Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2012; 8(8):1168-1177. doi:10.7150/ijbs.4778 This issue Cite
Research Paper
High Level of COP1 Expression is Associated with Poor Prognosis in Primary Gastric Cancer
1. State Key Laboratory of Oncology in South China and Department of Experimental Research, Cancer Center, Sun Yat-sen University, Guangzhou, People's Republic of China;
2. Department of Gastric and Pancreatic Surgery, Cancer Center, Sun Yat-sen University, Guangzhou, People's Republic of China.
# These authors contributed equally to this work.
Received 2012-6-25; Accepted 2012-9-18; Published 2012-10-2
Abstract
COP1 (constitutive photomorphogenic 1, also known as RFWD2) is a p53-targeting E3 ubiquitin ligase containing RING-finger, coiled-coil, and WD40-repeat domains. Recent studies have identified that COP1 is overexpressed in several cancer types and that increased COP1 expression promotes cell proliferation, cell transformation, and tumor progression. In the present study, we investigated the expression and prognostic value of COP1 in primary gastric cancer. To investigate the role of the COP1 gene in primary gastric cancer pathogenesis, real-time quantitative PCR and western blotting were performed to examine COP1 expression in paired cancerous and matched adjacent noncancerous gastric tissues. The results revealed high COP1 mRNA (P=0.030) and protein (P=0.008) expression in most tumor-bearing tissues compared with the matched adjacent non-tumor tissues. The correlated protein expression analysis revealed a negative correlation between COP1 and p53 in gastric cancer samples (P=0.005, r=-0.572). Immunohistochemical staining of gastric cancer tissues from the same patient showed a high COP1 expression and a low p53 expression. To further investigate the clinicopathological and prognostic roles of COP1 expression, we performed immunohistochemical analysis of 401 paraffin-embedded gastric cancer tissue blocks. The data revealed that high COP1 expression was significantly correlated with T stage (P=0.030), M stage (P=0.048) and TNM stage (P=0.022). Consistent with these results, we found that high expression of COP1 was significantly correlated with poor survival in gastric cancer patients (P<0.001). Cox regression analyses showed that COP1 expression was an independent predictor of overall survival (P<0.001). Our data suggest that COP1 could play an important role in gastric cancer and might serve as a valuable prognostic marker and potential target for gene therapy in the treatment of gastric cancer.
Keywords: COP1, gastric cancer, expression, immunohistochemistry, prognosis.
Introduction
Gastric cancer is the fourth most common malignant tumor worldwide and the second leading cause of cancer-related deaths each year (10.4% of cancer deaths) [1]. More new cases of gastric cancer are diagnosed in China each year than in any other country [2]. The treatment for gastric cancer includes a combination of surgery, chemotherapy, and radiation therapy. However, nearly 60% of patients succumb to gastric cancer after a curative resection alone or after adjuvant therapy [3]. It has long been known that gastric cancer is a multifactorial and multistep disease that involves the activation of oncogenes and the inactivation of tumor suppressor genes at different stages of gastric cancer progression [4, 5]. Genetic and genomic variations occurring in the genes and molecules that participate in proliferation, invasion and metastasis might influence the prognoses of patients with gastric cancer [6-10]. Understanding these genetic and genomic alterations and the molecular mechanisms involved in gastric carcinogenesis will be critical for the improvement of the diagnosis, therapy and prognostic prediction of this disease.
COP1 (constitutive photomorphogenic 1, also known as RFWD2) is a p53-targeting E3 ubiquitin ligase, containing RING-finger, coiled-coil, and WD40-repeat domains [11-13]. COP1 has been identified as a novel and critical negative regulator of the tumor suppressor protein p53, and it influences p53-dependent transcription, apoptosis, cell cycle arrest and DNA repair [6, 14]. Recently, COP1 has been found to be overexpressed in human hepatocellular carcinoma, breast cancer and ovarian adenocarcinomas [15, 16]. These observations indicate that COP1 could be a promising novel target for systemic therapy in cancer. On the other hand, some studies showed a tumor suppressor role of COP1 in mouse models [17, 18]. Vitari et al revealed that COP1 functions as a tumor suppressor whose downregulation promotes prostatic epithelial cell proliferation and tumorigenesis in an animal study. [17]. Another study by Migliorini et al demonstrated that COP1 functions, at least in part, by antagonizing c-Jun oncogenic activity [18]. Thus, COP1 expression and its functional role in different kinds of tumors remain controversial. Moreover, some mechanistic studies revealed interactive molecules of COP1 and its functional role in the DNA damage, c-Jun signaling passway, and ubiquitin-mediated degradations [11, 19-21]. These studies indicated the possible mechanism of COP1 in tumorigenesis and tumor progression. However, the role of COP1 in gastric cancer is unclear. Therefore, in the present study, we evaluated COP1 expression and its prognostic value in primary gastric adenocarcinomas.
In the present study, we aimed to analyze COP1 expression levels in gastric cancer using real-time quantitative RT-PCR (reverse transcription polymerase chain reaction), western blotting and immunohistochemistry. We investigated the correlated protein expression of COP1 and p53 in gastric cancer samples. Furthermore, for the first time, we identified the relationship between COP1 expression and clinicopathological features and evaluated its prognostic value for post-resection survival in gastric cancer.
Materials and Methods
Ethics statement
The research was approved by the Ethics Committee of Sun Yat-sen University Cancer Center, and written informed consent was obtained from each patient involved in the study.
Human tissue samples
A total of 40 paired cancerous and matched adjacent normal gastric mucosa tissues were collected from gastric cancer patients undergoing gastrectomy at Sun Yat-sen University Cancer Center between 2009 and 2011. After surgical resection, fresh tissues were immediately immersed in RNAlater (Ambion, Inc., USA) to avoid RNA degradation, were stored at 4°C overnight to allow thorough penetration of RNAlater into the tissue and then were frozen at -80°C until processed for RNA and protein extraction. Another 401 paraffin-embedded primary gastric cancer samples, which had been collected between 2000 and 2006, were obtained from the Sun Yat-sen University Cancer Center. None of these patients had received radiotherapy or chemotherapy prior to surgery. The histopathological type and the stage of gastric cancer were determined according to the criteria of the World Health Organization classification. All of the patients were staged using the 7th edition of the International Union Against Cancer (UICC) Tumor-Node-Metastasis (TNM) staging system. Gastric adenocarcinoma patients have been staged into four grades based on the percentage of glandular texture in the tumor tissues: well differentiated (percentage of glandular texture > 95%), moderately differentiated (percentage of glandular texture between 50% and 95%), poorly differentiated (percentage of glandular texture between 5% and 50%) and undifferentiated (percentage of glandular texture <5%) [22].
Extraction of total RNA and real-time quantitative RT-PCR
Total RNA was extracted using TRIzol (Invitrogen, USA) according to the manufacturer's instructions. Total RNA concentration was assessed by measuring absorbance at 260 nm using a NANO DROP spectrophotometer (ND-1000, Thermo Scientific, USA). Reverse transcription (RT) to synthesize the first strand of cDNA was performed in a 20-μl reaction system with 2 μg of total RNA treated with M-MLV reverse transcriptase (Promega, USA) according to the manufacturer's protocol. The resulting cDNA was then subjected to real-time quantitative PCR for the evaluation of the relative mRNA levels of COP1 and GAPDH (glyceraldehyde-3-phosphate dehydrogenase, as an internal control) with the following primers: COP1 forward: 5′-AGAATACAGCCAACCTCCAG-3′ and reverse: 5′-TCCACTGCATCCTGGATGAC-3′; GAPDH forward: 5′-CTCCTCCTGTTCGACAGTCAGC-3′ and reverse: 5′-CCCAATACGACCAAATCCGTT-3′. Gene-specific amplification was performed using an ABI 7900HT real-time PCR system (Life Technologies, Carlsbad, California, USA) with a 15-μl PCR mix containing 0.5 μl of cDNA, 7.5 μl of 2× SYBR Green master mix (Invitrogen, Carlsbad, California, USA), and 200 nM of the appropriate oligonucleotide primers. The mix was preheated at 95°C (10 min) and then was amplified at 95°C (30 sec) and 55°C (1 min) for 45 cycles. The resolution curve was measured at 95°C for 15 sec, 55°C for 15 sec and 95°C for 15 sec. The Ct (threshold cycle) value of each sample was calculated from the threshold cycles with the instrument's software (SDS 2.3), and the relative expression of COP1 mRNA was normalized to the GAPDH value. The data were analyzed using the comparative threshold cycle (2-ΔCT) method.
Western blotting analysis
The homogenized gastric cancer samples, including cancerous and noncancerous tissues, were lysed in RIPA lysis buffer, and the lysates were harvested by centrifugation (12,000 rpm) at 4°C for 30 min. Protein samples of approximately 20 μg were then separated by electrophoresis in a 12% sodium dodecyl sulfate polyacrylamide gel and were transferred onto a polyvinylidene fluoride membrane. After blocking the non-specific binding sites for 60 min with 5% non-fat milk, the membranes were incubated overnight at 4°C with a mouse monoclonal antibody against COP1 (Santa Cruz Biotechnology, USA; at a 1:1,000 dilution). The membranes were then washed three times with TBST (tris-buffered saline with tween-20) for 10 min and were probed with the horseradish peroxidase (HRP)-conjugated rabbit anti-mouse IgG antibody (Immunology Consultants Laboratory, USA, at a 1:2000 dilution) at room temperature for 1 hour. After three washes with TBST, the membranes were developed using an enhanced chemiluminescence system (Cell Signaling Technology, USA). p53 protein expression was detected as described above by using rabbit anti-human p53 polyclonal antibody (Proteintech group, USA, at a 1:1000 dilution) and HRP-conjugated goat anti-rabbit IgG antibody (Cell Signaling Technology, USA, at a 1:1000 dilution). The band intensity was measured by densitometry using Quantity One software (Bio-Rad Laboratories, USA). The COP1 and p53 protein levels were normalized to the level of GAPDH detected using mouse anti-human GAPDH monoclonal antibody (Shanghai Kangchen, China, at a 1:10,000 dilution).
Immunohistochemistry analysis
The tissue sections were deparaffinized with dimethylbenzene and rehydrated with 100%, 95%, 90%, 80% and 70% ethanol. After being washed three times in PBS (phosphate-buffered saline), the slides were boiled in antigen retrieval buffer containing 0.01 M sodium citrate-hydrochloric acid (pH=6.0) for 15 min in a microwave oven for antigen retrieval. After rinsing with PBS, the tissue sections were incubated with primary antibody, and the slides were then rinsed in 3% peroxidase quenching solution (Invitrogen) to block endogenous peroxidase. The sections were then incubated with a mouse monoclonal antibody against COP1 (Santa Cruz Biotechnology, USA; at a 1:300 dilution) at 4°C overnight and then were incubated with horseradish peroxidase (HRP) (ChemMateTM DAKO EnVisionTM Detection Kit) at room temperature for 30 min. After washing in PBS, the visualization signal was developed with 3, 3'-diaminobenzidine (DAB) solution, and all of the slides were counterstained with hematoxylin. As negative controls, tissue sections were processed under the same experimental conditions described above, except that they were incubated overnight at 4°C in blocking solution without the anti-COP1 antibody.
The total COP1 immunostaining score was calculated as the sum of the percentage of positively stained tumor cells and the staining intensity. The percentage of positive staining was scored as 0 (0%-9%, negative), 1 (10%-25%, sporadic), 2 (26%-50%, focal) or 3 (51%-100%, diffuse). The immunostaining intensity was determined as 0 (no staining), 1 (faint yellow, weak staining), 2 (claybank, moderate staining) or 3 (sepia, strong staining) under high magnification microscopy [23]. The total immunostaining score was calculated as the value of percent positivity score × staining intensity score and ranged from 0 to 9. The expression level of COP1 was defined as follows: “-” (negative, score 0), “+” (weakly positive, score 1-3), “++” (positive, score 4-6), or “+++” (strongly positive, score 7-9). Based on COP1 expression levels, the 401 gastric cancer patients were divided into two groups: low COP1 expression group (COP1 “-” and COP1 “+”) and high COP1 expression group (COP1 “++” and COP1 “+++”). The result assessment was performed independently by two pathologists blinded to the clinical parameters.
Statistical analysis
Differences in mRNA and protein expression between the gastric cancer samples and the paired adjacent noncancerous tissue samples were evaluated with a paired-samples t-test. P value less than 0.05 was deemed statistically significant. The relationships between COP1 expression and various clinicopathological parameters were tested using the χ2 test or Kruskal Wallis test. Survival curves were calculated using the Kaplan-Meier method and were compared with the log-rank test. The Cox proportional hazard regression model was used for univariate and multivariate analyses to explore the effects of the clinicopathological variables and the COP1 expression on survival. The statistical analyses were performed with the Statistical Package for the Social Sciences, version 17.0 (IBM SPSS, USA), and a two-sided P value less than 0.05 was considered to be statistically significant.
Results
COP1 mRNA expression analyzed by real-time quantitative RT-PCR
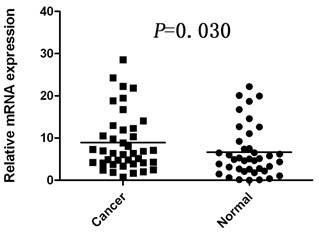
The mRNA level of COP1 was determined by real-time quantitative RT-PCR assays in 40 paired cancerous and matched adjacent noncancerous gastric tissues. The COP1 expression level was significantly higher in the 25 (62.5%) tumor-bearing tissues, compared with the adjacent non-tumor tissues (P=0.030, Figure 1).
Increased mRNA expression of COP1 in gastric cancer tissues as assessed by real time quantitative RT-PCR (n=40, P=0.030). Horizontal lines represent the mean.
COP1 protein expression analyzed by western blotting
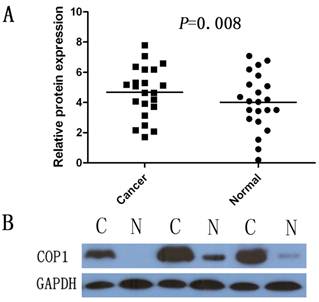
Western blotting was performed on 22 gastric cancer specimens and corresponding, adjacent, non-cancerous gastric mucosa tissues from the 40 paired samples. The results showed a COP1 band with the expected size of 90 kDa, and the amount of COP1 protein present was measured further by densitometry. Consistent with the real-time quantitative RT-PCR results, an increase in COP1 protein expression was observed in 12 (54.5%) of the gastric tumor tissues, compared with the matched adjacent non-tumor tissues (P=0.008, Figure 2A and Figure 2B).
Increased protein expression of COP1 in gastric cancer as assessed by Western blotting. (A) Relative COP1 protein expression levels in gastric cancer tissues and noncancerous tissues (COP1/GAPDH, n=22, P=0.008). Horizontal lines represent the mean. (B) Representative result of COP1 protein expression in 3 paired gastric tumorous and the matched adjacent nontumorous tissues (C, gastric cancer tissues; N, matched noncancerous gastric mucosa).
Correlated protein expression of COP1 and p53 in gastric cancer samples
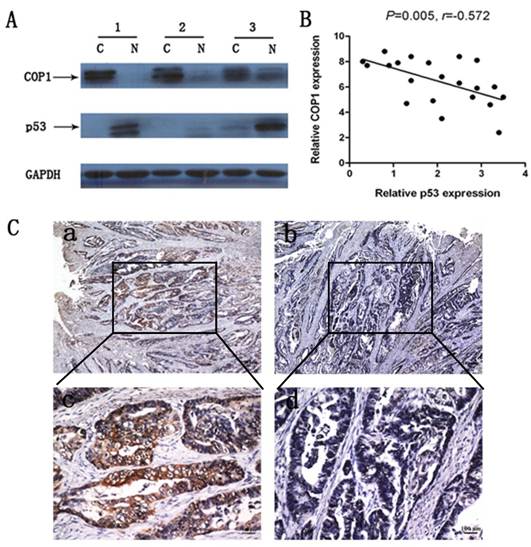
We examined COP1 and p53 protein expression in 22 cases of primary gastric cancer samples. Results revealed a negative correlation between COP1 and p53 protein expression in these gastric cancer samples (Figure 3A and Figure 3B, P=0.005, r=-0.572). Moreover, immunohistochemical staining of gastric cancer tissues from the same patient revealed that COP1 showed a high expression while p53 showed a low expression (Figure 3C).
Correlated protein expression of COP1 and p53 in gastric cancer samples. (A) Representative result of COP1 and p53 protein expression in 3 paired gastric tumors and the matched adjacent nontumorous tissues (C, gastric cancer tissues; N, matched noncancerous gastric mucosa). (B) Correlated protein expression of COP1 and p53 in 22 cases of primary gastric cancer samples. Data revealed a negative correlation between COP1 and p53 protein expression in the relevant gastric cancer samples (P=0.005, r=-0.572). (C) Immunohistochemical staining of gastric cancer tissues from the same patient showed a high COP1 expression and a low p53 expression. a: Immunohistochemical staining of COP1 (40×magnification); b: Immunohistochemical staining of p53 (40×magnification); c: Immunohistochemical staining of COP1 (200×magnification); d: Immunohistochemical staining of p53 (200×magnification).
Immunohistochemical analysis of COP1 expression in clinical samples and its relationship with clinicopathological parameters
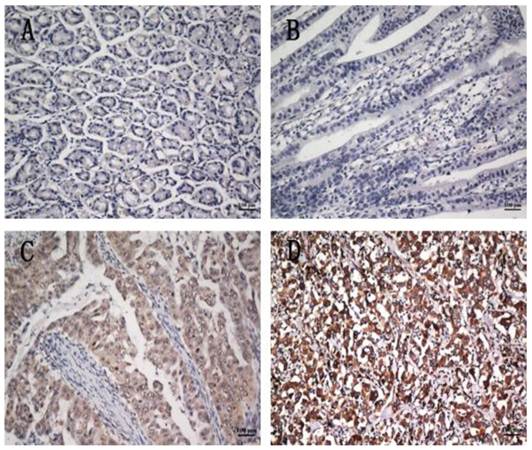
To further investigate the clinicopathological and prognostic roles of COP1 expression, we performed immunohistochemical analyses of the 401 paraffin-embedded gastric cancer tissue blocks. In total, 193 of 401 (48.1%) cases showed low COP1 expression in cancerous tissues (Figure 4B), whereas 208 (51.8%) cases showed high COP1 expression (Figure 4C and D). COP1 protein expression was positive staining in many gastric cancer tissues, and the results showed enhanced COP1 immunoreactivity in poorly differentiated gastric cancer tissues, compared to normal gastric tissues (Figure 4A). The correlations between the expression of COP1 and various clinicopathological parameters are listed in Table 1. The results showed that increased expression of COP1 was significantly correlated with the depth of tumor infiltration (T stage, P=0.030), M stage (P=0.048) and with TNM stage (P=0.022), whereas not with age, gender, tumor size, and tumor locus or local lymph node metastasis (N stage).
COP1 protein expression in gastric cancer surgical specimens shown by immunohistochemistry. (A) Weak COP1 staining was observed in noncancerous gastric mucosa. (B) Weak COP1 staining was observed in well-differentiated gastric cancer. (C) Strong COP1 staining was observed in moderately differentiated gastric cancer. (D) Strong COP1 staining was observed in poorly differentiated gastric cancer. All of these four pictures were taken under the same magnification (200×).
Correlation between COP1 expression and clinicopathological variables of 401 gastric cancer cases
| Clinicopathological parameters | na | COP1 expression | P value | |
|---|---|---|---|---|
| High | Low | |||
| All | 401 | 208(51.9%) | 193(48.1%) | |
| Age (years) | 0.546 | |||
| <55 | 174 | 87(21.7%) | 87(21.7%) | |
| ≥55 | 227 | 121(30.2%) | 106(26.4%) | |
| Gender | 0.465 | |||
| Male | 261 | 139(34.7%) | 122(30.4%) | |
| Female | 140 | 69(17.2%) | 71(17.7%) | |
| Tumor size | 0.783 | |||
| <3 cm | 62 | 31(7.7%) | 31(7.7%) | |
| ≥3 cm | 339 | 177(44.2%) | 162(40.4%) | |
| Tumor infiltration | 0.030* | |||
| T1 | 47 | 27(6.7%) | 20(5.0%) | |
| T2 | 38 | 13(3.2%) | 25(6.2%) | |
| T3 | 41 | 16(4.0%) | 25(6.2%) | |
| T4a | 213 | 112(27.9%) | 101(25.2%) | |
| T4b | 62 | 40(10.0%) | 22(5.5%) | |
| Local lymph node metastasis | 0.081 | |||
| N0 | 133 | 64(16.0%) | 69(17.2%) | |
| N1 | 71 | 35(8.7%) | 36(9.0%) | |
| N2 | 71 | 34(8.5%) | 37(9.2%) | |
| N3 | 126 | 75(18.7%) | 51(12.7%) | |
| Distant metastasis | 0.048* | |||
| M0 | 357 | 179(44.6%) | 178(44.4%) | |
| M1 | 44 | 29(7.2%) | 15(3.7%) | |
| TNM staging | 0.022* | |||
| 1 | 52 | 31(7.7%) | 21(5.2%) | |
| 2 | 138 | 59(14.7%) | 79(19.7%) | |
| 3 | 164 | 87(21.7%) | 77(19.2%) | |
| 4 | 47 | 31(7.7%) | 16(4.0%) | |
a Numbers of cases in each group. * Statistically significant (P<0.05).
Cox proportional-hazard model analysis of COP1 and clinical outcomes
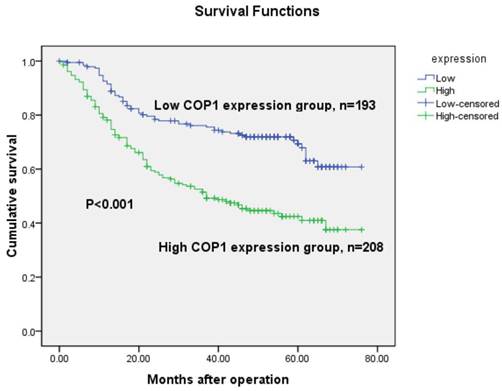
The 5-year overall survival rates in patients with increased and low COP1 expression were 48.1% and 71.0%, respectively. Consistent with the real-time quantitative RT-PCR and western blotting results, the overall survival of the patients with increased COP1 expression was shorter than that of the low COP1 expression patients (P<0.001, log-rank test, Figure 5). Univariate Cox regression analyses showed that depth of tumor infiltration, local lymph node metastasis, distant metastasis, TNM stage, tumor size and COP1 expression were significantly associated with overall survival (Table 2). Furthermore, a multivariate Cox regression analysis confirmed TNM stage (P<0.001) and COP1 (P<0.001) expression as independent predictors of the overall survival of gastric cancer patients (Table 2).
Kaplan-Meier survival curves of gastric cancer patients (n=401) after gastrectomy. The survival rate of patients in the COP1-high group was significantly lower than that of patients in the COP1-low group (log-rank test, P<0.001).
Univariate and multivariate analyses of overall survival of gastric cancer patients
| Variables | na | Univariate analyses | Multivariate analyses | ||||
|---|---|---|---|---|---|---|---|
| HR | (95% CI) | P value | HR | (95% CI) | P value | ||
| Age (years) | 0.101 | ||||||
| <55 | 174 | 1.000 | |||||
| ≥55 | 227 | 1.293 | 0.951-1.759 | ||||
| Gender | 0.991 | ||||||
| Female | 140 | 1.000 | |||||
| Male | 261 | 1.002 | 0.734-1.368 | ||||
| Tumor size | <0.001* | 0.627 | |||||
| <3 cm | 62 | 1.000 | 1.000 | ||||
| ≥3 cm | 339 | 4.419 | 2.172-8.990 | 1.196 | 0.564-2.537 | ||
| Tumor infiltration | <0.001* | 0.104 | |||||
| T1 | 47 | 1.000 | 1.000 | ||||
| T2 | 38 | 6.279 | 0.756-52.171 | 4.554 | 0.534-38.823 | ||
| T3 | 41 | 20.700 | 2.743-156.202 | 5.964 | 0.740-48.056 | ||
| T4a | 213 | 26.147 | 3.648-187.423 | 6.278 | 0.814-48.423 | ||
| T4b | 62 | 54.086 | 7.450-392.660 | 8.956 | 1.139-70.410 | ||
| Local lymph node metastasis | <0.001* | 0.927 | |||||
| N0 | 133 | 1.000 | 1.000 | ||||
| N1 | 71 | 2.537 | 1.525-4.220 | 0.934 | 0.519-1.680 | ||
| N2 | 71 | 2.588 | 1.544-4.338 | 0.938 | 0.515-1.711 | ||
| N3 | 126 | 4.425 | 2.857-6.854 | 1.052 | 0.603-1.837 | ||
| Distant metastasis | <0.001* | 0.736 | |||||
| M0 | 357 | 1.000 | 1.000 | ||||
| M1 | 44 | 4.556 | 3.121-6.651 | 1.142 | 0.528-2.470 | ||
| TNM staging | <0.001* | <0.001* | |||||
| 1 | 52 | 1.000 | 1.000 | ||||
| 2 | 138 | 14.387 | 1.971-105.030 | 6.912 | 0.862-55.396 | ||
| 3 | 164 | 40.292 | 5.616-289.072 | 16.184 | 1.974-132.656 | ||
| 4 | 47 | 115.650 | 15.876-842.473 | 35.159 | 3.848-320.385 | ||
| COP1 | <0.001* | <0.001* | |||||
| Low | 193 | 1.000 | 1.000 | ||||
| High | 208 | 2.274 | 1.654-3.126 | 2.175 | 1.571-3.012 | ||
HR, hazard ratio; CI, confidence interval; a Numbers of cases in each group; * Statistically significant (P < 0.05).
Discussion
Gastric cancer is one of the most deadly human carcinomas, and it has a dismal prognosis despite improved diagnosis and composite therapy [24, 25]. It has long been known that gastric cancer results from a combination of environmental factors and the accumulation of generalized and specific genetic alterations. Many of the genetic or epigenetic alterations associated with gastric cancer have been reported, including loss of heterozygosity, microsatellite and chromosomal instability and hypermethylation [3]. Therefore, identification of the gastric cancer-specific biomarkers involved in these procedures is very important for diagnosis, therapy and prognostic prediction in clinics.
Recently, COP1 overexpression has been found in many tumor types, including hepatocellular carcinoma, breast and ovarian adenocarcinomas [15, 16]. Furthermore, increased expression of COP1 has been found to be correlated strongly with these tumors [15, 16]. High expression of COP1 has been found to promote cell proliferation, cell transformation, and tumor progression, manifesting its role in cancer promotion [19]. These results suggest that COP1 might play an important role in promoting tumorigenesis or progression. However, thus far the expression and clinical significance of COP1 in primary gastric cancer have not been explored. Therefore, we evaluated the expression of COP1 in gastric cancer by real-time PCR, western blotting and immunohistochemistry, and analyzed its clinicopathological and prognostic significance in a large number of patient samples.
In the present study, we investigated COP1 mRNA expression in primary gastric cancer specimens by real-time quantitative RT-PCR, and protein expression in primary gastric cancer tissues by western blotting detection. Results showed that the COP1 mRNA and protein levels were significantly increased in 25 (62.5%) and 12 (54.5%) tumor tissue samples, respectively, compared with the levels in the adjacent non-tumor tissue samples (P=0.030 and 0.008, respectively), which was consistent with Lee's and Dornan's findings [15, 16]. Moreover, immunohistochemical analysis demonstrated high COP1 expression in 51.8% (208/401) cases of gastric cancer patients. These results support an earlier hypothesis that COP1 might be an oncogene by Dornan D et al [14, 15] and also suggest that COP1 might play an important role in the tumorigenesis or progression of gastric cancer.
Overexpression of COP1 has been found to contribute to the accelerated degradation of p53 protein in cancers and attenuates the tumor suppressor function of p53 [13]. p53 is a potent tumor-suppressor protein that is negatively regulated or mutated in some, if not all, cancers [25, 26]. p53 mutation rate was between 25% and 80% in different kinds of human cancers, and 41% in gastric cancer [27]. In a study by Lee et al, growth inhibition occurred in hepatocellular carcinoma cells that retained wild-type p53 or expressed mutant p53 (Y220C or R249S) after COP1 blockade by siRNA [16]. In the present study, we investigated correlated protein expression between COP1 and p53 in primary gastric cancer samples. Results revealed a negative correlation between COP1 and p53 protein expression in the gastric cancer samples (P=0.005, r=-0.572). Moreover, immunohistochemical staining of gastric cancer tissues from the same patient revealed that COP1 showed a high expression while p53 showed a low expression. These results indicated that the correlated expression of COP1 and p53 may play a role in the tumorigenesis or progression of primary gastric cancer. In future studies, the mechanism of their interaction and its correlation with gastric cancer will be examined.
Moreover, in a relatively large series of gastric cancer patients (401 cases), we found that high expression of COP1 was significantly correlated with a higher T stage of gastric cancer, implying that an increase in COP1 expression might promote tumor growth and invasion. In addition, we detected enhanced COP1 immunoreactivity in poorly differentiated gastric cancer tissues compare to normal gastric tissues and well-differentiated gastric cancer tissues, suggesting that high COP1 expression might play a role in tumor progression. These results are consistent with the findings of Dornan D et al. [15], who described an association between high COP1 expression and breast and ovarian adenocarcinomas.
In the Kaplan-Meier survival analysis, patients with high COP1 expression had significantly shorter overall survival than patients with low expressions. Univariate analyses showed that increased expression of COP1 in gastric cancer tissues was significantly correlated with overall survival rate. Cox hazard ratio regression analyses further demonstrated that COP1 expression, together with TNM stage, was an independent risk factor in the prognosis of gastric cancer patients. These results suggest that COP1 might serve as a valuable prognostic biomarker for gastric cancer patients after surgery and as a potential target for gene therapy in the treatment of gastric cancer.
Recently, some studies by Vitari et al and Migliorini et al showed a tumor suppressor role of COP1 in mouse models [17, 18]. Loss of function of COP1 has been found in human prostate cancer [17, 18]. However, our present study combined with some previous studies showed high expression of COP1 in several human cancer types and its increased expression has been found to be correlated strongly with these tumors [15, 16, and 19]. Thus, COP1 expression and its functional role in different kinds of human tumors remain controversial. Marine proposed that COP1 can function either as an oncogene or as a tumour suppressor, depending on the cellular context [28]. However, the interactive molecules of COP1 and its functional or mechanistic role in gastric cancer are unclear, which needs further investigation in the future research.
In conclusion, we have demonstrated that over expression of COP1 in gastric cancer is correlated with a more malignant phenotype and with a poorer prognosis in a large number of clinical samples for the first time. Our data suggested that COP1 may function as a valuable prognostic biomarker for gastric cancer.
Acknowledgements
This work was supported by the Guangdong Province Science and Technology Project (2009B080701006).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Parkin DM. International variation. Oncogene. 2004;23:6329-40
2. Jemal A, Siegel R, Ward E. et al. Cancer statistics. CA Cancer J Clin. 2009;59:225-49
3. Nobili S, Bruno L, Landini I. et al. Genomic and genetic alterations influence the progression of gastric cancer. World J Gastroenterol. 2011;17:290-9
4. Ajani JA, Barthel JS, Bekaii-Saab T. NCCN Clinical Practice Guidelines in Oncology. Gastric cancer, v.2.2010. www.nccn.org
5. Chen CN, Lin JJ, Chen JJ. et al. Gene expression profile predicts patient survival of gastric cancer after surgical resection. J Clin Oncol. 2005;23:7286-95
6. Dornan D, Shimizu H, Mah A. et al. ATM engages autodegradation of the E3 ubiquitin ligase COP1 after DNA damage. Science. 2006;313:1122-8
7. Yasui W, Oue N, Aung PP. et al. Molecularpathological prognostic factors of gastric cancer: a review. Gastric Cancer. 2005;8:86-94
8. Lee HS, Cho SB, Lee HE. et al. Protein expression profiling and molecular classification of gastric cancer by the tissue array method. Clin Cancer Res. 2007;13:4154-63
9. Oue N, Hamai Y, Mitani Y. et al. Gene expression profile of gastric carcinoma: identification of genes and tags potentially involved in invasion, metastasis, and carcinogenesis by serial analysis of gene expression. Cancer Res. 2004;64:2397-405
10. Hippo Y, Taniguchi H, Tsutsumi S. et al. Global gene expression analysis of gastric cancer by oligonucleotide microarrays. Cancer Res. 2002;62:233-40
11. Bianchi E, Denti S, Catena R. et al. Characterization of human constitutive photomorphogenesis protein 1, a RING finger ubiquitin ligase that interacts with Jun transcription factors and modulates their transcriptional activity. J Biol Chem. 2003;278:19682-90
12. Kato S, Ding J, Pisck E. et al. COP1 functions as a FoxO1 ubiquitin E3 ligase to regulate FoxO1-mediated gene expression. J Biol Chem. 2008;283:35464-73
13. Wang L, He G, Zhang P. et al. Interplay between MDM2, MDMX, Pirh2 and COP1: the negative regulators of p53. Mol Biol Rep. 2011;38:229-36
14. Dornan D, Wertz I, Shimizu H. et al. The ubiquitin ligase COP1 is a critical negative regulator of p53. Nature. 2004;429:86-92
15. Dornan D, Bheddah S, Newton K. et al. COP1, the Negative Regulator of p53, Is Overexpressed in Breast and Ovarian Adenocarcinomas. Cancer Res. 2004;64:7226-30
16. Lee YH, Andersen JB, Song HT. et al. Definition of ubiquitination modulator COP1 as a novel therapeutic target in human hepatocellular carcinoma. Cancer Res. 2010;70:8264-9
17. Vitari AC, Leong KG, Newton K. et al. COP1 is a tumour suppressor that causes degradation of ETS transcription factors. Nature. 2011;474:403-6
18. Migliorini D, Bogaerts S, Defever D. et al. Cop1 constitutively regulates c-Jun protein stability and functions as a tumor suppressor in mice. J Clin Invest. 2011;121:1329-43
19. Su CH, Zhao R, Zhang F. et al. 14-3-3σ exerts tumor-suppressor activity mediated by regulation of COP1 stability. Cancer Res. 2011;71:884-94
20. Baert JL, Monte D, Verreman K. et al. The E3 ubiquitin ligase complex component COP1 regulates PEA3 group member stability and transcriptional activity. Oncogene. 2010;29:1810-20
21. Choi HH, Gully C, Su CH. et al. COP9 signalosome subunit 6 stabilizes COP1, which functions as an E3 ubiquitin ligase for 14-3-3σ. Oncogene. 2011;30:4791-801
22. Stanley R. Hamilton, Lauri A. Aaltonen. Pathology and genetics of tumours of the digestive system. New York, USA: Oxford University Press. 2001
23. Xu LZ, Yang WT. Immunohistochemistry reaction results judgement standard. China Oncology. 1996;6:229-31
24. Jemal A, Bray F, Center MM. et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90
25. Hartgrink HH, Jansen EP, van Grieken NC. et al. Gastric cancer. Lancet. 2009;374:477-90
26. Jin S & Levine AJ. The p53 functional circuit. J. Cell Sci. 2001;114:4139-40
27. Hollstein M, Sidransky D, Vogelstein B. et al. p53 mutations in human cancers. Science. 1991;253:49-53
28. Marine JC. Spotlight on the role of COP1 in tumorigenesis. Nat Rev Cancer. 2012;12:455-64
Author contact
![]() Corresponding author: Jian-chuan Xia. State Key Laboratory of Oncology in Southern China and Department of Experimental Research, Sun Yat-sen University Cancer Center, 651 Dongfeng Road East, Guangzhou 510060, P. R. China. Tel: +86-20-87343173, Fax: +86-20-87343392, E-mail: xiajchsysu.edu.cn or Zhi-wei Zhou. State Key Laboratory of Oncology in South China and Department of Experimental Research, Department of Gastric and Pancreatic Surgery, Sun Yat-sen University Cancer Center, 651 Dongfeng Road East, Guangzhou 510060, P. R. China. E-mail: zhouzhworg.cn
Corresponding author: Jian-chuan Xia. State Key Laboratory of Oncology in Southern China and Department of Experimental Research, Sun Yat-sen University Cancer Center, 651 Dongfeng Road East, Guangzhou 510060, P. R. China. Tel: +86-20-87343173, Fax: +86-20-87343392, E-mail: xiajchsysu.edu.cn or Zhi-wei Zhou. State Key Laboratory of Oncology in South China and Department of Experimental Research, Department of Gastric and Pancreatic Surgery, Sun Yat-sen University Cancer Center, 651 Dongfeng Road East, Guangzhou 510060, P. R. China. E-mail: zhouzhworg.cn

 Global reach, higher impact
Global reach, higher impact