to the manufacturer's protocol. Protein samples were separated on 5%-12% gradient SDS-PAGE and transferred to PVDF membranes. The membranes were blocked in 5% skim milk for 1 h, and probed overnight at 4oC with primary antibodies in 5% skim milk. The membranes were washed with Triton X-100 in TBS 3 times for 5 min, and incubated for 1 h with appropriate HRP-conjugated secondary antibodies at 37oC. Blots were washed and developed by using an enhanced chemiluminescence system according to manufacturer's instruction and exposed to Hyperfilm-ECL (Amersham). The same blots were reprobed with β-actin antibody as a loading control. Densitometry analysis band intensity was performed using Image J software.
Statistical analysis
All in vitro experiments were reproduced at least in triplicate. Data were analyzed using SPSS software, version 11.0 (SPSS Inc., Chicago), and values were presented as mean ± standard deviation (S.D.). Differences between groups were considered statistically significant according to a one-way ANOVA followed by a parametric multiple comparison test (Dunnett's test). Significances were indicated with *p<0.05; **p<0.01.
Results
Pb concentration in blood and hippocampus
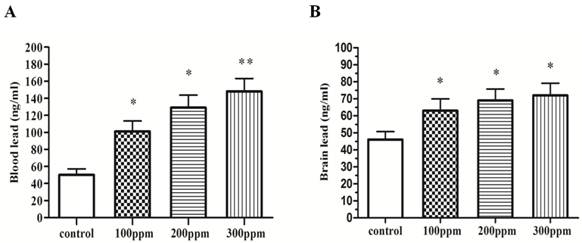
In order to verify the success of Pb-modeling, we tested the level of Pb in blood and brain in different groups of rats exposed for 8 weeks. We found the level of Pb in blood and brain of 100ppm, 200ppm, and 300ppm groups, was significantly higher than that of control group (Fig.1).
Pb exposure affected the ability of learning and memory in rats
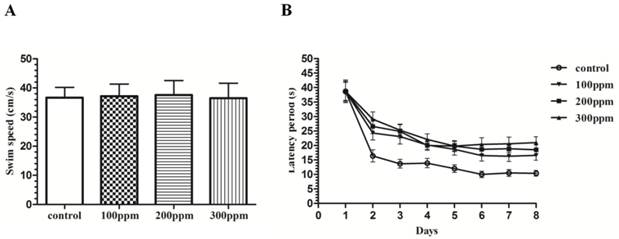
After 8 weeks of Pb exposure, we found that ability of learning and study in Pb exposure rats was obviously lower than those in control groups with Morris water maze test. During this experiment, there was no difference on the gloss of coat, moving activities, alertness, nose, eyes and limbs between Pb exposure groups and control group. And no significant difference in swimming velocity was observed among the different experimental groups (Fig. 2A). Pb exposure rats were impaired at acquiring the spatial reference memory (SRM) in water maze task. In the hidden platform test, rats of control group showed a progressive decline in escape latencies and pathlengths over the 8 training days. In contrast, the rats of different Pb exposure groups showed little decline (Figure 2B). A further day-by-day analysis revealed that the latencies of Pb exposure groups were significantly longer than that of the control group on day 2 (P<0.05), day 3 (P<0.01), day 4 (P<0.01), day 5 (P<0.01), day 6 (P<0.01), day 7 (P<0.01) and day 8 (P<0.01).
The Pb concentration in blood and hippocampus. Different doses of Pb in blood and hippocampus after exposure for 8 weeks. (A) Blood Pb (n=10). (B) Brain Pb (n=10). Significance (Bonferroni /Dunn post hoc comparisons after ANOVA): *p<0.05, ** p <0.01 vs the vehicle control group. Graphs show mean± SD.
The effect of Pb exposure on spatial reference memory. (A): Swimming speed in the water maze on the first day of the training showed no significant difference among the groups. (B): Performance of rats in Pb-exposed groups in terms of escape latency across eight blocks of training was obviously impaired compared with rats in the control group. Analysis of escape latencies revealed that these abilities were affected by Pb exposure (treatment effect, p<0.05).
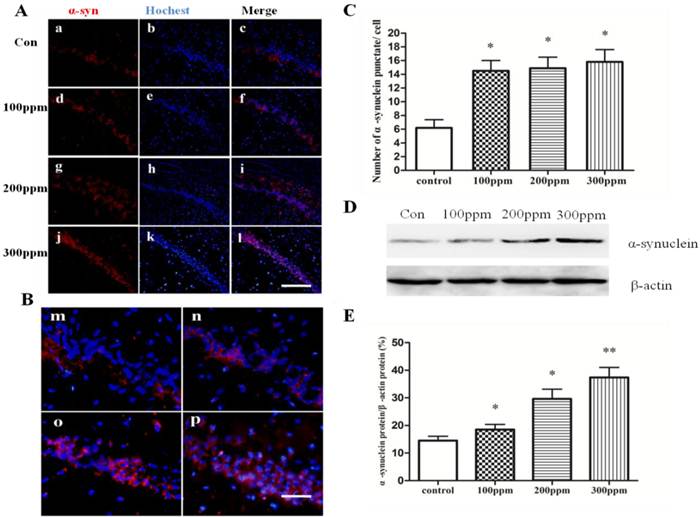
Accumulation of α-synuclein was induced by Pb exposure
We used double immunofluorescence to examine whether the accumulation of α-synuclein was induced in Pb-treated groups. We found that α-synuclein aberrantly accumulated in Pb treated groups, but not in control group. Interestingly, with the increase of the Pb concentration, the numbers of α-synuclein stained puncta contemporary increased (Fig.3C). The Western blotting results also illustrated the same consequence (Fig.3D). These results show that Pb promoted aberrant accumulation of α-synuclein in the hippocampus of adult rats.
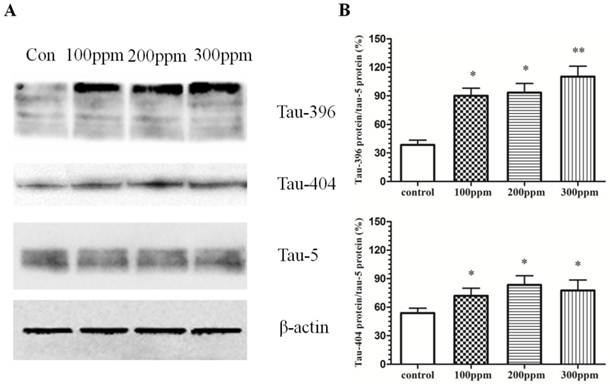
Hyperphosphorylation of tau in the hippocampus of the rats in Pb-exposed groups
The phosphorylation of tau is mostly at S396 and S404 sites. Abnormal phosphorylation of tau occurs before the onset of NFTs. In our studies, we found that excessive phosphorylation of tau at both 396 and 404 sites in the Pb-exposed groups (Fig.4). With the increased concentration of Pb exposure, tau phosphorylation levels at both 396 and 404 sites were also enhanced. Our results suggested that Pb exposure promoted the tau hyperphosphorylation in hippocampus in rats.
The effect of Pb exposure on accumulation of α-synuclein in CA1 in hippocampus. Immunofluorescence and Western blotting were conducted to measure α-synuclein immunoreactivity and α-synuclein protein expression in CA1 in hippocampus. (A, B) Immunofluorescence observation on α-synuclein protein expression in CA1 in hippocampus. (a-c) control; (d-f) 100ppm, (g-i) 200ppm, (j-l) 300ppm (A, Bars: 100μm; B, Bars:50μm). (C) Quantitative analysis of the effect of Pb exposure on the number of α-synuclein puncta per cell. *p<0.05, ** p <0.01 vs the vehicle control group (D) The effect of Pb on protein expression of α-synuclein protein. (E) Densitometry analysis of α-synuclein protein levels was performed using three independent experiments. β-actin was used as the control for protein loading (mean± SD; one-way ANOVA with Newman-Keuls post hoc analysis, *p<0.05 and **p<0.01 vs the vehicle control group).
The effect of Pb exposure on tau phosphorylation in CA1 in hippocampus. Western blotting was conducted to mesure phosphorylation of tau at 396 and 404 sites. (A) The effect of Pb on tau phosphorylation at 396 and 404 sites. (B, C) Densitometry analysis of tau phosphorylation at 396 and 404 sites were performed using three independent experiments. Tau-5 was used as the control for protein loading (mean±SD; one-way ANOVA with Newman-Keuls post hoc analysis, *p<0.05 and **p<0.01 vs the vehicle control group).
Both autophagy and apoptosis were obviously heightened by Pb exposure
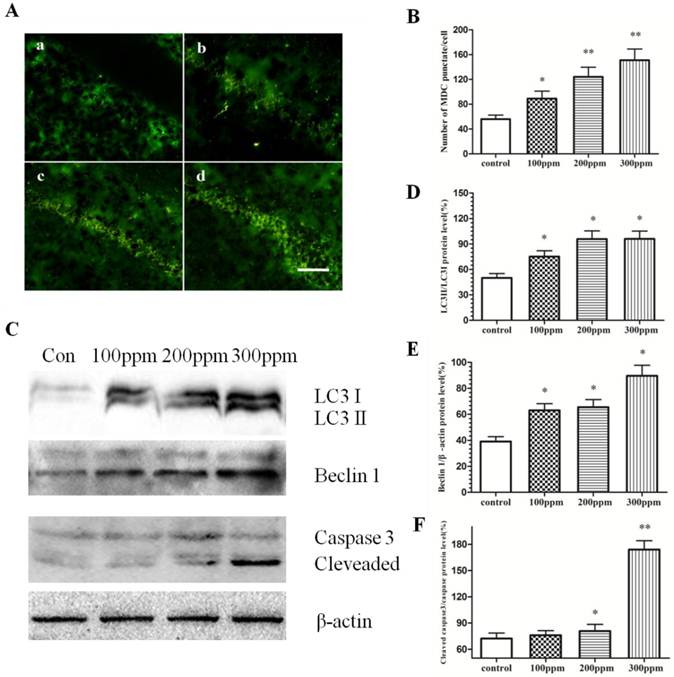
To evaluate the effect of Pb on autophagy and apoptosis, immunohistochemical staining and Western blotting studies were carried out. As shown in Fig. 5A, the number of autophgosomes marked by MDC in CA1 in hippocampus of Pb exposure was obviously higher than that in control group. Further Western blotting experiments established a dose-dependent effect of Pb exposure on the protein level of Beclin1 and microtubule-associated protein 1 light chain 3 (LC3). With the increase of Pb concertration, the autophagy level was also increased, as evidenced by the levels of LC3II to LC3I and Beclin1. In addition, Western blotting experiments also confirmed that Pb exposure can activate caspase-3, from which we can judge that Pb exposure could promote the occurrence of apoptosis. These findings suggest that autophagy and apoptosis may be the reasons for impaired learning and memory after Pb exposure.
Pb exposure promoted endoplasmic reticulum stress and inhibited Akt/mTOR signaling pathway
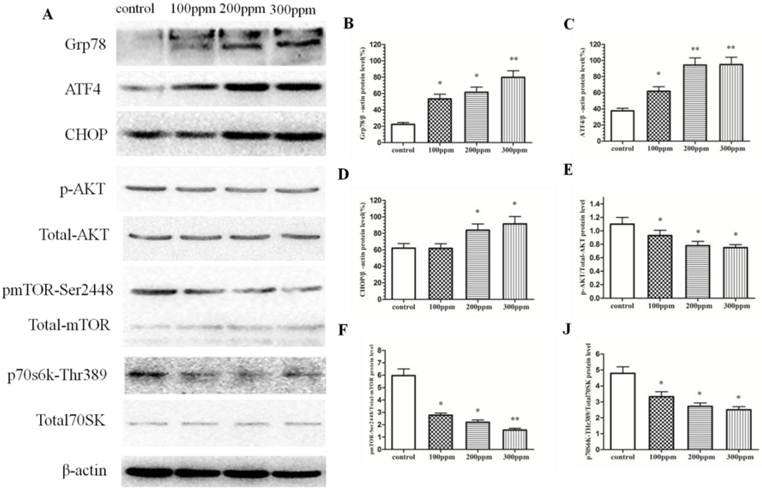
Grp78 is an ER stress-related protein. In our study, we detected the level of Grp78 protein in CA1 of hippocampus in rats exposed to Pb for 8 weeks by Western blotting, and found that Pb exposure can significantly promote the level of Grp78 protein, compared with that of control group (Figure 6A). During ER stress, the levels of CHOP and ATF4 proteins in CA1 of hippocampus in Pb exposure rats were also increased, compared with that of control group (Figure 6A). At the same time, we also detected the Akt/mTOR signal pathway. Akt/mTOR pathway was suppressed in Pb-exposed groups, compared with that of control group. With the increase of Pb concentration, Akt/mTOR pathway was inhibited more apparently (Fig.6). The inhibition of Akt/mTOR pathway may be the reason for promoting the occurrence of autophagy and apoptosis, and leading to cutdown number and impaired function of neuron in CA1 of hippocampus in Pb-exposed rats.
Discussion
Recent studies show that environmental Pb exposure has certain toxicity to nervous system of animals and humans. Pb expose can injure the ability of learning and memory. In central nervous system (CNS), the major function of hippocampus is learning and memory. When hippocampus is damaged, the ability of learning and memory will be injured. The mechanisms underlying this effect have not been fully clarified. In our study, we confirmed the effects of Pb exposure on the ability of learning and memory in rats and further determined possible mechanisms involved in this process.
The function of α-synuclein in CNS neurons is modulating synaptic transmission, the density of synaptic vesicles, and neuronal plasticity. But aberrantly α-synuclein polymerize with typical amyloid properties can cause Parkinson's disease or related disorder dementia with Lewy bodies. Previous studies show that manganese can induce the accumulation of α-synuclein and is toxic to neurons [16] . In our study, the results showed that Pb exposure also can cause excessive accumulation of α-synuclein in CA1 of hippocampus (Fig. 3). Maybe the increase of α-synuclein is one of the reasons leading to damage on ability of learning and memory of Pb exposure in rats.
The effect of Pb exposure on autophagy and apoptosis. Immunofluorescence and Western blotting were conducted to measure autophagy level in CA1 in hippocampus. (A) Immunofluorescence observation on autophagosome formation in CA1 in hippocampus. (a) control; (b) 100ppm, (c) 200ppm, (d) 300ppm (Bars: 50μm). (B) Quantitative analysis of the effect of Pb on the number of autophagosome in CA1 in hippocampus. *p<0.05 and **p<0.01 vs the vehicle control group. (C) The effect of Pb on Beclin1, LC3 II and LC3 I protein expression, and caspase-3 cleavage. (D, E, F) Densitometry analysis of the ratio of LC 3 II to LC 3 I (D) Beclin1 (E) relative to β-actin (loading control), and the cleavage of caspase-3 (F) were performed in three independent experiments (mean± SD; one-way ANOVA with Newman-Keuls post hoc analysis, *p<0.05 and **p<0.01 vs the vehicle control group).
The effect of Pb exposure on ER stress and Akt/mTOR pathway. (A) The effect of Pb on protein expression of Grp78, ATF4, CHOP, and Akt/mTOR/p70s6k phosphorylation. (B-H) Densitometry analysis of Grp78 (B) ATF4, (C) CHOP, (D) relative to β-actin (loading control), and the phosphorylation of Akt (E), mTOR (F) and p70s6k (J) were performed in three independent experiments (mean±SD; one-way ANOVA with Newman-Keuls post hoc analysis, *p<0.05 and **p<0.01).
Tau protein is also an important protein related to the neuronal degeneration in CNS. Pathological hyperphosphorylation and aggregation of the tau protein can cause nervous system diseases. The phosphorylation of tau is mostly at S396 and S404 sites. Abnormal phosphorylation of tau occurs before the onset of NFTs. Our results showed that Pb exposure can cause hyperphosphorylation of tau in CA1 in hippocampus (Fig. 3, Fig.4).
ER is an important organelle that regulates the metabolism of abnormal protein intracellular. When there were a large number of abnormal proteins intracellular, such as α-synuclein and tau, ER will occur. In our study, we found that Pb exposure can include the accumulation of α-synuclein and excessive phosphorylation of tau, so we want to know the relationship between them with ER stress, and we detected the level of Grp78, ATF4, and CHOP protein as the ER stress-related proteins by Western blotting. We found that the level of ER stress-related proteins increased (Fig 6), indicated that Pb exposure could induce ER stress in hippocampal neurons. There are two ways to cause ER stress, one is direct effects on hippocampal neurons, and the other is indirect effect. So, Pb as an exogenous toxic metal ions into the neurons can cause endoplasmic reticulum stress (Fig.6). Taken together, Pb can lead to excessive accumulation of α-synuclein and hyperphosphorylation of tau protein which indirectly cause ER stress (Fig. 3, Fig. 4).
Severe ER stress can cause cell death, usually by activating intrinsic apoptosis. Moreover, the ER stress also can upregulate the autophagy machinery [22,23]. Autophagy is a general term for pathways by which cytoplasmic materials, including soluble macromolecules and organelles, are delivered to lysosomes for degradation [30]. Although autophagy can protect neurons through regulating the environment within the cell. However, the occurrence of excessive autophagy can lead to autophagic cell death, also known as programmed cell death II. In this study, we also found that Pb could induce apoptosis and autophagy, the activation of caspase-3. The ratio of LC3II to LC3I and the level of beclin1 protein were also increased in Pb-exposed rats (Fig. 5). Maybe those results were induced by ER stress.
It is well established that mTOR kinase, which integrates upstream signaling pathways, serves as a key signaling molecule in the suppression of autophagy [31,32] . It has also been reported that inhibition of p70s6k, a downstream target of mTOR signal, is involved in the enhancement of autophagy. Overall, the inhibition of autophagy is dependent on the activation of mTOR and p70s6k signaling [33,34]. In our study, the Akt/mTOR/p70s6k signaling pathway was suppressed in Pb-exposed groups, compared with that in control group, and with the increase of Pb concentration, Akt/mTOR pathway was inhibited more apparently (Fig.6).
The results of this study indicate that Pb-induced neurotoxicity may be mediated by ER stress, which could be indirectly induced by excess accumulation of α-synuclein and hyperphosphorylation of tau. ER stress can promote expression of Beclin1 and changed ration of LC3II/LC3I (Fig.5C), and inhibit the Akt/mTOR pathway, suggesting that Pb can cause autophagic cell death. ER stress can also promote the activation of caspase-3, which causes apoptotic cell death. These may be the reasons for hippocampal neuronal injury and key reasons leading to injured learning and memory in response to Pb exposure.
Acknowledgements
This work was supported by Key Project of Natural Science Foundation of China (30830087); National Basic Research Program of China (973 Program, 2012CB525004); Program for New Century Excellent Talents in University; National Natural Science Foundation of China (30972499, 30771768, 30800898, 30901176, 81001256, 81001233); and Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT).
Abbreviations
ATG, autophagy-related genes; CNS, central nervous system; ER, endoplasmic reticulum; LC3, microtubule-associated protein light chain 3; MDC, Monodansylcadaverine; mTOR, mammalian target of rapamycin; NFTs, neurofibrillary tangles; p70s6k, p70 ribosomal protein S6 kinase; Pb, lead; tau, tubulin-associated unit; UPR, unfolded protein response
Competing Interests
The authors have declared that no competing interest exists.
References
1. Kosnett MJ, Wedeen RP, Rothenberg SJ, Hipkins KL, Materna BL. et al. Recommendations for medical management of adult lead exposure. Environ Health Perspect. 2007;115:463-471
2. Finkelstein Y, Markowitz ME, Rosen JF. Low-level lead-induced neurotoxicity in children: an update on central nervous system effects. Brain Res Brain Res Rev. 1998;27:168-176
3. Rosen JF. Adverse health effects of lead at low exposure levels: trends in the management of childhood lead poisoning. Toxicology. 1995;97:11-17
4. KAPLAN E, SHAULL RS. Determination of lead in paint scrapings as an aid in the control of lead paint poisoning in young children. Am J Public Health Nations Health. 1961;51:65-69
5. Baker EL, Folland DS, Taylor TA, Frank M, Peterson W. et al. Lead poisoning in children of lead workers: home contamination with industrial dust. N Engl J Med. 1977;296:260-261
6. Nriagu JO, Pacyna JM. Quantitative assessment of worldwide contamination of air, water and soils by trace metals. Nature. 1988;333:134-139
7. Edwards M, Triantafyllidou S, Best D. Elevated blood lead in young children due to lead-contaminated drinking water: Washington, DC, 2001-2004. Environ Sci Technol. 2009;43:1618-1623
8. Davidson WS, Jonas A, Clayton DF, George JM. Stabilization of alpha-synuclein secondary structure upon binding to synthetic membranes. J Biol Chem. 1998;273:9443-9449
9. Jensen PH, Nielsen MS, Jakes R, Dotti CG, Goedert M. Binding of alpha-synuclein to brain vesicles is abolished by familial Parkinson's disease mutation. J Biol Chem. 1998;273:26292-26294
10. Perrin RJ, Woods WS, Clayton DF, George JM. Interaction of human alpha-Synuclein and Parkinson's disease variants with phospholipids. Structural analysis using site-directed mutagenesis. J Biol Chem. 2000;275:34393-34398
11. McLean PJ, Kawamata H, Ribich S, Hyman BT. Membrane association and protein conformation of alpha-synuclein in intact neurons. Effect of Parkinson's disease-linked mutations. J Biol Chem. 2000;275:8812-8816
12. Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A. et al. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045-2047
13. Spillantini MG, Goedert M, Crowther RA, Murrell JR, Farlow MR. et al. Familial multiple system tauopathy with presenile dementia: a disease with abundant neuronal and glial tau filaments. Proc Natl Acad Sci U S A. 1997;94:4113-4118
14. Gupta A, Dawson VL, Dawson TM. What causes cell death in Parkinson's disease? Ann Neurol 64 Suppl. 2008;2:3-15
15. Popescu A, Lippa CF, Lee VM, Trojanowski JQ. Lewy bodies in the amygdala: increase of alpha-synuclein aggregates in neurodegenerative diseases with tau-based inclusions. Arch Neurol. 2004;61:1915-1919
16. Guilarte TR. APLP1, Alzheimer's-like pathology and neurodegeneration in the frontal cortex of manganese-exposed non-human primates. Neurotoxicology. 2010;31:572-574
17. Weingarten MD, Lockwood AH, Hwo SY, Kirschner MW. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A. 1975;72:1858-1862
18. Maccioni RB, Cambiazo V. Role of microtubule-associated proteins in the control of microtubule assembly. Physiol Rev. 1995;75:835-864
19. Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer's disease. Neuron. 1989;3:519-526
20. Berridge MJ. The endoplasmic reticulum: a multifunctional signaling organelle. Cell Calcium. 2002;32:235-249
21. Gorlach A, Klappa P, Kietzmann T. The endoplasmic reticulum: folding, calcium homeostasis, signaling, and redox control. Antioxid Redox Signal. 2006;8:1391-1418
22. Ogata M, Hino S, Saito A, Morikawa K, Kondo S. et al. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol. 2006;26:9220-9231
23. Kouroku Y, Fujita E, Tanida I, Ueno T, Isoai A. et al. ER stress (PERK/eIF2alpha phosphorylation) mediates the polyglutamine-induced LC3 conversion, an essential step for autophagy formation. Cell Death Differ. 2007;14:230-239
24. Yang Z, Klionsky DJ. Eaten alive: a history of macroautophagy. Nat Cell Biol. 2010;12:814-822
25. Rosenfeldt MT, Ryan KM. The role of autophagy in tumour development and cancer therapy. Expert Rev Mol Med. 2009;11:36
26. Reggiori F, Klionsky DJ. Autophagosomes: biogenesis from scratch? Curr Opin Cell Biol. 2005;17:415-422
27. Norris CM, Foster TC. MK-801 improves retention in aged rats: implications for altered neural plasticity in age-related memory deficits. Neurobiol Learn Mem. 1999;71:194-206
28. Foster TC, Sharrow KM, Masse JR, Norris CM, Kumar A. Calcineurin links Ca2+ dysregulation with brain aging. J Neurosci. 2001;21:4066-4073
29. Biederbick A, Kern HF, Elsasser HP. Monodansylcadaverine (MDC) is a specific in vivo marker for autophagic vacuoles. Eur J Cell Biol. 1995;66:3-14
30. Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011;469:323-335
31. Jung CH, Ro SH, Cao J, Otto NM, Kim DH. mTOR regulation of autophagy. FEBS Lett. 2010;584:1287-1295
32. Chen N, Karantza-Wadsworth V. Role and regulation of autophagy in cancer. Biochim Biophys Acta. 2009;1793:1516-1523
33. Jung CH, Jun CB, Ro SH, Kim YM, Otto NM. et al. ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol Biol Cell. 2009;20:1992-2003
34. Mehrpour M, Esclatine A, Beau I, Codogno P. Overview of macroautophagy regulation in mammalian cells. Cell Res. 2010;20:748-762
Author contact
![]() Corresponding author: Dr. Jingyuan Chen, Department of Occupational and Environmental Health, Fourth Military Medical University, 169 Changlexi Road, Xi'an, China 710032 Tel: +86 29 84774401, Fax: +86 29 84774862, E-mail: jy_chenedu.cn. Or to: Dr. Wenjing Luo, E-mail: luowenjedu.cn.
Corresponding author: Dr. Jingyuan Chen, Department of Occupational and Environmental Health, Fourth Military Medical University, 169 Changlexi Road, Xi'an, China 710032 Tel: +86 29 84774401, Fax: +86 29 84774862, E-mail: jy_chenedu.cn. Or to: Dr. Wenjing Luo, E-mail: luowenjedu.cn.

 Global reach, higher impact
Global reach, higher impact