10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2013; 9(5):509-517. doi:10.7150/ijbs.5220 This issue Cite
Research Paper
Akt Activation Protects Liver Cells from Apoptosis in Rats during Acute Cold Exposure
Department of Occupational and Environmental Health, Faculty of Military Preventive Medicine, Fourth Military Medical University, 169 Changle West Road, Xi'an, Shaanxi 710032, China.
Received 2012-9-13; Accepted 2013-2-20; Published 2013-5-25
Abstract
Accidental deaths due to exposure to extremely low natural temperature happen every winter. Exposure to extreme cold causes injury of multiple organs. However, early responses of the bodies to acute extreme cold exposure remain incompletely understood. In this study, we found that hepatic glycogen was rapidly reduced in rats exposed to -15°C, and the key enzymes required for glycogenesis were upregulated in the livers of the cold-exposed rats. In line with the rapid consumption of glycogen, acute cold exposure induced a transient elevation of cellular ATP level, which lasted about one hour. The ATP level went back to basal level after two hours of cold exposure. Four hours of cold exposure resulted in cellular ATP depletion and cell apoptosis. The dynamic change of cellular ATP levels was well associated with Akt activation in cold-exposed liver cells. The activation of Akt was required for cold exposure-induced ATP elevation. Blockade of Akt activation diminished the transient increase of intracellular ATP content and exacerbated cell apoptosis during acute cold exposure. These results suggest that Akt activation plays a pivotal role in maintaining cellular bioenergy balance and promoting liver cell survival during acute cold exposure.
Keywords: Cold exposure, Rat, Liver, Apoptosis, ATP, Akt.
Introduction
Occupational and accidental cold exposure is a significant risk for people who are unable to maintain their core body temperature [1]. In the United States, there are hundreds of deaths per year from hypothermia according to the reports from the Centers for Disease Control and Prevention. Accidental deaths due to extreme low natural temperature happen in North of China every winter as well. Human bodies can adapt to the low ambient temperature by insulative and metabolic responses. The insulative response, characterized by a decrease in mean skin temperature, in particular of the extremities, restricts heat loss of internal organs [2, 3]. The metabolic response increases energy expenditure by shivering and non-shivering thermogenesis using glucose and lipids of adipose tissue and liver [2-4]. But nonetheless, prolonged exposure to extreme cold environment has been shown to damage a variety of vital organs, including the liver. Previous studies by using rats have demonstrated that four hours of exposure to -8°C depletes liver glycogen [5], five minutes of exposure to extreme cold temperature, such as -20°C, causes liver edema and histopathological damages [6]. Cold exposure was reported to induce liver metabolic changes and oxidative damage as well [7]. However, it remains incompletely understood how acute cold exposure initially affects liver cells, thereby causes liver injury.
Bioenergetic homeostasis is essential for cell survival. ATP, the main cellular energy source, is produced primarily from glycolysis and mitochondrial respiration. It is well known that intracellular ATP level plays a pivotal role in the regulation of cell fate [8]. A previous in vitro study suggested that ATP depletion is a major contributor to intracellular organelle damage and cell apoptosis upon exposure to low-temperature [9]. Five and ten days of cold exposure lead to a significant increase of hepatic mitochondrial mass and ATP production in isolated mitochondria [10]. However, it is unclear how cellular ATP level in liver cells is regulated during acute cold exposure.
The kinase Akt, also called protein kinase B, is a central signaling molecule that links multiple signaling pathways to control cell growth, proliferation, metabolism, and survival [11]. Akt is a member of the AGC (cAMP-dependent, cGMP-dependent, and protein kinase C) kinase family [12]. Full activation of Akt depends on phophorylation of Thr-308 in its activation loop and Ser-473 in its hydrophobic motif [13, 14]. A recent study showed that Akt, Foxos and mTORC1 form a regulatory circuit controlling the bioenergetic balance in which Akt acts as a major cell energy producer [15]. Previous study showed that Akt is favorably activated in heat-producing brown adipose tissue by insulin [16]. Adipose-specific deletion of PTEN, the negative regulator of PI3K/Akt signaling pathway, enhances insulin sensitivity, energy expenditure and thermogenesis in mice [17]. However, it remains unknown whether Akt can be activated in the liver to promote cold resistance during acute cold exposure.
In this study we showed that acute cold exposure of rats to -15°C, an extremely low temperature that is similar to the natural ambient temperature in winter of some areas of North of China, led to a rapid reduction of hepatic glycogen, an increase of key enzymes required for glycogenesis in the livers. In line with the rapid consumption of glycogen, acute cold exposure induced a transient elevation of cellular ATP level, lasting about one hour. Four hours of cold exposure resulted in cellular ATP depletion and cell apoptosis. Acute cold exposure also transiently activated Akt. Blockade of Akt activation diminished the transient ATP increase and exaggerated cell apoptosis during acute cold exposure. These results suggest that Akt activation plays a critical role in maintaining bioenergetic balance and promoting liver cell survival during acute cold exposure.
Materials and methods
Animals
Male Sprague-Dawley rats, weighing 220-230 g, were used. Animals were maintained on a 12-h light-dark cycle at 25°C with free access to food and water. All experimental procedures involving animal studies were carried out in accordance with the guidelines of the local Animal Care and Use Committee.
Acute cold exposure
Rats were exposed to cold at -15°C for 4 hours by keeping them in a low temperature simulation chamber. Each rat in the control and cold exposure groups was placed in a metal cage (20×20×18 cm) alone and deprived of food and water during exposure.
Drug administration
The selective inhibitor of PI3K, Wortmannin (15 μg/kg body weight, LC Laboratories, Massachusetts, USA), was added to sterile physiological saline and injected intraperitoneally (i.p.) 1 hour before the onset of cold exposure [18]. The rats in the control group were injected with sterile physiological saline.
Tissue preparation
Rats were sacrificed by decapitation immediately after cold exposure, and the left lobe of the liver was removed and immediately stored at -80°C for glycogen and ATP detection, as well as RT-PCR and Western blot analysis.
Measurement of liver glycogen
Rat liver glycogen was detected by sulfuric anthrone methods using liver/muscle glycogen detection kit (Jiancheng, Nanjing, China). Briefly, 75 mg liver tissue was hydrolyzed with alkali liquor and boiled with reaction buffer. The OD value was detected by multimode plate reader (Infinite 200, Tecan, Männedorf, Switzerland) at 620 nm. The tissue glycogen content was normalized to the concurrent standard cure.
Real Time-PCR
Total RNA was isolated from rat livers (50 mg) by using Trizol (Invitrogen Corporation, Barcelona, Spain) according to the instructions of the manufacturer. Total RNA concentration was determined by measuring the absorbance at 260 and 280 nm using a UV-vis spectrophotometer (Nano 2000c, Thermo, USA). RNA (500ng) was transcribed to cDNA using the One-Step cDNA Reverse Transcription Kit with RNase Inhibitor (DRR036A, TAKARA, Japan) according to the manufacturer's instructions. Quantitative real-time PCR was performed using real-time PCR System (7500 Fast Real-time PCR System, Applied Biosystems, USA) with sets of primers as listed below:
G6Pase: 5'-AACGTCTGTCTGTCCCGGATCTA-3' and 5'-CCTCTGGAGGCTGGCATTGTA-3';
Pepck: 5'-AAAGCATTCAACGCCAGGTTC-3' and 5'-CACCACATAGGGCGAGTCTGTC-3';
Actin: 5'-GGAGATTACTGCCCTGGCTCCTA-3' and 5'-GACTCATCGTACTCCTGCTTGCTG-3'. Actin was used as a control.
Measurement of liver ATP content
The liver cell ATP level was detected as previously described [19]. Approximately 70 mg liver tissue was homogenized in 700 μl ice-cold tissue lysis buffer for ATP detection (Beyotime, Jiangsu, China) with protease inhibitor cocktail (Roche, Basel, Switzerland) and PMSF (Beyotime, Jiangsu, China). After centrifuged at 12,000 g for 10 minutes at 4°C, ATP was extracted using 1.5% trichloroacetic acid and supernatants was then neutralized with 1:150 Tris-acetate [pH 7.85]. 100 μl working solution (ATP Assay Kit, Beyotime, Jiangsu, China) was added to a 10 μl diluted sample, the luciferase activity was immediately evaluated by multimode plate reader (Infinite 200, Tecan, Männedorf, Switzerland). ATP content was determined by comparison to a concurrent standard curve and was then normalized by protein concentration and expressed as μmol/mg protein.
Western blots
Liver samples were homogenized on ice in lysis buffer (Beyotime, Jiangsu, China) containing a protease inhibitor cocktail (Roche, Basel, Switzerland) and PMSF (Beyotime, Jiangsu, China). After quantification of protein concentration, samples were diluted to equal concentration. 3 samples in each group were mixed with equal volume and boiled with SDS sample buffer. Equal volumes of sample buffer were separated in a 5-12% Tris-HCl polyacrylamide gel and transferred to PVDF membranes (Millipore, Darmstadt, Germany). After incubation with a blocking buffer (5% nonfat dry milk in 20 mM Tris-HCl [pH 7.5], 137 mM NaCl, and 0.1% Tween 20) for 1 h at room temperature, the membranes were incubated sequentially with primary antibodies against Caspase-3, Akt, phosphor-Akt, GSK-3β, phosphor-GSK-3β (Cell Signaling Technology, Massachusetts, USA), and β-actin (Sigma-Aldrich, Missouri, USA). After incubation with respective horseradish peroxidase-conjugated secondary antibodies in blocking buffer for 1 h at room temperature, immunoreactive proteins were detected via enhanced chemiluminescence.
TUNEL Assay
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) staining was performed using an in situ cell death detection kit according to the manufacturers' protocol (Roche, Basel, Switzerland). Paraffin-embedded liver sections were dewaxed in xylene, and rehydrated in decreasing ethanol solutions. Permeabilization was done by 60 s microwave irradiation in 0.1 M Citrate buffer [pH 6.0] followed by washing three times in PBS (137 mM NaCl, 4.0 mM KCl, 0.6 mM Na2HPO4, 0.15 mM KH2PO4, [pH7.2]). Sections were then incubated with reaction mix (enzyme:label solution = 1:9) at 37°C for 1 h. After incubation and three washes in PBS, slides was incubated with Hoechst 33342 (Beyotime, Jiangsu, China) for 5 min. Apoptotic cells were stained green by the detection kit, while all nuclei were stained blue by Hoechst when observed with a fluorescence microscope (BX51FL, Olympus, Tokyo, Japan). TUNEL-positive cells were quantified microscopically in 8 random fields of each slice section at a magnification of 400×.
Statistical analysis
All values are expressed as means ± s.d.. One-way repeated-measures ANOVAs were applied to assess differences in cellular ATP level and TUNEL positive cells between experimental and control values. Probability values (P) <0.05 were considered significant.
Results
Acute cold exposure led to reduction of liver glycogen and induction of glycogenesis enzymes
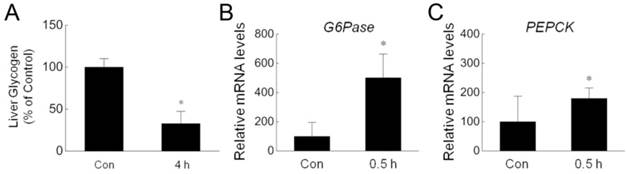
Nonshivering thermogenesis in the liver is critical for the body to adapt to low ambient temperature. To investigate the effect of acute cold exposure on the liver, we measured hepatic glycogen in rats after cold exposure. As shown in Fig. 1A, acute cold exposure resulted in a dramatic reduction of hepatic glycogen after exposure to -15ºC for 4 hours. This result is consistent with previous studies in which rats were exposed to -8ºC [5] or relatively mild 4ºC [20]. In addition to glycogenolysis, glucose can be generated by gluconeogenesis in liver [21, 22]. To determine whether acute cold exposure can promote gluconeogenesis, we assessed the expression of G6Pase and PEPCK, two critical enzymes for gluconeogenesis [23], by quantitative RT-PCR. The expression of these genes were significantly upregulated in the livers isolated from cold-exposed rats (Fig. 1B,C). These data suggest that the body tried to increase glucose output to meet the energy demand during acute cold exposure.
Reduction of hepatic glycogen and increase of gluconeogenesis enzymes during cold exposure. (A) Hepatic glycogen of four pairs of control (Con) and 4 h cold-exposed (4 h) rats was detected by using liver/muscle glycogen assay kit. (B,C) The mRNA amount of G6Pase (B) and PEPCK (C) in the livers isolated from three pairs of control (Con) and 0.5 h cold-exposed (0.5 h) rats was determined by quantitative PCR. * depicts significant difference between the control and cold exposure groups (p<0.05).
Acute cold exposure induced a transient increase of ATP level in rat liver cells
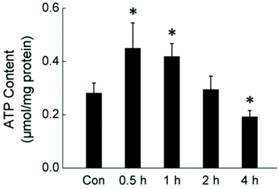
During cold exposure, the body needs to produce more energy to keep the energetic balance. The rapid consumption of glycogen suggests that the liver is trying to maintain the energetic balance through metabolic response. To determine the energetic status of liver cells in rats during acute cold exposure, we measured the intracellular ATP level in liver cells isolated from rats exposed to -15°C for 0.5-4 hours. As shown in Fig. 2, acute cold exposure caused a transient ATP increase, which lasted for about one hour. The ATP level went back to normal after 2 hours cold exposure, and then further declined as the cold exposure extended to 4 hours.
Dynamic change of intracellular ATP level in rat liver cells during four hours of cold exposure. Liver tissues were isolated from rats exposed to -15 ºC for 0.5-4 hours. ATP content of liver cells was measured luminometrically. * depicts significant difference between cold-exposed and the control groups (n = 6, p< 0.05).
Akt activation promoted ATP generation in rat liver cells during acute cold exposure
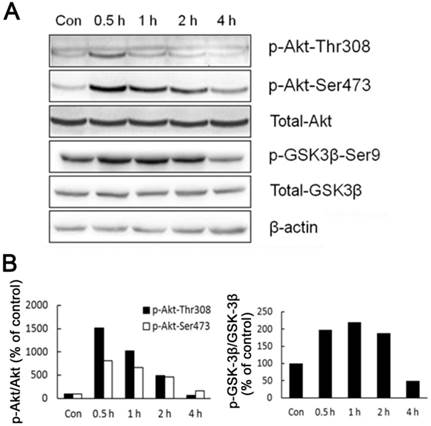
As the main source of cellular energy, ATP is produced primarily by glycolysis and mitochondrial oxidation. Recently, Hay group showed that Akt, Foxos and mTORC1 form a regulatory circuit controlling the bioenergetic balance in which Akt acts as a major cell energy producer [15]. By measuring Akt phosphorylated at Thr-308 and Ser-473 residuals, we showed that Akt was activated in liver cells of rats shortly after acute cold exposure (Fig. 3). The activation of Akt reached the peak after half hour cold exposure, and went back to basal level after four hours cold exposure (Fig. 3). In line with Akt activation, GSK-3β, an Akt kinase substrate showed a similar pattern of phosphoryaltion (Fig. 3).
Activation of Akt and GSK-3β during acute cold exposure. (A) The phosphorylation of Akt and GSK-3β was detected by western blot. β-actin was used as a loading control. A representative of three independent experiments is shown. (B) Quantification of p-Akt (left) and p-GSK3β (right). The density of immunoblot bands was quantified, and the amount of p-Akt (left) and p-GSK3β was normalized to total Akt and GSK3β, respectively. The change of Akt and GSK3β phosphorylation was expressed as percentage of the controls.
Akt activation promoted the generation of cellular ATP during cold exposure. (A) Wortmannin (Wort) was injected intraperitoneally into rats one hour before cold exposure. Ser473 phosphorylation and total Akt were assessed by Western blot. Data shown is a representative of three independent experiments. (B) Quantification of p-Akt. The density of immunoblot bands was quantified, and the amount of p-Akt was normalized to total Akt. The change of Akt phosphorylation was expressed as percentage of the control. (C) Liver tissues were isolated from rats exposed to -15 ºC for 0.5 and 4 hours with or without pretreatment of Wort. ATP content of liver cells was measured luminometrically. * and # depict significant difference between Wort treated and the control groups after 0.5 and 4 hours cold exposure respectively. (n =6, p < 0.05).
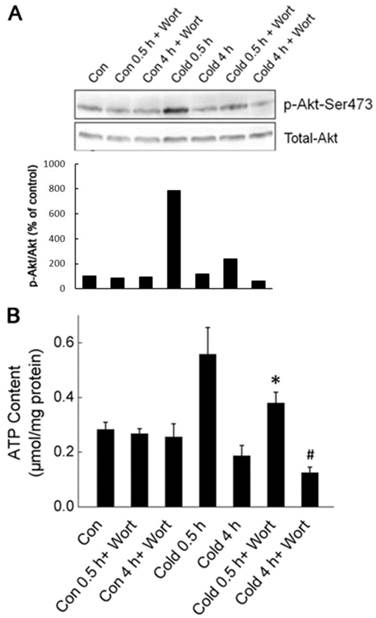
To determine whether Akt activation was required for acute cold exposure-induced transient increase of ATP, we injected the Wortmannin, a selective inhibitor of PI3K-Akt pathway, intraperitoneally (i.p.) into rats to block Akt activation one hour before the onset of cold exposure [18]. As shown in Fig. 4, Wortmannin treatment abolished cold exposure-induced Akt activation. Inhibition of Akt diminished the transient ATP increase in liver cells after half hour of cold exposure, and caused further decrease of intracellular ATP content in liver cells after four hours of cold exposure. These data suggest that Akt activation is an early response of liver cells to keep bioenergetic balance by promoting ATP generation.
Blockade of Akt activation exacerbated liver cell apoptosis in rats during acute cold exposure
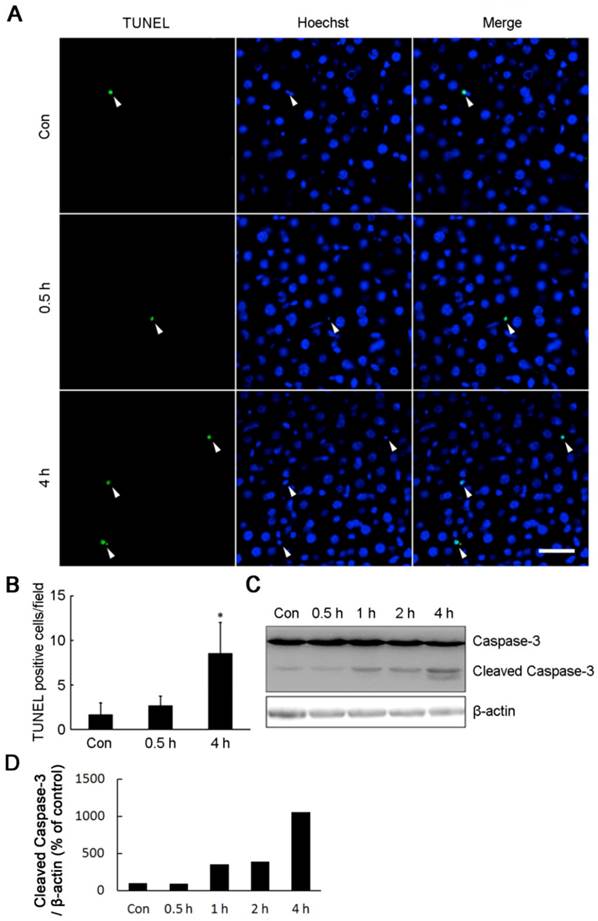
Maintenance of intracellular ATP under bioenergrtic stress is critical for cell survival. In line with the decrease of intracellular ATP content, four hours of cold exposure caused a significant increase of apoptosis cells (Fig. 5). TUNEL positive cells were markedly increased in the livers isolated from rats exposed to -15°C for 4 hours (Fig. 5A,B). The cell apoptosis was confirmed by Western blot showing a 12 kDa cleaved band of Caspase-3 in liver cells after four hours of cold exposure (Fig. 5C,D).
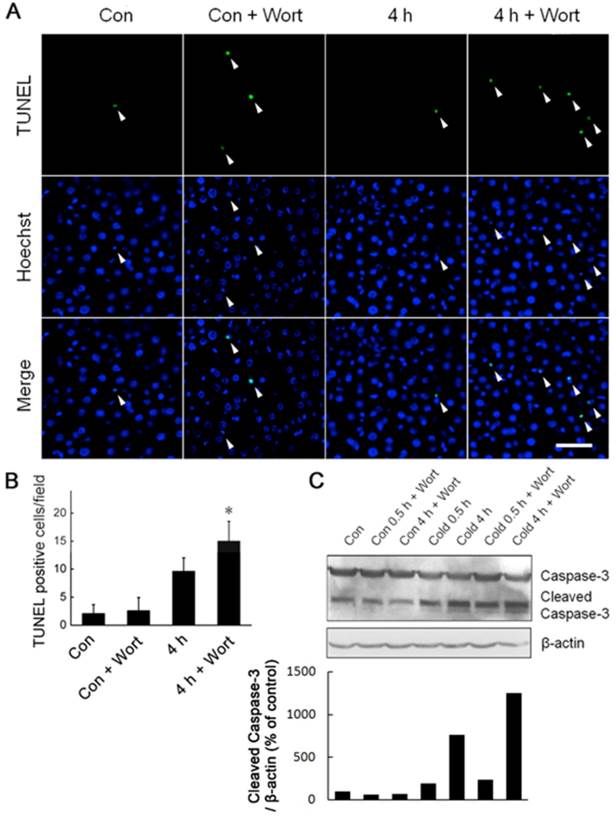
Under cold stress, Akt was activated and required for liver cells to generate more ATP to keep the bioenergetic balance (Figs. 3,4). We speculated that Akt activation may play a critical role in promoting liver cell survival during acute cold exposure. Indeed, blockade of Akt activation by injection of Wortmannin heightened cell apoptosis in the livers of rats after acute cold exposure indicated by increase of TUNEL-positive cells (Fig. 6A,B) and Caspase-3 cleavage (Fig. 6C,D).
Discussion
Maintenance of an energy balance under bioenergetic stress is critical for cell survival [24]. In this study, we investigated the initiate responses of liver cells of rats exposed to extremely low temperature. Our data showed that cold exposure led to a rapid depletion of hepatic glycogen and upregulation of enzymes critical for gluconeogenesis in the liver. Akt was activated shortly after cold exposure, which promoted ATP generation, leading to a transient increase of intracellular ATP level, and protected liver cell from cell apoptosis.
As the center of intermediary metabolism, the liver releases glucose and lipids into the circulation to meet the energy demands [25]. However, excessive energy demand may cause the loss of liver function. It has been reported that five minutes of -20°C freezing cold exposure can induce rat liver edema and histopathological damage [6]. It suggests that the liver maintains circulation energy substrate to meet the energy demand at the expense of its functional integrity during cold exposure. At the early time of cold exposure, increased glucose output from the liver is largely responsible for the elevation of circulating glucose [26]. As the time of cold exposure going on, this might lead to liver energy depletion, which in turn could lead to cellular ATP depletion and cell injury. It has been reported that four hours of -8°C exposure causes glycogen depletion in the livers of rats [5], which might be the cause of liver injury. The data from this study indicated that the liver of cold-exposed rats tried to maintain an intracellular energy balance through increasing circulating glucose by glucogenolysis and gluconeogenesis, and activating Akt pathway that promotes ATP generation. But nonetheless, acute cold exposure-induced elevation of intracellular ATP is very transient, lasting only about one hour. The level of intracellular ATP content of liver cells was significantly reduced after four hours of cold exposure. The decline of ATP leve1 is probably due to extended cold exposure-induced depletion of liver glycogen as reported in this and previous studies [5]. It was reported that decreases in ATP content could potentially promote heat production [27]. However, cellular ATP level plays a pivotal role in cell survival, the decrease of ATP level can also lead to cell apoptosis [8]. In line with the depletion of cellular ATP, we found that four hours of cold exposure caused a significant increase of scattered distributed TUNEL positive apoptotic cells in the liver, and the cleavage of Caspase-3 in the liver cells.
Acute cold exposure caused rat liver cell apoptosis. (A) Rats in cold exposure groups were maintained at -15°C for 0.5, 1, 2, and 4 h, while rats in control group (Con) were kept at room temperature. TUNEL assay was performed with an in situ cell death detection kit. White arrow heads indicated the apoptotic nuclei stained by the TUNEL reagent, bar “—” = 50 μm. (B) Quantification of TUNEL-positive cells in Figure 5A. * depicts significant difference between cold-exposed and the control groups (n = 3, p< 0.05). (C) Western bolt analysis of Caspase-3 cleavage in rat liver cells after cold exposure. β-actin was used as a loading control. A representative of three independent experiments is shown. (D) Quantification of cleaved Caspase-3. The density of immunoblot bands was quantified, and the amount of cleaved Caspase-3 was normalized to β-actin. The change of cleaved Caspase-3 was expressed as percentage of the control.
Blockade of Akt activation exaggerated liver cell apoptosis during acute cold exposure. Wortmannin (Wort) was injected into rats intraperitoneally 1 h before cold exposure. The apoptotic cells were assessed by TUNEL staining (A). White arrow heads indicated the apoptotic nuclei stained by the TUNEL reagent, bar “—” = 50 μm. TUNEL positive cells in Figure 6A were quantified (B). * depicts significant difference between Wort treated and the control groups after 4 hours cold exposure (n =3, p < 0.05). (C) Western bolt analysis of Caspase-3 cleavage in rat liver cells after 0.5 and 4 hours of cold exposure with or without Wort pretreatment. β-actin was used as a loading control. A representative of three independent experiments is shown. (D) Quantification of cleaved Caspase-3. The density of immunoblot bands was quantified, and the amount of cleaved Caspase-3 was normalized to β-actin. The change of cleaved Caspase-3 was expressed as percentage of the control.
Metabolic response of the bodies is critical for acclimatization. In addition to the nonshivering thermogenesis in the liver, the response includes shivering thermogenesis of the skeleton muscle [2-4, 28-30]. Currently, it remains unclear how acute exposure of rats to -15ºC affects skeleton muscle cells. It's a very interesting topic currently under investigation in our group.
Akt, a major cell energy producer, was activated in liver cells of rats shortly after acute cold exposure. Similar results have been reported that Akt can be transiently activated during 1 hour of exposure to 4°C [31], and phosphorylation of the activating residue of Akt, Ser473 and Thr308, occurs in response to low temperatures in cell cultures [32]. In our study, blockade of Akt activation diminished the transient increase of ATP. These data suggest a critical role of Akt activation in promoting ATP generation and maintaining a cell energy balance under cold stress which were in accordance with previously reported results that Akt exerts its protective role through regulation of cellular ATP level [33]. Interestingly, blockade of Akt signal pathway only partially abolished acute cold exposure-induced ATP elevation, suggesting other signal pathway may also be activated to promote ATP generation. MEK-Erk1/2 signal pathway has been reported to be activated in the adipose tissue after cold exposure [34, 35]. Our previous study also showed that cold exposure induces Erk1/2 activation in brain [36]. But nonetheless, Erk1/2 was not activated in rat liver by acute cold exposure (data not shown). The additional signal pathway promoting ATP generation during cold exposure warrants future study.
In addition to its critical role in promoting ATP generation, Akt was recently shown to protect cancer cells from bioenergetic stress by enhancing glucose uptake and glycogen storage that allows the maintenance of ATP levels during bioenergetic stress [24]. Akt activation may also promote glucose uptake under cold stress if there is sufficient glucose. It will be an interest topic to investigate whether Akt-dependent cold exposure-induced ATP elevation can last longer and have a better protection of liver cells from apoptosis when give rats exogenous glucose.
In conclusion, this study revealed that Akt activation, as an early response of host liver cells to cold stress, plays a critical role in maintaining bioenergetic balance and promoting liver cell survival during acute cold exposure.
Abbreviations
PI3K, phosphoinositide 3-kinase; Akt, protein kinase B; GSK, glycogen synthase kinase; PVDF, polyvinylidene difluoride; Wort, wortmannin; TUNEL, terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling.
Acknowledgements
This study was sponsored by the National Key Technology R&D Program [2009BAI85B04]; National Nature Science Foundation of China [30872077, 30901173, 81072285, and 81001232]; and Program for Changjiang Scholars and Innovative Research Team in University [PCSIRT].
Competing Interests
The authors have declared that no competing interest exists.
References
1. Imray CH, Richards P, Greeves J. et al. Nonfreezing cold-induced injuries. J R Army Med Corps. 2011;157:79-84
2. Cabanac M. Thermiametrics and behavior. In: (ed.) Blatteis CM. Physiology and pathophysiology of temperature regulation. New York: World Scientific. 1998:108-126
3. van Marken Lichtenbelt WD, Daanen HA. Cold-induced metabolism. Curr Opin Clin Nutr Metab Care. 2003;6(4):469-75
4. Makinen TM. Different types of cold adaptation in humans. Front Biosci (Schol Ed). 2010;2:1047-67
5. Meneghini A, Ferreira C, Abreu LC. et al. Cold stress effects on cardiomyocytes nuclear size in rats: light microscopic evaluation. Rev Bras Cir Cardiovasc. 2008;23:530-3
6. Bozkurt A, Ghandour S, Okboy N. et al. Inflammatory response to cold injury in remote organs is reduced by corticotropin-releasing factor. Regul Pept. 2001;99:131-9
7. P. Venditti, R. Pamplona, V. Ayala, et al. Differential effects of experimental and cold-induced hyperthyroidism on factors inducing rat liver oxidative damage. J Exp Biol. 2006;209:817-825
8. Richter C, Schweizer M, Cossarizza A. et al. Control of apoptosis by the cellular ATP level. FEBS Lett. 1996;378:107-10
9. Clarke DM, Baust JM, Van Buskirk RG. et al. Addition of anticancer agents enhances freezing-induced prostate cancer cell death: implications of mitochondrial involvement. Cryobiology. 2004;49:45-61
10. Iossa S, Barletta A, Liverini G. Hepatic selective adjustments in short-term cold exposed rats. Cell Biochem Funct. 1991;9:275-80
11. Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261-74
12. Woodgett JR. Recent advances in the protein kinase B signaling pathway. Curr Opin Cell Biol. 2005;17:150-7
13. Alessi DR, James SR, Downes CP. et al. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7:261-9
14. Cho H, Thorvaldsen JL, Chu Q. et al. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J Biol Chem. 2001;276:38349-52
15. Chen CC, Jeon SM, Bhaskar PT. et al. FoxOs inhibit mTORC1 and activate Akt by inducing the expression of Sestrin3 and Rictor. Dev Cell. 2010;18:592-604
16. Gasparetti AL, de Souza CT, Pereira-da-Silva M. et al. Cold exposure induces tissue-specific modulation of the insulin-signalling pathway in Rattus norvegicus. J Physiol. 2003;552(Pt 1):149-62
17. Komazawa N, Matsuda M, Kondoh G. et al. Enhanced insulin sensitivity, energy expenditure and thermogenesis in adipose-specific Pten suppression in mice. Nat Med. 2004;10(11):1208-15
18. Gurusamy N, Lekli I, Gorbunov NV. et al. Cardioprotection by adaptation to ischaemia augments autophagy in association with BAG-1 protein. J Cell Mol Med. 2009;13:373-87
19. He S, Atkinson C, Qiao F. et al. A complement-dependent balance between hepatic ischemia/reperfusion injury and liver regeneration in mice. J Clin Invest. 2009;119:2304-16
20. Iossa S, Lionetti L, Mollica MP. et al. Effect of cold exposure on energy balance and liver respiratory capacity in post-weaning rats fed a high-fat diet. Br J Nutr. 2001;85(1):89-96
21. Boden G. Gluconeogenesis and glycogenolysis in health and diabetes. J Investig Med. 2004;52(6):375-8
22. [22] Jitrapakdee S. Transcription factors and coactivators controlling nutrient and hormonal regulation of hepatic gluconeogenesis. Int J Biochem Cell Biol. 2012;44(1):33-45
23. Foretz M, Hébrard S, Leclerc J. et al. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest. 2010;120(7):2355-69
24. Cheng KW, Agarwal R, Mitra S. et al. Rab25 increases cellular ATP and glycogen stores protecting cancer cells from bioenergetic stress. EMBO Mol Med. 2012;4:125-41
25. Louzao MC, Vieytes MR, Botana LM. Effect of okadaic acid on glucose regulation. Mini Rev Med Chem. 2005;5:207-15
26. REID R. L. Studies on the carbohydrate metabolism of sheep. XV. The adrenal response to the climatic stresses of cold, wind and rain. Australian Journal of Agricultural Research. 1962;13:296-306
27. Bobyleva V, Pazienza L, Muscatello U. et al. Short-term hypothermia activates hepatic mitochondrial sn-glycerol-3-phosphate dehydrogenase and thermogenic systems. Arch Biochem Biophys. 2000;380(2):367-72
28. Tikuisis P, Bell DG, Jacobs I. Shivering onset, metabolic response, and convective heat transfer during cold air exposure. J Appl Physiol. 1991;70(5):1996-2002
29. Haman F, Peronnet F, Kenny GP. et al. Effects of carbohydrate availability on sustained shivering I. Oxidation of plasma glucose, muscle glycogen, and proteins. J Appl Physiol. 2004;96(1):32-40
30. Morrison SF, Nakamura K, Madden CJ. Central control of thermogenesis in mammals. Exp Physiol. 2008;93(7):773-97
31. Deiuliis JA, Liu LF, Belury MA. et al. Beta(3)-adrenergic signaling acutely down regulates adipose triglyceride lipase in brown adipocytes. Lipids. 2010;45(6):479-89
32. Shao ZH, Sharp WW, Wojcik KR. et al. Therapeutic hypothermia cardioprotection via Akt- and nitric oxide-mediated attenuation of mitochondrial oxidants. Am J Physiol Heart Circ Physiol. 2010;298(6):H2164-73
33. Hahn-Windgassen A, Nogueira V, Chen CC. et al. Akt activates the mammalian target of rapamycin by regulating cellular ATP level and AMPK activity. J Biol Chem. 2005;280(37):32081-9
34. Lindquist JM, Rehnmark S. Ambient temperature regulation of apoptosis in brown adipose tissue. Erk1/2 promotes norepinephrine-dependent cell survival. J Biol Chem. 1998;273(46):30147-56
35. Gasparetti AL, de Souza CT, Pereira-da-Silva M. et al. Cold exposure induces tissue-specific modulation of the insulin-signalling pathway in Rattus norvegicus. J Physiol. 2003;552(Pt 1):149-62
36. Zheng G, Chen Y, Zhang X. et al. Acute cold exposure and rewarming enhanced spatial memory and activated the MAPK cascades in the rat brain. Brain Res. 2008;1239:171-80
Author contact
![]() Corresponding author: Tel: +86 29 84774863 Fax: +86 29 84774863 E-mail: luowenjedu.cn (W. Luo); or jy_chenedu.cn (J. Chen).
Corresponding author: Tel: +86 29 84774863 Fax: +86 29 84774863 E-mail: luowenjedu.cn (W. Luo); or jy_chenedu.cn (J. Chen).

 Global reach, higher impact
Global reach, higher impact