10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2015; 11(3):324-334. doi:10.7150/ijbs.10567 This issue Cite
Review
Optimization of Pre-transplantation Conditions to Enhance the Efficacy of Mesenchymal Stem Cells
1. Department of Restorative Dentistry, Faculty of Dentistry, University of Malaya, Kuala Lumpur, Malaysia.
2. Regenerative Dentistry Research Group, Faculty of Dentistry, University of Malaya, Kuala Lumpur, Malaysia.
3. Department of Biotechnology, Faculty of Science, International Islamic University Malaysia, Kuantan, Malaysia.
Received 2014-9-17; Accepted 2014-12-20; Published 2015-2-5
Abstract
Mesenchymal stem cells (MSCs) are considered a potential tool for cell based regenerative therapy due to their immunomodulatory property, differentiation potentials, trophic activity as well as large donor pool. Poor engraftment and short term survival of transplanted MSCs are recognized as major limitations which were linked to early cellular ageing, loss of chemokine markers during ex vivo expansion, and hyper-immunogenicity to xeno-contaminated MSCs. These problems can be minimized by ex vivo expansion of MSCs in hypoxic culture condition using well defined or xeno-free media i.e., media supplemented with growth factors, human serum or platelet lysate. In addition to ex vivo expansion in hypoxic culture condition using well defined media, this review article describes the potentials of transient adaptation of expanded MSCs in autologous serum supplemented medium prior to transplantation for long term regenerative benefits. Such transient adaptation in autologous serum supplemented medium may help to increase chemokine receptor expression and tissue specific differentiation of ex vivo expanded MSCs, thus would provide long term regenerative benefits.
Keywords: Mesenchymal stem cell, hyper-immunogenicity, chemokine receptors, xenogenic, autologous, allogeneic.
Introduction
The lineage committed progenitor cells or unipotent stem cells maintain cellular homeostasis [1]. Mesenchymal stem cells or mesenchymal stromal cells (MSCs) originated in bone-marrow, adipose tissue, dental pulp are involved in such homeostasis [2]. The number of MSCs increases in the peripheral blood during skeletal muscle injury [3] and osteoporosis [4]. Higher numbers of circulatory MSCs are also observed immediately after ischemic stroke and myocardial infarction [5, 6]. However, natural regenerative process alone is insufficient to repair a diseased or injured organ in case of myocardial infarction, stroke and spinal cord injuries because of the limited indigenous supply of the stem cells [7, 8]. Hence, adjunctive treatment such as stem cell based regenerative therapy has been given considerable attentions [7].
Due to pluripotency, embryonic stem cells (ESCs) are initially considered as the best source of stem cells for regenerative therapy [9]. Ethical issues over the use of ESCs compel researchers to search for suitable alternative [10]. In recent years, researchers developed a technology to generate induced pluripotent stem cells (iPSCs) that share characteristics of ESCs [11, 12]. Epigenetic memory, teratoma formation and immunogenicity related to the therapeutic potentials of iPSCs are yet to be resolved [13, 14]. Meanwhile, due to multi-differentiation potential, immunomodulatory effects, trophic functions, vasculogenesis potential of MSCs as well as its large donor pool make MSCs as the potential source for regenerative therapy [2, 15, 16].
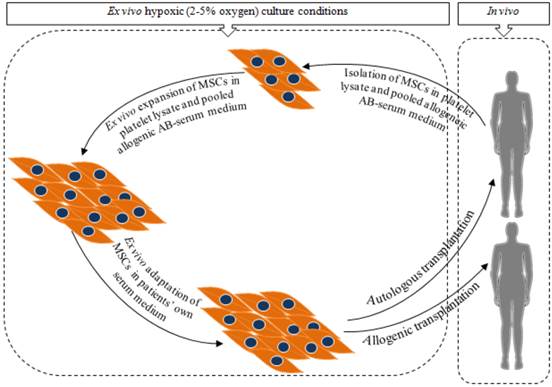
Steps to produce clinical grade MSCs for long term regenerative benefits. Isolation and expansion of MSCs in platelet lysate or pooled allogeneic AB-serum supplemented medium followed by adaptation in autologous serum (patients' own serum) supplemented medium. Hypoxic (2-5% oxygen) culture condition will be favourable for both the initial isolation and expansion later for adaptation [18, 36, 37, 39, 40].
For each regenerative therapy, 50-400 million MSCs are required [17, 18]. The presence of very low number of MSCs within the tissues makes it impossible to isolate such a large number of MSCs from a single donor. Recently, derivation of MSCs from ESCs and iPSCs has been reported [19-23]. MSCs from these sources can also be used for cell based therapy and tissue engineering. Thus iPSCs may resolve patient-specific MSCs scarcity [20, 21, 23]. However, regardless of the sources, ex vivo expansion of MSCs prior transplantation is required to yield enough MSCs for cell based therapy [18, 24].
Several in vitro, in vivo and clinical studies reported encouraging regenerative potentials of MSCs [25-28]. However, low number of engrafted MSCs is considered as a major drawback for long term functional benefits [29, 30]. Different strategies were attempted to minimize such drawback such as intra-arterial delivery instead of intravenous delivery to avoid accumulation of MSCs in the lung [31, 32]; and modification of cell surface molecules through chemical, genetic and coating techniques to promote selective adherence to particular organs or tissues [33]. Several modifications in ex vivo or in vitro culture environment have also given due attention to overcome insufficient engraftment of MSCs such as culturing MSCs in hypoxic environment for partial [34] or entire [35] period of time; and culturing MSCs in medium that mimics the hypoxic condition [36]. Culture environment have an influential effect on cellular ageing and chemokine marker expression that may affect trafficking and engraftment of MSCs following transplantation [17, 18, 37]. In addition, there are safety concerns regarding hyper-immunogenicity to MSCs expanded in xenogenic serum [38] that might be a cause of acute rejection of transplanted MSCs.
To resolve the issue of poor engraftment of MSCs, this article elaborates the advantages and drawbacks of different approaches of ex vivo MSCs culture techniques. Finally a two phase ex vivo MSCs culture strategy is proposed as a possible way to produce clinical grade MSCs to enhance engraftment and regenerative outcomes. In phase 1, MSCs are initially isolated and expanded in human platelet lysate or pooled allogeneic AB-serum supplemented medium followed by the phase 2 where the expanded MSCs are cultured in autologus serum (patients' own) supplemented medium mainly to adapt the MSCs prior to transplantation (Figure 1).
Causes behind Poor Engraftment of MSCs Following Transplantation
For clinical trials, MSCs are mostly expanded in xenogenic serum supplemented media and incubated in ambient oxygen condition (Table 1). Use of MSCs (both autologous and allogeneic) for therapeutic purposes has been proven safe [41-55]. Clinical trials that used autologous MSCs to treat multiple system atrophy, renal transplant rejection, multiple sclerosis, ischemic cardiomyopathy, spinal cord injury and liver failure shown to have short term regenerative benefits or partial improvement [41, 42, 44, 46, 47, 50, 53, 55]. Clinical trials with allogeneic MSCs have also been shown significantly increased overall survival of graft-versus-host disease patients; improved forced expiration volume and global symptom score, and reduced infarct size in cardiovascular disease patients; improved Ankel Brachial Pressure Index in critical limb ischemia patient; and increased osteopoetic cell engraftment in patient with osteogenesis imperfecta [43-45, 48, 49, 54]. However, none of them have been reported the long term benefits from MSCs therapy.
List of completed clinical trials using ex vivo expanded MSCs.
| Clinical trial No. | Source of MSCS | Serum Supplement | Disease Treated | Dose No. of treatment | Route of Administration | Phase | Design | References |
|---|---|---|---|---|---|---|---|---|
| NCT00395200 | Au BM | FBS | Multiple Sclerosis | 1-2 ×106 cells/ kg BW Single | Intravenous | I & II | Non-randomized, Safety/efficacy study, Single group assignment, Open label | [41, 42] |
| NCT00504803 | Allo BM | Irradiated FBS | Graft-versus-host-disease | - Single | Intravenous | II | Non-randomized, Safety/efficacy study, Single group assignment, Open label | [43] |
| NCT01087996 | Au BM Allo BM | - | Ischemic cardiomyopathy | 20/100/200 ×106 cells Single | Transendocardial | I & II | Randomized, Safety/efficacy study, Parallel assignment, Open label | [44] |
| NCT00114452 | Allo BM | - | Myocardial infarction | 0.5/1.6/5 ×106 cells / kg BW Single | Intravenous | I | Randomized, Safety study, Parallel assignment, Double blind (Subject, Caregiver, Investigator, Outcomes assessor) | [45] |
| NCT00658073 | Au BM | - | Renal transplant rejection | 1-2×106 cells/ kg BW Twice | Intravenous | - | Randomized, Efficacy study, Parallel assignment, Open label | [46] |
| NCT00734396 | Au BM | FBS | Renal transplant rejection | 1×106 cells/ kg BW Twice | Intravenous | I & II | Non-randomized, Safety/efficacy study, Single group assignment, Open label | [47] |
| NCT00883870 | Allo BM | - | Critical limb ischemia | 2×106 cells/kg BW Single | Intramuscular (gastrocnemius muscle) | I & II | Randomized, Safety/efficacy study, Parallel assignment, Double blind (Subject, caregiver, investigator) | [48] |
| NCT00823316 | Allo UCB | FBS | Graft rejection and graft-versus-host-disease | 1 & 5 ×106 cells/ kg BW Single | Intravenous | I & II | Randomized, Safety/efficacy study, Parallel assignment, Open label | [49] |
| NCT00911365 | Au BM | FBS | Multiple system atrophy | 40×106 cells Multiple | Intra arterial (1 time) Intravenous (3 times) | II | Randomized, Parallel assignment, Single blind (subject) | [50] |
| NCT01274975 | Au AD | FBS | Spinal cord injury | 400×106 cells Single | Intravenous | I | Randomized, Safety study, Single group assignment, Open label | [51] |
| NCT00683722 | Allo BM | - | Coronary obstructive pulmonary disorder. | 100×106 cells Multiple | Intravenous | II | Randomized, Safety/efficacy study, Parallel assignment, Double blind (subject, caregiver, investigator, outcomes assessor) | [52] |
| NCT00956891 | Au BM | FBS | Liver failure | ≈100×106 cells Single | Hepatic artery | - | Case Control, retrospective | [53] |
| NCT00187018 | Allo BM | FBS | Osteogenesis imperfecta | 0.68-2.75×103 cells/kg BW Single | Intravenous | - | Non-Randomized, Safety/Efficacy Study, Single Group Assignment, Open Label | [54] |
| NCT00816803 | Au BM | Serum free | Spinal cord injury | 2×106 cells/ kg BW Multiple | Lumbar puncture | I & II | Safety/Efficacy Study, Parallel Assignment, Single Blind (Outcomes Assessor) | [55] |
Au, Autologous; Allo, Allogeneic; BM, Bone marrow; UCB, Umbilical cord blood; AD, Adipose derived.
Prior to transplantation, MSCs are generally expanded in ex vivo culture conditions. Oxygen concentration of this culture environment is higher than MSCs' natural niche and the media contains xenoantigen [56, 57]. This culture conditions resulted in telomere shortening, early senescence, loss of chemokine receptors, and xeno-contamination in cultured MSCs [18, 37, 38]. Use of these ex vivo expanded MSCs may exhibit post-transplantation hyper-immunogenicity, improper trafficking and poor engraftment which in turn might result in failure of long term regenerative benefits.
Post-transplantation hyper-immunogenicity to MSCs cultured in xenogenic serum
MSCs are able to prevent expression of co-stimulatory molecules such as CD40, CD80, CD83 and CD86 and induce expression of inhibitory molecules such as B7-H1, B7-H4 and human leukocyte antigen G (HLA-G). At the same time, MSCs were reported to secrete soluble factors such as prostaglandin E2 (PGE2), transforming growth factor (TGF)-β, interleukin 10 (IL-10), nitric oxide (NO), hepatocyte growth factor (HGF) and indolamin-2,3-dioxygenase (IDO). These properties help MSCs to inhibit proliferation and function of cytotoxic T cells (TC), natural killer (NK) cells and B cells, as well as prevent differentiation of monocytes into antigen-presenting dendritic cells (DCs). Notably, IDO plays an important role in activating immunosuppressive regulatory T cells (Tregs), facilitating differentiation of monocytes into M2 macrophages, and inhibit helper T cells (TH) and TC cells [58-60]. These immunomodulatory properties, makes MSCs a “universal donor” for stem cell based regenerative therapy [61].
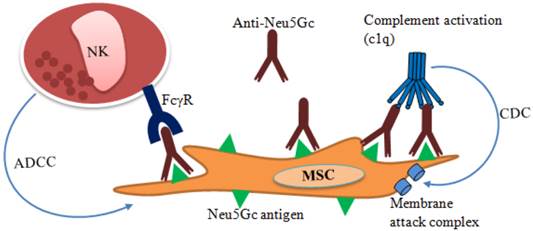
In contrast, MSCs are described as immune evasive rather than immune privileged since differentiated MSCs or MSCs treated with interferon gamma (INF-γ) exhibit significantly higher expression of MHC class I and MHC class II. If mismatched, these MHC class I and MHC class II act as a source of hyper-immunogenicity thus the “universal donor” role of MSCs remains questionable [62, 63]. Besides, MSCs expanded in fetal bovine serum (FBS) supplemented media can be contaminated with bovine proteins that remains after multiple washings [64]. MSCs contaminated with N-glycolylneuraminic acid (Neu5Gc) xenoantigen [65, 66] originating from FBS potentially cause immunological reaction after transplantation with anti-Neu5Gc antibodies present in human serum [67, 68]. Binding of anti-Neu5Gc antibody present in the human serum to xenoantigen Neu5Gc may cause post-transplantation lysis of the MSCs (Figure 2). Antibody dependent lysis of MSCs may take place in two ways: (i) complement-dependent cytotoxicity (CDC) and (ii) NK cell based antibody dependent cell-mediated cytotoxicity (ADCC).
MSCs cytotoxicity by complement activated membrane attack complex regardless of their source (autologous or allogeneic) has been reported in both in vivo and in vitro studies [66, 69]. However, CDC was less to autologous MSCs and this effect was greatly reduced when CD55 was highly expressed by MSCs [69]. In contrast, MSCs that show expression of complement regulatory proteins such as CD46, CD55, and CD59 are reported to be resistant to CDC [66]. The role of MSCs secreted factor H on inhibiting complement activation has also been reported [70]. For cell mediated cytotoxicity, higher phagocytic activity and ADCC was reported for the Neu5Gc-contaminated MSCs. In addition, reduced Neu5Gc contamination was reported to reduce cell mediated phagocytosis and lysis of the MSCs expanded in human serum supplemented medium [66]. Thus, CDC and ADCC to xeno-contaminated MSCs may lead to the acute rejection of transplanted cells [65, 66, 71]. Therefore, the effect of xenogenic serum on poor engraftment of transplanted MSCs regardless of autologous or allogeneic source should not be overlooked. Moreover, FBS supplemented media are potential source of viral or bacterial infections [72] and prions transmission [73].
Immune response to transplanted xeno-contaminated MSCs. N-glycolylneuraminic acid (Neu5Gc) in FBS contaminates MSCs during ex vivo expansion. Anti-Neu5Gc antibody present in human serum may bind to the xeno-contaminated MSCs following transplantation. As a result, natural killer (NK) cells may bind to the antibody coated cells through Fc-gamma receptors (FcγR) and cause lysis by antibody dependent cell mediated cytotoxicity (ADCC). Anti-Neu5Gc antibody may also activate complement-dependent cytotoxicity (CDC) and cause lysis through membrane attack complex.
Aging of MSCs during in vitro or ex vivo expansion
In standard culture conditions, MSCs reach senescence after a limited number of cell division [17]. Cellular ageing or replicative senescence affects proliferation and differentiation potentials of stem cells [74-77]. Senescence can be triggered by gradual loss of telomere repeat sequences, DNA damage and de-repression of the INK4/ARF locus [78]. Without any detectable telomere loss, oxidative stress-induced premature senescence may also take place in cultured cells [79, 80].
Among the different mechanisms of cellular aging, gradual loss of telomere sequence has been studied the most. Telomere is a guanine-rich DNA repeat sequence of the chromosomal end [81]. A reverse transcriptase named 'telomerase' plays key role in maintaining the telomeric repeats. Usually in rapidly proliferating germ cells and ES cells telomerase is highly expressed. After birth, telomerase level within cells including in MSCs gradually diminishes [81]. As a result, telomere repeat sequences in MSCs is gradually lost at a similar rate to non-stem cells [82]. Basic fibroblast growth factor (bFGF) was reported to maintain long telomeres without up-regulation of telomerase expression [83, 84]. However, the possible effect of bFGF on reduced differentiation potential and priming of MSCs should be taken into consideration when used in regenerative therapy [85].
Previous study has also shown that highly confluenced MSCs (100%) aged faster than the cells passaged at lower confluency (60-70%). During in vitro culture of MSCs, initial dense population showed prolonged population doubling time, higher expression of senescence associated β-galactosidase, and increased cell cycle arrest along with increased intracellular reactive oxygen species (ROS). However, difference in telomere length and alteration in p53 expression was not observed [80]. Contrary to this observation, the presence of ROS causes Wharton's jelly derived MSCs to be irregularly enlarged and flattened with granular cytoplasm and induce higher expression of other senescent markers namely p53, p21, p16 and lysosomal β-galactosidase [86]. Studies have also been reported that ambient culture environment cause higher ROS generation within cultured cells including MSCs compared to hypoxic culture environment (2-5%), and ROS is also responsible for faster telomere shortening and cellular senescence [17, 37].
These evidences suggest that aging of MSCs in culture is inevitable. It might not be possible to stop the aging process completely, yet it can be delayed and reduced by using proper growth factors and manipulating the culture practice and environment. As the success of stem cell based therapy depends on both the self-renewal and differentiation (towards the target cell populations) of the transplanted cells following engraftment [87], it is important to produce higher number of MSCs with longer telomere and regenerative potentials for successful regenerative therapy.
Ex vivo expansion in xenogenic serum may lead to improper trafficking and engraftment of the transplanted MSCs
Site specific trafficking and engraftment of transplanted MSCs are important in cell based regenerative therapy. These events are assisted by the affinity of chemokine receptors on MSCs (CXCR4, CXCR7, CX3CR1) to the chemokines (SDF-1, fractalkine) [34, 88-92]. Loss of these chemokine receptors during their in vitro or ex vivo expansion [93] is thought to affect the regenerative outcomes. Growth factors like platelet-derived growth factor (PDGF)-AB, PDGF-BB, insulin-like growth factor-1 (IGF-1), HGF, epidermal growth factor (EGF) and angiopoietin-1 (Ang-1) work as chemoattractants for MSCs [90, 94-97]. Inflammatory cytokine such as tumor necrosis factor alpha (TNF-α) also helps migration of MSCs towards the site of chemokines [90, 92]. All these paracrine signalling molecules are of primary importance for tissue specific migration and engraftment of MSCs. In vivo composition of these cytokines may vary depending on the type and stage of pathological conditions. Once isolated and expanded in ex vivo culture media, MSCs could embrace different cytokine composition, depending on the type of serum supplement. In other words, media supplementation with xenogenic serum, allogeneic human serum, platelet lysate or growth factors do not represent in situ cytokine composition of the serum of the patients undergoing stem cell based regenerative therapy. Therefore, paracrine signals to ex vivo expanded MSCs in those media supplement might cause improper trafficking consequently poor homing and engraftment.
Approaches to Enhance Engraftment and Regenerative Benefits of Cultured MSCs
In recent years, researchers have modified the culture media and environment (Figure 3) to improve engraftment efficiency of transplanted MSCs. Such modifications have shown partial improvement in the characteristics of MSCs. These modified ex vivo culture techniques have both advantages and limitations in producing clinical grade MSCs with higher engraftment potential.
Culture of MSCs in xeno-free media
From the very beginning of the development of synthetic cell culture medium by Harry Eagle in 1955, researchers were looking for suitable supplement to support cell viability and expansion. Animal serum especially FBS have been widely using to supplement media, as it provides almost all the necessary nutrients needed for the survival and proliferation of cells in culture condition [98, 99]. However, the uncertainty over the composition and concentration of cytokines and growth factors of FBS, possibility of disease transmission, and Neu5GC mediated hyper-immunogenicity [99, 100] are considered as drawbacks of FBS when used for isolation and expansion of stem cells for therapeutic purpose [64, 66, 101]. Hence, xeno-free media or well defined serum free media are being used as alternative [102-104]. Usually xeno-free media require different types of growth factors as supplement: recombinant human PDGF-BB, bFGF and TGF-β1 [105]. However, MSCs in both growth factors supplemented serum free media and FBS supplemented media showed similar growth kinetics and differentiation potential during in vitro expansion [105-107]. While, xeno-free media were found suitable for isolation and expansion of MSCs to maintain their multipotent differentiation capacity [102, 103], on the other hand there are also evidence that xeno-free medium does not support primary culture or isolation of MSCs. Indeed, after isolation of MSCs in any serum supplemented medium, MSCs can be further expanded and differentiated in xeno-free media [106, 107]. Moreover, xeno-free media does not offer solutions for early senescence, telomere shortening, and loss of chemokine receptors that are needed for site specific migration, engraftment and long term regeneration benefits.
Human serum and platelet lysate as alternative to growth factors and FBS
In the search for a solution to the problems related to severe immunogenicity to xeno-contamination caused by FBS, and limited isolation and expansion of MSCs in serum free media, researchers have proposed to use human serum, plasma and/or platelet lysate as possible replacement [56, 108-110]. The potential of autologous human serum in supporting the in vitro isolation and expansion of MSCs has gained considerable attention [56, 111-113]. Autologous human serum has been reported to have positive effect on the proliferation [112, 114] and differentiation potential of MSCs [56, 111, 114]. MSCs cultured in autologous human serum have shown more stable gene expression [56, 115] and higher motility [114] compared to MSCs cultured in FBS. Moreover, MSCs cultured in autologous serum supposed to be more effective in immunomodulation as it significantly decreased the percentage of INF-γ producing activated T cells compared to MSC cultured in FBS [113]. Nonetheless, collection of blood from elderly, diseased and inflamed patients could be a limiting factor for serum preparation for the ex vivo expansion of MSCs prior to transplantation [111, 116, 117].
In addition to the autologous serum, allogeneic human serum and human cord blood serum has also been considered as suitable alternative to FBS [108, 118, 119]. However, it has been reported that allogeneic serum supplement during in vitro expansion of MSCs could cause over expression of genes that are responsible for growth arrest and cell death [56]. As opposed to that pooled allogeneic serum from adult AB-blood donors and pooled cord blood serum support isolation and expansion of MSCs while maintaining its differentiation potentials, motility and immunosuppressive property [114, 117, 120-124]. Lower level of hemagglutinin in pooled cord blood serum compared to adult serum, and lack of A and B hemagglutinin in pooled allogeneic AB- serum was attributed to be behind the success [120].
Among the different types of supplement from human source, platelet lysate was considered to be the best alternative to FBS because of its superiority in maintaining growth potential, genetic stability, immunomodulatory properties, and differentiation potential [110, 113, 125-130]. However, to produce clinical grade MSCs platelets free of infectious agents are of vital importance to prevent any possibility of disease transmission.
Transient adaptation of expanded MSCs in autologous serum supplemented media prior to transplantation
Despite the advantages of using the platelet lysate or allogeneic serum for ex vivo expansion, the microenvironment of the culture media with those supplement vary significantly compared to that of the patients' diseased organ. Hence, to make the ex vivo expanded MSCs accustomed with new microenvironment upon transplantation, incubation of the MSCs in well-defined or xeno-free media supplemented with freshly prepared autologous serum might be proven useful (Figure 4). Regeneration is a complex process and a large number of autocrine and paracrine signalling factors play a vital role in promoting this [131, 132]. Effect of cytokines, chemokines and growth factors on enhancing the chemotaxis and site specific migration of MSCs have been reported [90, 95, 133, 134]. Furthermore, enhanced site specific migration potential has been shown in MSCs pre-incubated with inflammatory cytokine TNF-α [90, 92]. In recent years researchers have acknowledged that the regenerative properties of microvesicles have been overlooked for years [135, 136]. Microvesicles are small (30-1000 nm) membranous vesicles released from the activated healthy cells or demaged cells during membrane blebbing [135, 137-139]. Rozmyslowicz et al. reported the transfer of CXCR4 receptor from the surface of platelets or megakaryocytes to the surface of CD4+/CXCR4-null cells through microvesicles [140]. Microvesicles are also able to transfer mRNA and miRNA from the cell of origin to the receiver cells [135, 141-143]. Induced epigenetic changes following internalization of microvesicles by receiver cells have been recognized as a universal phenomenon [135, 139, 144-146].
Several human and animal studies reported the increase of inflammatory cytokines, chemokines, growth factors and microvesicles in blood circulation following stroke and ischemic heart disease [5, 136, 147-151]. If the expanded MSCs are meant for transplantation in such pathological conditions where inflammatory cytokines, chemokines, growth factors and circulatory microvesicles are increased, positive response of the transplanted cells to the host microenvironment is highly important for successful regenerative therapy.
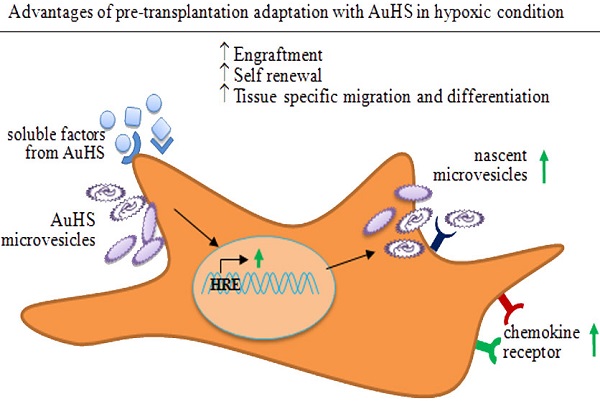
Notably, chemokines and inflammatory cytokines in the patients' freshly prepared autologous serum have the potential to enhance migratory potential of MSCs by inducing the expression of chemokine markers during incubation [5, 90, 92, 148]. Meanwhile, microvesicles present in the patients' autologous serum could enhance MSCs' migratory properties by delivering chemokine markers and as well as potentially cause epigenetic changes of MSCs by transferring host mRNA or miRNA [135, 137-139, 142-146]. Expression of chemokine markers on MSCs, transiently incubated in autologus serum, may facilitate tissue specific migration and engraftment. At the same time, the tissue specific modified cell population may produce microvesicles similar to that of injured tissues and organs [144] following engraftment. In turn, it might facilitate the migration and homing of circulatory MSCs and prevent apoptosis of cells in injured tissues or organs [136]. Since the number of circulatory MSCs and progenitor cells in circulation was found to be increased within 24 hours following stroke and myocardial infarction [5, 6, 8], incubation of MSCs for similar time period, i.e., 24 hours, would be considered sufficient for the transient ex vivo adaptation of the expanded MSCs.
Maintenance of hypoxic condition for genetic stability and stemness of MSCs
Tissues where the MSCs reside are hypoxic in nature [57, 152-154]. In vitro hypoxic culture conditions (2-5% oxygen) help MSCs to grow faster while maintaining homogeneity, differentiation potential, increased chemokine receptors expression and retard the cellular ageing process as well [17, 18, 35, 37, 39]. Biosafety issue related to aneuploidy in expanded MSCs caused by oxidative stress [17] can be resolved by using hypoxic culture conditions [18]. Hypoxia inducible factor (HIF) especially HIF-1 plays an important role in maintaining the regenerative potential at hypoxic environment. Under hypoxic conditions, the lack of O2 causes the prolyl-hydroxylation process to be suppressed resulting in stability of HIF-1α and this will facilitate translocation of its to nucleus. After nuclear translocation, it binds with HIF-1β to form the heterodimer. Then the HIF-1 heterodimer binds to a hypoxia-response element (HRE) in the target genes, associated with co-activators such as CBP/p300, and regulates the transcription of genes involved in metabolism, angiogenesis, cell migration and cell fate. Besides, through Notch signalling, HIF-1α regulate the expression of genes (e.g. HES and HEY) that maintain proliferation of cells [18]. To provide MSCs natural niche like oxygen concentration isolation, expansion and adaptation of MSCs should be done in hypoxic (2-5% oxygen) conditions. This culture environment will facilitate proliferation, site specific migration, and prevent early aging of MSCs. Moreover, hypoxic culture environment may increase biosafety by reducing aneuploidy [17, 18].
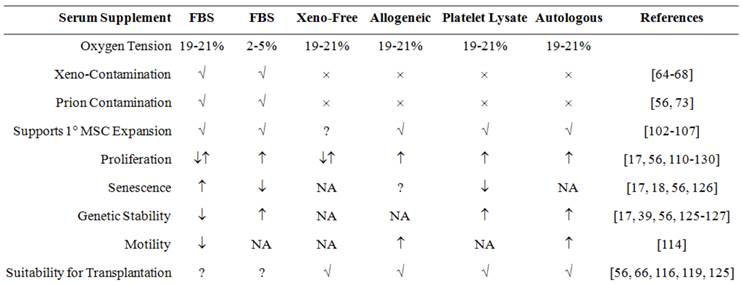
Effect of culture media supplement on in vitro or ex vivo expansion of MSCs, and their suitability for clinical applications. FBS, allogeneic serum (pooled AB-serum), platelet lysate and autologous serum supplemented media support isolation and expansion of MSCs. Presence of xenoantigen in FBS make its use controversial. Although xeno-free media do not support isolation, they support further expansion of MSCs isolated in any serum supplemented media. MSCs expanded in xeno-free media and media supplemented with platelet lysate, pooled allogeneic AB-serum or autologous serum are considered appropriate for regenerative therapy as they are free from any xeno-contamination. Abbreviations are: MSC, Mesenchymal stem cells; FBS, fetal bovine serum. [↓= decrease; ↑= increase; ↓↑= regular/unchanged; ×=absent; √ = present; ?= controversial; NA= data not available]
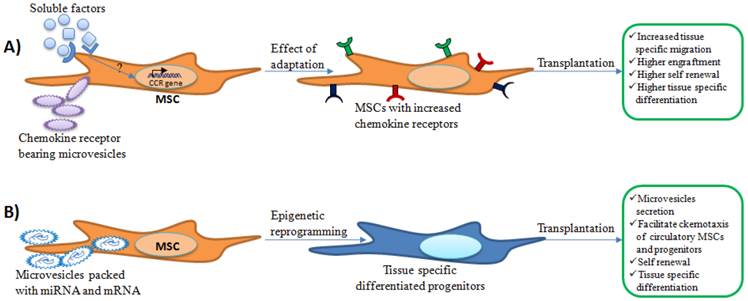
Possible effects of adaptation of expanded MSCs in autologous serum supplemented media on engraftment and regenerative eficiency. A) Cytokines and other soluble factors present in the freshly prepared autologous serum may increase chemokine receptor (CCR) expression on MSCs. Microvesicles present in the serum may deliver chemokine receptors that might enhance chemotactic properties of incubated MSCs. Expression of chemokine receptors may facilitate tissue specific migration and further regenerative benefits. B) In addition, mRNA or miRNA packed in microvesicle may be delivered to MSCs during incubation that could aid in tissue specific differentiation. Upon transplantation, these tissue specific differentiated cells may produce microvesicles similar to the cells within the injured tissues. This may help tissue specific migration of circulatory progenitors or MSCs and enhance regenerative outcomes.
Conclusion
MSCs have tremendous potential in regenerative medicine. It is the store house of several cytokines and paracrine signalling factors that facilitates the process of regeneration. For successful translation of the use of MSCs from bench side to bedside, ex vivo expansion of MSCs prior to transplantation requires appropriate supplement to minimize the impact of xenogenic serum. This article highlights comparative benefits of human platelet lysate and pooled human-AB serum as supplement for expansion of MSCs and subsequent transient ex vivo adaptation of the expanded MSCs in autologus serum supplement media prior to transplantation. Hypoxic culture environment must be maintained both for ex vivo expansion and adaptation. Collectively, ex vivo expansion using human platelet lysate and pooled human-AB serum and transient adaptation in autologus serum in hypoxic condition might prove useful in enhancing the regenerative potential of MSCs.
Acknowledgements
The authors thank Dr Aied Mohammed, Department of Oral Biology & Biomedical Sciences, Faculty of Dentistry, University of Malaya for giving his opinion during the early stage of manuscript preparation. This work was supported by High Impact Research MoE Grant UM.C/625/1/HIR/MOHE/DENT/01 from the Ministry of Education Malaysia.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Fuchs E, Chen T. A matter of life and death: self-renewal in stem cells. EMBO Rep. 2013;14:39-48
2. Caplan AI. MSCs as Therapeutics. Mesenchymal Stromal Cells. Springer. 2013:79-90
3. Ramírez M, Lucia A, Gómez-Gallego F, Esteve-Lanao J, Pérez-Martínez A, Foster C. et al. Mobilisation of mesenchymal cells into blood in response to skeletal muscle injury. British journal of sports medicine. 2006;40:719-22
4. Carbonare LD, Valenti MT, Zanatta M, Donatelli L, Lo Cascio V. Circulating mesenchymal stem cells with abnormal osteogenic differentiation in patients with osteoporosis. Arthritis & Rheumatism. 2009;60:3356-65
5. Wang Y, Johnsen HE, Mortensen S, Bindslev L, Ripa RS, HaacK-Sørensen M. et al. Changes in circulating mesenchymal stem cells, stem cell homing factor, and vascular growth factors in patients with acute ST elevation myocardial infarction treated with primary percutaneous coronary intervention. Heart. 2006;92:768-74
6. Paczkowska E, Kucia M, Koziarska D, Halasa M, Safranow K, Masiuk M. et al. Clinical evidence that very small embryonic-like stem cells are mobilized into peripheral blood in patients after stroke. Stroke. 2009;40:1237-44
7. Hatzistergos KE, Quevedo H, Oskouei BN, Hu Q, Feigenbaum GS, Margitich IS. et al. Bone Marrow Mesenchymal Stem Cells Stimulate Cardiac Stem Cell Proliferation and DifferentiationNovelty and Significance. Circulation research. 2010;107:913-22
8. Kucia M, Zhang Y, Reca R, Wysoczynski M, Machalinski B, Majka M. et al. Cells enriched in markers of neural tissue-committed stem cells reside in the bone marrow and are mobilized into the peripheral blood following stroke. Leukemia. 2006;20:18-28
9. Levi B, Hyun JS, Montoro DT, Lo DD, Chan CK, Hu S. et al. In vivo directed differentiation of pluripotent stem cells for skeletal regeneration. Proceedings of the National Academy of Sciences. 2012;109:20379-84
10. Robertson JA. Embryo stem cell research: ten years of controversy. The Journal of Law, Medicine & Ethics. 2010;38:191-203
11. Okita K, Yamakawa T, Matsumura Y, Sato Y, Amano N, Watanabe A. et al. An efficient nonviral method to generate integration-free human-induced pluripotent stem cells from cord blood and peripheral blood cells. Stem Cells. 2013;31:458-66
12. Wang Y, Liu J, Tan X, Li G, Gao Y, Liu X. et al. Induced Pluripotent Stem Cells from Human Hair Follicle Mesenchymal Stem Cells. Stem Cell Reviews and Reports. 2012:1-10
13. Polo JM, Liu S, Figueroa ME, Kulalert W, Eminli S, Tan KY. et al. Cell type of origin influences the molecular and functional properties of mouse induced pluripotent stem cells. Nat Biotechnol. 2010;28:848-55
14. Liang G, Zhang Y. Genetic and epigenetic variations in iPSCs: potential causes and implications for application. Cell stem cell. 2013;13:149-59
15. Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011;9:11-5
16. Govindasamy V, Ronald V, Abdullah A, Nathan KRG, Aziz ZACA, Abdullah M. et al. Differentiation of dental pulp stem cells into islet-like aggregates. Journal of dental research. 2011;90:646-52
17. Estrada J, Torres Y, Benguría A, Dopazo A, Roche E, Carrera-Quintanar L. et al. Human mesenchymal stem cell-replicative senescence and oxidative stress are closely linked to aneuploidy. Cell death & disease. 2013;4:e691
18. Haque N, Rahman MT, Abu Kasim NH, Alabsi AM. Hypoxic Culture Conditions as a Solution for Mesenchymal Stem Cell Based Regenerative Therapy. The Scientific World Journal. 2013;2013:12
19. Brown SE, Tong W, Krebsbach PH. The derivation of mesenchymal stem cells from human embryonic stem cells. Cells Tissues Organs. 2009;189:256-60
20. Lian Q, Zhang Y, Zhang J, Zhang HK, Wu X, Zhang Y. et al. Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation. 2010;121:1113-23
21. Villa-Diaz LG, Brown SE, Liu Y, Ross AM, Lahann J, Parent JM. et al. Derivation of mesenchymal stem cells from human induced pluripotent stem cells cultured on synthetic substrates. Stem Cells. 2012;30:1174-81
22. Liu Y, Goldberg AJ, Dennis JE, Gronowicz GA, Kuhn LT. One-Step Derivation of Mesenchymal Stem Cell (MSC)-Like Cells from Human Pluripotent Stem Cells on a Fibrillar Collagen Coating. PLoS ONE. 2012;7:e33225
23. Zou L, Luo Y, Chen M, Wang G, Ding M, Petersen CC. et al. A simple method for deriving functional MSCs and applied for osteogenesis in 3D scaffolds. Sci Rep. 2013 3
24. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD. et al. Multilineage potential of adult human mesenchymal stem cells. science. 1999;284:143-7
25. Azizi SA, Stokes D, Augelli BJ, DiGirolamo C, Prockop DJ. Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats—similarities to astrocyte grafts. Proceedings of the National Academy of Sciences. 1998;95:3908-13
26. Jin HK, Carter JE, Huntley GW, Schuchman EH. Intracerebral transplantation of mesenchymal stem cells into acid sphingomyelinase-deficient mice delays the onset of neurological abnormalities and extends their life span. Journal of Clinical Investigation. 2002;109:1183-92
27. Shake JG, Gruber PJ, Baumgartner WA, Senechal G, Meyers J, Redmond JM. et al. Mesenchymal stem cell implantation in a swine myocardial infarct model: engraftment and functional effects. The Annals of thoracic surgery. 2002;73:1919-26
28. Kim N, Cho S-G. Clinical applications of mesenchymal stem cells. The Korean journal of internal medicine. 2013;28:387-402
29. Volarevic V, Arsenijevic N, Lukic ML, Stojkovic M. Concise review: Mesenchymal stem cell treatment of the complications of diabetes mellitus. Stem Cells. 2011;29:5-10
30. Malliaras K, Marban E. Cardiac cell therapy: where we've been, where we are, and where we should be headed. Br Med Bull. 2011;98:161-85
31. Guo L, Ge J, Wang S, Zhou Y, Wang X, Wu Y. A novel method for efficient delivery of stem cells to the ischemic brain. Stem Cell Research & Therapy. 2013;4:116
32. Lu S-s, Liu S, Zu Q-q, Xu X-q, Yu J, Wang J-w. et al. In Vivo MR Imaging of Intraarterially Delivered Magnetically Labeled Mesenchymal Stem Cells in a Canine Stroke Model. PloS one. 2013;8:e54963
33. Kean TJ, Lin P, Caplan AI, Dennis JE. MSCs: Delivery Routes and Engraftment, Cell-Targeting Strategies, and Immune Modulation. Stem Cells International. 2013;2013:13
34. Liu H, Liu S, Li Y, Wang X, Xue W, Ge G. et al. The role of SDF-1-CXCR4/CXCR7 axis in the therapeutic effects of hypoxia-preconditioned mesenchymal stem cells for renal ischemia/reperfusion injury. PloS one. 2012;7:e34608
35. Saller MM, Prall WC, Docheva D, Schonitzer V, Popov T, Anz D. et al. Increased stemness and migration of human mesenchymal stem cells in hypoxia is associated with altered integrin expression. Biochemical and Biophysical Research Communications. 2012;423:379-85
36. Hung SP, Ho JH, Shih YRV, Lo T, Lee OK. Hypoxia promotes proliferation and osteogenic differentiation potentials of human mesenchymal stem cells. Journal of Orthopaedic Research. 2012;30:260-6
37. Estrada J, Albo C, Benguría A, Dopazo A, López-Romero P, Carrera-Quintanar L. et al. Culture of human mesenchymal stem cells at low oxygen tension improves growth and genetic stability by activating glycolysis. Cell Death & Differentiation. 2012;19:743-55
38. Sakamoto N, Tsuji K, Muul LM, Lawler AM, Petricoin EF, Candotti F. et al. Bovine apolipoprotein B-100 is a dominant immunogen in therapeutic cell populations cultured in fetal calf serum in mice and humans. Blood. 2007;110:501-8
39. Basciano L, Nemos C, Foliguet B, de Isla N, de Carvalho M, Tran N. et al. Long term culture of mesenchymal stem cells in hypoxia promotes a genetic program maintaining their undifferentiated and multipotent status. BMC cell biology. 2011;12:12
40. Grayson WL, Zhao F, Bunnell B, Ma T. Hypoxia enhances proliferation and tissue formation of human mesenchymal stem cells. Biochemical and Biophysical Research Communications. 2007;358:948-53
41. Connick P, Kolappan M, Patani R, Scott MA, Crawley C, He XL. et al. The mesenchymal stem cells in multiple sclerosis (MSCIMS) trial protocol and baseline cohort characteristics: an open-label pre-test: post-test study with blinded outcome assessments. Trials. 2011;12:62
42. Connick P, Kolappan M, Crawley C, Webber DJ, Patani R, Michell AW. et al. Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: an open-label phase 2a proof-of-concept study. Lancet Neurol. 2012;11:150-6
43. Baron F, Lechanteur C, Willems E, Bruck F, Baudoux E, Seidel L. et al. Cotransplantation of mesenchymal stem cells might prevent death from graft-versus-host disease (GVHD) without abrogating graft-versus-tumor effects after HLA-mismatched allogeneic transplantation following nonmyeloablative conditioning. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2010;16:838
44. Hare JM, Fishman JE, Gerstenblith G, Velazquez DLDF, Zambrano JP, Suncion VY. et al. Comparison of Allogeneic vs Autologous Bone Marrow-Derived Mesenchymal Stem Cells Delivered by Transendocardial Injection in Patients With Ischemic CardiomyopathyThe POSEIDON Randomized TrialMesenchymal Stem Cells and Ischemic Cardiomyopathy. JAMA: The Journal of the American Medical Association. 2012;308:2369-79
45. Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP. et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. Journal of the American College of Cardiology. 2009;54:2277-86
46. Tan J, Wu W, Xu X, Liao L, Zheng F, Messinger S. et al. Induction Therapy With Autologous Mesenchymal Stem Cells in Living-Related Kidney Transplants A Randomized Controlled Trial. JAMA: The Journal of the American Medical Association. 2012;307:1169-77
47. Reinders ME, de Fijter JW, Roelofs H, Bajema IM, de Vries DK, Schaapherder AF. et al. Autologous bone marrow-derived mesenchymal stromal cells for the treatment of allograft rejection after renal transplantation: results of a phase I study. Stem cells translational medicine. 2013;2:107-11
48. Gupta PK, Chullikana A, Parakh R, Desai S, Das A, Gottipamula S. et al. A double blind randomized placebo controlled phase I/II study assessing the safety and efficacy of allogeneic bone marrow derived mesenchymal stem cell in critical limb ischemia. Journal of translational medicine. 2013;11:143
49. Lee SH, Lee MW, Yoo KH, Kim DS, Son MH, Sung KW. et al. Co-transplantation of third-party umbilical cord blood-derived MSCs promotes engraftment in children undergoing unrelated umbilical cord blood transplantation. Bone marrow transplantation. 2013;48:1040-5
50. Lee PH, Lee JE, Kim HS, Song SK, Lee HS, Nam HS. et al. A randomized trial of mesenchymal stem cells in multiple system atrophy. Ann Neurol. 2012;72:32-40
51. Ra JC, Shin IS, Kim SH, Kang SK, Kang BC, Lee HY. et al. Safety of intravenous infusion of human adipose tissue-derived mesenchymal stem cells in animals and humans. Stem Cells Dev. 2011;20:1297-308
52. Weiss DJ, Casaburi R, Flannery R, LeRoux-Williams M, Tashkin DP. A placebo-controlled, randomized trial of mesenchymal stem cells in COPD. Chest. 2013;143:1590-8
53. Peng L, Xie DY, Lin BL, Liu J, Zhu HP, Xie C. et al. Autologous bone marrow mesenchymal stem cell transplantation in liver failure patients caused by hepatitis B: short-term and long-term outcomes. Hepatology. 2011;54:820-8
54. Otsuru S, Gordon PL, Shimono K, Jethva R, Marino R, Phillips CL. et al. Transplanted bone marrow mononuclear cells and MSCs impart clinical benefit to children with osteogenesis imperfecta through different mechanisms. Blood. 2012;120:1933-41
55. El-Kheir WA, Gabr H, Awad MR, Ghannam O, Barakat Y, Farghali HA. et al. Autologous bone marrow-derived cell therapy combined with physical therapy induces functional improvement in chronic spinal cord injury patients. Cell transplantation. 2013
56. Shahdadfar A, Frønsdal K, Haug T, Reinholt FP, Brinchmann JE. In vitro expansion of human mesenchymal stem cells: choice of serum is a determinant of cell proliferation, differentiation, gene expression, and transcriptome stability. Stem cells. 2005;23:1357-66
57. Mohyeldin A, Garzón-Muvdi T, Quiñones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell stem cell. 2010;7:150
58. Gebler A, Zabel O, Seliger B. The immunomodulatory capacity of mesenchymal stem cells. Trends Mol Med. 2012;18:128-34
59. Plock JA, Schnider JT, Solari MG, Zheng XX, Gorantla VS. Perspectives on the use of mesenchymal stem cells in vascularized composite allotransplantation. Frontiers in immunology. 2013;4:175
60. Francois M, Romieu-Mourez R, Li M, Galipeau J. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Molecular therapy: the journal of the American Society of Gene Therapy. 2012;20:187-95
61. Klyushnenkova E, Mosca JD, Zernetkina V, Majumdar MK, Beggs KJ, Simonetti DW. et al. T cell responses to allogeneic human mesenchymal stem cells: immunogenicity, tolerance, and suppression. J Biomed Sci. 2005;12:47-57
62. Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol. 2014;32:252-60
63. Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringden O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Experimental hematology. 2003;31:890-6
64. Spees JL, Gregory CA, Singh H, Tucker HA, Peister A, Lynch PJ. et al. Internalized Antigens Must Be Removed to Prepare Hypoimmunogenic Mesenchymal Stem Cells for Cell and Gene Therapy&ast. Molecular Therapy. 2004;9:747-56
65. Heiskanen A, Satomaa T, Tiitinen S, Laitinen A, Mannelin S, Impola U. et al. N-Glycolylneuraminic Acid Xenoantigen Contamination of Human Embryonic and Mesenchymal Stem Cells Is Substantially Reversible. Stem Cells. 2006;25:197-202
66. Komoda H, Okura H, Lee C, Sougawa N, Iwayama T, Hashikawa T. et al. Reduction of N-glycolylneuraminic acid xenoantigen on human adipose tissue-derived stromal cells/mesenchymal stem cells leads to safer and more useful cell sources for various stem cell therapies. Tissue engineering Part A. 2010;16:1143
67. Ghaderi D, Taylor RE, Padler-Karavani V, Diaz S, Varki A. Implications of the presence of N-glycolylneuraminic acid in recombinant therapeutic glycoproteins. Nat Biotechnol. 2010;28:863-7
68. Zhu A, Hurst R. Anti-N-glycolylneuraminic acid antibodies identified in healthy human serum. Xenotransplantation. 2002;9:376-81
69. Li Y, Lin F. Mesenchymal stem cells are injured by complement after their contact with serum. Blood. 2012;120:3436-43
70. Tu Z, Li Q, Bu H, Lin F. Mesenchymal stem cells inhibit complement activation by secreting factor H. Stem Cells Dev. 2010;19:1803-9
71. Padler-Karavani V, Varki A. Potential impact of the non-human sialic acid N-glycolylneuraminic acid on transplant rejection risk. Xenotransplantation. 2011;18:1-5
72. Dedrick V. Determining the safety of medical devices containing animal tissue: the new European standards. J Regul Affairs Prof Soc. 1997;4:2-20
73. Cobo F, Talavera P, Concha A. Diagnostic approaches for viruses and prions in stem cell banks. Virology. 2006;347:1-10
74. Lepperdinger G, Brunauer R, Jamnig A, Laschober G, Kassem M. Controversial issue: Is it safe to employ mesenchymal stem cells in cell-based therapies? Experimental Gerontology. 2008;43:1018-23
75. Bonab MM, Alimoghaddam K, Talebian F, Ghaffari SH, Ghavamzadeh A, Nikbin B. Aging of mesenchymal stem cell in vitro. BMC cell biology. 2006;7:14
76. Wagner W, Horn P, Castoldi M, Diehlmann A, Bork S, Saffrich R. et al. Replicative senescence of mesenchymal stem cells: a continuous and organized process. PloS one. 2008;3:e2213
77. Schellenberg A, Lin Q, Schüler H, Koch CM, Joussen S, Denecke B. et al. Replicative senescence of mesenchymal stem cells causes DNA-methylation changes which correlate with repressive histone marks. Aging (Albany NY). 2011;3:873
78. Collado M, Blasco MA, Serrano M. Cellular Senescence in Cancer and Aging. Cell. 2007;130:223-33
79. Kuilman T, Michaloglou C, Mooi WJ, Peeper DS. The essence of senescence. Genes & development. 2010;24:2463-79
80. Ho JH, Chen YF, Ma WH, Tseng TC, Chen MH, Lee OK. Cell contact accelerates replicative senescence of human mesenchymal stem cells independent of telomere shortening and p53 activation: roles of Ras and oxidative stress. Cell transplantation. 2011;20:1209-20
81. Hiyama E, Hiyama K. Telomere and telomerase in stem cells. Br J Cancer. 2007;96:1020-4
82. Fehrer C, Lepperdinger G. Mesenchymal stem cell aging. Experimental Gerontology. 2005;40:926-30
83. Yanada S, Ochi M, Kojima K, Sharman P, Yasunaga Y, Hiyama E. Possibility of selection of chondrogenic progenitor cells by telomere length in FGF-2-expanded mesenchymal stromal cells. Cell Proliferation. 2006;39:575-84
84. Bianchi G, Banfi A, Mastrogiacomo M, Notaro R, Luzzatto L, Cancedda R. et al. Ex vivo enrichment of mesenchymal cell progenitors by fibroblast growth factor 2. Experimental Cell Research. 2003;287:98-105
85. Handorf AM, Li W-J. Fibroblast Growth Factor-2 Primes Human Mesenchymal Stem Cells for Enhanced Chondrogenesis. PLoS ONE. 2011;6:e22887
86. Choo KB, Tai L, Hymavathee KS, Wong CY, Nguyen PNN, Huang C-J. et al. Oxidative Stress-Induced Premature Senescence in Wharton's Jelly-Derived Mesenchymal Stem Cells. International journal of medical sciences. 2014;11:1201
87. Limb GA, Daniels JT. Ocular regeneration by stem cells: present status and future prospects. British medical bulletin. 2008;85:47-61
88. Song CH, Honmou O, Furuoka H, Horiuchi M. Identification of chemoattractive factors involved in the migration of bone marrow-derived mesenchymal stem cells to brain lesions caused by prions. Journal of virology. 2011;85:11069-78
89. Wu Y, Zhao RCH. The role of chemokines in mesenchymal stem cell homing to myocardium. Stem Cell Reviews and Reports. 2012;8:243-50
90. Ponte AL, Marais E, Gallay N, Langonne A, Delorme B, Herault O. et al. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25:1737-45
91. Smith H, Whittall C, Weksler B, Middleton J. Chemokines stimulate bidirectional migration of human mesenchymal stem cells across bone marrow endothelial cells. Stem Cells Dev. 2012;21:476-86
92. Baek SJ, Kang SK, Ra JC. In vitro migration capacity of human adipose tissue-derived mesenchymal stem cells reflects their expression of receptors for chemokines and growth factors. Exp Mol Med. 2011;43:596-603
93. Honczarenko M, Le Y, Swierkowski M, Ghiran I, Glodek AM, Silberstein LE. Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells. 2006;24:1030-41
94. Fiedler J, Brill C, Blum WF, Brenner RE. IGF-I and IGF-II stimulate directed cell migration of bone-marrow-derived human mesenchymal progenitor cells. Biochemical and biophysical research communications. 2006;345:1177-83
95. Forte G, Minieri M, Cossa P, Antenucci D, Sala M, Gnocchi V. et al. Hepatocyte growth factor effects on mesenchymal stem cells: proliferation, migration, and differentiation. Stem cells. 2006;24:23-33
96. Tamama K, Fan VH, Griffith LG, Blair HC, Wells A. Epidermal growth factor as a candidate for ex vivo expansion of bone marrow-derived mesenchymal stem cells. Stem cells. 2006;24:686-95
97. Phipps MC, Xu Y, Bellis SL. Delivery of platelet-derived growth factor as a chemotactic factor for mesenchymal stem cells by bone-mimetic electrospun scaffolds. PloS one. 2012;7:e40831
98. Whitford WG. Supplementation of animal cell culture media. BioProcess Int. 2005 3
99. Gstraunthaler G. Alternatives to the use of fetal bovine serum: serum-free cell culture. Altex. 2003;20:275-81
100. Lindroos B, Boucher S, Chase L, Kuokkanen H, Huhtala H, Haataja R. et al. Serum-free, xeno-free culture media maintain the proliferation rate and multipotentiality of adipose stem cells in vitro. Cytotherapy. 2009;11:958-72
101. Schallmoser K, Bartmann C, Rohde E, Reinisch A, Kashofer K, Stadelmeyer E. et al. Human platelet lysate can replace fetal bovine serum for clinical-scale expansion of functional mesenchymal stromal cells. Transfusion. 2007;47:1436-46
102. Simoes IN, Boura JS, dos Santos F, Andrade PZ, Cardoso CM, Gimble JM. et al. Human mesenchymal stem cells from the umbilical cord matrix: successful isolation and ex vivo expansion using serum-/xeno-free culture media. Biotechnology journal. 2013;8:448-58
103. Corotchi MC, Popa MA, Remes A, Sima LE, Gussi I, Plesu M. Isolation method and xeno-free culture conditions influence multipotent differentiation capacity of human Wharton's jelly-derived mesenchymal stem cells. Stem Cell Res Ther. 2013;4:81
104. Patrikoski M, Juntunen M, Boucher S, Campbell A, Vemuri MC, Mannerström B. et al. Development of fully defined xeno-free culture system for the preparation and propagation of cell therapy-compliant human adipose stem cells. Stem cell research & therapy. 2013;4:27
105. Chase LG, Lakshmipathy U, Solchaga LA, Rao MS, Vemuri MC. A novel serum-free medium for the expansion of human mesenchymal stem cells. Stem Cell Res Ther. 2010;1:1549-53
106. Rashi K-J. Growth and Differentiation of Human Dental Pulp Stem Cells Maintained in Fetal Bovine Serum, Human Serum and Serum-free/Xeno-free Culture Media. Journal of Stem Cell Research & Therapy. 2012;2:4
107. Crapnell K, Blaesius R, Hastings A, Lennon DP, Bruder SP. Growth, Differentiation Capacity, and Function of Mesenchymal Stem Cells Expanded in Serum-Free Medium Developed Via Combinatorial Screening. Experimental cell research. 2013;319:1409-18
108. Aldahmash A, Haack-Sorensen M, Al-Nbaheen M, Harkness L, Abdallah BM, Kassem M. Human Serum is as Efficient as Fetal Bovine Serum in Supporting Proliferation and Differentiation of Human Multipotent Stromal (Mesenchymal) Stem Cells In Vitro and In Vivo. Stem Cell Reviews and Reports. 2011;7:860-8
109. Lin HT, Tarng YW, Chen YC, Kao CL, Hsu CJ, Shyr YM. et al. Using Human Plasma Supplemented Medium to Cultivate Human Bone Marrow-Derived Mesenchymal Stem Cell and Evaluation of Its Multiple-Lineage Potential. Transplantation proceedings: Elsevier. 2005:4504-5
110. Jonsdottir-Buch SM, Lieder R, Sigurjonsson OE. Platelet lysates produced from expired platelet concentrates support growth and osteogenic differentiation of mesenchymal stem cells. PLoS One. 2013;8:e68984
111. Stute N, Holtz K, Bubenheim M, Lange C, Blake F, Zander AR. Autologous serum for isolation and expansion of human mesenchymal stem cells for clinical use. Experimental hematology. 2004;32:1212-25
112. Mizuno N, Shiba H, Ozeki Y, Mouri Y, Niitani M, Inui T. et al. Human autologous serum obtained using a completely closed bag system as a substitute for foetal calf serum in human mesenchymal stem cell cultures. Cell biology international. 2006;30:521-4
113. Perez-Ilzarbe M, Diez-Campelo M, Aranda P, Tabera S, Lopez T, del Canizo C. et al. Comparison of ex vivo expansion culture conditions of mesenchymal stem cells for human cell therapy. Transfusion. 2009;49:1901-10
114. Kobayashi T, Watanabe H, Yanagawa T, Tsutsumi S, Kayakabe M, Shinozaki T. et al. Motility and growth of human bone-marrow mesenchymal stem cells during ex vivo expansion in autologous serum. Journal of Bone & Joint Surgery, British Volume. 2005;87:1426-33
115. Dahl J-A, Duggal S, Coulston N, Millar D, Melki J, Shandadfar A. et al. Genetic and epigenetic instability of human bone marrow mesenchymal stem cells expanded in autologous serum or fetal bovine serum. International Journal of Developmental Biology. 2008;52:1033
116. Jung S, Panchalingam KM, Rosenberg L, Behie LA. Ex Vivo Expansion of Human Mesenchymal Stem Cells in Defined Serum-Free Media. Stem Cells International. 2012;2012:21
117. Cooper K, SenMajumdar A, Viswanathan C. Derivation, expansion and characterization of clinical grade mesenchymal stem cells from umbilical cord matrix using cord blood serum. International journal of stem cells. 2010;3:119
118. Jung J, Moon N, Ahn JY, Oh EJ, Kim M, Cho CS. et al. Mesenchymal stromal cells expanded in human allogenic cord blood serum display higher self-renewal and enhanced osteogenic potential. Stem cells and development. 2009;18:559-72
119. Bieback K, Hecker A, Schlechter T, Hofmann I, Brousos N, Redmer T. et al. Replicative aging and differentiation potential of human adipose tissue-derived mesenchymal stromal cells expanded in pooled human or fetal bovine serum. Cytotherapy. 2012;14:570-83
120. Turnovcova K, Ruzickova K, Vanecek V, Sykova E, Jendelova P. Properties and growth of human bone marrow mesenchymal stromal cells cultivated in different media. Cytotherapy. 2009;11:874-85
121. Tateishi K, Ando W, Higuchi C, Hart D, Hashimoto J, Nakata K. et al. Comparison of human serum with fetal bovine serum for expansion and differentiation of human synovial MSC: potential feasibility for clinical applications. Cell transplantation. 2008;17:549-57
122. Le Blanc K, Samuelsson H, Lönnies L, Sundin M, Ringdén O. Generation of immunosuppressive mesenchymal stem cells in allogeneic human serum. Transplantation. 2007;84:1055-9
123. Poloni A, Maurizi G, Rosini V, Mondini E, Mancini S, Discepoli G. et al. Selection of CD271+ cells and human AB serum allows a large expansion of mesenchymal stromal cells from human bone marrow. Cytotherapy. 2009;11:153-62
124. Phadnis SM, Joglekar MV, Venkateshan V, Ghaskadbi SM, Hardikar AA, Bhonde RR. Human umbilical cord blood serum promotes growth, proliferation, as well as differentiation of human bone marrow-derived progenitor cells. In vitro cellular & developmental biology Animal. 2006;42:283-6
125. Crespo-Diaz R, Behfar A, Butler GW, Padley DJ, Sarr MG, Bartunek J. et al. Platelet lysate consisting of a natural repair proteome supports human mesenchymal stem cell proliferation and chromosomal stability. Cell transplantation. 2011;20:797-811
126. Griffiths S, Baraniak PR, Copland IB, Nerem RM, McDevitt TC. Human platelet lysate stimulates high-passage and senescent human multipotent mesenchymal stromal cell growth and rejuvenation in vitro. Cytotherapy. 2013;15:1469-83
127. Trojahn Kolle SF, Oliveri RS, Glovinski PV, Kirchhoff M, Mathiasen AB, Elberg JJ. et al. Pooled human platelet lysate versus fetal bovine serum-investigating the proliferation rate, chromosome stability and angiogenic potential of human adipose tissue-derived stem cells intended for clinical use. Cytotherapy. 2013;15:1086-97
128. Capelli C, Domenghini M, Borleri G, Bellavita P, Poma R, Carobbio A. et al. Human platelet lysate allows expansion and clinical grade production of mesenchymal stromal cells from small samples of bone marrow aspirates or marrow filter washouts. Bone marrow transplantation. 2007;40:785-91
129. Govindasamy V, Ronald VS, Abdullah ANB, Ganesan Nathan KR, Aziz ZACA, Abdullah M. et al. Human platelet lysate permits scale-up of dental pulp stromal cells for clinical applications. Cytotherapy. 2011;13:1221-33
130. Vasanthan P, Gnanasegaran N, Govindasamy V, Abdullah AN, Jayaraman P, Ronald VS. et al. Comparison of fetal bovine serum and human platelet lysate in cultivation and differentiation of dental pulp stem cells into hepatic lineage cells. Biochemical Engineering Journal. 2014;88:142-53
131. Vunjak-Novakovic G, Scadden DT. Biomimetic platforms for human stem cell research. Cell stem cell. 2011;8:252-61
132. Wagers AJ. The Stem Cell Niche in Regenerative Medicine. Cell stem cell. 2012;10:362-9
133. Fiedler J, Roderer G, Gunther KP, Brenner RE. BMP-2, BMP-4, and PDGF-bb stimulate chemotactic migration of primary human mesenchymal progenitor cells. J Cell Biochem. 2002;87:305-12
134. Ji JF, He BP, Dheen ST, Tay SS. Interactions of chemokines and chemokine receptors mediate the migration of mesenchymal stem cells to the impaired site in the brain after hypoglossal nerve injury. Stem Cells. 2004;22:415-27
135. Aliotta JM, Pereira M, Johnson KW, de Paz N, Dooner MS, Puente N. et al. Microvesicle entry into marrow cells mediates tissue-specific changes in mRNA by direct delivery of mRNA and induction of transcription. Experimental hematology. 2010;38:233-45
136. Ratajczak M, Kucia M, Jadczyk T, Greco N, Wojakowski W, Tendera M. et al. Pivotal role of paracrine effects in stem cell therapies in regenerative medicine: can we translate stem cell-secreted paracrine factors and microvesicles into better therapeutic strategies&quest. Leukemia. 2012;26:1166-73
137. Sabin K, Kikyo N. Microvesicles as mediators of tissue regeneration. Translational research: the journal of laboratory and clinical medicine. 2014;163:286-95
138. Yuan A, Farber EL, Rapoport AL, Tejada D, Deniskin R, Akhmedov NB. et al. Transfer of MicroRNAs by Embryonic Stem Cell Microvesicles. PLoS ONE. 2009;4:e4722
139. Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P. et al. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20:847-56
140. Rozmyslowicz T, Majka M, Kijowski J, Murphy SL, Conover DO, Poncz M. et al. Platelet- and megakaryocyte-derived microparticles transfer CXCR4 receptor to CXCR4-null cells and make them susceptible to infection by X4-HIV. AIDS (London, England). 2003;17:33-42
141. Biancone L, Bruno S, Deregibus MC, Tetta C, Camussi G. Therapeutic potential of mesenchymal stem cell-derived microvesicles. Nephrology Dialysis Transplantation. 2012;27:3037-42
142. Camussi G, Deregibus MC, Bruno S, Cantaluppi V, Biancone L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 2010;78:838-48
143. Mrvar-Brečko A, Šuštar V, Janša V, Štukelj R, Janša R, Mujagić E. et al. Isolated microvesicles from peripheral blood and body fluids as observed by scanning electron microscope. Blood Cells, Molecules, and Diseases. 2010;44:307-12
144. Quesenberry PJ, Dooner MS, Aliotta JM. Stem cell plasticity revisited: the continuum marrow model and phenotypic changes mediated by microvesicles. Experimental hematology. 2010;38:581-92
145. Deregibus MC, Cantaluppi V, Calogero R, Lo Iacono M, Tetta C, Biancone L. et al. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood. 2007;110:2440-8
146. Lee Y, Andaloussi SE, Wood MJ. Exosomes and microvesicles: extracellular vesicles for genetic information transfer and gene therapy. Human molecular genetics. 2012;21:R125-R34
147. Ripa RS, Wang Y, Goetze JP, Jørgensen E, Johnsen HE, Tägil K. et al. Circulating angiogenic cytokines and stem cells in patients with severe chronic ischemic heart disease—Indicators of myocardial ischemic burden? International journal of cardiology. 2007;120:181-7
148. Ferrarese C, Mascarucci P, Zoia C, Cavarretta R, Frigo M, Begni B. et al. Increased cytokine release from peripheral blood cells after acute stroke. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 1999;19:1004-9
149. Intiso D, Zarrelli MM, Lagioia G, Di Rienzo F, Checchia De Ambrosio C, Simone P. et al. Tumor necrosis factor alpha serum levels and inflammatory response in acute ischemic stroke patients. Neurological sciences: official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2004;24:390-6
150. Drimal J, Knezl V, Paulovicova E, Drimal D. Enhanced early after-myocardial infarction concentration of TNF-alpha subsequently increased circulating and myocardial adrenomedullin in spontaneously hypertensive rats. General physiology and biophysics. 2008;27:12-8
151. Lionetti V, Bianchi G, Recchia FA, Ventura C. Control of autocrine and paracrine myocardial signals: an emerging therapeutic strategy in heart failure. Heart failure reviews. 2010;15:531-42
152. Harrison JS, Rameshwar P, Chang V, Bandari P. Oxygen saturation in the bone marrow of healthy volunteers. Blood. 2002;99:394
153. Panchision DM. The role of oxygen in regulating neural stem cells in development and disease. Journal of cellular physiology. 2009;220:562-8
154. Eliasson P, Jönsson JI. The hematopoietic stem cell niche: low in oxygen but a nice place to be. Journal of cellular physiology. 2010;222:17-22
Author contact
![]() Corresponding authors: Mohammad Tariqur Rahman, Ph.D., Associate Professor, Department of Biotechnology, Faculty of Science, International Islamic University Malaysia, Bandar Indera Mahkota, Kuantan 25200, Pahang, Malaysia. Phone: +601399494741 (Mobile), +6095705042 (Office); Fax: +6095726781; Email: tariqueedu.my | m.tariqur.rahmancom or Noor Hayaty Abu Kasim, Professor, Department of Restorative Dentistry, Faculty of Dentistry, University of Malaya, 50603 Kuala Lumpur, Malaysia, Tel. No. (Office): +6-03-79674806, Fax No.: +6-03-79674533, E-mail: nhayatyedu.my
Corresponding authors: Mohammad Tariqur Rahman, Ph.D., Associate Professor, Department of Biotechnology, Faculty of Science, International Islamic University Malaysia, Bandar Indera Mahkota, Kuantan 25200, Pahang, Malaysia. Phone: +601399494741 (Mobile), +6095705042 (Office); Fax: +6095726781; Email: tariqueedu.my | m.tariqur.rahmancom or Noor Hayaty Abu Kasim, Professor, Department of Restorative Dentistry, Faculty of Dentistry, University of Malaya, 50603 Kuala Lumpur, Malaysia, Tel. No. (Office): +6-03-79674806, Fax No.: +6-03-79674533, E-mail: nhayatyedu.my

 Global reach, higher impact
Global reach, higher impact