10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2012; 8(9):1227-1236. doi:10.7150/ijbs.4666 This issue Cite
Review
Targeting Interleukin-6: All the Way to Treat Autoimmune and Inflammatory Diseases
1. Department of Clinical Application of Biologics, Osaka University Graduate School of Medicine, Osaka University, Osaka, Japan;
2. Department of Respiratory Medicine, Allergy and Rheumatic Diseases, Osaka University Graduate School of Medicine, Osaka University, Osaka, Japan;
3. Department of Immunopathology, Immunology Frontier Research Center, Osaka University, Osaka, Japan;
4. Laboratory of Immunoregulation, Immunology Frontier Research Center, Osaka University, Osaka, Japan.
Received 2012-5-29; Accepted 2012-7-14; Published 2012-10-24
Abstract
Interleukin (IL)-6, a cytokine featuring redundancy and pleiotropic activity, contributes to host defense against acute environmental stress, while dysregulated persistent IL-6 production has been demonstrated to play a pathological role in various autoimmune and chronic inflammatory diseases. Targeting IL-6 is thus a rational approach to the treatment of these diseases. Indeed, clinical trials of tocilizumab, a humanized anti-IL-6 receptor antibody have verified its efficacy and tolerable safety for patients with rheumatoid arthritis, Castleman's disease and systemic juvenile idiopathic arthritis, resulting in approval of this innovative biologic for treatment of these diseases. Moreover, a considerable number of case reports and pilot studies of off-label use of tocilizumab point to the beneficial effects of tocilizumab for a variety of other phenotypically different autoimmune and chronic inflammatory diseases. Elucidation of the source of IL-6 and of mechanisms through which IL-6 production is dysregulated can thus be expected to lead to clarification of the pathogenesis of various diseases.
Keywords: interleukin-6, a humanized anti-interleukin-6 receptor antibody, tocilizumab, autoimmune, inflammation.
Introduction
Interleukin-6 (IL-6), initially designated as a B cell differentiation factor [1], is a representative cytokine featuring redundancy and pleiotropic activity [2-4]. In the early phase of infectious inflammation, IL-6 is produced by monocytes and macrophages immediately after the stimulation of Toll-like receptors (TLRs) with distinct pathogen-associated molecular patterns (PAMPs) [5]. In noninfectious inflammations, such as burn or traumatic injury, damage-associated molecular patterns (DAMPs) from damaged or dying cells stimulate TLRs to produce IL-6 [6]. This acute IL-6 expression plays a central role in host defense by stimulating various cell populations. When acting on hapatocytes, IL-6 strongly induces a broad spectrum of acute-phase proteins such as C-reactive protein (CRP), serum amyloid A (SAA), fibrinogen, hepcidin, haptoglobin, and antichymotrypsin, whereas it reduces albumin, cytochrome P 450, fibronectin, and transferrin [7, 8] (Figure 1).
IL-6 has a pleiotropic effect but its dysregulated persistent production causes the onset and development of various autoimmune and chronic inflammatory diseases. IL-6 is originally found as a B cell stimulatory factor-2, which induces activated B cells into antibody production. IL-6, combined with TGF-β, preferentially induces the differentiation of naïve CD4 positive T cells into Th17 cells whereas IL-6 inhibits TGF-β induced regulatory T cell (Treg) development. As a consequence, Th17/Treg imbalance may cause the onset and progression of autoimmune and chronic inflammatory diseases. IL-6 induces production of acute-phase proteins such as CRP, fibrinogen, serum amyloid A, and hepcidin, whereas it reduces synthesis of albumin in hepatocytes. High persistent levels of serum amyloid A and hepcidin lead to amyloid A amyloidosis and anemia of inflammation, respectively. In bone marrow, IL-6 induces maturation of megakaryocytes into platelets and activation of hematopoietic stem cells. In addition, IL-6 promotes the differentiation of osteoclasts and angiogenesis, the proliferation of keratinocytes and mesangial cells, and the growth of myeloma and plasmacytoma cells. Treg: regulatory T cells; CRP: C-reactive protein; VEGF: vascular endothelial growth factor; RANKL: receptor activator of NF-kappaB ligand.
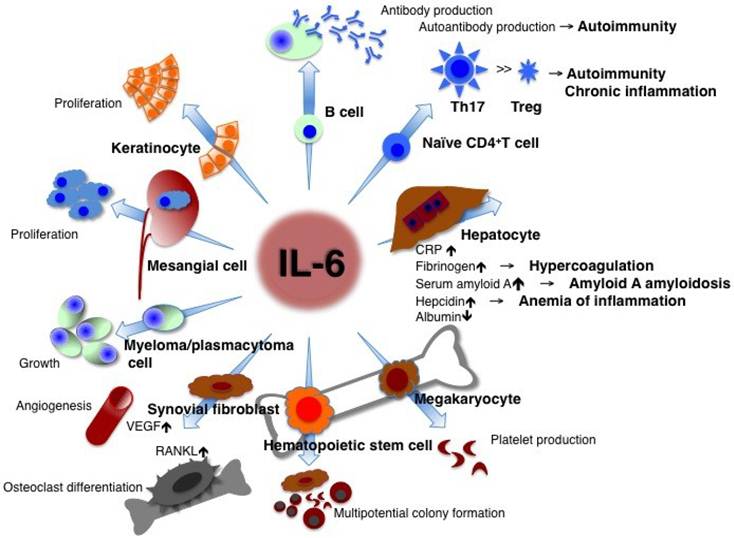
CRP is a good biomarker of inflammation and is used as such in clinical laboratory tests. Its expression mainly depends on IL-6 [9]. If the free concentration of the anti-interleukin 6 receptor antibody, tocilizumab is maintained in serum at more than 1 μg/ml, CRP remains negative [10], so that the serum CRP level is a hallmark for checking whether IL-6 activity is completely blocked in vivo. Continuously high levels of hepcidin induced by IL-6 block iron transporter ferroportin 1 in macrophages, hepatocytes, and gut epithelial cells and lead to hypoferremia and anemia of chronic inflammation [11], whereas long-term high levels of SAA result in amyloid A amyloidosis [12]. In lymphocytes, IL-6 induces B cell differentiation into immunoglobulin-producing cells [1]. When CD4-positive naïve T cells are primed, a specific cytokine prompts their differentiation into an effector T cell subset. IL-6 together with TGF-β preferentially promotes differentiation of IL-17-producing T helper cells (Th17) that play a crucial role in the induction of autoimmune tissue injury, whereas IL-6 inhibits TGF-β-induced regulatory T cell (Treg) differentiation [13, 14]. The resultant Th17/Treg imbalance leads to breakage of immunological tolerance and is of pathological importance for the development of various autoimmune and chronic inflammatory diseases [15]. IL-6 also induces CD8-positive T cells to generate cytotoxic T cells [16]. The function of IL-6 in hematopoiesis is to induce maturation of megakaryocytes into platelets as well as activation of hematopoietic stem cells [17]. IL-6 production in bone marrow stromal cells generates the receptor activator of NF-kappaB ligand (RANKL), which is an essential factor for the differentiation and activation of osteoclasts and bone resorption, thus leading to osteoporosis [18]. Enhanced angiogenesis and increased vascular permeability are pathological features of inflammation, and these characteristics are due to the excess production of vascular endothelial growth factor (VEGF), which is induced by IL-6 in inflamed lesions such as seen in synovium tissue of rheumatoid arthritis [19]. The promotional activities of IL-6, such as the proliferation of keratinocytes or collagen production in dermal fibroblasts, may contribute to autoimmune skin diseases including psoriasis and systemic sclerosis [20, 21]. Furthermore, IL-6 stimulates the growth of cells such as myeloma/plasmacytoma cells and mesangial cells [22-24].
IL-6 triggers signal transduction after binding to the IL-6 receptor (IL-6R) [25, 26]. There are two forms of IL-6R, a transmembrane 80-kDa form with a short cytoplasmic domain and a soluble form (sIL-6R). After binding of IL-6 to transmembrane IL-6R, the resultant IL-6/IL-6R complex associates with gp130 [27-29], and the activated IL-6 receptor complex is formed as a hexameric structure consisting of two molecules each of IL-6, IL-6R and gp130 (so-called a classical signaling) [30, 31]. The expression of transmembrane IL-6R is limited to a few cell types but the IL-6/sIL-6R complex can also transduce the IL-6 signal to various cells which do not express transmembrane IL-6R but express gp130 (known as a trans-signaling mechanism) [32], so that IL-6 affects a wide variety of cells.
Pathological Role of IL-6 in Development of Diseases
When IL-6 is synthesized transiently, it promptly participates in the host defense against environmental stress such as infection and injury and at the same time provides an SOS (warning) signal by triggering a broad spectrum of biological events. Once the source of stress is removed from the host, IL-6-mediated activation of the signal transduction cascade is terminated by negatively regulatory systems in conjunction with the normalization of serum IL-6 and CRP levels. However, dysregulated persistent IL-6 production has been implicated in the development of various autoimmune, chronic inflammatory diseases and even cancers [2-4, 33]. The reason(s) why such dysregulated continuous IL-6 production is induced remains to be clarified and elucidation of the mechanism(s) underlying persistent IL-6 synthesis in diseases is of particular importance to make their pathogenesis clear. It was found that in human immunodeficiency virus (HIV)-positive cases of multicentric Castleman's diseases all patients were infected with the Kaposi sarcoma-associated herpes virus (KSHV) and that sustained synthesis of both virus-derived IL-6, which directly binds to and stimulates human gp130, and of host-derived human IL-6 contribute to the development of the disease [34].
Moreover, numerous animal models of diseases have also disclosed the pathologic role of IL-6 in disease development and that IL-6 blockade by means of gene-knockout or administration of anti-IL-6 or anti-IL-6R antibody can suppress such disease development either preventively or therapeutically. For example, IL-6 blockade strategy demonstrably limited susceptibility to Castleman's disease-like symptoms in IL-6 transgenic mice [35], as well as in various mouse models of rheumatoid arthritis [36-48], systemic lupus erythematosus [49-51], scleroderma [52, 53], C-peptide-induced myositis [54], experimental autoimmune uveoretinitis [55, 56], experimental autoimmune encephalomyelitis [57], and many other diseases.
Targeting IL-6: All the Way to Treat Autoimmune and Inflammatory Diseases
Because of the pathological role of IL-6 in various diseases, blockade of IL-6 was expected to constitute a novel treatment strategy for these diseases [3, 58, 59]. Consequently, a humanized anti-human IL-6R monoclonal antibody (chemical name: tocilizumab, generic name: Actemra outside of the EU or RoActemra inside the EU) was developed, by grafting the complementarity-determining regions of a mouse anti-human IL-6R antibody onto human IgG1. The resultant tocilizumab then blocks IL-6-mediated signal transduction by inhibiting IL-6 binding to transmembrane and soluble IL-6 receptors.
Clinical trials of tocilizumab have demonstrated its outstanding efficacy for rheumatoid arthritis [60-66], systemic juvenile idiopathic arthritis [67-71] and Castleman's disease [72, 73]. For patients with moderately to severely active rheumatoid arthritis, tocilizumab is now being used as an innovative drug in more than 90 countries worldwide. As a monotherapy or in combination with disease-modifying antirheumatic drugs, it has significantly suppressed the disease activity and radiographically detected progression of joint deformity, thus improving daily functional activity. Tocilizumab was also approved as the first line biologic for the treatment of systemic juvenile idiopathic arthritis in Japan, India, USA and EU and for Castleman's disease in Japan and India.
Furthermore, favorable results of recent pilot studies, case series or case studies have suggested that tocilizumab may have broad application for other diseases. These diseases include systemic autoimmune diseases such as systemic lupus erythematosus [74-76], systemic sclerosis [77], polymyositis [78], vasculitis syndrome including giant cell arteritis [79-84], Takayasu arteritis [79, 82, 85-87], cryoglobulinemia [88], myeloperoxidase-antineutrophil cytoplasmic antibody-associated crescentic glomerulonephritis [89] and rheumatoid vasculitis [90]. The application of tocilizumab may also extend to organ-specific autoimmune diseases including Crohn's disease [91], relapsing polychondritis [92, 93], acquired hemophilia A [94], autoimmune hemolytic anemia [95, 96], as well as to chronic inflammatory diseases such as adult-onset Still's disease [97-113], amyloid A amyloidosis [114-120], polymyalgia rheumatica [79, 84, 121], remitting seronegative symmetrical synovitis with pitting edema [122], Behcet's disease [123, 124], uveitis [125], graft-versus-host diseases [126, 127], and tumor necrosis factor receptor-associated periodic syndrome [128] (Table 1). Some studies have reported that tocilizumab is efficacious for spondyloarthritis [129-135], although others observed only minor effects [136-138]. In addition, tocilizumab is reportedly effective for pulmonary arterial hypertension [139-141], atopic dermatitis [142], and sciatica [143]. Finally, it was observed that during tocilizumab treatment of patients with rheumatoid arthritis, HbA1c levels and insulin resistance indices such as the homeostasis model assessment of insulin resistance (HOMA-IR) and the leptin-to-adiponectin ratio improved [144, 145], while serum levels of reactive oxygen metabolites decreased [146]. It can thus be expected that long-term tocilizumab treatment may offer protection against the progression of atherosclerosis leading to cardiovascular events [147]. Indeed, large-scale genetic analyses demonstrated a causal association between IL-6R-related pathways and coronary heart disease [148, 149], and a randomized, open-label, parallel-group, multicenter study to evaluate the rate of cardiovascular events of tocilizumab in comparison to a TNF inhibitor, etanercept in patients with rheumatoid arthritis (ClinicalTrials.gov, Identifier: NCT01331837) is now in progress. To establish broad clinical indications for tocilizumab for various diseases, however, further clinical studies will be needed to verify its efficacy and safety. The current clinical trials are listed in Table 2.
Application of IL-6 blockade strategy for various autoimmune and chronic inflammatory diseases.
| Approved or candidate diseases | References | |
|---|---|---|
| RA | > 90 countries worldwide* | 60-66 |
| Systemic JIA | Japan, India, USA and EU* | 67-71 |
| Castleman's disease | Japan and India* | 72,73 |
| SLE | Phase I, case reports | 74-76 |
| Systemic sclerosis | Case series | 77 |
| Polymyositis | Case series | 78 |
| Vasculitis syndrome | Case report & series | 79-90 |
| Crohn's disease | Pilot randomized trial | 91 |
| Relapsing polychondritis | Case reports | 92,93 |
| Acquired hemophilia A | Case report | 94 |
| Autoimmune hemolytic anemia | Case reports | 95,96 |
| Adult-onset Still's disease | Case reports & series | 97-113 |
| Amyloid A amyloidosis | Case reports | 114-120 |
| Polymyalgia rheumatica | Case reports & series | 79,84,121 |
| RS3PE | Case report | 122 |
| Behcet's disease | Case reports | 123,124 |
| Uveitis | Case report | 125 |
| GVHD | Case report & series | 126,127 |
| TRAPS | Case report | 128 |
| Spondyloarthritides | Case reports | 129-135 |
| Pulmonary arterial hypertension | Case reports | 139-141 |
| Atopic dermatitis | Case series | 142 |
| Sciatica | Case series | 143 |
Tocilizumab, a humanized anti-IL-6 receptor antibody, has been approved* as a biological drug for the treatment of RA, Castleman's disease and systemic JIA, and is expected to be applicable to various other autoimmune and chronic inflammatory diseases. RA: rheumatoid arthritis; JIA: juvenile idiopathic arthritis; SLE: systemic lupus erythematosus; RS3PE: remitting seronegative, symmetrical synovitis with pitting edema; GVHD: graft-versus-host disease; TRAPS: tumor necrosis factor-associated periodic syndrome.
Clinical trials of tocilizumab for diseases other than RA.
| Targeted diseases | Identifier |
|---|---|
| ClinicalTrials.gov (USA) | |
| Adult-onset Still's disease | NCT01002781 |
| Relapsing polychondritis | NCT01041248 |
| Type II diabetes, obesity | NCT01073826 |
| Ankylosing spondylitis | NCT01209702 |
| Graves' ophthalmopathy | NCT01297609 |
| Cardiovascular disease in rheumatoid arthritis | NCT01331837 |
| Polymyalgia rheumatica | NCT01396317 |
| Giant cell arteritis | NCT01450137 |
| Acute GVHD | NCT01475162 |
| Non-ST elevation myocardial infarction | NCT01491074 |
| Systemic sclerosis | NCT01532869 |
| Transplant rates in highly sensitized patients awaiting kidney transplantation | NCT01594424 |
| EU Clinical Trials Registry | |
| Ankylosing spondylitis | 2009-017488-40 |
| 2009-017443-34 | |
| Cardiovascular disease in rheumatoid arthritis | 2010-020065-24 |
| Graves' ophthalmopathy | 2010-023841-31 |
| Systemic sclerosis | 2011-001460-22 |
| UMIN-CTR clinical trials (Japan) | |
| ANCA-associated vasculitis | UMIN000002892 |
| Systemic sclerosis | UMIN000005550 |
| Neuromyelitis optica | UMIN000005889 |
| UMIN000007866 | |
| Chronic glomerulonephritis | UMIN000006080 |
| Colorectal cancer | UMIN000007493 |
| Takayasu arteritis | UMIN000007845 |
Current clinical trials of tocilizumab in the USA, EU and Japan are listed. GVHD: graft-versus-host disease; ANCA: anti-neutrophil cytoplasmic antibody.
On the basis of the pathologic role of IL-6 and the outstanding beneficial effect of tocilizumab, targeting IL-6 is a rational strategy for the treatment of various diseases and other biologics of IL-6 inhibitors are also being developed [32]. These include fully human anti-IL-6R, anti-IL-6R nanobody, anti-IL-6 antibody, and anti-IL-6/anti-IgG avimer protein consisting of the IgG-binding domain fused to the N-terminus of a 3-domain IL-6 binding region, which results in a 19-kDa heterotetrameric avimer. These novel biologics block IL-6-mediated both classical and trans-signaling pathway by inhibiting IL-6 binding to both transmembrane and soluble IL-6R. By contrast, the fusion protein soluble gp130-Fc selectively targets IL-6/sIL-6R trans-signaling pathway. It is hypothesized that IL-6 trans-signaling is a local and temporal danger signal with fewer and less important physiological functions under non-stressed conditions than classical signaling on the basis of several animal models [32, 150].
Future Directions
IL-6 participates in the host defense against environmental pathogens, whereas dysregulation of IL-6 production has been implicated in the development of various autoimmune and chronic inflammatory diseases [2-4, 33]. The pleiotropic activity of IL-6 also indicates that IL-6 blockade represents a rational treatment strategy for various diseases. A good example of the efficacy of such treatment is the dramatic improvement engendered by tocilizumab in amyloid A amyloidosis and anemia of inflammation through inhibition of their respective responsible proteins, SAA and hepcidin synthesis [151, 152]. However, the mechanisms through which tocilizumab exerts its therapeutic effects on various phenotypically different autoimmune and inflammatory diseases are not yet well understood. In recent years, it has been shown that Th17 and/or Th1 >> Treg causes the onset of various autoimmune and chronic inflammatory diseases [14, 15]. IL-6, in combination with TGF-β, promotes the differentiation of naïve T cells into Th17, but inhibits TGF-β-induced Treg differentiation, indicating that IL-6 is a very important factor for determining the Th17/Treg balance [14]. Dysregulated IL-6 production leads to predominance of Th17 over Treg but anti-IL-6R antibody can repair this imbalance. It has been demonstrated in several animal disease models that IL-6 blocking suppresses antigen-specific Th17 and/or Th1 differentiation but induces antigen-specific Treg [47, 48, 55-57]. Furthermore, it has been shown that tocilizumab in fact corrects Th17/Treg imbalance in rheumatoid arthritis patients [153]. In another study, it was found that tocilizumab induced a significant reduction in the peripheral pre-switch and post-switch memory B cells of rheumatoid arthritis patients [154] and that tocilizumab but not the TNF inhibitor significantly reduced somatic hypermutation in immunoglobulin gene rearrangements in pre-switch memory B cells [155], thus suggesting that modulation of memory B cells may be one possible target for tocilizumab. Moreover, tocilizumab treatment led to a reduction in the pathologic CD38highCD19lowIgDnegative plasma cells of SLE patients [74] and could lessen the survival of plasmablasts, which produce the anti-aquaporin 4 antibody in neuromyelitis optica [156]. These findings suggest that the clinical effect of tocilizumab is also mediated through its inhibition of pathological autoantibody production.
Because of the therapeutic efficacy of tocilizumab, IL-6 plays a major role in the onset or development of various phenotypically different diseases. IL-6 is produced by a panoply of cells including monocytes, macrophages, dendritic cells, T and B cells, neutrophils, mast cells, fibroblasts, synovial cells, keratinocytes, endothelial cells, stromal cells, mesangial cells, glial cells, neurons, chondrocytes, osteoblasts, smooth muscle cells, and others in response to various stimuli [2-4, 33]. Such phenotypic difference of diseases is conceivably due to differences in cells which generate IL-6 through abnormal transcriptional activation of the IL-6 gene [157, 158] and/or inhibition of IL-6 mRNA degradation [159, 160], or dose-dependent effects of IL-6 produced by cells recruited into the organs. Some virus products from KSHV, human immunodeficiency virus (HIV), human lymphotropic virus-1 (HTLV-1) and hepatitis B virus have been reported to affect IL-6 gene activation and/or mRNA degradation [161-168]. Therefore clarification of the cell source of IL-6 production and of the mechanism(s) through which dysregulated continuous IL-6 synthesis is induced constitutes an important issue for future studies into the pathogenesis of diseases.
Conflict of Interests
Tadamitsu Kishimoto holds a patent for tocilizumab and receives royalties for Actemra. Toshio Tanaka declares no conflict of interest. No assistance was provided for the writing of this manuscript.
References
1. Hirano T, Yasukawa K, Harada H. et al. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986;324:73-6
2. Kishimoto T. The biology of interleukin-6. Blood. 1989;74:1-10
3. Kishimoto T. Interleukin-6: from basic science to medicine-40 years in immunology. Annu Rev Immunol. 2005;23:1-21
4. Tanaka T, Katada Y, Suemura M. et al. Interleukin-6. In: (ed.) Snapper CM. Cytokine regulation of humoral immunity: basic and clinical aspects. New Jersey: John Wiley & Sons Ltd. 1996:251-72
5. Janeway CA Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197-216
6. Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301-5
7. Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990;265:621-36
8. Siewert E, Bort R, Kluge R. et al. Hepatic cytochrome p450 down-regulation during aseptic inflammation in the mouse is interleukin 6 dependent. Hepatology. 2000;32:49-55
9. Nishikawa T, Hagihara K, Serada S. et al. Transcriptional complex formation of c-Fos, STAT3, and Hepatocyte NF-1alpha is essential for cytokine-driven C-reactive protein gene expression. J Immunol. 2008;180:3492-501
10. Nishimoto N, Terao K, Mima T. et al. Mechanisms and pathologic significances in increase in serum interleukin-6 (IL-6) and soluble IL-6 receptor after administration of an anti-IL-6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and Castleman disease. Blood. 2008;112:3959-64
11. Nemeth E, Rivera S, Gabayan V. et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271-6
12. Obici L, Perfetti V, Palladini G. et al. Clinical aspects of systemic amyloid diseases. Biochim Biophys Acta. 2005;1753:11-22
13. Korn T, Bettelli E, Oukka M. et al. IL-17 and Th17 cells. Annu Rev Immunol. 2009;27:485-517
14. Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol. 2010;40:1830-35
15. Miyara M, Gorochov G, Ehrenstein M. et al. Human FoxP3+ regulatory T cells in systemic autoimmune diseases. Autoimmun Rev. 2011;10:744-55
16. Okada M, Kitahara M, Kishimoto S. et al. IL-6/BSF-2 functions as a killer helper factor in the in vitro induction of cytotoxic T cells. J Immunol. 1988;141:1543-9
17. Ishibashi T, Kimura H, Shikama Y. et al. Interleukin-6 is a potent thrombopoietic factor in vivo in mice. Blood. 1989;74:1241-4
18. Kotake S, Sato K, Kim KJ. et al. Interleukin-6 and soluble interleukin-6 receptors in the synovial fluids from rheumatoid arthritis patients are responsible for osteoclast-like cell formation. J Bone Miner Res. 1996;11:88-95
19. Nakahara H, Song J, Sugimoto M. et al. Anti-interleukin-6 receptor antibody therapy reduces vascular endothelial growth factor production in rheumatoid arthritis. Arthritis Rheum. 2003;48:1521-9
20. Grossman RM, Krueger J, Yourish D. et al. Interleukin 6 is expressed in high levels in psoriatic skin and stimulates proliferation of cultured human keratinocytes. Proc Natl Acad Sci USA. 1989;86:6367-71
21. Duncan MR, Berman B. Stimulation of collagen and glycosaminoglycan production in cultured human adult dermal fibroblasts by recombinant human interleukin 6. J Invest Dermatol. 1991;97:686-92
22. Kawano M, Hirano T, Matsuda T. et al. Autocrine generation and requirement of BSF-2/IL-6 for human multiple myelomas. Nature. 1988;332:83-5
23. Suematsu S, Matsuda T, Aozasa K. et al. IgG1 plasmacytosis in interleukin 6 transgenic mice. Proc Natl Acad Sci USA. 1989;86:7547-51
24. Horii Y, Muraguchi A, Iwano M. et al. Involvement of IL-6 in mesangial proliferative glomerulonephritis. J Immunol. 1989;143:3949-55
25. Yamasaki K, Taga T, Hirata Y. et al. Cloning and expression of the human interleukin-6 (BSF-2/IFN beta 2) receptor. Science. 1988;241:825-8
26. Kishimoto T, Akira S, Taga T. Interleukin-6 and its receptor: a paradigm for cytokines. Science. 1992;258:593-7
27. Hibi M, Murakami M, Saito M. et al. Molecular cloning and expression of an IL-6 signal transducer, gp130. Cell. 1990;63:1149-57
28. Kishimoto T, Akira S, Narazaki M. et al. Interleukin-6 family of cytokines and gp130. Blood. 1995;86:1243-54
29. Taga T, Kishimoto T. Gp130 and the interleukin-6 family of cytokines. Annu Rev Immunol. 1997;15:797-819
30. Murakami M, Hibi M, Nakagawa N. et al. IL-6-induced homodimerization of gp130 and associated activation of a tyrosine kinase. Science. 1993;260:1808-10
31. Boulanger MJ, Chow DC, Brevnova EE. et al. Hexameric structure and assembly of the interleukin-6/IL-6 alpha-receptor/gp130 complex. Science. 2003;300:2101-4
32. Jones SA, Scheller J, Rose-John S. Therapeutic strategies for the clinical blockade of IL-6/gp130 signaling. J Clin Invest. 2011;121:3375-83
33. Akira S, Taga T, Kishimoto T. Interleukin-6 in biology and medicine. Adv Immunol. 1993;54:1-78
34. Aoki Y, Narazaki M, Kishimoto T. et al. Receptor engagement by viral interleukin-6 encoded by Kaposi sarcoma-associated herpes virus. Blood. 2001;98:3042-9
35. Brandt SJ, Bodine DM, Dunbar CE. et al. Dysregulated interleukin 6 expression produces a syndrome resembling Castleman's disease in mice. J Clin Invest. 1990;86:592-9
36. Tanaka T, Kishimoto T. Immunotherapy of tocilizumab for rheumatoid arthritis. J Clin Cell Immunol. 2011:S6-001
37. Alonzi T, Fattori E, Lazzaro D. et al. Interleukin 6 is required for the development of collagen-induced arthritis. J Exp Med. 1998;187:461-8
38. Takagi N, Mihara M, Moriya Y. et al. Blockade of interleukin-6 receptor ameliorates joint disease in murine collagen-induced arthritis. Arthritis Rheum. 1998;41:2117-21
39. Ohshima S, Saeki Y, Mima T. et al. Interleukin 6 plays a key role in the development of antigen-induced arthritis. Proc Natl Acad Sci USA. 1998;95:8222-6
40. Sasai M, Saeki Y, Ohshima S. et al. Delayed onset and reduced severity of collagen-induced arthritis in interleukin-6-deficient mice. Arthritis Rheum. 1999;42:1635-43
41. Kobayashi H, Ohshima S, Nishioka K. et al. Antigen induced arthritis (AIA) can be transferred by bone marrow transplantation: evidence that interleukin 6 is essential for induction of AIA. J Rheumatol. 2002;29:1176-82
42. Wong PK, Quinn JM, Sims NA. et al. Interleukin-6 modulates production of T lymphocyte-derived cytokines in antigen-induced arthritis and drives inflammation-induced osteoclastogenesis. Arthritis Rheum. 2006;54:158-68
43. Hata H, Sakaguchi N, Yoshitomi H. et al. Distinct contribution of IL-6, TNF-alpha, IL-1, and IL-10 to T cell-mediated spontaneous autoimmune arthritis in mice. J Clin Invest. 2004;114:582-8
44. Hirota K, Hashimoto M, Yoshitomi H. et al. T cell self-reactivity forms a cytokine milieu for spontaneous development of IL-17+ Th cells that cause autoimmune arthritis. J Exp Med. 2007;204:41-7
45. Iwakura Y, Saijo S, Kioka Y. et al. Autoimmunity induction by human T cell leukemia virus type 1 in transgenic mice that develop chronic inflammatory arthropathy resembling rheumatoid arthritis in humans. J Immunol. 1995;155:1588-98
46. Atsumi T, Ishihara K, Kamimura D. et al. A point mutation of Tyr-759 in interleukin 6 family cytokine receptor subunit gp130 causes autoimmune arthritis. J Exp Med. 2002;196:979-90
47. Fujimoto M, Serada S, Mihara M. et al. Interleukin-6 blockade suppresses autoimmune arthritis in mice by the inhibition of inflammatory Th17 responses. Arthritis Rheum. 2008;58:3710-9
48. Iwanami K, Matsumoto I, Tanaka-Watanabe Y. et al. Crucial role of the interleukin-6/interleukin-17 cytokine axis in the induction of arthritis by glucose-6-phosphate isomerase. Arthritis Rheum. 2008;58:754-63
49. Mihara M, Takagi N, Takeda Y. et al. IL-6 receptor blockage inhibits the onset of autoimmune kidney disease in NZB/W F1 mice. Clin Exp Immunol. 1998;112:397-402
50. Liang B, Gardner DB, Griswold DE. et al. Anti-interleukin-6 monoclonal antibody inhibits autoimmune responses in a murine model of systemic lupus erythematosus. Immunology. 2006;119:296-305
51. Pflegerl P, Vesely P, Hantusch B. et al. Epidermal loss of JunB leads to a SLE phenotype due to hyper IL-6 signaling. Proc Natl Acad Sci USA. 2009;106:20423-8
52. Kitaba S, Murota H, Terao M. et al. Blockade of interleukin-6 receptor alleviates disease in mouse model of scleroderma. Am J Pathol. 2012;80:165-76
53. Yoshizaki A, Yanaba K, Ogawa A. et al. Immunization with DNA topoisomerase I and Freund's complete adjuvant induces skin and lung fibrosis and autoimmunity via interleukin-6 signaling. Arthritis Rheum. 2011;63:3575-85
54. Okiyama N, Sugihara T, Iwakura Y. et al. Therapeutic effects of interleukin-6 blockade in a murine model of polymyositis that does not require interleukin-17A. Arthritis Rheum. 2009;60:2505-12
55. Hohki S, Ohguro N, Haruta H. et al. Blockade of interleukin-6 signaling suppresses experimental autoimmune uveoretinitis by the inhibition of inflammatory Th17 responses. Exp Eye Res. 2010;91:162-70
56. Haruta H, Ohguro N, Fujimoto M. et al. Blockade of interleukin-6 signaling suppresses not only Th17 but also interphotoreceptor retinoid binding protein-specific Th1 by promoting regulatory T cells in experimental autoimmune uveoretinitis. Invest Ophthalmol Vis Sci. 2011;52:3264-71
57. Serada S, Fujimoto M, Mihara M. et al. IL-6 blockade inhibits the induction of myelin antigen-specific Th17 cells and Th1 cells in experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2008;105:9041-6
58. Tanaka T, Narazaki M, Kishimoto T. Anti-interleukin-6 receptor antibody, tocilizumab, for the treatment of autoimmune diseases. FEBS lett. 2011;585:3699-709
59. Tanaka T, Narazaki M, Kishimoto T. Therapeutic targeting of the interleukin-6 receptor. Annu Rev Pharmacol Toxicol. 2012;52:199-219
60. Nishimoto N, Yoshizaki K, Miyasaka N. et al. Treatment of rheumatoid arthritis with humanized anti-interleukin-6 receptor antibody: a multicenter, double-blind, placebo-controlled trial. Arthritis Rheum. 2004;50:1761-9
61. Maini RN, Taylor PC, Szechinski J, et al; CHARISMA Study Group. Double-blind randomized controlled clinical trial of the interleukin-6 receptor antagonist, tocilizumab, in European patients with rheumatoid arthritis who had an incomplete response to methotrexate. Arthritis Rheum. 2006;54:2817-29
62. Nishimoto N, Miyasaka N, Yamamoto K. et al. Long-term safety and efficacy of tocilizumab, an anti-IL-6 receptor monoclonal antibody, in monotherapy, in patients with rheumatoid arthritis (the STREAM study): evidence of safety and efficacy in a 5-year extension study. Ann Rheum Dis. 2009;68:1580-4
63. Jones G, Sebba A, Gu J. et al. Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: the AMBITION study. Ann Rheum Dis. 2010;69:88-96
64. Smolen JS, Beaulieu A, Rubbert-Roth A, et al; OPTION Investigators. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomized trial. Lancet. 2008;371:987-97
65. Genovese MC, McKay JD, Nasonov EL. et al. Interleukin-6 receptor inhibition with TCZ reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: the TCZ in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis Rheum. 2008;58:2968-80
66. Emery P, Keystone E, Tony HP. et al. IL-6 receptor inhibition with TCZ improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-TNF biologics: results from a 24-week multicentre randomized placebo controlled trial. Ann Rheum Dis. 2008;67:1516-23
67. Woo P, Wilkinson N, Prieur AM. et al. Open label phase II trial of single, ascending doses of MRA in Caucasian children with severe systemic juvenile idiopathic arthritis: proof of principle of the efficacy of IL-6 receptor blockade in this type of arthritis and demonstration of prolonged clinical improvement. Arthritis Res Ther. 2005;7:R1281-8
68. Yokota S, Miyamae T, Imagawa T. et al. Therapeutic efficacy of humanized recombinant anti-interleukin-6 receptor antibody in children with systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 2005;52:818-25
69. Yokota S, Imagawa T, Mori M. et al. Efficacy and safety of tocilizumab in patients with systemic-onset juvenile idiopathic arthritis: a randomized, double-blind, placebo-controlled, withdrawal phase III trial. Lancet. 2008;371:998-1006
70. De Benedetti F, Brunner H, Ruperto N. et al. Tocilizumab in patients with systemic juvenile idiopathic arthritis: efficacy data from the placebo-controlled 12-week part of the phase 3 TENDER trial. Arthritis Rheum. 2010;62(Suppl 10):1434
71. De Benedetti F, Brunner H, Ruperto N. et al. Efficacy and safety of tocilizumab (TCZ) in patients with systemic juvenile idiopathic arthritis (SJIA): tender 52-week data. Pediat Rheumatol. 2011;9(Suppl 1):164
72. Nishimoto N, Sasai M, Shima Y. et al. Improvement in Castleman's disease by humanized anti-interleukin-6 receptor antibody therapy. Blood. 2000;95:56-61
73. Nishimoto N, Kanakura Y, Aozasa K. et al. Humanized anti-interleukin-6 receptor antibody treatment of multicentric Castleman disease. Blood. 2005;106:2627-32
74. Illei GG, Shirota Y, Yarboro CH. et al. Tocilizumab in systemic lupus erythematosus: data on safety, preliminary efficacy, and impact on circulating plasma cells from an open-label phase I dosage-escalation study. Arthritis Rheum. 2010;62:542-52
75. Maeshima K, Ishii K, Torigoe M. et al. Successful tocilizumab and tacrolimus treatment in a patient with rheumatoid arthritis complicated by systemic lupus erythematosus. Lupus. 2012 [Epub ahead of print]
76. Makol A, Gibson LE, Michet CJ. Successful use of interleukin 6 antagonist tocilizumab in a patient with refractory cutaneous lupus and urticarial vasculitis. J Clin Rheumatol. 2012;18:92-5
77. Shima Y, Kuwahara Y, Murota H. et al. The skin of patients with systemic sclerosis softened during the treatment with anti-IL-6 receptor antibody tocilizumab. Rheumatology (Oxford). 2010;49:2408-12
78. Narazaki M, Hagihara K, Shima Y. et al. Therapeutic effect of tocilizumab on two patients with polymyositis. Rheumatology (Oxford). 2011;50:1344-6
79. Seitz M, Reichenbach S, Bonel HM. et al. Rapid induction of remission in large vessel vasculitis by IL-6 blockade. A case series. Swiss Med Wkly. 2011;141:w13156
80. Beyer C, Axmann R, Sahinbegovic E. et al. Anti-interleukin 6 receptor therapy as rescue treatment for giant cell arteritis. Ann Rheum Dis. 2011;70:1874-5
81. Sciascia S, Rossi D, Roccatello D. Interleukin 6 blockade as steroid-sparing treatment for 2 patients with giant cell arteritis. J Rheumatol. 2011;38:2080-1
82. Salvarani C, Magnani L, Catanoso M. et al. Tocilizumab: a novel therapy for patients with large-vessel vasculitis. Rheumatology (0xford). 2012;51:151-6
83. Vinit J, Bielefeld P, Muller G. et al. Efficacy of tocilizumab in refractory giant cell arteritis. Joint Bone Spine. 2012;79:317-8
84. Christidis D, Jain S, Das Gupta B. Successful use of tocilizumab in polymyalgic onset biopsy positive GCA with large vessel involvement. BMJ Case Reports. 2011 [Epub ahead of print]
85. Nishimoto N, Nakahara H, Yoshio-Hoshino N. et al. Successful treatment of a patient with Takayasu arteritis using a humanized anti-interleukin-6 receptor antibody. Arthritis Rheum. 2008;58:1197-200
86. Salvarani C, Magnani L, Catanoso MG. et al. Rescue treatment with tocilizumab for Takayasu arteritis resistant to TNF-α blockers. Clin Exp Rheumatol. 2012 [Epub ahead of print]
87. Bredemeier M, Rocha CM, Barbosa MV. et al. One-year clinical and radiological evolution of a patient with refractory Takayasu's arteritis under treatment with tocilizumab. Clin Exp Rheumatol. 2012 [Epub ahead of print]
88. Cohen C, Mekinian A, Saidenberg-Kermanach N. et al. Efficacy of tocilizumab in rituximab-refractory cryoglobulinemia vasculitis. Ann Rheum Dis. 2012;71:628-9
89. Sumida K, Ubara Y, Suwabe T. et al. Complete remission of myeloperoxidase-antineutrophil cytoplasmic antibody-associated crescentic glomerulonephritis complicated with rheumatoid arthritis using a humanized anti-interleukin 6 receptor antibody. Rheumatology (0xford). 2011;50:1928-30
90. Sumida K, Ubara Y, Takemoto F. et al. Successful treatment with humanized anti-interleukin 6 receptor antibody for multidrug-refractory and anti-tumour necrosis factor-resistant systemic rheumatoid vasculitis. Clin Exp Rheumatol. 2011;29(1 Suppl 64):S133
91. Ito H, Takazoe M, Fukuda Y. et al. A pilot randomized trial of a human anti-interleukin-6 receptor monoclonal antibody in active Crohn's disease. Gastroenterology. 2004;126:989-96
92. Kawai M, Hagihara K, Hirano T. et al. Sustained response to tocilizumab, anti-interleukin-6 receptor antibody, in two patients with refractory relapsing polychondritis. Rheumatology (Oxford). 2009;48:318-9
93. Narshi CB, Allard SA. Sustained response to tocilizumab, anti-IL-6 antibody, following anti-TNF-α failure in a patient with relapsing polychondritis complicated by aortitis. Rheumatology (Oxford). 2012;51:952-3
94. Nishida S, Kawasaki T, Kashiwagi H. et al. Successful treatment of acquired hemophilia A, complicated by chronic GVHD, with tocilizumab. Mod Rheumatol. 2011;21:420-2
95. Yuzuriha A, Saitoh T, Koiso H. et al. Successful treatment of autoimmune hemolytic anemia associated with multicentric Castleman disease by anti-interleukin-6 receptor antibody (tocilizumab) therapy. Acta Haematol. 2011;126:147-50
96. Garcia-Hernandez FJ, Gonzalez-Leon R, Castillo-Palma MJ. et al. Tocilizumab for treating refractory haemolytic anemia in a patient with systemic lupus erythematosus. Rheumatology (Oxford). 2012 [Epub ahead of print]
97. Iwamoto M, Nara H, Hirata D. et al. Humanized monoclonal anti-interleukin-6 receptor antibody for treatment of intractable adult-onset Still's disease. Arthritis Rheum. 2002;46:3388-9
98. Nakahara H, Mima T, Yoshino-Hoshino N. et al. A case report of a patient with refractory adult-onset Still's disease who was successfully treated with tocilizumab over 6 years. Mod Rheumatol. 2009;19:69-72
99. De Bandt M. Saint-Marcoux B. Tocilizumab for multirefractory adult-onset Still's disease. Ann Rheum Dis. 2009;68:153-4
100. Matsumoto K, Nagashima T, Takatori S. et al. Glucocorticoid and cyclosporine refractory adult onset Still's disease successfully treated with tocilizumab. Clin Rheumatol. 2009;28:485-7
101. Cunha ML, Wagner J, Osawa A. et al. The effect of tocilizumab on the uptake of 18FDG-PET imaging in patients with adult-onset Still's disease. Rheumatology (Oxford). 2010;49:1014-6
102. Sumida K, Ubara Y, Hoshino J. et al. Etanercept-refractory adult-onset Still's disease with thrombotic thrombocytopenic purpura successfully treated with tocilizumab. Clin Rheumatol. 2010;29:1191-4
103. Yoshimura M, Makiyama J, Koga T. et al. Successful treatment with tocilizumab in a patient with refractory adult-onset Still's disease (AOSD). Clin Exp Rheumatol. 2010;28:141-2
104. Perdan-Pirkmajer K, Praprotnik S, Tomsic M. A case of refractory adult-onset Still's disease successfully controlled with tocilizumab and a review of the literature. Clin Rheumatol. 2010;29:1465-7
105. Naniwa T, Ito R, Watanabe M. et al. Case report: successful use of short-term add-on tocilizumab for multirefractory systemic flare of adult-onset Still's disease. Clin Rheumatol. 2010 [Epub ahead of print]
106. Kishida D, Okuda Y, Ohnishi M. et al. Successful tocilizumab treatment in a patient with adult-onset Still's disease complicated by chronic active hepatitis B and amyloid A amyloidosis. Mod Rheumatol. 2011;21:215-8
107. Thonhofer R, Hiller M, Just H. et al. Treatment of refractory adult-onset Still's disease with tocilizumab: report of two cases and review of the literature. Rheumatol Int. 2011;31:1653-6
108. Sabnis GR, Gokhale YA, Kulkarni UP. Tocilizumab in refractory adult-onset Still's disease with aseptic meningitis-efficacy of interleukin-6 blockade and review of the literature. Semin Arthritis Rheum. 2011;40:365-8
109. Rech J, Ronneberger M, Englbrecht M. et al. Successful treatment of adult-onset Still's disease refractory to TNF and IL-1 blockade by IL-6 blockade. Ann Rheum Dis. 2011;70:390-2
110. Kobayashi M, Takahashi Y, Yamashita H. et al. Benefit and a possible risk of tocilizumab therapy for adult-onset Still's disease accompanied by macrophage-activation syndrome. Mod Rheumatol. 2011;21:92-6
111. Puechal X, DeBandt M, Berthelot JM. et al. Tocilizumab in refractory adult Still's disease. Arthritis Care Res. 2011;63:155-9
112. Sekkach Y, Elqatni M, Khattabi AE. et al. Antagonists of interleukin-6 (tocilizumab), in adult refractory still disease. Presse Med. 2011;40:e333-7
113. Suematsu R, Ohta A, Matsuura E. et al. Therapeutic response of patients with adult Still's disease to biologic agents: multicenter results in Japan. Mod Rheumatol. 2011 [Epub ahead of print]
114. Okuda Y, Takasugi K. Successful use of a humanized anti-interleukin-6 receptor antibody, tocilizumab, to treat amyloid A amyloidosis complicating juvenile idiopathic arthritis. Arthritis Rheum. 2006;54:2997-3000
115. Nishida S, Hagihara K, Shima Y. et al. Rapid improvement of AA amyloidosis with humanised anti-interleukin 6 receptor antibody treatment. Ann Rheum Dis. 2009;68:1235-6
116. Sato H, Sakai T, Sugaya T. et al. Tocilizumab dramatically ameliorated life-threatening diarrhea due to secondary amyloidosis associated with rheumatoid arthritis. Clin Rheumatol. 2009;28:1113-6
117. Inoue D, Arima H, Kawanami C. et al. Excellent therapeutic effect of tocilizumab on intestinal amyloid a deposition secondary to active rheumatoid arthritis. Clin Rheumatol. 2010;29:1195-7
118. De La Torre M, Arboleya L, Pozo S. et al. Rapid and sustained response to tocilizumab, anti-interleukin-6 receptor antibody, in a patient with nephritic syndrome secondary to systemic juvenile idiopathic arthritis-related amyloidosis. NDT Plus. 2011;4:178-80
119. Magro-Checa C, Navas-Parejo Casado A, Borrego-Garcia E. et al. Successful use of tocilizumab in a patient with nephritic syndrome due to a rapidly progressing AA amyloidosis to latent tuberculosis. Amyloid. 2011;18:235-9
120. Hattori Y, Ubara Y, Sumida K. et al. Tocilizumab improves cardiac disease in a hemodialysis patient with AA amyloidosis secondary to rheumatoid arthritis. Amyloid. 2012;19:37-40
121. Hagihara K, Kawase I, Tanaka T. et al. Tocilizumab ameliorates clinical symptoms in polymyalgia rheumatica. J Rheumatol. 2010;37:1075-6
122. Tanaka T, Hagihara K, Shima Y. et al. Treatment of a patient with remitting seronegative, symmetrical synovitis with pitting oedema with a humanized anti-interleukin-6 receptor antibody, tocilizumab. Rheumatology (Oxford). 2010;49:824-6
123. Hirano T, Ohguro N, Hohki S. et al. A case of Behcet's disease treated with a humanized anti-interleukin-6 receptor antibody, tocilizumab. Mod Rheumatol. 2012;22:298-302
124. Shapiro LS, Farrell J, Haghighi AB. Tocilizumab treatment for neuro-Behcet's disease, the first report. Clin Neurol Neurosurgery. 2011;114:297-8
125. Muselier A, Bielefeld P, Bidot S. et al. Efficacy of tocilizumab in two patients with anti-TNF-alpha refractory uveitis. Ocular Immunol Inflamm. 2011;19:382-3
126. Gergis U, Arnason J, Yantiss R. et al. Effectiveness and safety of tocilizumab, an anti-interleukin-6 receptor monoclonal antibody, in a patient with refractory GI graft-versus-host disease. J Clin Onocol. 2010;28:e602-4
127. Drobyski WR, Pasquini M, Kovatovic K. et al. Tocilizumab for the treatment of steroid refractory graft versus host disease. Biol Blood Marrow Transplant. 2011;17:1862-8
128. Vaitla PM, Radford PM, Tighe PJ. et al. Role of interleukin-6 in a patient with tumor necrosis factor receptor-associated periodic syndrome: Assessment of outcomes following treatment with the anti-interleukin-6 receptor monoclonal antibody tocilizumab. Arthritis Rheum. 2011;63:1151-5
129. Tanaka T, Kuwahara Y, Shima Y. et al. Successful treatment of reactive arthritis with a humanized anti-interleukin-6 receptor antibody, tocilizumab. Arthritis Rheum. 2009;61:1762-4
130. Henes JC, Horger M, Guenaydin I. et al. Mixed response to tocilizumab for ankylosing spondylitis. Ann Rheum Dis. 2010;69:2217-8
131. Wendling D, Bossert M, Prati C. Short-term effect of IL-6 inhibition in spondylarthritis. Joint Bone Spine. 2010;77:624-5
132. Brulhart L, Nissen MJ, Chevallier P. et al. Tocilizumab in a patient with ankylosing spondylitis and Crohn's disease refractory to TNF antagonists. Joint Bone Spine. 2010;77:625-6
133. Shima Y, Tomita T, Ishii T. et al. Tocilizumab, a humanized anti-interleukin-6 receptor antibody, ameliorated clinical symptoms and MRI findings of a patient with ankylosing spondylitis. Mod Rheumatol. 2011;21:436-9
134. Cohen JD, Ferreira R, Jorgensen C. Ankylosing spondylitis refractory to tumor necrosis factor blockade responds to tocilizumab. J Rheumatol. 2011;38:1527
135. Koumakis E, Feydy A, Kahan A. et al. Interleukin 6 blockade in spondyloarthritis. J Rheumatol. 2012;39:1097-8
136. Dudler J, Aubry-Rozier B. Tocilizumab in axial spondylarthropathies: about 18 cases. Ann Rheum Dis. 2010;70:128
137. Del Castillo Pinol N, Gossec L, Sparsa L. et al. Tocilizumab for treatment of refractory spondyarthritis: report of 5 patients. Ann Rheum Dis. 2011;70:343
138. Ogata A, Umegaki N, Katayama I. et al. Psoriatic arthritis in two patients with an inadequate response to treatment with tocilizumab. Joint Bone Spine. 2012;79:85-7
139. Taniguchi K, Shimazaki C, Fujimoto Y. et al. Tocilizumab is effective for pulmonary hypertension associated with multicentric Castleman's disease. Int J Hematol. 2009;90:99-102
140. Arita Y, Sakata Y, Sudo T. et al. The efficacy of tocilizumab in a patient with pulmonary arterial hypertension associated with Castleman's disease. Heart Vessels. 2010;25:444-7
141. Furuya Y, Satoh T, Kuwana M. Interleukin-6 as a potential therapeutic target for pulmonary arterial hypertension. Int J Rheumatol. 2010 Article ID 720305
142. Navarini AA, French LE, Hofbauer GFL. Interrupting IL-6-receptor signaling improves atopic dermatitis but associates with bacterial superinfection. J Allergy Clin Immunol. 2011;128:1128-30
143. Ohtori S, Miyagi M, Eguchi Y. et al. Efficacy of epidural administration of anti-interleukin-6 receptor antibody onto spinal nerve for treatment of sciatica. Eur Spine J. 2012 [Epub ahead of print]
144. Ogata A, Morishima A, Hirano T. et al. Improvement of HbA1c during treatment with humanised anti-interleukin 6 receptor antibody, tocilizumab. Ann Rheum Dis. 2011;70:1164-5
145. Schultz O, Oberhauser F, Saech J. et al. Effects of inhibition of interleukin-6 signalling on insulin sensitivity and lipoprotein (a) levels in human subjects with rheumatoid diseases. PLoS One. 2010;5:e14328
146. Hirao M, Yamasaki N, Oze H. et al. Serum level of oxidative stress marker is dramatically low in patients with rheumatoid arthritis treated with tocilizumab. Rheumatol Int. 2011 [Epub ahead of print]
147. Matthijs Boekholdt S, Stores ESG. The interleukin-6 pathway and atherosclerosis. Lancet. 2012;379:1176-8
148. IL-6R Genetics Consortium Emerging Risk Factors Collaboration, Sarwar N, Butterworth AS, Freitag DF. et al. Interleukin-6 receptor pathways in coronary heart disease: a collaborative meta-analysis of 82 studies. Lancet. 2012;379:1205-13
149. The Interleukin-6 Receptor Mendelian Randomization Analysis (IL-6R MR) Consortium, Hingorani AD, Casas JP. The interleukin-6 receptor as a target for prevention of coronary heart disease: a mendelian randomisation analysis. Lancet. 2012;379:1214-24
150. Waetzig GH, Rose-John S. Hitting a complex target: an update on interleukin-6 trans-signaling. Expert Opin Ther Targets. 2012;16:225-36
151. Tanaka T, Hagihara K, Hishitani Y. et al. Tocilizumab for the treatment of AA amyloidosis. In: (ed.) Guvenc IA. Amyloidosis - An insight to disease of systems and novel therapies. Croatia: INTECH Open Access Publisher. 2011:155-70
152. Song SN, Tomosugi N, Kawabata H. et al. Down-regulation of hepcidin resulting from long-term treatment with an anti-IL-6 receptor antibody (tocilizumab) improves anemia of inflammation in multicentric Castleman disease. Blood. 2010;116:3627-34
153. Samson M, Audia S, Janikashvili N. et al. Inhibition of IL-6 function corrects Th17/Treg imbalance in rheumatoid arthritis patients. Arthritis Rheum. 2012 [Epub ahead of print]
154. Roll P, Muhammad K, Schumann M. et al. In vivo effects of the anti-interleukin-6 receptor inhibitor tocilizumab on the B cell compartment. Arthritis Rheum. 2011;63:1255-64
155. Muhammad K, Roll P, Seibold T. et al. Impact of IL-6 receptor inhibition on human memory B cells in vivo: impaired somatic hypermutation in preswitch memory B cells and mutational targeting in memory B cells. Ann Rheum Dis. 2011;70:1507-10
156. Chihara N, Aranami T, Sato W. et al. Interleukin 6 signaling promotes anti-aquaporin 4 autoantibody production from plasmablasts in neuromyelitis optica. Proc Natl Acad Sci USA. 2011;108:3701-6
157. Akira S, Kishimoto T. IL-6 and NF-IL6 in acute-phase response and viral infection. Immunol Rev. 1992;127:25-50
158. Matsusaka T, Fujikawa K, Nishio Y. et al. Transcriptional factor NF-IL-6 and NF-κB synergistically activate transcription of the inflammatory cytokines, interleukin 6 and interleukin 8. Proc Natl Acad Sci USA. 1993;90:10193-7
159. Zhao W, Liu M, Kirkwood KL. P38alpha stabilizes interleukin-6 mRNA via multiple AU-rich elements. J Biol Chem. 2008;283:1778-85
160. Iwasaki H, Takeuchi O, Teraguchi S. et al. The IκB kinase complex regulates the stability of cytokine-encoding mRNA induced by TLR-IL-1R by controlling degradation of regnase-1. Nat Immunol. 2011;12:1167-75
161. Kang JG, Pripuzova N, Majerciak V. et al. Kaposi's sarcoma-associated herpesvirus ORF57 promotes escape of viral and human interleukin-6 from microRNA-mediated suppression. J Virol. 2011;85:2620-30
162. Qin Z, Keamey P, Plaisance K. et al. Pivotal advance: Kaposi's sarcoma-associated herpesvirus (KSHV)-encoded microRNA specifically induce IL-6 and IL-10 secretion by macrophages and monocytes. J Leukoc Biol. 2010;87:25-34
163. Leung K, Nabel GJ. HTLV-1 transactivator induces interleukin-2 receptor expression through an NF-kappa B-like factor. Nature. 1988;333:776-8
164. Ballard DW, Bohnlein E, Lowenthal JW. et al. HTLV-1 tax induces cellular proteins that activate the kappa B element in the IL-2 receptor alpha gene. Science. 1988;241:1652-5
165. Scala G, Ruocco MR, Ambrosino C. et al. The expression of the interleukin 6 gene is induced by the human immunodeficiency virus 1 TAT protein. J Exp Med. 1994;179:961-71
166. Ambrosino C, Ruocco MR, Chen X. et al. HIV-1 Tat induces the expression of the interleukin-6 (IL6) gene by binding to the IL6 leader RNA and by interacting with CAAT enhancer-binding protein beta (NF-IL6) transcription factors. J Biol Chem. 1997;272:14883-92
167. Mahe Y, Mukaida N, Kuno K. et al. Hepatitis B virus X protein transactivates human interleukin-8 gene through acting on nuclear factor kB and CCAAT /enhancer-binding protein-like cis-elements. J Biol Chem. 1991;266:13759-63
168. Ohno H, Kaneko S, Lin Y. et al. Human hepatitis B virus X protein augments the DNA binding of nuclear factor for IL-6 through its basic-leucine zipper domain. J Med Virol. 1999;58:11-8
Author contact
![]() Corresponding author: Tadamitsu Kishimoto, Laboratory of Immunoregulation, Immunology Frontier Research Center, Osaka University, 8F IFReC Building, 3-1 Yamada-oka, Suita City, Osaka 565-0871, Japan. Tel.: +81-6-6879-4957 Fax: +81-6-6879-4958 E-mail: kishimotoosaka-u.ac.jp.
Corresponding author: Tadamitsu Kishimoto, Laboratory of Immunoregulation, Immunology Frontier Research Center, Osaka University, 8F IFReC Building, 3-1 Yamada-oka, Suita City, Osaka 565-0871, Japan. Tel.: +81-6-6879-4957 Fax: +81-6-6879-4958 E-mail: kishimotoosaka-u.ac.jp.

 Global reach, higher impact
Global reach, higher impact