10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2012; 8(7):1005-1012. doi:10.7150/ijbs.4800 This issue Cite
Review
Preservation of Fertility in Females Treated for Cancer
1. Department of Obstetrics and Gynecology, Navy General Hospital, Beijing, China;
2. Department of Anesthesia, Navy General Hospital, Beijing, China.
* Authors contributed equally to this paper.
Received 2012-7-1; Accepted 2012-7-29; Published 2012-8-1
Abstract
Advancements of diagnosis and treatment have substantially improved cancer survival rates in the last few decades. The increasing number of survivors focuses attention on long-term effects caused by cancer treatment and its impact on quality of life. Ovarian failure is one of the major sequelae of cytotoxic chemotherapy and/or radiotherapy in female children and reproductive-age women. Oncologists should address the patients about fertility preservation options before therapy. Embryo cryopreservation is the only well-established method for females in preserving fertility; however other strategies including ovarian suppression, ovarian transposition and cryopreservation of oocytes and ovarian tissue are still experimental. Patients need advice and to know which are the most practical options for them. This article reviews the available fertility preservation methods in women, and the related issues including normal physiology of the ovary, effect of anticancer therapy on fertility, role of the oncologist and ethics. We performed a MEDLINE search from 1971 to 2011 in a similar way as Jensen et al. 2011, using the following MeSH terms: antineoplastic agents; ovarian failure; premature; infertility, female; fertility preservation; child and cancer; reproductive technologies, assisted.
Keywords: Fertility Preservation, Cancer patient, Females
Introduction
Over the past two decades, cancer incidence rates continue to increase [1]. Among women, under the age of 40, approximately 1 in 47 will develop cancer [2]. Due to advancements in cancer therapy, the overall death rate decreased by 1.5% per year in females from 2002 through 2007 and 0.8% per year from 1994 through 2002 [2]. Between 1990/1991 and 2007, cancer death rates decreased by 13.9% in women, the reduction in death rates for breast, colorectal, ovarian and cervical cancers and non-Hodgkin lymphoma and leukemia accounted for almost 75% of the decrease [2]. However, the treatments of cancer often cause infertility or premature gonadal failure [3-5]. An increasing proportion of reproductive-age women who undergo anticancer therapy have concerns about their future fertility and parenthood [6].
Clinical infertility is defined as the inability to conceive after one year or more of intercourse without contraception during the fertile phase of the menstrual cycle [7]. The baseline incidence of sterility is estimated at 8% for women aged 19 to 26 years, and 18% for women aged 35 to 39 years [7]. Risks of permanent infertility and compromised fertility after cancer treatment vary and depend on the type of cancer, the major treatments, age of the patient, the time available before treatments, and the risk of ovarian metastasis [8]. Current techniques of fertility preservation, such as cryopreservation of embryos, oocytes and ovaries, ovarian transposition, ovarian suppression, conservative surgery and gonadal shielding are often available in female cancer patients [9]. Fertility preservation should be considered as early during treatment plan as possible [9]. Oncologists should inform young patients about these options in a comprehensive pattern with focus on medical and ethical issues [9].
Normal physiology of the ovary
Oogenesis is a limited event which ceases during fetal development with a finite number of oocytes present at birth [7,10,11]. At about 20 weeks in utero, a peak in a number of oogonia approximately 6-7 million is seen in a female infant's ovaries. This number is reduced after follicular assembly. Some of the germ cells do not get into follicular structures and are lost [12,13]. There is a abruptly drops in this number by birth to about 1-2 million, at puberty only about 200,000-500,000 oocytes remain, but at menopause less than 1000 remain [14-19]. Only a few oocytes will be mature and undergo ovulation during reproductive years [20]. The majority of oocytes will be lost due to the atresia [20], which is a inherent process until menopause in spite of pregnancy, amenorrhea or contraceptive use [10], however mediated by aging, disease mechanism like premature ovarian failure, cancer, or iatrogenic causes [12]. In the prepubescent period, primordial germ cells generate oogonia, these cells in turn undergo mitosis to produce oocytes, which will be arrested in meiosis I as primordial follicles until puberty [20]. Once puberty ensues, meiosis is resumed, a monthly cohort of follicles is selected to grow and develop, but only one of these oocytes will complete meiosis I and be arrested in metaphase II until fertilization [21,22].
Recent studies suggested that primordial germ cells in vitro are capable of forming oogonia, some species of mammals a type of ovarian stem cell may persist throughout adulthood, and ovarian regeneration may occur from stem cells in the bone marrow [23-25]. Since all the findings are performed in animal models and have not yet been replicated in other laboratories, the fertility potential of stem cells in humans remains answered [26]. At present there is no evidence that primordial germ cells that can produce new oocytes are present in ovaries of women. [27].
Effect of anticancer therapy/cancer on fertility
Multi-agent chemotherapy, radiotherapy, ovarian metastasis and surgical removal of the adnexa could result in premature ovarian failure, which is attributed to decreased ovarian reserve [10]. Ovarian reserve reflects the number of oocytes in a woman's ovaries, and is highly associated with fertility [18]. The side effect of therapies depend on the type of cancer, drug, method of administration, size/location of the irradiation field, dose/dose-intensity, age, and the prescribed treatment [15].
Chemotherapy
Most multi-chemotherapeutic agents can affect oocytes, granulosa and theca cells, it will be detrimental to ovarian reserve and induce amenorrhea which may often be reversible [28]. The alkylating agents (cyclophosphamide, procarbazine, ifosfamide, nitrosoureas, chlorambucil, melphalan, busulfan) are more likely to pose this risk to gametes, because they are non-cell-cycle specific and could cause damage even to “resting” oocytes [29-34]. Cyclophosphamide is the most commonly implicated agent in causing damage to ovaries, particularly to the primordial follicles [35]. The odds ratio for inducing premature ovarian failure with cyclophosphamide exposure were reported between 4-9.3 compared to those in unexposed patients [35]. Kenney et al. [36] reported that cyclophosphamide caused follicular damage in a dose-dependent manner, however, Meirow et al. [37] described the destructions of primordial follicles were observed at all levels of cyclophosphamide exposure, even at low doses of 20 mg/kg. Cyclophosphamide and related combination routinely used for breast cancer and hematopoietic stem cell transplantation (HSCT) [38]. Platinum-based compounds and taxanes are also often applied in breast cancer, cervical cancer and germ cell tumors-all common in premenopausal women [38]. Age plays an important role in the risk of chemotherapy-induced ovarian failure. Older women (with low primordial follicle pool) appear more susceptible to permanent ovarian damage compared with young women (with higher primordial follicle number) [39]. This explains why the incidence of ovarian failure after chemotherapy in breast cancer patients is more than 50% in age over 40 compared to 30% in those less than age 35 [40].
Radiotherapy
Radiotherapy may affected fertility not only by direct total body, low abdominal, pelvic, or spinal radiation, but also scattered radiation which can cause gonadal failure even if ovaries are outside of the radiation field [5,6]. Human oocyte is extremely sensitive to ionizing radiation, direct radiation cause a dose and age-related reduction in the ovarian follicular pool, dose of less than 2 Gy is enough to destroy half of the oocyte population, dose of more than 6 Gy usually causes irreversible ovarian failure [5,6]. Compared with younger women, older patients are at higher risk for developing infertility after radiotherapy due to the low primordial follicle pool and the high radiation-induced chromosome damage in the oocytes [41]. It appears to be more toxic when ionizing radiation is given in a single dose compared to fractionated doses [9]. Anatomic or vascular changes to the uterus, cervix or vagina and interferes with the hormonal balance from ionizing radiation may also affected fertility [42,43]. In addition, radiotherapy has a potential deleterious effects on the uterus (as consequence of uterine vasculature damage, endometrium damage and myometrial fibrosis) and the hypothalamic-pituitary axis (as consequence of cranial irradiation in central nervous tumours or total body irradiation in hematological malignancies) [42,43].
Metastasis and surgery
It is logical that there is a risk of ovarian metastasis if the patient survives malignant tumor. Hematologic diseases such as leukaemias and Burkitt's lymphoma are high risk in ovarian involvement, some advanced stage solid tumours such as breast and colon cancers are moderate risk [5,6]. Most other cancers in reproductive age women have low risk of ovarian metastasis, including breast cancer in stage I-III, squamous cell carcinoma of the cervix, Non-Hodgkin's lymphoma, Hodgkin's lymphoma, osteogenic sarcoma, Ewing's sarcoma, etc. [6] However, in spite of the risk of metastatic disease, histopathological or molecular biological analysis should be performed to determine the presence of cancer cells in the ovarian tissue before and after cryopreservation of ovaries [6].
Gynecologic surgery is the major therapy for gynecologic malignancies, the radical operations remove the key fertility organs (cervix, uterus, fallopian tubes and ovaries) and cause permanent damage to fertility and endocrine function [10]. The current trend of treatment is the conservative surgery with the intent of maintain organs as much as possible for reproductive function. It has been suggested in the stage 1A2-IB cervical cancers, the recurrence rates of radical trachelectomy have no significant higher than that of radical hysterectomy [44]. Studies also showed in the ovarian germ cell tumors, conservative surgery will not significant affect the risk of recurrence compared with historical controls [45,46]. However, the view of fertility-sparing surgery has not received wide acceptance, for these trials are often limited in size and lack randomized controls [9].
Fertility preservation options in females
Each individual has her own situation; this section reviewed the currently available fertility preservation options in female and the relevant clinical applied scope (Figure 1A and B). Before cancer treatments, if the circumstances permit, women can be treated with in vitro fertilization to create embryos for cryopreservation or with surgically moving ovaries out of the radiation field [9,13]. Embryo cryopreservation is the only well-established technique used for fertility preservation in female, however, it may not be appropriate for every patient due to age, delay in therapy, type and stage of cancer, and her partner [13]. Most other options such as cryopreservation of oocyte and ovarian tissue, and ovarian suppression remain in the area of research [5]. Conservative surgical techniques (preserving ovaries in early ovarian tumors, preserving uterus in early cervical cancers) and gonadal shielding are also available approaches but will not be further discussed in this paper.
Ovarian suppression
Clinical data support a viewpoint that the prepubertal girls have greater tolerance than postpubertal women during gonadotoxic treatments [47]. Ovarian suppression with gonadotropin-releasing hormone agonist (GnRH-a) during chemotherapy has been advocated by some oncologists [47]. One hypothesis is that the administration of GnRH-a could make the ovary an endocrine prepubertal state with less susceptible to cancer treatments [47]. Studies in monkeys successful demonstrated the protective role of GnRH-a against the primordial follicle loss associated with cyclophosphamide, however failed to found the same role in ionizing radiation-induced ovarian injuries [48,49]. The available clinical researches are highly controversial, some meta-analysis showed a benefit of ovarian suppression by GnRH-a, but other studies did not show a benefit of this approach [50,51]. Oncologists discussed that there was little possible for GnRH-a to preserve ovarian reserve due to the total 90% of follicle pool in an adult was primordial follicles which recruited through a follicle stimulating hormone (FSH) independent manner [9,52]. There was a theoretical higher risk of hormone receptor-positive cancers recurrence, including breast, endometrial and ovarian cancer [9,52]. The American Society of Clinical Oncology concludes there is insufficient evidence that ovarian suppression protects fertility from gonadotoxic therapies [9]. However, GnRH-a administration has been used widely due to the readily available [9]. A large randomized clinical study with long-term follow-up should be performed to clear the safety and efficacy of GnRH-a treatment.
Summary of fertility preservation strategies in female cancer patients before (A) and after (B) treatment.
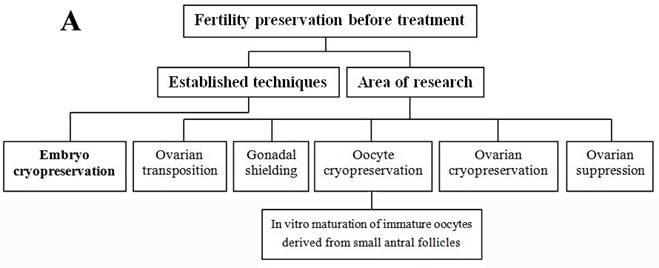
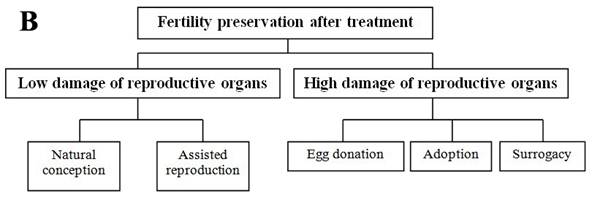
Ovarian transposition
A standard radiation dose often causes premature ovarian failure in the treatment of genital, intestinal or urinary tract tumors [10]. Ovarian transposition (oophoropexy) can be used in the pelvic radiotherapy through surgically moving the ovaries above the pelvic brim and out of the planned exposure field to avoid radiation injuries [9,10]. This procedure should be done as close as possible to the radiotherapy for the possibility of migration of the ovaries [10]. If laparotomy is needed for tumor removal, oophoropexy can be performed simultaneously, or if it not need, this operation can be accomplished laparoscopically [53-55]. The success with resumption of menstruation by oophoropexy before radiation varies between 16 and 90% [53-55]. This variation is mainly affected by scatter radiation which is approximately 8-15% of the radiation dose in pelvic region, and by ovarian vascular change caused by surgical procedure in separation of utero ovarian ligament and mesovarium [56]. Spontaneous pregnancy is generally not happen when ovaries were transposed out of their original sites [9]. Ovarian repositioning or transabdominal oocyte retrieval may often be needed to restore fertility [9]. If the radiotherapy is whole-body radiation or it combines with chemotherapy, the strategy will not work [9,10]. As a result, the patients should be selected carefully before oophoropexy to maximize their benefits.
Cryopreservation options
Embryos Embryo cryopreservation is used routinely for storage of surplus embryos during in vitro fertilization [9,10]. This procedure involves 2-6 weeks of ovarian stimulation [daily injections of follicle stimulating hormone (FSH) from the onset of menses, to collect mature oocytes with an injection of human chorionic gonadotropin (GSH) to start ovulation], in vitro fertilization (a partner or donor sperm is required) and cryopreservation [57]. The delay of 2-6 weeks in therapy initiation may make it not applicable for women with highly aggressive malignancies, such as leukemia and sarcomas, which require immediate treatment [58]. It might also be noted that ovarian stimulation causes high serum concentrations of estrogen in some hormone-sensitive tumors [59]. For these patients, alternative protocols have been investigated to reduce the potential risk of estrogen exposure, such as the use of tamoxifen in breast cancer and the use of letrozole in endometrial cancer, and showed some individual successes [6]. Despite these limitations, reported survival rates per thawed embryo range from 35-90%, implantation rates from 8-30%, and cumulative pregnancy rates of 30-40% [60-62]. Although the pregnancy rates are encouraging, this technique may be impossible because of lack of partner and the patient not wanting a sperm donor [6,9].
Oocytes Oocytes cryopreservation (unfertilized) is an alternative option for fertility preservation in patients who do not wish to use donor sperm [6]. The process requires ovarian stimulation, therefore, it has similar limitations as embryo cryopreservation, including impossibility for girls before puberty, delay in therapy and high hormonal levels exposure [6]. The oocytes can be aspirated in the operating theatre or directly from ovaries [6]. Unfertilized oocytes are more sensitive to cryodamage than embryos due to their large size, water content, high-lipid content and temperature-sensitive spindle [63]. The overall pregnancy rate per cryopreserved oocyte is only about 2%, which is lower than the rate using embryo cryopreservation and much lower than the rate using fresh oocytes [64-66]. Studies showed the improvement of cryopreservation could increase success rates, there is success in oocyte freezing by vitrification but slow freezing is also used [67,68]. Compared with slow freezing, vitrification is a more efficient method for freezing the oocytes produced from in vitro maturation [67,68]. Collection of immature oocytes with in vitro maturation and subsequent cryopreservation could be done with mild ovarian stimulation, thus avoiding the delay in cancer treatment and high hormonal levels exposure [69,70]. Immature oocytes seem more resistant to cryodamage due to their lower cell volume and lack of metaphase spindle, however the inefficiency of in vitro maturation also results in low success rates [71]. At present, oocyte cryopreservation should only be operated in the centers with necessary expertises, a much higher success rate must be obtained so that this option can be widely offered to females [9].
Ovarian tissue Ovarian tissue cryopreservation is an experimental technique, but it precludes the ovarian stimulation and sperm donor in embryo/oocyte cryopreservation [5,6]. It may be the only acceptable method that can be offered to prepubertal patients, and a preferred method for women who cannot delay their therapies for the hormonal stimulation to harvest oocytes and/or create embryos [8]. Generally, ovarian cortical tissue is removed by a laparoscope followed by subsequent cryopreservation, ovarian cortex (contains primordial follicles) can be cryopreserved with great efficiency [9]. After treatment, ovarian tissue is thawed and reimplanted orthotopically in the ovarian fossa or heterotopically in the forearm or abdominal wall [10]. Although studies are case reports, there have been successful live births after orthotopic transplantation in cancer patients, to date, human livebirths have only been achieved after grafting to the ovary [72]. Same as a common transplantation, the interval between transplant and revascularization will cause ischemia-reperfusion injury after ovarian transplantation [10]. Most primordial follicles could survive the freeze-thaw cycle, however, nearly a two-thirds or more of these follicles are lost after transplantation [13]. Recent studies suggest that host treatment with melatonin or graft incubation with HA-rich biological glue, especially when combined with VEGF-A and vitamin E improves graft survival [73,74]. The main concern with re-implanted tissue is the theoretical risk of ovarian contamination in some malignancies [75-78]. There is more and more evidence of possible reseeding of cancer as malignant cells might exist in the ovary, especially in lymphomas and leukemia [75-78]. In the future, in vitro culture of primordial follicles may eliminate this risk, at present it had been successful only in the mouse but small antral follicles have been obtained in humans [79,80].
Role of the oncologist
Infertility is often low understood by patients, oncologists have a responsibility to inform them that the cancer therapy may permanently affect their fertility [9]. Fertility preservation has positive influences on the mental health of a patient as it is a way of saying there is normal life after cancer [5,6]. There is no evidence that currently used fertility preservation options decrease the success of cancer therapy, adversely affect a survivor's health, increase the risk of maternal or perinatal complications, or compromise the health of subsequent children [9,81,82]. Oncologists must pay attention to the plans for fertility preservation as medical necessity.
Some fertility preservation methods in women need timing because of menstruation [9], if gonadal toxicity is unavoidable, oncologists should refer the candidates to fertility specialists as soon as possible, so that avoid missing opportunities. Fertility specialists will help females to perform cryopreservation of oocytes, embryos or ovarian tissue, and provide other assisted reproductive technologies [83]. The most used methods for cryopreservation are vitrification and slow freezing, in the process, cryoprotectant, such as dimethyl sulfoxide, polyethylene glycol and glycerine etc., is needed to prevent frozen injury [84]. A psychologist should be available to help a woman has moderate to severe distress about potential infertility, since structured and cognitive behavioral counseling can reduce anxiety and depression [85-87].
Ethics
Harvesting ovarian tissues will raise a wide range of ethical issues which include the available assisted techniques, safety of the tissue harvesting, subsequent use, possible implications for the progeny, as well as some cases that might be posed by special religious beliefs or cultural values that preclude assisted reproductive techniques [70]. When treating cancer survivors, the most ethical consideration is whether assisted reproduction techniques pose risks to the potential children [88], especially whether offsprings are themselves at increased risk of cancer (a hereditary cancer risk should not be regarded). Some large registry studies give us encouragement; they revealed there is no clear increased risk to cancers, genetic abnormalities, or birth defects in the children of cancer survivors [9]. There are some ethicists suggested it unethical to enable reproduction for cancer survivors whose lifetime may be reduced by diseases, for it unfairly makes a child bereft of a parent [88]. However, the Ethics Committee of the American Society for Reproductive Medicine concludes the concern is an insufficient argument to deny cancer patients assistance in reproducing [88]. Quite different from fertility preservation in adult females, there are special ethical issues in pediatric and adolescent patients [89]. Fertility preservation in a minor is only allowed: firstly, when appropriate permission of a parent and assent of a child with the ability to understand are both obtained; secondly, assisted procedures are justified by the anticipated benefit which is at least as favorable as that provided by alternatives [89]. Regardless of whether the patient lives or dies, the disposition of gametes must be considered [88]. Oocytes and ovarian tissue are the sole possessions of a person from whom they were removed, however, an embryo could belong to both parties, and the embryo should not be used for reproduction unless they both agree [90]. If the patient dies, an extreme controversial issue is the use of her gametes, and this issue is acceptable only if the decision is made by the surviving partner and the written permission given by the deceased [91].
Conclusions
Unlike sperm production may recover, the loss of oocytes is permanent [92]. Fertility preservation is often available but ignored in the female undergoing cancer treatment [5,9]. Embryo cryopreservation is considered standard practice, other methods such as ovarian suppression, ovarian transposition and cryopreservation of oocytes and ovarian tissue should be considered investigational, they could only be performed in specific centers [6,9]. The fertility preservation approaches vary depending on the patient's age, the type of cancer, the time available and the risk of ovarian metastasis [6]. At present, the primary purpose of medical intervention on malignancy is to cure the diseases, however, the generally trend is that oncologists should pay attention to the damage of reproductive system and consider fertility preservation options to help the patients preserve fertility during treatment.
Acknowledgements
This work was supported by a grant from the Ministry of Science and Technology of China (No. 2012BAI32B05).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10-29
2. Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011. CA Cancer J Clin. 2011;61(4):212-36
3. Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics, 2003. CA Cancer J Clin. 2003;53(1):5-26
4. Siegel R, Desantis C, Virgo K, Stein K, Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, Lin C, Leach C, Cannady RS, Cho H, Scoppa S, Hachey M, Kirch R, Jemal A, Ward E. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62(4):220-41
5. Jensen JR, Morbeck DE. Fertility Preservation. Mayo Clin Proc. 2011;86(1):45-49
6. Sonmezer M, Oktay K. Fertility preservation in female patients. Hum Reprod Update. 2004;10:251-266
7. Fallat ME, Hutter J. Preservation of fertility in pediatric and adolescent patients with cancer. Pediatrics. 2008;121(5):1461-9
8. Jacqueline SJ, Teresa KW. Preservation of Fertility in Patients with Cancer. N Engl J Med. 2009;360(9):902-911
9. Lee SJ, Schover LR, Partridge AH. et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24(18):2917-2931
10. Noyes N, Knopman JM, Long K, Coletta JM, Abu-Rustum NR. Fertility considerations in the management of gynecologic malignancies. Gynecol Oncol. 2011;120(3):326-33
11. Jahnukainen K, Ehmcke J, Soder O, Schlatt S. Clinical potential and putative risks of fertility preservation in children utilizing gonadal tissue or germline stem cells. Pediatr Res. 2006;59(2):40-47
12. Anchan RM, Ginsburg ES. Fertility concerns and preservation in younger women with breast cancer. Crit Rev Oncol Hematol. 2010;74(3):175-92
13. Lobo RA. Potential options for preservation of fertility in women. N Engl J Med. 2005;353(1):64-73
14. Derman SG, Seifer DB. In vitro fertilization in the older patient. Curr Womens Health Rep. 2003;3(5):375-83
15. Lobo RA. Menopause and aging. Strauss JF3rd, Barbieri RL, editors. Yen & Jaffe's reproductive endocrinology. Philadelphia, PA: Saunders, Elsevier Inc. 2004:421-52
16. Bendsen E, Byskov AG, Andersen CY, Westergaard LG. Number of germ cells and somatic cells in human fetal ovaries during the first weeks after sex differentiation. Hum Reprod. 2006;21(1):30-5
17. Faddy MJ, Gosden RG, Gougeon A, Richardson SJ, Nelson JF. Accelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopause. Hum Reprod. 1992;7(10):1342-6
18. Gosden R, Nagano M. Preservation of fertility in nature and ART. Reproduction. 2002;123(1):3-11
19. McLaren A, Southee D. Entry of mouse embryonic germ cells into meiosis. Dev Biol. 1997;187(1):107-113
20. Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev. 1996;17(2):121-55
21. Gougeon A. Ovarian follicular growth in humans: ovarian ageing and population of growing follicles. Maturitas. 1998;30(2):137-42
22. Gougeon A. The biological aspects of risks of infertility due to age: the female side. Rev Epidemiol Sante Publique. 2005;2:2S37-45
23. Hubner K, Fuhrmann G, Christenson LK. et al. Derivation of oocytes from mouse embryonic stem cells. Science. 2003;300(5623):1251-1256
24. Johnson J, Canning J, Kaneko T, Pru JK, Tilly JL. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature. 2004;428(6979):145-50
25. Johnson J, Bagley J, Skaznik-Wikiel M. et al. Oocyte generation in adult mammalian ovaries by putative germ cells in bone marrow and peripheral blood. Cell. 2005;122(2):303-315
26. Telfer EE, Gosden RG, Byskov AG. et al. On regenerating the ovary and generating controversy. Cell. 2005;122(6):821-822
27. Tilly JL, Niikura Y, Rueda BR. The current status of evidence for and against postnatal oogenesis in mammals: a case of ovarian optimism versus pessimism. Biol Reprod. 2009;80(1):2-12
28. Oktay K, Kan MT, Rosenwaks Z. Recent progress in oocyte and ovarian tissue cryopreservation and transplantation. Curr Opin Obstet Gynecol. 2001;13:263-268
29. Warne GL, Fairley KF, Hobbs JB, Martin FI. Cyclophosphamide induced ovarian failure. N Engl J Med. 1973;289:1159-1162
30. Koyama H, Wada T, Nishizawa Y, Iwanaga T, Aoki Y. Cyclophosphamide-induced ovarian failure and its therapeutic significance in patients with breast cancer. Cancer. 1977;39:1403-1409
31. Fisher B, Sherman B, Rockette H, Redmond C, Margolese R, Fisher ER. 1-phenylalanine mustard (L-PAM) in the management of premenopausal patients with primary breast cancer: lack of association of disease-free survival with depression of ovarian function. Cancer. 1979;44:847-857
32. Viviani S, Santoro A, Ragni G, Bonfante V, Bestetti O, Bonadonna G. Gonadal toxicity after combination chemotherapy for Hodgkin's disease. Eur J Cancer Clin Oncol. 1985;21:601-605
33. Mackie EJ, Radford M, Shalet SM. Gonadal function following chemotherapy for childhood Hodgkin's disease. Med Pediatr Oncol. 1996;27:74-78
34. Blumenfeld Z, Shapiro D, Shteinberg M, Avivi I, Nahir M. Preservation of fertility and ovarian function and minimizing gonadotoxicity in young women with systemic lupus erythematosus treated by chemotherapy. Lupus. 2000;9:401-405
35. Meirow D, Nugent D. The effects of radiotherapy and chemotherapy on female reproduction. Hum Reprod Update. 2001;7:535-543
36. Kenney LB, Laufer MR, Grant FD, Grier H, Diller L. High risk of infertility and long term gonadal damage in males treated with high dose cyclophosphamide for sarcoma during childhood. Cancer. 2001;91:613-621
37. Meirow D, Lewis H, Nugent D, Epstein M. Subclinical depletion of primordial follicular reserve in mice treated with cyclophosphamide: clinical importance and proposed accurate investigative tool. Hum Reprod. 1999;14:1903-1907
38. Jemal A, Siegel R, Ward E. et al. Cancer statistics. CA Cancer J Clin. 2008;58:71-96
39. Tauchmanova L, Selleri C, Rosa GD, Pagano L, Orio F, Lombardi G, Rotoli B, Colao A. High prevalence of endocrine dysfunction in long- term survivors after allogeneic bone marrow transplantation for hematologic diseases. Cancer. 2002;95:1076-1084
40. Goodwin P, Ennis M, Pritchard K, Trudeau M, Hood N. Risk of menopause during the first year after breast cancer diagnosis. J Clin Oncol. 1999;17:2365-9
41. Tease C, Fisher G. The influence of maternal age on radiation induced chromosome aberrations in mouse oocytes. Mutat Res. 1991;262:57-62
42. Hawkins MM, Smith RA. Pregnancy outcomes in childhood cancer survivors: probable effects of abdominal irradiation. Int J Cancer. 1989;43:399-402
43. Critchley HO, Bath LE, Wallace WH. Radiation damage to the uterus—review of the effects of treatment of childhood cancer. Hum Fertil (Camb). 2002;5:61-6
44. Covens A, Shaw P, Murphy J. et al. Is radical trachelectomy a safe alternative to radical hysterectomy for patients with stage IA-B carcinoma of the cervix? Cancer. 1999;86:2273-2279
45. Shusterman S, Meadows AT. Long term survivors of childhood leukemia. Curr Opin Hematol. 2000;7:217-222
46. Zanetta G, Bonazzi C, Cantu M. et al. Survival and reproductive function after treatment of malignant germ cell ovarian tumors. J Clin Oncol. 2001;19:1015-1020
47. Wallace W, Thomson A. Preservation of fertility in children treated for cancer. Arch Dis Child. 2003;88:493-496
48. Ataya K, Rao LV, Lawrence E, Kimmel R. Luteinizing hormonereleasing hormone agonist inhibits cyclophosphamide-induced ovarian follicular depletion in rhesus monkeys. Biol Reprod. 1995;52:365-372
49. Ataya K, Pydyn E, Ramahi-Ataya A, Orton CG. Is radiation induced ovarian failure in rhesus monkeys preventable by luteinizing hormone-releasing hormone agonists: Preliminary observations. J Clin Endocrinol Metab. 1995;8:790-795
50. Waxman JH, Ahmed R, Smith D. et al. Failure to preserve fertility in patients with Hodgkin's disease. Cancer Chemother Pharmacol. 1987;19:159-162
51. Blumenfeld Z, Avivi I, Linn S. et al. Prevention of irreversible chemotherapy-induced ovarian damage in young women with lymphoma by a gonadotrophin-releasing hormone agonist in parallel to chemotherapy. Hum Reprod. 1996;11:1620-1626
52. Oktay K, Sönmezer M, Oktem O, Fox K, Emons G, Bang H. Absence of conclusive evidence for the safety and efficacy of gonadotropin-releasing hormone analogue treatment in protecting against chemotherapy-induced gonadal injury. Oncologist. 2007;12(9):1055-66
53. Clough KB, Goffinet F, Labib A. et al. Laparoscopic unilateral ovarian transposition prior to irradiation: Prospective study of 20 cases. Cancer. 1996;77:2638-2645
54. Covens AL, van der Putten HW, Fyles AW. et al. Laparoscopic ovarian transposition. Eur J Gynaecol Oncol. 1996;17:177-182
55. Visvanathan DK, Cutner AS, Cassoni AM. et al. A new technique of laparoscopic ovariopexy before irradiation. Fertil Steril. 2003;79:1204-1206
56. Zinger M, Liu JH, Husseinzadeh N. et al. Successful surrogate pregnancy after ovarian transposition, pelvic irradiation and hysterectomy. J Reprod Med. 2004;49:573-574
57. West ER, Zelinski MB, Kondapalli LA. et al. Preserving female fertility following cancer treatment: current options and future possibilities. Pediatr Blood Cancer. 2009;53(2):289-295
58. Stern CJ, Toledo MG, Gook DA. et al. Fertility preservation in female oncology patients. Aust NZ J Obstet Gynaecol. 2006;46:15-23
59. Pena JE, Chang PL, Chan LK. et al. Supraphysiological estradiol levels do not affect oocyte and embryo quality in oocyte donation cycles. Hum Reprod. 2002;17:83-87
60. Senn A, Vozzi C, Chanson A, De Grandi P, Germond M. Prospective randomized study of two cryopreservation policies avoiding embryo selection: the pronucleate stage leads to a higher cumulative delivery rate than the early cleavage stage. Fertil Steril. 2000;74:946-952
61. Wang JX, Yap YY, Matthews CD. Frozen-thawed embryo transfer: influence of clinical factors on implantation rate and risk of multiple conception. Hum Reprod. 2001;16:2316-2319
62. Son WY, Yoon SH, Yoon HJ, Lee SM, Lim JH. Pregnancy outcome following transfer of human blastocysts vitrified on electron microscopy grids after induced collapse of the blastocoele. Hum Reprod. 2003;18:137-139
63. Baka SG, Toth TL, Veeck LL. et al. Evaluation of the spindle apparatus of in-vitro matured human oocytes following cryopreservation. Hum Reprod. 1995;10:1816-1820
64. Gosden RG. Prospects for oocyte banking and in vitro maturation. J Natl Cancer Inst Monogr. 2005;34:60-63
65. Kuwayama M, Vajta G, Kato O. et al. Highly efficient vitrification method for cryopreservation of human oocytes. Reprod Biomed Online. 2005;11:300-308
66. Borini A, Sciajno R, Bianchi V. et al. Clinical outcome of oocyte cryopreservation after slow cooling with a protocol utilizing a high sucrose concentration. Hum Reprod. 2006;21:512-517
67. Son WY, Tan SL. Laboratory and embryological aspects of hCG-primed in vitro maturation cycles for patients with polycystic ovaries. Hum Reprod Update. 2010;16(6):675-89
68. Tulandi T, Huang JY, Tan SL. Preservation of female fertility: an essential progress. Obstet Gynecol. 2008;112(5):1160-72
69. Ata B, Shalom-Paz E, Chian RC, Tan SL. In vitro maturation of oocytes as a strategy for fertility preservation. Clin Obstet Gynecol. 2010;53(4):775-86
70. Shah DK, Goldman E, Fisseha S. Medical, ethical, and legal considerations in fertility preservation. Int J Gynaecol Obstet. 2011;115(1):11-5
71. Chian RC, Huang JY, Gilbert L, Son WY, Holzer H, Cui SJ. et al. Obstetric outcomes following vitrification of in vitro and in vivo matured oocytes. Fertil Steril. 2009;91(6):2391-8
72. Donnez J, Silber S, Andersen CY, Demeestere I, Piver P, Meirow D, Pellicer A, Dolmans MM. Children born after autotransplantation of cryopreserved ovarian tissue. a review of 13 live births. Ann Med. 2011;43(6):437-50
73. Friedman O, Orvieto R, Fisch B, Felz C, Freud E, Ben-Haroush A, Abir R. Possible improvements in human ovarian grafting by various host and graft treatments. Hum Reprod. 2012;27(2):474-82
74. Abir R, Fisch B, Jessel S, Felz C, Ben-Haroush A, Orvieto R. Improving posttransplantation survival of human ovarian tissue by treating the host and graft. Fertil Steril. 2011;95(4):1205-10
75. Dolmans MM, Marinescu C, Saussoy P, Van Langendonckt A, Amorim C, Donnez J. Reimplantation of cryopreserved ovarian tissue from patients with acute lymphoblastic leukemia is potentially unsafe. Blood. 2010;116(16):2908-14
76. Bittinger SE, Nazaretian SP, Gook DA, Parmar C, Harrup RA, Stern CJ. Detection of Hodgkin lymphoma within ovarian tissue. Fertil Steril. 2011;95(2):803.e3-6
77. Meirow D, Hardan I, Dor J, Fridman E, Elizur S, Ra'anani H, Slyusarevsky E, Amariglio N, Schiff E, Rechavi G, Nagler A, Ben Yehuda D. Searching for evidence of disease and malignant cell contamination in ovarian tissue stored from hematologic cancer patients. Hum Reprod. 2008;23(5):1007-13
78. Kyono K, Doshida M, Toya M, Sato Y, Akahira J, Sasano H. Potential indications for ovarian autotransplantation based on the analysis of 5,571 autopsy findings of females under the age of 40 in Japan. Fertil Steril. 2010May1;93(7):2429-30
79. Latham KE, Wigglesworth K, McMenamin M, Eppig JJ. Stage-dependent effects of oocytes and growth differentiation factor 9 on mouse granulosa cell development: advance programming and subsequent control of the transition from preantral secondary follicles to early antral tertiary follicles. Biol Reprod. 2004;70(5):1253-62
80. O'Brien MJ, Pendola JK, Eppig JJ. A revised protocol for in vitro development of mouse oocytes from primordial follicles dramatically improves their developmental competence. Biol Reprod. 2003;68(5):1682-6
81. Hawkins MM, Draper GJ, Smith RA. Cancer among 1,348 offspring of survivors of childhood cancer. Int J Cancer. 1989;43(6):975-978
82. Schover LR, Agarwal A, Thomas AJ Jr. Cryopreservation of gametes in young patients with cancer. J Pediatr Hematol Oncol. 1998;20(5):426-428
83. Ethics Committee of the American Society for Reproductive Medicine. Fertility preservation and reproduction in cancer patients. Fertil Steril. 2005;83(6):1622-8
84. Boldt J. Current results with slow freezing and vitrification of the human oocyte. Reprod Biomed Online. 2011;23(3):314-22
85. Domar AD, Clapp D, Slawsby E. et al. The impact of group psychological interventions on distress in infertile women. Health Psychol. 2000;19:568-575
86. McQueeney DA, Stanton AL, Sigmon S. Efficacy of emotion-focused and problem-focused group therapies for women with fertility problems. J Behav Med. 1997;20:313-331
87. Stewart DE, Boydell KM, McCarthy K. A prospective study of the effectiveness of brief professionally-led support groups for infertility patients. Int J Psychiatry Med. 1992;22:173-182
88. Ethics Committee of the American Society for Reproductive Medicine. Fertility preservation and reproduction in cancer patients. Fertil Steril. 2005;83(6):1622-8
89. Committee on Bioethics, American Academy of Pediatrics. Informed consent, parental permission, and assent in pediatric practice. Pediatrics. 1995;95(2):314-7
90. Davis v. Davis, 842 S.W.2d 588, 597, 1992.
91. Pennings G, de Wert G, Shenfield F, Cohen J, Devroey P. ESHRE Task Force on Ethics and Law 11: Posthumous assisted reproduction. Hum Reprod. 2006;21(12):3050-3
92. Chuai Y, Gao F, Li B, Zhao L, Qian L, Cao F, Wang L, Sun X, Cui J, Cai J. Hydrogen-rich saline attenuates radiation-induced male germ cell loss in mice through reducing hydroxyl radicals. Biochem J. 2012;442:49-56
Author contact
![]() Corresponding author: Dr. Aiming Wang, Department of Obstetrics and Gynecology, Navy General Hospital, Fucheng Road, 100048, Beijing, PR China. Tel: +86-13381207220. Fax: +86-21-81871101. E-mail: one_armycom.
Corresponding author: Dr. Aiming Wang, Department of Obstetrics and Gynecology, Navy General Hospital, Fucheng Road, 100048, Beijing, PR China. Tel: +86-13381207220. Fax: +86-21-81871101. E-mail: one_armycom.

 Global reach, higher impact
Global reach, higher impact