Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2015; 11(5):508-524. doi:10.7150/ijbs.11241 This issue Cite
Review
Modulation of Glucose Transporter Protein by Dietary Flavonoids in Type 2 Diabetes Mellitus
1. Department of Pharmacy, Faculty of Medicine, University of Malaya, 50603 Kuala Lumpur, Malaysia;
2. Faculty of Pharmacy, Universiti Teknologi MARA (UiTM), 42300 Bandar Puncak Alam, Selangor Darul Ehsan, Malaysia.
* These authors had equal contribution in this work.
Received 2014-12-5; Accepted 2015-2-8; Published 2015-3-19
Abstract
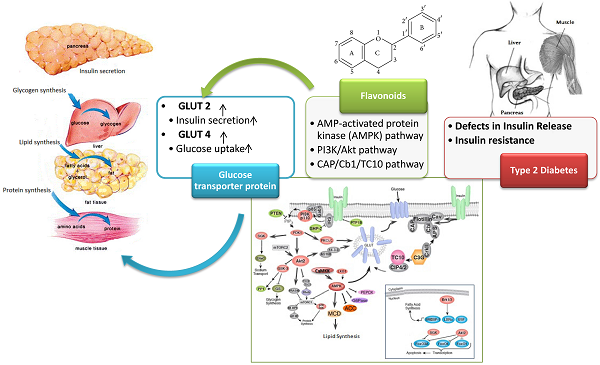
Diabetes mellitus (DM) is a metabolic diseases characterized by hyperglycemia due to insufficient or inefficient insulin secretory response. This chronic disease is a global problem and there is a need for greater emphasis on therapeutic strategies in the health system. Phytochemicals such as flavonoids have recently attracted attention as source materials for the development of new antidiabetic drugs or alternative therapy for the management of diabetes and its related complications. The antidiabetic potential of flavonoids are mainly through their modulatory effects on glucose transporter by enhancing GLUT-2 expression in pancreatic β cells and increasing expression and promoting translocation of GLUT-4 via PI3K/AKT, CAP/Cb1/TC10 and AMPK pathways. This review highlights the recent findings on beneficial effects of flavonoids in the management of diabetes with particular emphasis on the investigations that explore the role of these compounds in modulating glucose transporter proteins at cellular and molecular level.
Keywords: Glucose transporter protein, insulin, type 2 diabetes mellitus, flavonoids, glucose uptake.
Introduction
Diabetes mellitus (DM) is a metabolic disease marked by a high level of blood glucose due to insufficient or inefficient insulin secretory response [1, 2]. This disease has been considered as the fast growing epidemic worldwide. It is estimated that the number of people with DM will rise from 381.8 million in 2013 to 591.9 million in 2035 [3, 4]. Genetic condition (e.g., monogenic and polygenic mutations) and environmental factor (e.g., overweight, obesity, and inactivity), and their complex interaction can contribute to development of DM. Type 2 DM (T2DM) or non-insulin-dependent diabetes mellitus (NIDDM) is one of the most common types of DM, accounted for 90-95% of the diabetic cases worldwide. This common disease mainly occurs at the age over 40, caused by either deficiency in insulin secretion in the pancreatic beta cells or insulin resistance in the body [5, 6]. T2DM affects several major organs, including heart, blood vessels, nerves, eyes and kidneys leading to disabling or even life-threatening complications such as cardiac dysfunction, atherosclerosis, and nephropathy [7]. Hence, T2DM is a global problem which needs to a greater emphasis on its prevention and therapeutic strategies in the health system.
Although T2DM cannot be cured, it can be treated successfully. A healthy lifestyle such as diet, exercise and weight control can provide the foundation for managing of T2DM, however anti-diabetic agents are required to regulate blood glucose levels in the serious conditions. These drugs can cause side effects for instance, weight gain which consequently increases the risk of insulin resistance leading to a further enhance in drug dose. Administration of neutral anti-diabetic drugs derived from plants has broken this scenario. These medicinal herbs have been traditionally used for the treatment of T2DM since 1550 BC. Approximately, 80% of the people around the world rely on traditional medicinal plants to meet their primary health care needs. Amongst the phytochemical compounds, flavonoids and their derivatives are more under attention due to their hypoglycemic activity [8]. Flavonoids have antioxidative properties which protect the body against the deleterious effects of hyperglycemia in T2DM, through acting on the biological targets such as α-glucosidase, glucose co-transporter or aldose reductase. These antioxidants have been proposed as potential anti-diabetic drugs by acting as biological targets involved in T2DM development.
In this review, we have focused on the structure and function of the flavonoids. Moreover, we highlighted the anti-diabetic effects of the flavonoids in the management of T2DM, through modulating glucose transporters, with particular emphasis on the investigations and recent findings.
Type 2 diabetes
Type 2 diabetes is a progressive disease characterized by hyperglycemia with antecedent phase of insulin resistance. However, insulin resistance alone does not lead to diabetes and the disease develops when the insulin resistance is associated with deficit β-cell function. In fact, insulin resistance and defects in insulin release are considered as key pathophysiologic abnormalities in development of T2DM [9-10].
Insulin resistance
Resistance to insulin or less sensitivity of β-cell to insulin is caused by several different metabolic abnormalities including obesity which more or less effects the function abnormalities to the pancreas. The primarily metabolic abnormality in insulin-resistant type 2 diabetics is the defect in glucose uptake due to defective regulation of glucose transporter-4 (GLUT-4) protein [11]. The defect in translocation of GLUT-4 protein takes place due to the inhibition of tyrosine phosphorylation of insulin receptor substrate-1 (IRS-1). This follows serine phosphorylation IRS-1 which inhibits binding and activation of phosphatidylinositol 3-kinase (PI3K) and initiation of downstream signaling events [12].
It has been proven that exposure of cells to pro-inflammatory cytokine such as tumor necrosis factor alpha (TNF-α) or high levels of free fatty acids (FFAs) has inhibitory effect on phosphorylation of IRS-1 which inhibits downstream signaling and insulin action [13-15]. Metabolic stresses originated from intracellular and extracellular signaling molecules, leads to activation of inflammatory signaling pathways. Enormous evidence suggested that obesity activates inflammatory signaling pathway by mediating functional capacity of the endoplasmic reticulum (ER) and induction of ER stress. The change caused to the inflammatory signaling pathway then contributes to insulin resistance [16-20].
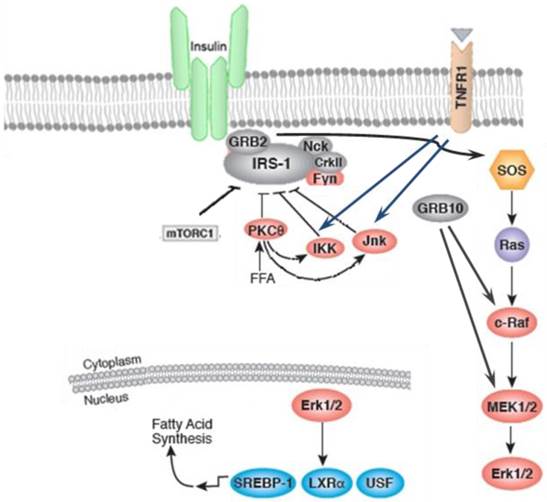
Inflammation and stressful stimuli activates protein kinase Cθ (PKC-θ) and IκB kinase (IKK) which results in inhibition of insulin signaling [21]. These serine/threonine kinase, particularly IKK and c-jun amino terminal kinase (JNK), are also activated in obesity which highlights the overlap of metabolic and immune pathways [16-18]. As shown in figure 1, IKK and JNK pathways are activated in response to stimuli during metabolic dysregulation including ligands for TNF-α, interleukin-1 (IL-1), Toll, or advanced glycation end products receptors (RAGE), intracellular stresses including reactive oxygen species (ROS) and ER stress, ceramide, and various PKC isoforms [22, 23]. Upon activation of both JNK and IKK, IRS-1 phosphorylation takes place on Ser307 and Ser302 which results in impairment of insulin action [15, 16, 24-26]. JNK and IKK pathways lead to the production of additional inflammatory mediators via transcriptional regulation of inflammatory genes by phosphorylating activator protein-1 (AP-1) and nuclear factor -kappa B (NF-κB), respectively [27]. IKKβ activates NF-κB by phosphorylation of NF-κB inhibitor and consequently stimulates production of multiple inflammatory mediators, including TNF-α and IL-6 [28].
On the other hand, lipids-regulated transcription factors, e.g. peroxisome proliferator-activated receptor (PPAR) and liver X receptor (LXR) families) are promoter of nutrient transport and lipid metabolism thereby moderating inflammatory response. However, it has been demonstrated that the expression of fatty acid-binding proteins (FABPs) is antagonist to these transcription factors and promotes a more inflammatory environment [23]. It has been reported that the PPAR-γ activation by insulin sensitizers, enhance the expression and translocation of GLUT-1 and GLUT-4, which results in increased glucose uptake in adipocytes and muscle cells and subsequent reduction in plasma glucose levels [29]. Insulin action can be disturbed by other inflammatory kinases, PKC-θ. Activation of PKC-θ and increased Ser307 phosphorylation of IRS-1 is correlated with lipid infusion and rise in levels of intracellular fatty acid metabolites, such as diacylglycerol (DAG) and fatty acyl CoAs. PKC-θ may also cause insulin resistance by activation of IKKβ, or JNK [30, 31]. The role of other PKC isoforms in inhibition of insulin signaling has also been reported [32].
The insulin action can be inhibited by inflammatory signaling pathways. Inflammation and stressful stimuli activates c-jun amino terminal kinase (JNK), IκB kinase (IKK), and protein kinase Cθ (PKC-θ) which result in inhibition of insulin signaling. The activation of sterol regulatory element binding protein-1c (SREBP-1C), upstream stimulatory factor 1 (USF1), and liver X receptor (LXR)induces fatty acid synthesis.
Defects in Insulin Release
The pathogenesis of T2DM is characterized by alteration in β-cell function and mass in the presence of hyperglycemia and relatively constant insulin resistance. In response to insulin resistance β-cell compensate with increased insulin production to maintain euglycemia. The increased insulin production is accompanied by increased islet size and pancreatic proportion of β-cells [33]. At this stage, β-cells weaken the insulin secretion and gradually the over worked β-cells and their mass diminished.
Decreased β-cells mass is due to apoptosis of β-cells mainly caused by glucotoxicity, lipotoxicity, and deposits of islets amyloid polypeptide (IAPP) [34-36]. The islet in T2DM is characterized by amyloid deposit that derived from islet amyloid polypeptide is co-stored and co-secreted with insulin by pancreatic β-cells. It has been proven that in addition to extracellular IAPP deposit, IAPP toxic oligomers are present intracellulary in β-cells of type 2 diabetic patients which induces β-cells apoptosis [37].
Clinical and experimental animal studies have documented the deleterious role of chronic hyperglycemia in β-cell function and induction of cell apoptosis. The mechanisms involved include mitochondrial dysfunction caused by production of ROS, ER stress, and elevated levels of intracellular calcium [38-40]. In addition, increased FFAs has been demonstrated to induce pro-apoptotic effects on β-cells through ER stress, suppression of the mitogen activated protein kinase (MAPK cascade) [41, 42]. Moreover, intracellular accumulation of triglycerides due to the activation of the sterol regulatory element binding proteins (SREBP) may also contribute to β-cell dysfunction [43].
Role of glucagon, incretin hormones and oxidative stress in the pathogenesis of T2DM
In addition to insulin, secretion of glucagon by pancreatic α-cells has a critical role in glucose hemostasis. These hormones have opposite effects on glycaemia where low blood glucose level induces α-cell secretion while β-cells release insulin in high blood glucose levels. In diabetic condition glucagon secretion is not suppressed at high glucose level and the secretion is inadequate at low glucose level [44-46]. Moreover, the impact of gut on insulin or glucagon secretion by giving rise to a number of peptide hormones such as glucagon-like peptide 1 (GLP-1), gastric inhibitory polypeptide (GIP) and gastrin has been demonstrated. The secretion and insulinotropic action of the two major incretin hormones, GIP and GLP-1, are markedly decreased in T2DM. The reduced incretin action can precede the onset of hyperglycaemia in patients with T2DM [47-49].
Another factor that can lead to insulin resistance, β-cell dysfunction, impaired glucose tolerance and eventually T2DM is oxidative stress. Oxidative stress is defined as imbalance between formation and removal of highly reactive molecules, e.g., reactive oxygen species (ROS) and reactive nitrogen species (RNS) [50]. In oxidative stress condition reactive oxygen species such as superoxide (O.2¯), hydroxyl (.OH), peroxyl (.RO2), hydroperoxyl (.HRO2¯) and reactive nitrogen species such as nitric oxide (.NO) are responsible for lipid and protein modifications [51]. For instance the role of ROS such as O.2¯ and H2O2 in stimulation of stress-related signaling mechanisms including NF-κB, p38-MAP and janus kinase signal transducer and activator of transcription (JAK-STAT) has been well documented [52]. In T2DM, such activation of stress-sensitive pathways and elevations in glucose and free fatty acid (FFA) levels lead to both insulin resistance and impaired insulin secretion [53]. The production of ROS combined with a decreased antioxidant enzymes level leads to development of diabetes complications [50]. Many studies shown the key role of dietary antioxidants to neutralize or trap reactive oxygen species (ROS); therefore, antioxidants can act as antidiabetic agents [54-56]. Many studies have shown that oxidative stress increases the progression of the disorder, whereas an antioxidant-rich diet reduces the risk of diabetes [57, 58].
Dietary Flavonoids
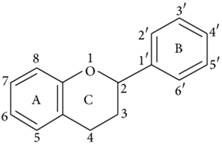
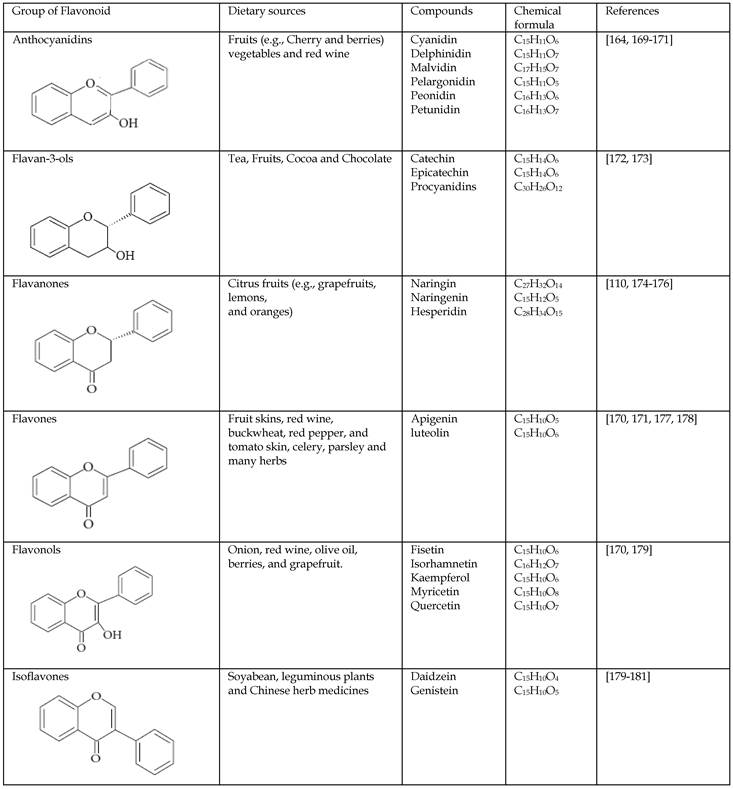
Flavonoids represent a biologically active class of secondary metabolite plant compounds that constitute an important part of the human diet. Till date, about 8,000 different members have been identified in a wide variety of fruit, vegetables and other plant-based food and beverage products [59]. The basic structure of flavonoids consist of 2 phenyl rings (A and B rings) linked by a 3-carbon unit that forms an oxygenated heterocyclic ring (C ring) (Figure 2). Based on differences in generic structure of the C ring, functional groups on the rings and the position at which the B ring is attached to the C ring flavonoids are classified into six subgroups; namely anthocyanidins, flavan-3-ols, flavanones, flavones, flavonols and isoflavones [60, 61]. The chemical structures and individual compounds along with the dietary source of these subgroups are shown in Table 1.
The antidiabetic properties of flavonoids are mainly through their effect on a number of molecular targets and regulation of several pathways such as reducing apoptosis, improving proliferation of pancreatic β-cell and promoting insulin secretion; regulation of glucose metabolism in hepatocytes and subsequent improvement of hyperglycemia; decreasing insulin resistant, inflammation and oxidative stress in adipocytes and skeletal myofibers; enhancing glucose uptake in skeletal muscle and adipose tissues. Some epidemiological studies have suggested some undesirable effects on the consumption of flavonoid-rich diets and development of certain ailments [62, 63]. However, many in vitro and in vivo studies on animal have shown convincing evidences regarding beneficial effects of flavonoids on glucose homeostasis [64].
Uptake of glucose by the cells is an important phenomena in maintain the blood glucose level and there are convincing evidences regarding beneficial effects of flavonoids on peripheral glucose uptake in both insulin sensitive and non-insulin sensitive tissues (Table 2). A study conducted by Prabhakar and Doble [1], showed comparable performance of phenolic compounds to common hypoglycemic drugs in enhancing glucose uptake. In line with the phenolic compounds, epicatechin has been known to possess antidiabetic effects by reducing blood glucose levels [65, 66] and improving the insulin sensitivity and secretion [66-69]. Similarly, the protective effect of epigallocatechin gallate (EGCG), the major polyphenol in green tea, on diabetes and obesity has been extensively studied. It has been indicated that EGCG possess insulin-potentiating activity on the utilization of glucose [70-74]. Many studies on polyphenolic flavonoid, quercetin have identified it as a natural immunity booster and showed to possess α-glucosidase inhibitory activity in vitro [75, 76]. In addition, administration of quercetin has been found to attenuate fasting and postprandial blood glucose level in diabetic mice and rats [77].
Basic structure of flavonoid.
The chemical structures and classification of the flavonoids dietary sources.
List of flavonoids targeting signaling pathways in diabetes.
| Flavonoids | Glucose transporter isoforms | Pathways/target molecules | Experimental model | Targets | Comments | References |
|---|---|---|---|---|---|---|
| Anthocyanins | ||||||
| Anthocyanin | GLUT-4 | IRS1, PI3k/AKT pathway, Antiinflamtroy pathway | HFD-treated mice | Liver | Suppressed reactive oxygen species, restored the impairment of PI3k/AKT pathway, suppressed the JNK, NF- κB and IKKβ activation. | [78] |
| Anthocyanin | GLUT-4 | AMPK pathway, glucose uptake | In vitro | Adipocyte 3T3-L1, C2C12 muscle cells and β TC-tetcells | Enhanced glucose uptake, insulin-like activities, insulin-sensitizing properties, PPARγ agonist activity, increased insulin secretion. | [182] |
| Anthocyanin | GLUT-4 | Glucose uptake | STZ-induced diabetic rats | Heart, skeletal muscle, pancreatic tissues and serum | Antioxidant activity, prevent pancreatic apoptosis, decreased glucose levels, Increased insulin secretion, activated insulin receptor phosphorylation and increased GLUT-4 expression. | [163] |
| Anthocyanin | GLUT-4 | AMPK, insulin sensitivity, PPAR | diabetic mic | White adipose tissue, skeletal muscle, and the liver | hyperglycemia and insulin sensitivity via activation of AMPK, upregulation of glucose transporter 4 in WAT and skeletal muscle, suppression of glucose production and inactivation of acetyl-CoA carboxylase in the liver. | [164] |
| Cyanidin 3-glucoside | GLUT-4 | Antiinflamtroy pathway, glucose uptake, GLUT 4 regulation, | KK-Ay diabetic mice | White adipose tissue | Ameliorated hyperglycemia and insulin sensitivity, upregulated the GLUT 4,downregulated the inflammatory adipocytokines (TNF-α and MCP-1). | [183] |
| Cyanidin 3-glucoside | GLUT-4 | Antiinflamtroy pathway, modulating the JNK/FoxO1 signaling pathway | C57BL/Ks db/db | White adipose tissue and blood | Lowered fasting glucose levels, improved the insulin sensitivity, reduced inflammatory cytokines (TNF-α, IL-6, and MCP-1). | [184] |
| Cyanidin 3-Glucoside | GLUT-4 | Glucose uptake | In vitro | Adipocyte 3T3-L1 | Insulin-like activities, increased adipocyte glucose uptake, GLUT-4 expression and translocation, increased nuclear PPARg activity, improve insulin resistance. | [161, 185] |
| Flavon-3-ols | ||||||
| Catechin | GLUT-4 | Enhanced GLUT4 mRNA and protein expression | STZ-induced diabetic rats | Liver, muscle and blood | Hypo-glycemic, Glucose oxidizing and insulin mimetic activities. | [186] |
| (-)-epicatechin(EP) | GLUT-4 | Glucose uptake, PI3K | In vitro | 3T3-L1 adipocytes | Promote the translocation of GLUT-4 through activation of PI3K, increased phosphorylation of PKCλ/ζ. | [187] |
| (-)-epigallocatechin (EGC) | GLUT-4 | Glucose uptake, PI3K | In vitro | 3T3-L1 adipocytes | Promote the translocation of GLUT-4 through activation of PI3K, increased phosphorylation of PKCλ/ζ. | [187] |
| (-)-epigallocatechin-3-gallate (EGCG) | GLUT-4 | Suppressed JNK pathway | In vitro and obese KK-ay mice,HFD-induced obese rats | Adipocytes tissue, 3T3-L1 adipocytes | Decreased JNK phosphorylation and promoted GLUT-4 translocation. | [188] |
| (-)-epigallocatechin-3-gallate (EGCG) | GLUT-4 | AMPK, insulin signaling pathway | In vivo | Skeletal muscle and adipose tissue | Activated AMPK pathway, improving insulin signaling pathway, decrease oxidative stress, membrane translocation and Ser307 phosphorylation of IRS-1, increase in Ser473 phosphorylation of Akt and GLUT-4 translocation in skeletal muscle and adipose tissue. | [150] |
| (-)-epigallocatechin-3-gallate (EGCG) | GLUT-4 | PI3K/aPKCλ, AMPK and NF- κB signaling pathways | In vitro | H4IIE cells | Stimulates the PI3K/aPKCλ, AMPK and NF- κB pathways. | [149] |
| (-)-epigallocatechin-3-gallate (EGCG) | GLUT-4 | inflammatory response pathway | HFD rats | adipose tissues | Decreasing the levels of toll-like receptor 4, IKKβ, NF- κB, TNF-α, and IL-6, decreased the level of phosphorylated insulin receptor substrate 1 and increased phosphoinositide-3-kinase and GLUT-4 in adipose tissues of HFD rats. | [189] |
| (-)-epigallocatechin-3-gallate (EGCG) | GLUT-4 | Glucose uptake and GLUT-4 translocation | In vitro and ratEx vivo | Insulin-resistant L6 myotubes and skeletal muscle of mice | Increased glucose uptake and promoted translocation of GLUT-4 to plasma membrane in skeletal muscle. | [131] |
| (-)-epigallocatechin-3-gallate (EGCG) | GLUT-4 | IRS1, AMPK | In vitro | HepG2 | Attenuates insulin signaling blockade by reducing IRS-1 Ser307 phosphorylation through the AMPK activation pathway. | [139] |
| (-)-epigallocatechin-3-gallate (EGCG) | GLUT-4 | AMPK, insulin signaling pathway | FFAs-induced peripheral insulin resistance in rats | Skeletal muscle cells and adipose tissue | Decreased oxidative stress and PKCθ membrane translocation activated the AMPK pathway and improved insulin signaling pathway. | [140] |
| (-)-epigallocatechin-3-gallate (EGCG) | - | AMPK pathway | In vitro and BALB/c mice | Mouse hepatoma (Hepa 1-6) cells, rat myotube L6 cells, and 3T3-L1 adipocytes cells and liver of mice | Increased in AMPK and the downstream target acetyl-CoA carboxylase (ACC) phosphorylation. | [151] |
| (-)-epigallocatechin-3-gallate (EGCG) | GLUT-4 | AMPK and PI3K/Akt pathway. | In vitro | L6 cells | Improved insulin-stimulated glucose uptake by increasing GLUT-4 translocation to plasma membrane. | [141] |
| Procyanidins | GLUT-4 | signaling pathway (PI3K and p38 MAPK), glucose uptake, glucose transporter-4 translocation | In vitro and STZ-induced diabetic rats | L6E9 myotubes and 3T3-L1 adipocytes, Blood sample | Antihyperglycemic effect, insulinomimetic activity, stimulated glucose uptake, stimulated GLUT-4 expression and translocation. | [147] |
| Flavanones | ||||||
| Hesperidin | GLUT-2 and GLUT-4 | GLUT 4 expression, increasing hepatic glycolysis and lowering hepatic gluconeogenesis | C57BL/KsJ-db/db mice | Liver and adipocyte | Reduced blood glucose, reduced protein expression of GLUT-2 in hepatocyte, elevated GLUT-4 in adipocyte and hepatocyte. | [80, 110] |
| Naringenin | GLUT-4 | AMPK, glucose uptake, | In vitro | L6 myotubes | Stimulated glucose uptake, increased AMPK phosphorylation/activation. | [132] |
| Naringin | GLUT-2 and GLUT-4 | GLUT 4 expression, increasing hepatic glycolysis and lowering hepatic gluconeogenesis | C57BL/KsJ-db/db mice | Liver and adipocyte | Reduced blood glucose, lowered the mRNA expression of PEPCKand G6Pase in the liver, reduced protein expression of GLUT-2 in hepatocyte, elevated GLUT-4 in adipocyte and hepatocyte. | [80, 110] |
| Naringin | GlUT-4 | AMPK-dependent mechanism and Anti-oxidative stress | HFD in C57BL/6 mice | Liver, white adipose tissue and Blood | Regulated of PEPCK and G6pase, improvement of insulin resistance, protective by phosphorylating AMPKa and IRS1. | [152] |
| Naringin | GLUT-4 | AMPK Pathway, glucose uptake | In vitro | L6 myotubes | Stimulated glucose uptake, increased AMPK phosphorylation/activation. | [132] |
| Flavones | ||||||
| Apigenin | GLUT-4 | GLUT-4 translocation | STZ-induced diabetic rats | Liver and pancreas | Antioxidant effect, effective control of blood glucose level along, enhanced GLUT-4 translocation. | [190] |
| Apigenin-6-C-β-L-fucopyranoside | GLUT-2 | MAPK-PP1 and PI3K-GSK3 pathways, Insulin secretion | In vivo and in vitro | rat soleus muscle, hyperglycemic rats serum, | Stimulated insulin secretion and glycogen synthesis, reduce blood glucose level and insulin mimetic effects. | [158] |
| luteolin-7-O-glucoside | GlUT-4 | Glucose uptake, suppressed gluconeogenic enzymes | STZ-induced diabetic rats | Rat skeletal muscle | Protected pancreatic β-cells, stimulated glucose uptake, increased GLUT-4 expression and translocation, suppressed G6Pase. | [191] |
| Tangeritin | GLUT-4 | AMPK, glucose uptake | In vitro and HFD mice | C2C12 myotubes, muscle tissue | Phosphorylated AMPK and AS160, glucose uptake, Glut4 translocation. | [192] |
| Tangeritin | GLUT-4 | PI3K/Akt1/2, glucose uptake | In vitro | 3T3-F442A adipocytes | Increased in glucose uptake. | [193] |
| Flavonols | ||||||
| Fisetin | GlUT-4 | AMPK | STZ-induced diabetic rats | Liver | Decreased the expression levels of gluconeogenic genes, such as PEPCKand G6Pase. | [194] |
| Kaempferitrin | GlUT-4 | glucose uptake, insulin receptor, PI3K, atypical PKC activity | In vitro | Rat soleus muscle | Stimulated glucose uptake, involved in translocation of GLUT-4, glucose homeostasis, insulin-mimetic role. | [83] |
| Kaempferol | GlUT-4 | Glucose uptake, PPARγ | In vitro | 3T3-L1 adipocytes | Improved glucose uptake, ameliorated hyperglycemia, PPARγ agonist activity. | [195] |
| Kaempferol 3-neohesperidoside | GLUT-4 | PI3K - GSK-3 pathway and MAPK - PP1 pathway, glycogen synthesis | In vitro | Rat soleus muscle | Stimulated glycogen synthesis. | [196] |
| Morin | GlUT-4 | Insulin receptor, IRS, PI3K/Akt, FOXO1 | In vitro | HepG2 | Insulin-mimetic effect, increases the phosphorylation of the insulin receptor and Akt, FOXO1 phosphorylation, inhibits gluconeogenesis and enhances glycogen synthesis. | [197] |
| Myricetin | GLUT-4 | IRS1, PI3k/AKT, p85 | fructose chow-fed rats | Soleus muscles, plasma | Improved insulin sensitivity through the enhancement of insulin action on IRS-1-associated PI3K and GLUT 4 activity. | [145, 146] |
| Myricetin | GLUT-2 and GLUT-4 | insulin signaling pathway | STZ-Cd induced diabetic nephrotoxic rats | Plasma, Liver, pancreas and skeletal muscle | Increased glycogen phosphorylase, glycogen synthase and G6pase, increased insulin and insulin signaling molecule expression like GLUT-2, GLUT-4, IRS-1, IRS-2 and PKB. | [198] |
| Pentamethylquercetin | GLUT-4 | GLUT 4, PPAR | In vitro | 3T3-L1 cell | Increased mRNA levels of GLUT-4, and PPAR. | [129] |
| Quercetin | GlUT-4 | Glucose uptake, PPARγ | In vitro | 3T3-L1 adipocytes | Improved glucose uptake, ameliorated hyperglycemia, PPARγ agonist activity. | [195] |
| Rutin | GLUT-4 | PI3K, atypical PkC and MAPK pathways | Wistar rats | Isolated soleus muscles from rats | Insulin-mimetic role, stimulated glucose uptake via the PI3K, atypical PKC, CaMKII and MAPK pathways, increased GLUT-4 translocation, stimulated calcium uptake. | [143, 144] |
| Tetramethylkaempferol | GLUT-4 | GLUT-4, PPAR | In vitro | 3T3-L1 cell | Increased mRNA levels of GLUT-4, and PPAR. | [129] |
| Isoflavones | ||||||
| Genistein | GLUT-2 | CaMK II and Ca2+ signaling, insulin secretion | In vitro | INS-1 rat insulinoma cells | Stimulated insulin secretion via activation of Ca2+/CaMK II. | [100] |
Lot of studies on vegetables enrich with flavonoids have been reported to mediates blood glucose levels and are helpful in the management of T2DM. One of the example is purple sweet potato which significantly ameliorate the fasting blood glucose level, glucose and insulin tolerance by blocking oxidative stress and enhancing activity of antioxidant enzymes [78, 79]. A study conducted by Jung Lee [80], showed beneficial effects of hesperidin and naringin in treatment of diabetes. The improved hyperglycemia was observed in diabetic mice, evidenced by regulation of the activities of the hepatic glucose metabolic enzymes involved in glycolysis and gluconeogenesis.
It has been shown that berberine is effective in the treatment of diabetes in human by lowering blood insulin level through enhancing insulin sensitivity. A similar study has proven the beneficial effect of berberine by improving insulin secretion in patients with impaired β-cell function [81, 82]. Kaempferitrin has also been demonstrated to possess antidiabetic effects by stimulating GLUT-4 translocation and synthesis in adipocytes [83-85].
Glucose transporter proteins
One of the most vital cellular nutrient transport in eukaryotic cells is the transport of glucose across plasma membrane which is catalyzed by a family of glucose transporter proteins (GLUT). GLUT family, encoded by SLC2 genes, are integral membrane proteins that meditate transport of monosaccharides, polyols, and other small carbon compounds across the membranes of eukaryotic cells. In human, fourteen GLUT proteins are expressed, named GLUT-1-12 and 14 as well as myo-inositol transporter (HMIT). These proteins are comprised of ~500 amino acid residues and based on their amino acid sequence similarity are characterized into three classes namely Class 1 (GLUTs 1-4, 14); Class 2 (GLUTs 5, 7, 9, and 11); and Class 3 (GLUTs 6, 8, 10, 12, and HMIT) [86, 87].
The fourteen GLUT proteins are composed of 12 transmembrane segments, a single site of N-linked glycosylation, a relatively large, central, cytoplasmic linker domain, and exhibit topologies with both their N and C termini positioned in the cytoplasm [87]. In almost every human cell types, GLUT proteins are expressed. The rate of glucose entry into the cell is determined by tissue-specific expression of one or more GLUT proteins [86]. Among all glucose transporters, GLUTs 1- 4 are most widely studied and their roles have been well documented as glucose and/or fructose transporters in different tissues and cell types.
GLUT-1
GLUT-1, the major membrane protein, was the first purified membrane transporter. This glucose transporter is encoded by SLC2A1 gene, comprising 3-5% of total membrane protein. Gene transcription of GLUT-1 is stimulated by glucose deprivation, as well as most mitogens [87-89]. GLUT is found predominantly at the endothelial of barrier tissues such as blood vessels and the blood-brain barrier (BBB), however, it is expressed in many other tissues such as kidney and colon with minimal expression in the liver [87, 90].
GLUT-1 may express in conjunction with one or more GLUT isoforms. For instant, in adipose tissue, GLUT-1 is expressed along with GLUT-4. Glucose, mannose, galactose, glucosamine, and reduced ascorbate can be transported by GLUT-1, although glucose is the main physiological substrate of GLUT-1[87]. The activation of GLUT-1 is mainly occurred by the cell stressors such as azide [91, 92], osmotic stress [93, 94], methylene blue [95], and glucose deprivation [96, 97].
Medicinal plants rich in flavonoids have shown promising effects in up-regulation of GLUT-1 expression levels. Berberin, the major active component of Rhizoma Coptidis, has been reported to enhance GLUT-1 expression and promotes its activities [98, 99]. In addition, genistein derivatives have been demonstrated promising effects in the treatment of T2DM. since the tested compounds significantly stimulated the glucose uptake through elevating GLUT-4 and GLUT-1 mRNA expressions levels in L6 myotubes [100].
GLUT-2
GLUT-2 encoded by SLC2A2, is predominantly expressed in hepatocytes. However, it is also expressed by kidney proximal convoluted tubule cells, intestinal absorptive cells and pancreatic β-cells [101, 102]. This isoform of glucose transporter is involved in glucose-sensing in pancreatic β-cells, liver, and the hypothalamus as well as triggering the glucose-mediated insulin secretion cascade [103-106]. Among all glucose transporters, GLUT-2 has the lowest apparent affinity for glucose. It has low affinity to other monosaccharaides such as galactose, mannose and fructose. However, glucosamine can be transported with a very high affinity [107].
In recent years GLUT-2 has drawn attentions as a molecule that could be involved in the pathogenesis of diabetes mellitus. Studies have proven that the GLUT-2 expression is down-regulated in pancreatic β-cells [108], while hepatic expression of this glucose transporter is enhanced in diabetic animal models [109]. Hesperidin and naringin have been demonstrated to reduce protein expression of GLUT-2 in the liver of experimental animals [80, 110]. Epicatechin has also been reported to protect HepG2 functionality in high glucose concentration by restoring GLUT-2 expression level and modulating glucose production and uptake [111, 112].
GLUT-3
GLUT-3 also known as neuron specific glucose transporter, was first characterized by cloning SLC2A3 gene from human fetal skeletal muscle cell line. This glucose transporter is expressed abundantly in mammalian neurons and trophoblasts, with less expression in the cell body [87, 113]. GLUT-3 plays an important role in the alterations of placental function observed in diabetic pregnancies [114]. GLUT-3 has found to be present in early gestation, proposing its important role in glucose uptake [115]. Among other GLUT proteins present in class I, GLUT-3 has the highest apparent affinity and highest maximum turnover number of glucose. The predominant site of expression of GLUT-3 is brain. However, lower amounts are expressed in placenta, liver, heart and kidney [86, 87]. This tissue distribution is apparently due to the fact that GLUT-3 protein expression occurs more in tissues which exhibit high demand of glucose such as nerve and brain.
The main glucose transporter expressed at the blood-nerve and blood-brain barrier is GLUT-1 while GLUT-3 is responsible for uptake of glucose into the neurons. In fact, GLUT-3 acts in tandem with GLUT-1 to meet the high energy demand of these tissues [116]. In addition, 64% sequence similarity has been reported between GLUT-1 and GLUT-3 proteins [87]. Although GLUT-3 has been proposed to have role in gestation diabetes and alterations of placental function in diabetic pregnancies, there is insufficient information on the expression pattern of GLUT-3 and the role of flavonoids in modulating this glucose transporter isoform.
GLUT-4
GLUT-4 encoded by Slc2a4 gene, is mostly expressed in adipocytes, skeletal muscle, and cardiomyocytes. The presence of this glucose transporter has also been identified in the brain and kidney [117-119]. GLUT-4 is unique isoform among glucose transporters due to its insulin- sensitive regulation while exhibiting 65, 54 and 58% protein sequence similarity with GLUT-1, 2 and 3, respectively. The expression of GLUT-4 gene is subject to both tissue-specific and hormonal/metabolic regulation. Muscle and adipose tissue has shown a large similarity in cellular GLUT-4 regulation, although some minor and major differences have been observed. GLUT-4 plays a vital role in glucose-sensing although only 15% of the blood glucose is absorbed by adipose tissue and the remaining 85% by muscle in healthy individuals [120, 121].
GLUT-4 exist in small vesicles in cytoplasm and can be stimulated by both insulin and muscle contraction which induce the translocation of this glucose transporter isoform to the plasma membrane where it serves as portal though which glucose uptake takes place. Impaired translocation of intracellular GLUT-4 to the plasma membrane refers to insulin resistant. Development of insulin resistance in conjunction with impaired insulin secretion and insulin resistance in the liver plays an important role in the pathogenesis of T2DM [122, 123].
Numerous studies have suggested the role of flavonoids and phenolic compound in enhancement of GLUT-4 expression and glucose uptake. Quercetin and procyanidins have been reported to possess antidiabetic properties by up-regulation of mRNA level of GLUT-4 and its translocation to the cell membrane in adipocytes and skeletal muscle cells [119, 124-128]. Various flavonoids have shown antihyperglycemic effects by increasing mRNA expression levels of GLUT-4 in murine embryonic fibroblast line [129, 130]. EGCG has also been suggested to increase glucose uptake and promote translocation of GLUT-4 to plasma membrane in skeletal muscle cells [131]. Same effects have been observed in adipocyte and skeletal muscle cells by both hesperidin and naringin treatment [80, 110, 132].
Mechanism of Action of dietary flavonoids on glucose transporter proteins
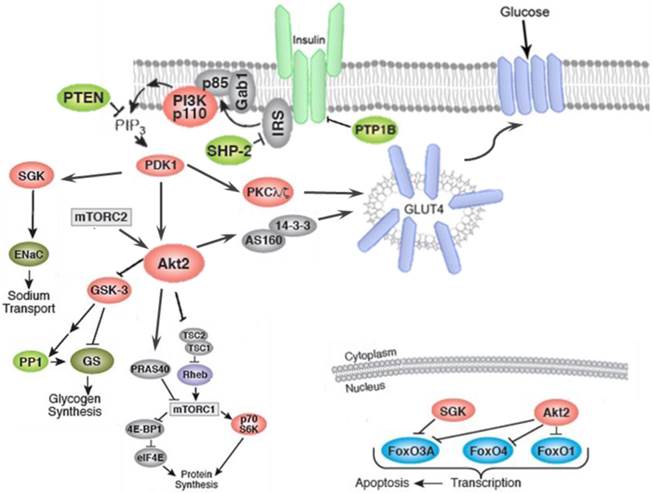
Insulin-induced translocation of GLUT-4 in fat and muscle cells, takes place by two parallel signaling pathways namely, PI3K/AKT and CAP/Cb1/TC10 pathways as shown in figure 3 and figure 4, respectively. Activation of insulin receptor (IR), leads to phosphorylation of insulin receptor substrate (IRS), which in turn triggers the activation of phosphoinositide 3-kinase (PI3K). PI3k then phosphorylates the lipid phosphatidylinositol 4, 5-bisphosphate (PIP2) to yield phosphatidylinositol 3,4,5-trisphosphate (PIP3). PIP3 then activates phosphoinositide-dependent protein kinase 1 (PDK). PDK1-mediated phosphorylation of protein kinase B (Akt), in turn allows phosphorylation of the Rab GTPase-activating protein AS160 and leads to translocation of GLUT-4 from intracellular storage vesicles to plasma membrane and enhances the glucose uptake (Figure 3).
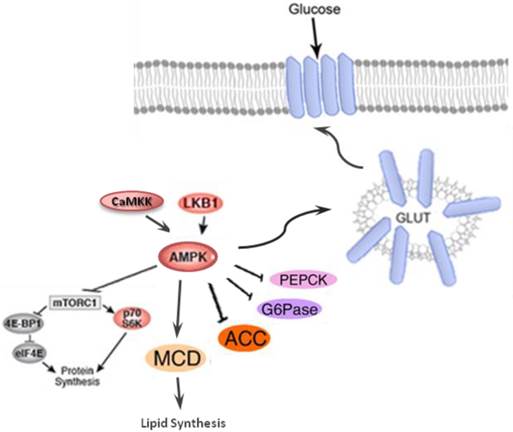
Activation of AKT leads to the inhibition of glycogen synthase kinase-3 (GSK-3), which then phosphorylates and deactivates glycogen synthase (GS). Furthermore, AKT phosphorylates the forkhead box protein O1 (FOXO1), which deactivates the expression of phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6Pase), and suppresses gluconeogenesis in the hepatocyte [133]. AMP-activated protein kinase (AMPK) is an important regulator of the cellular metabolism which is also represses the hepatic glucose production through the modulation of PEPCK and G6Pase (Figure 5) [134].
AMPK, a serine/threonine kinase is responsible for regulating anabolic and catabolic processes and maintaining cellular energy balance. The activation of AMPK, is mainly through the cellular energy changes (Figure 5). However, unknown factors from both immune and metabolic tissues/cells can coordinate to regulate this protein kinase [135]. Under high cellular energy demands, the intracellular ATP is reduced and AMP levels is increased. The increased AMP/ATP ratio activates LKB1:STRAD:MO25 complex which in turn phosphorylates Thr172. The phosphorylation of Thr172 eventually leads to AMPK activation. The alternate pathway to activate AMPK is through activation of Ca2+/calmodulin-dependent protein kinase (CaMKKβ) in response to elevation of Ca2+ level in cell cytoplasm. The Activated AMPK, decrease hepatic glucose production by inhibiting gluconeogenic enzymes phosphoenolpyruvate carboxykinase (PEPCK) and G6Pase and translocates GLUT-4 and GLUT-1 which ultimately increase glucose uptake (Figure 5). Lipid metabolism is also stimulated by AMPK through decreasing malonyl CoA levels in response to inhibition of acetyl CoA carboxylate (ACC) and activation of malonyl CoA decarboxylase (MCD)[133, 135].
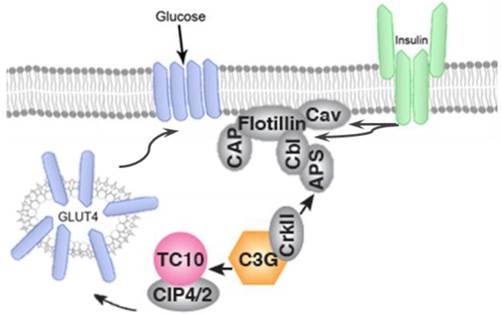
In addition to PI3K/AKT, participation of CAP/Cb1/TC10 pathway has been well documented to have an important role in translocation of GLUT-4 to plasma membrane. Activation of insulin receptor, recruits the adapter protein APS to the insulin receptor β subunit and subsequently phosphorylates Cb1 proto-oncogene. Cb1 and Cb1associated protein (CAP) then interact and bind to the lipid raft protein flotillin in the plasma membrane resulting in the recruitment of CrKII. CrKII then binds to the exchange factor C3G which catalyze the exchange of GDP to GTP on the lipid-raft-associated protein TC10. The TC10 downstream effectors are known to translocate GLUT-4 on plasma membrane and facilitate glucose uptake (Figure 4).
Insulin is secreted from pancreatic β-cells in response to enhanced glucose levels in blood circulation. Glucose is transported into the β-cells through GLUT-2. The increased intracellular concentrations of glucose leads to increase in the production of ATP, and an increase in the ATP/ADP ratio; which ultimately leads to closure of potassium channels on the cell membrane. The membrane depolarizes and leads to voltage-dependent calcium influx. The increased Ca2+ concentration eventually promotes insulin secretion [136-138].
Insulin binding causes activation of the insulin receptor (IR), which phosphorylates different substrate adaptors such as the insulin receptor substrate (IRS). Upon tyrosine phosphorylation, IRS displays binding sites for many signaling partners. Among this signaling pathways PI3K has the main role in insulin function, through the activation of the Akt/PKB and the PKCζ cascades. Activated Akt leads to induction of glycogen synthesis via inhibition of glycogen synthase kinase (GSK-3); protein synthesis through mammalian target of rapamycin (mTOR) and downstream molecules; and regulation of cell proliferation via inhibition of pro-apoptotic agents such as forkhead family transcription factors, bcl-2-associated death promoter (Bad) and GSK-3. The activated Akt eventually leads to translocation GLUT4 to plasma membrane and glucose uptake. In addition to PI3K/Akt pathway.
GLUT-4 translocation takes place by IR-mediated phosphorylation of CAP, and formation of the CAP:Cbl:CrkII complex.
AMP-activated protein kinase (AMPK) which is the central energy leads to decreases hepatic glucose production via inhibiting the activation of gluconeogenic enzymes phosphoenolpyruvate carboxykinase (PEPCK) and glucose 6-phosphatase (G6Pase); induction of glucose uptake through inducing GLUT-4 and GLUT-1 and stimulation of lipid metabolism via declining malonyl CoA levels by inhibiting acetyl CoA carboxylate (ACC) and activation of malonyl CoA decarboxylase.
Recent studies on flavonoids modulating glucose transporter proteins
A number of studies hypothesized that some flavonoids may increase GLUT-4 expression and PI3K/Akt activity leading to restore insulin sensitivity and might be a viable therapeutic avenue for treating diabetes. It has been reported that epicatechin reinforce the insulin signaling pathway by activating key proteins, such as IR/IRS, PI3K/AKT pathway and AMPK, and regulates the glucose production through AKT and AMPK modulation in HepG2 cells [112]. A more recent study on antihyperglycaemic effect of epicatechin, demonstrated that this flavonoid compound reduced the high glucose-induced insulin signaling blockade by preventing the decrease of tyrosine phosphorylated and total IR, IRS-1 and IRS-2 levels, the inhibition of PI3K/AKT and AMPK pathways, and the increase of IRS-1 Ser636/639 phosphorylation values in HepG2 cell line [111]. In addition, it has been reported that EGCG attenuated insulin resistance by increasing IRS-1 serine phosphorylation in vitro [139] and in vivo [140, 141].
Anthocyanins derived from purple sweet potato has shown to remarkably restore the impairment of the IRS1/PI3K /Akt insulin signaling pathway in the livers of high fat diet-treated mice [78]. Lou, Zhang [142], claimed that IL-6 and TNF-α are involved in the development of insulin resistance in hepatocytes. The result of this study confirmed that berberine effectively inhibited palmitate-induced IL-6 and TNF-α and attenuated insulin signaling cascade by modification of Serin/Threonine phosphorylation of insulin receptor substrate-1(IRS-1) and downstream Akt. Kaempferitrin and rutin have been identified to stimulate AKT phosphorylation as well as synthesis and translocation of GLUT-4 in adipocytes and skeletal muscle cells [83, 143, 144]. Myricetin has been evaluated to possess promising activities on improving insulin sensitivity by phosphorylation IR/IRS-1 and PI3K/AKT, which subsequently effect translocation of GLUT-4 in soleus muscles of fructose chow-fed rats [145, 146].
In recent years, studies have most often focused on the effect of flavonoids in stimulating CAP/Cb1/TC10 pathway. For instant, an in vitro study has demonstrated the effect of gallic acid in elevating glucose up take by activation of Cb1-TC10 pathway in parallel to PI3K/AKT pathway in 3T3-L1 adipocytes [130].
The importance of AMPK pathway in controlling cellular metabolism and regulating energy status has drawn the attention of scientists to target activation of this pathway as a new treatment for obesity and diabetes. For instant, Procyanidins, the oligomers and polymers of flavan-3-ols and consist of epicatechin and catechin subunits, have been reported to effectively increase glucose uptake through activation of this pathway [127, 128, 147]. In vitro studies have indicated the role of polyphenolic compounds such as quercetin and resveratrol in increasing glucose uptake in muscle cells and adipocytes by promoting translocation of GLUT-4, mainly via induction of the AMP-activated protein kinase [126, 148]. Epigallocatechin gallate has also been shown to possess beneficial effect on insulin signaling through activation of the AMPK pathway [139, 141, 149-151]. It has been reported that naringin exert hypoglycemic effect in hepatocytes exposed to high glucose by phosphorylating AMPKα and IRS1. The result of the same study also revealed that naringin protected against metabolic syndrome through an AMPK-dependent mechanism in high-fat diet mice [152].
Many studies have demonstrated the ability of berberine to induce Thr-172 phosphorylation of AMPK [81, 99, 153-156]. Berberine has also been reported to be responsible for moderate inhibition of mitochondrial function, decreased oxygen consumption and enhanced glycolysis which lead to increased AMP/ATP ratio and subsequent activation of AMPK pathway [81, 154, 156]. In a study conducted by Ding and his group [157], myricetin intervention revealed the attenuation for the inhibitory effect of hyperinsulinemia on glucose uptake through increasing AMP-activated protein kinase activity in C2C12 myotubes.
The significant insulin secretagogue effect of flavonoids and their potential role in the treatment of diabetes have been widely studied. For instant, Apigenin-6-C-β-L-fucopyranoside and quercetin have been reported to reduce blood glucose level and improve insulin secretion in hyperglycemic rats [158-160].
Antioxidant and anti-inflammatory properties of flavonoids have been well documented, and might be serve as potential therapeutic agents against diabetes by avoiding of insulin resistance. Cyanidin-3-O-b-glucoside and its metabolite protocatechuic acid have been demonstrated to exert insulin-like effects by up-regulating PPARγ activity which results in regulation of adiponectin and translocation of GLUT-4 in human omental adipocytes [161]. In addition, the positive effect of anthocyanins in glucose homeostasis has been investigated in vivo and in vitro [162-164]. For instant, studies conducted by Zhang et al [78, 165, 166] demonstrated the antidiabetic activity of anthocyanins derived from purple sweet potato through inhibition of JNK and IKKβ activation caused by oxidative and ER stress in the liver of high-fat-diet mice [166]. Yan Li and colleges [140], suggested the protective effect of epigallocatechin gallate from FFAs-induced peripheral insulin resistance through inhibition of oxidative stress and PKC activity. In addition, the ability of epigallocatechin gallate to improve insulin signaling by decreasing the levels of toll-like receptor 4, IKKβ, NF- κB, TNF-α, and IL-6 has been reported in high-fat diet rats [129, 149]. Emerging evidence indicated the hypoglycemic effect of naringin by regulation of PEPCK and G6pase as well as anti-oxidative stress property of this flavanone glycoside antioxidant in the improvement of insulin resistance and lipogenesis [152, 167]. It has been reported that naringin and hesperidin attenuate hyperglycemia-mediated oxidative stress and pro-inflammatory cytokines production where, the increased levels of MDA, NO, TNF-α and IL-6 have been reversed in HFD/STZ-induced diabetic rats after administration of naringin and hesperidin [167, 168].
Conclusion
Diabetes mellitus is now a major global public health problem. Currently available drugs for the management of diabetes are costly and produce adverse effects. Flavonoids have recently attracted attention as source materials for development of new antidiabetic drugs. Emerging evidence from various epidemiological, animal and in vitro studies have confirmed the beneficial effects of many dietary flavonoids in the treatment and management of type 2 diabetes and its related complications. Flavonoid mediates glucose metabolism by a number of mechanisms and pathways such as influencing insulin secretion, energy metabolism and insulin sensitivity in peripheral tissues. Not only through antioxidant and anti-inflammatory but different enzyme inhibition, receptor agonist or antagonist activities have shown the protective and curative properties of flavonoids against diabetes. Flavonoid may act through multiple components of signaling cascades to exert their modulatory effects in different cell types. Most of the studies discussed in this review have suggested the important role of flavonoids in enhancing glucose uptake and expression of glucose transporter proteins in particular up-regulation and translocation of GLUT-4. Although to date considerable scientific progress has been made in understanding the mechanism of action of flavonoids, we are still short of having a complete picture of the mechanisms involved in cross-talk based on the insulin action in order to provide new insights into the potential role of flavonoids in the treatment of type 2 diabetes.
Abbreviations
DM: Diabetes mellitus; T2DM: Type 2 Diabetes mellitus; GLP-1: glucagon-like peptide 1; GIP: gastric inhibitory polypeptide; ACC: Acetyl Co-A carboxylase AMPK: Adenosine monophosphate-activated protein kinase; ATP: Adenosine triphosphate cAMP: cyclic adenosine mono phosphate; ECG: Epicatechingallate; EGC: Epigallocatechin; EGCG: Epigallocatechingallate; ER: Endoplasmic reticulum; FFA: Free fatty acid; GSK-3: glycogen synthase kinase; mTOR: mammalian target of rapamycin; Bad: bcl-2-associated death promoter; SREBP-1C: sterol regulatory element binding protein-1c; USF1: upstream stimulatory factor 1; LXR: liver X receptor; G6Pase: glucose 6-phosphatase; IKK: IκB kinase; FoXO1: Forkhead box protein O1; G6Pase: Glucose-6-phosphatase; GK: Glucokinase; GLUT: Glucose transporter; GSIS: Glucose-stimulated insulin secretion; IR: Insulin receptor; IRS: Insulin receptor Substrate; JNK: c-jun amino terminal kinase MAPK: Mitogen activated protein kinase; NF-κB: Nuclear factor-κB; TNFα: Tumor necrosis factor α; PEPCK: Phosphoenolpyruvatecarboxykinase; ROS: Reactive oxygen species; STZ: Streptozotocin; PGC-1α: Peroxisome proliferator-activated receptor gamma coactivator-1-α; PKC: Protein kinase C; PPAR: peroxisome proliferator activated receptor; CaMK II: Calmodulin kinase II; STZ-induced: Streptozotocin-induced; STZ-Cd-induced: Streptozotocin-cadmium-induced; HFD: high-fat diet; FFAs-induced: free fatty acids-induced; NA-STZ-induced: Nicotinamide-streptozotocin-induced.
Acknowledgements
This work was supported by the Fundamental Research Grant (FRGS Grant No: FP021-2014A) from the Ministry of Higher Education, Malaysia and the University of Malaya Research Grant (UMRG Grant No: RP001D-13BIO).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Prabhakar PK, Doble M. Synergistic effect of phytochemicals in combination with hypoglycemic drugs on glucose uptake in myotubes. Phytomedicine: international journal of phytotherapy and phytopharmacology. 2009;16:1119-26
2. Mohler ML, He Y, Wu Z, Hwang DJ, Miller DD. Recent and emerging anti-diabetes targets. Medicinal research reviews. 2009;29:125-95
3. Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes research and clinical practice. 2014;103:137-49
4. Beagley J, Guariguata L, Weil C, Motala AA. Global estimates of undiagnosed diabetes in adults. Diabetes research and clinical practice. 2014;103:150-60
5. Lyssenko V, Laakso M. Genetic screening for the risk of type 2 diabetes: worthless or valuable? Diabetes care. 2013;36(Suppl 2):S120-6
6. Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992;35:595-601
7. Sarje SK, Ghiware NB, Kawade RM, Gunjkar VN, Vadvalkar SM. ssociation of chronic complications of type 2 diabetes with the biochemical and physical estimations in subjects attending single visit screening for complications. International journal of research in pharmacy and chemistry. 2013;3:842-5
8. Bello SO, Chika A, Jimoh AO, Abubakar K, Adebisi I. Evaluation of hypoglycaemic and antihyperglycaemic activity of methanolic wholeplant extract of Schwenckia americana (Solanacae) in normal and alloxan-induced diabetic rats. African journal of pharmacy and pharmacology. 2013;7:2662-6
9. Nicolle E, Souard F, Faure P, Boumendjel A. Flavonoids as promising lead compounds in type 2 diabetes mellitus: molecules of interest and structure-activity relationship. Current medicinal chemistry. 2011;18:2661-72
10. Kahn SE. The importance of the beta-cell in the pathogenesis of type 2 diabetes mellitus. The American journal of medicine. 2000;108(Suppl 6a):2S-8S
11. Zierath JR, He L, Gumà A, Wahlström EO, Klip A, Wallberg-Henriksson H. Insulin action on glucose transport and plasma membrane GLUT4 content in skeletal muscle from patients with NIDDM. Diabetologia. 1996;39:1180-9
12. Dresner A, Laurent D, Marcucci M, Griffin ME, Dufour S, Cline GW. et al. Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. The Journal of clinical investigation. 1999;103:253-9
13. Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science (New York, NY). 1993;259:87-91
14. Paz K, Hemi R, LeRoith D, Karasik A, Elhanany E, Kanety H. et al. A molecular basis for insulin resistance. Elevated serine/threonine phosphorylation of IRS-1 and IRS-2 inhibits their binding to the juxtamembrane region of the insulin receptor and impairs their ability to undergo insulin-induced tyrosine phosphorylation. The Journal of biological chemistry. 1997;272:29911-8
15. Aguirre V, Uchida T, Yenush L, Davis R, White MF. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307). The Journal of biological chemistry. 2000;275:9047-54
16. Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E. et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science (New York, NY). 2004;306:457-61
17. Nakatani Y, Kaneto H, Kawamori D, Yoshiuchi K, Hatazaki M, Matsuoka TA. et al. Involvement of endoplasmic reticulum stress in insulin resistance and diabetes. The Journal of biological chemistry. 2005;280:847-51
18. Ozawa K, Miyazaki M, Matsuhisa M, Takano K, Nakatani Y, Hatazaki M. et al. The endoplasmic reticulum chaperone improves insulin resistance in type 2 diabetes. Diabetes. 2005;54:657-63
19. Lin Y, Berg AH, Iyengar P, Lam TK, Giacca A, Combs TP. et al. The hyperglycemia-induced inflammatory response in adipocytes: the role of reactive oxygen species. The Journal of biological chemistry. 2005;280:4617-26
20. Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y. et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. The Journal of clinical investigation. 2004;114:1752-61
21. Zick Y. Role of Ser/Thr kinases in the uncoupling of insulin signaling. International journal of obesity and related metabolic disorders: journal of the International Association for the Study of Obesity. 2003;27(Suppl 3):S56-60
22. Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783-801
23. Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. The Journal of clinical investigation. 2006;116:1793-801
24. Gao Z, Hwang D, Bataille F, Lefevre M, York D, Quon MJ. et al. Serine phosphorylation of insulin receptor substrate 1 by inhibitor kappa B kinase complex. The Journal of biological chemistry. 2002;277:48115-21
25. Gao Z, Zhang X, Zuberi A, Hwang D, Quon MJ, Lefevre M. et al. Inhibition of insulin sensitivity by free fatty acids requires activation of multiple serine kinases in 3T3-L1 adipocytes. Molecular endocrinology (Baltimore, Md). 2004;18:2024-34
26. Yin MJ, Yamamoto Y, Gaynor RB. The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-beta. Nature. 1998;396:77-80
27. Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239-52
28. Shoelson SE, Lee J, Yuan M. Inflammation and the IKK beta/I kappa B/NF-kappa B axis in obesity- and diet-induced insulin resistance. International journal of obesity and related metabolic disorders: journal of the International Association for the Study of Obesity. 2003;27(Suppl 3):S49-52
29. Kramer D, Shapiro R, Adler A, Bush E, Rondinone CM. Insulin-sensitizing effect of rosiglitazone (BRL-49653) by regulation of glucose transporters in muscle and fat of Zucker rats. Metabolism: clinical and experimental. 2001;50:1294-300
30. Yu C, Chen Y, Cline GW, Zhang D, Zong H, Wang Y. et al. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. The Journal of biological chemistry. 2002;277:50230-6
31. Perseghin G, Petersen K, Shulman GI. Cellular mechanism of insulin resistance: potential links with inflammation. International journal of obesity and related metabolic disorders: journal of the International Association for the Study of Obesity. 2003;27(Suppl 3):S6-11
32. Schmitz-Peiffer C. Protein kinase C and lipid-induced insulin resistance in skeletal muscle. Annals of the New York Academy of Sciences. 2002;967:146-57
33. Guillausseau PJ, Meas T, Virally M, Laloi-Michelin M, Medeau V, Kevorkian JP. Abnormalities in insulin secretion in type 2 diabetes mellitus. Diabetes & metabolism. 2008;34(Suppl 2):S43-8
34. Cernea S, Dobreanu M. Diabetes and beta cell function: from mechanisms to evaluation and clinical implications. Biochemia Medica. 2013:266-80
35. Haataja L, Gurlo T, Huang CJ, Butler PC. Islet amyloid in type 2 diabetes, and the toxic oligomer hypothesis. Endocrine reviews. 2008;29:303-16
36. Del Prato S. Role of glucotoxicity and lipotoxicity in the pathophysiology of Type 2 diabetes mellitus and emerging treatment strategies. Diabetic Medicine. 2009;26:1185-92
37. Rivera JF, Costes S, Gurlo T, Glabe CG, Butler PC. Autophagy defends pancreatic β cells from human islet amyloid polypeptide-induced toxicity. The Journal of clinical investigation. 2014;124:3489-500
38. Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocrine reviews. 2008;29:351-66
39. Wajchenberg BL. beta-cell failure in diabetes and preservation by clinical treatment. Endocrine reviews. 2007;28:187-218
40. Yan LJ. Pathogenesis of chronic hyperglycemia: from reductive stress to oxidative stress. Journal of diabetes research. 2014;2014:137919
41. Unger RH, Zhou Y-T, Orci L. Regulation of fatty acid homeostasis in cells: Novel role of leptin. Proceedings of the National Academy of Sciences. 1999;96:2327-32
42. Cnop M, Welsh N, Jonas J-C, Jörns A, Lenzen S, Eizirik DL. Mechanisms of Pancreatic β-Cell Death in Type 1 and Type 2 Diabetes: Many Differences, Few Similarities. Diabetes. 2005;54:S97-S107
43. Yamashita T, Eto K, Okazaki Y, Yamashita S, Yamauchi T, Sekine N. et al. Role of uncoupling protein-2 up-regulation and triglyceride accumulation in impaired glucose-stimulated insulin secretion in a beta-cell lipotoxicity model overexpressing sterol regulatory element-binding protein-1c. Endocrinology. 2004;145:3566-77
44. Zhang Q, Ramracheya R, Lahmann C, Tarasov A, Bengtsson M, Braha O. et al. Role of KATP channels in glucose-regulated glucagon secretion and impaired counterregulation in type 2 diabetes. Cell metabolism. 2013;18:871-82
45. Gromada J, Franklin I, Wollheim CB. Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocrine reviews. 2007;28:84-116
46. D'Alessio D. The role of dysregulated glucagon secretion in type 2 diabetes. Diabetes, obesity & metabolism. 2011;13(Suppl 1):126-32
47. Meier JJ. The contribution of incretin hormones to the pathogenesis of type 2 diabetes. Best practice & research Clinical endocrinology & metabolism. 2009;23:433-41
48. Vilsbøll T, Holst JJ. Incretins, insulin secretion and Type 2 diabetes mellitus. Diabetologia. 2004;47:357-66
49. Litwack G. Incretins and Insulin Secretion: Academic Press; 2010.
50. Banerjee M, Vats P. Reactive metabolites and antioxidant gene polymorphisms in Type 2 diabetes mellitus. Redox biology. 2013;2C:170-7
51. Kassab A, Piwowar A. Cell oxidant stress delivery and cell dysfunction onset in type 2 diabetes. Biochimie. 2012;94:1837-48
52. Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circulation research. 2010;107:1058-70
53. Aronson D. Hyperglycemia and the pathobiology of diabetic complications. Advances in cardiology. 2008;45:1-16
54. Cao H, Xie Y, Chen X. Type 2 diabetes diminishes the benefits of dietary antioxidants: Evidence from the different free radical scavenging potential. Food chemistry. 2014
55. Liu YJ, Zhan J, Liu XL, Wang Y, Ji J, He QQ. Dietary flavonoids intake and risk of type 2 diabetes: a meta-analysis of prospective cohort studies. Clinical nutrition (Edinburgh, Scotland). 2014;33:59-63
56. van Dam RM, Naidoo N, Landberg R. Dietary flavonoids and the development of type 2 diabetes and cardiovascular diseases: review of recent findings. Current opinion in lipidology. 2013;24:25-33
57. Knekt P, Kumpulainen J, Järvinen R, Rissanen H, Heliövaara M, Reunanen A. et al. Flavonoid intake and risk of chronic diseases. The American Journal of Clinical Nutrition. 2002;76:560-8
58. Wedick NM, Pan A, Cassidy A, Rimm EB, Sampson L, Rosner B. et al. Dietary flavonoid intakes and risk of type 2 diabetes in US men and women. The American Journal of Clinical Nutrition. 2012;95:925-33
59. Cushnie TPT, Lamb AJ. Antimicrobial activity of flavonoids. International Journal of Antimicrobial Agents. 2005;26:343-56
60. Beecher GR. Overview of Dietary Flavonoids: Nomenclature, Occurrence and Intake. The Journal of nutrition. 2003;133:3248S-54S
61. Castellano G, Gonzalez-Santander JL, Lara A, Torrens F. Classification of flavonoid compounds by using entropy of information theory. Phytochemistry. 2013;93:182-91
62. Graf BA, Milbury PE, Blumberg JB. Flavonols, flavones, flavanones, and human health: epidemiological evidence. Journal of medicinal food. 2005;8:281-90
63. Arts CI, Hollman CP. Polyphenols and disease risk in epidemiologic studies. The American Journal of Clinical Nutrition. 2005;81:317-25
64. de Bock M, Derraik JG, Cutfield WS. Polyphenols and glucose homeostasis in humans. Journal of the Academy of Nutrition and Dietetics. 2012;112:808-15
65. Igarashi K, Honma K, Yoshinari O, Nanjo F, Hara Y. Effects of dietary catechins on glucose tolerance, blood pressure and oxidative status in Goto-Kakizaki rats. Journal of nutritional science and vitaminology. 2007;53:496-500
66. Grassi D, Desideri G, Necozione S, Lippi C, Casale R, Properzi G. et al. Blood pressure is reduced and insulin sensitivity increased in glucose-intolerant, hypertensive subjects after 15 days of consuming high-polyphenol dark chocolate. The Journal of nutrition. 2008;138:1671-6
67. Ruzaidi AMM, Abbe MMJ, Amin I, Nawalyah AG, Muhajir H. Protective effect of polyphenol-rich extract prepared from Malaysian cocoa (Theobroma cacao) on glucose levels and lipid profiles in streptozotocin-induced diabetic rats. Journal of the Science of Food and Agriculture. 2008;88:1442-7
68. Vazquez-Prieto MA, Bettaieb A, Haj FG, Fraga CG, Oteiza PI. (-)-Epicatechin prevents TNFalpha-induced activation of signaling cascades involved in inflammation and insulin sensitivity in 3T3-L1 adipocytes. Archives of biochemistry and biophysics. 2012;527:113-8
69. Martin MA, Fernandez-Millan E, Ramos S, Bravo L, Goya L. Cocoa flavonoid epicatechin protects pancreatic beta cell viability and function against oxidative stress. Molecular nutrition & food research. 2014;58:447-56
70. Anderson RA, Polansky MM. Tea enhances insulin activity. Journal of agricultural and food chemistry. 2002;50:7182-6
71. Tipoe GL, Leung TM, Hung MW, Fung ML. Green tea polyphenols as an anti-oxidant and anti-inflammatory agent for cardiovascular protection. Cardiovascular & hematological disorders drug targets. 2007;7:135-44
72. Kao YH, Chang HH, Lee MJ, Chen CL. Tea, obesity, and diabetes. Molecular nutrition & food research. 2006;50:188-210
73. Klaus S, Pultz S, Thone-Reineke C, Wolfram S. Epigallocatechin gallate attenuates diet-induced obesity in mice by decreasing energy absorption and increasing fat oxidation. International journal of obesity (2005). 2005;29:615-23
74. Wolfram S, Wang Y, Thielecke F. Anti-obesity effects of green tea: from bedside to bench. Molecular nutrition & food research. 2006;50:176-87
75. Ishikawa A, Yamashita H, Hiemori M, Inagaki E, Kimoto M, Okamoto M. et al. Characterization of inhibitors of postprandial hyperglycemia from the leaves of Nerium indicum. Journal of nutritional science and vitaminology. 2007;53:166-73
76. Jo SH, Ka EH, Lee HS, Apostolidis E, Jang HD, Kwon YI. Comparison of Antioxidant Potential and Rat intestinal a-Glucosidases inhibitory Activities of Quercetin, Rutin, and Isoquercetin. International Journal of Applied Research in Natural Products; Vol 2, No 4 (2009). 2009
77. Kim JH, Kang MJ, Choi HN, Jeong SM, Lee YM, Kim JI. Quercetin attenuates fasting and postprandial hyperglycemia in animal models of diabetes mellitus. Nutrition research and practice. 2011;5:107-11
78. Zhang ZF, Lu J, Zheng YL, Wu DM, Hu B, Shan Q. et al. Purple sweet potato color attenuates hepatic insulin resistance via blocking oxidative stress and endoplasmic reticulum stress in high-fat-diet-treated mice. The Journal of nutritional biochemistry. 2013;24:1008-18
79. Matsui T, Ebuchi S, Kobayashi M, Fukui K, Sugita K, Terahara N. et al. Anti-hyperglycemic effect of diacylated anthocyanin derived from Ipomoea batatas cultivar Ayamurasaki can be achieved through the alpha-glucosidase inhibitory action. Journal of agricultural and food chemistry. 2002;50:7244-8
80. Jung UJ, Lee MK, Jeong KS, Choi MS. The hypoglycemic effects of hesperidin and naringin are partly mediated by hepatic glucose-regulating enzymes in C57BL/KsJ-db/db mice. The Journal of nutrition. 2004;134:2499-503
81. Yin J, Ye J, Jia W. Effects and mechanisms of berberine in diabetes treatment. Acta Pharmaceutica Sinica B. 2012;2:327-34
82. Yin J, Xing H, Ye J. Efficacy of berberine in patients with type 2 diabetes mellitus. Metabolism: clinical and experimental. 2008;57:712-7
83. Cazarolli LH, Pereira DF, Kappel VD, Folador P, Figueiredo Mdos S, Pizzolatti MG. et al. Insulin signaling: a potential signaling pathway for the stimulatory effect of kaempferitrin on glucose uptake in skeletal muscle. European journal of pharmacology. 2013;712:1-7
84. Ong KC, Khoo HE. Effects of myricetin on glycemia and glycogen metabolism in diabetic rats. Life sciences. 2000;67:1695-705
85. Jorge AP, Horst H, de Sousa E, Pizzolatti MG, Silva FR. Insulinomimetic effects of kaempferitrin on glycaemia and on 14C-glucose uptake in rat soleus muscle. Chemico-biological interactions. 2004;149:89-96
86. Mueckler M, Caruso C, Baldwin SA, Panico M, Blench I, Morris HR. et al. Sequence and structure of a human glucose transporter. Science (New York, NY). 1985;229:941-5
87. Mueckler M, Thorens B. The SLC2 (GLUT) family of membrane transporters. Mol Aspects Med. 2013;34:121-38
88. Baldwin SA, Lienhard GE. Purification and reconstitution of glucose transporter from human erythrocytes. Methods in enzymology. 1989;174:39-50
89. Kasahara M, Hinkle PC. Reconstitution and purification of the D-glucose transporter from human erythrocytes. The Journal of biological chemistry. 1977;252:7384-90
90. Birnbaum MJ, Haspel HC, Rosen OM. Cloning and characterization of a cDNA encoding the rat brain glucose-transporter protein. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:5784-8
91. Shetty M, Loeb JN, Vikstrom K, Ismail-Beigi F. Rapid activation of GLUT-1 glucose transporter following inhibition of oxidative phosphorylation in clone 9 cells. The Journal of biological chemistry. 1993;268:17225-32
92. Rubin D, Ismail-Beigi F. Distribution of Glut1 in detergent-resistant membranes (DRMs) and non-DRM domains: effect of treatment with azide. American journal of physiology Cell physiology. 2003;285:C377-83
93. Barros LF, Barnes K, Ingram JC, Castro J, Porras OH, Baldwin SA. Hyperosmotic shock induces both activation and translocation of glucose transporters in mammalian cells. Pflugers Archiv: European journal of physiology. 2001;442:614-21
94. Barnes K, Ingram JC, Porras OH, Barros LF, Hudson ER, Fryer LG. et al. Activation of GLUT1 by metabolic and osmotic stress: potential involvement of AMP-activated protein kinase (AMPK). Journal of cell science. 2002;115:2433-42
95. Louters LL, Dyste SG, Frieswyk D, Tenharmsel A, Vander Kooy TO, Walters L. et al. Methylene blue stimulates 2-deoxyglucose uptake in L929 fibroblast cells. Life sciences. 2006;78:586-91
96. Kumar A, Xiao YP, Laipis PJ, Fletcher BS, Frost SC. Glucose deprivation enhances targeting of GLUT1 to lipid rafts in 3T3-L1 adipocytes. American journal of physiology Endocrinology and metabolism. 2004;286:E568-76
97. Roelofs B, Tidball A, Lindborg AE, TenHarmsel A, Vander Kooy TO, Louters LL. Acute activation of glucose uptake by glucose deprivation in L929 fibroblast cells. Biochimie. 2006;88:1941-6
98. Cok A, Plaisier C, Salie MJ, Oram DS, Chenge J, Louters LL. Berberine acutely activates the glucose transport activity of GLUT1. Biochimie. 2011;93:1187-92
99. Kim SH, Shin EJ, Kim ED, Bayaraa T, Frost SC, Hyun CK. Berberine activates GLUT1-mediated glucose uptake in 3T3-L1 adipocytes. Biological & pharmaceutical bulletin. 2007;30:2120-5
100. Lee MS, Kim CH, Hoang DM, Kim BY, Sohn CB, Kim MR. et al. Genistein-derivatives from Tetracera scandens stimulate glucose-uptake in L6 myotubes. Biological & pharmaceutical bulletin. 2009;32:504-8
101. Kellett GL, Brot-Laroche E, Mace OJ, Leturque A. Sugar absorption in the intestine: the role of GLUT2. Annual review of nutrition. 2008;28:35-54
102. Cramer SC, Pardridge WM, Hirayama BA, Wright EM. Colocalization of GLUT2 glucose transporter, sodium/glucose cotransporter, and gamma-glutamyl transpeptidase in rat kidney with double-peroxidase immunocytochemistry. Diabetes. 1992;41:766-70
103. Mounien L, Marty N, Tarussio D, Metref S, Genoux D, Preitner F. et al. Glut2-dependent glucose-sensing controls thermoregulation by enhancing the leptin sensitivity of NPY and POMC neurons. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2010;24:1747-58
104. Garcia M, Millan C, Balmaceda-Aguilera C, Castro T, Pastor P, Montecinos H. et al. Hypothalamic ependymal-glial cells express the glucose transporter GLUT2, a protein involved in glucose sensing. Journal of neurochemistry. 2003;86:709-24
105. Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson AU. et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nature genetics. 2010;42:105-16
106. Burcelin R, Dolci W, Thorens B. Glucose sensing by the hepatoportal sensor is GLUT2-dependent: in vivo analysis in GLUT2-null mice. Diabetes. 2000;49:1643-8
107. Uldry M, Ibberson M, Hosokawa M, Thorens B. GLUT2 is a high affinity glucosamine transporter. FEBS letters. 2002;524:199-203
108. Bonny C, Roduit R, Gremlich S, Nicod P, Thorens B, Waeber G. The loss of GLUT2 expression in the pancreatic beta-cells of diabetic db/db mice is associated with an impaired DNA-binding activity of islet-specific trans-acting factors. Molecular and cellular endocrinology. 1997;135:59-65
109. Okamoto Y, Tanaka S, Haga Y. Enhanced GLUT2 gene expression in an oleic acid-induced in vitro fatty liver model. Hepatology Research. 2002;23:138-44
110. Jung UJ, Lee MK, Park YB, Kang MA, Choi MS. Effect of citrus flavonoids on lipid metabolism and glucose-regulating enzyme mRNA levels in type-2 diabetic mice. The international journal of biochemistry & cell biology. 2006;38:1134-45
111. Cordero-Herrera I, Martin MA, Goya L, Ramos S. Cocoa flavonoids attenuate high glucose-induced insulin signalling blockade and modulate glucose uptake and production in human HepG2 cells. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association. 2014;64:10-9
112. Cordero-Herrera I, Martin MA, Bravo L, Goya L, Ramos S. Cocoa flavonoids improve insulin signalling and modulate glucose production via AKT and AMPK in HepG2 cells. Molecular nutrition & food research. 2013;57:974-85
113. Simpson IA, Dwyer D, Malide D, Moley KH, Travis A, Vannucci SJ. The facilitative glucose transporter GLUT3: 20 years of distinction. American journal of physiology Endocrinology and metabolism. 2008;295:E242-53
114. Boileau P, Mrejen C, Girard J, Hauguel-de Mouzon S. Overexpression of GLUT3 placental glucose transporter in diabetic rats. The Journal of clinical investigation. 1995;96:309-17
115. Brown K, Heller DS, Zamudio S, Illsley NP. Glucose transporter 3 (GLUT3) protein expression in human placenta across gestation. Placenta. 2011;32:1041-9
116. Gould GW, Holman GD. The glucose transporter family: structure, function and tissue-specific expression. Biochemical Journal. 1993;295:329-41
117. Apelt J, Mehlhorn G, Schliebs R. Insulin-sensitive GLUT4 glucose transporters are colocalized with GLUT3-expressing cells and demonstrate a chemically distinct neuron-specific localization in rat brain. Journal of Neuroscience Research. 1999;57:693-705
118. Brosius FC 3rd, Briggs JP, Marcus RG, Barac-Nieto M, Charron MJ. Insulin-responsive glucose transporter expression in renal microvessels and glomeruli. Kidney international. 1992;42:1086-92
119. Huang S, Czech MP. The GLUT4 Glucose Transporter. Cell metabolism. 2007;5:237-52
120. Abel ED, Peroni O, Kim JK, Kim YB, Boss O, Hadro E. et al. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature. 2001;409:729-33
121. Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM. et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356-62
122. Kahn BB. Glucose Transport: Pivotal Step in Insulin Action. American Diabetes Association. 1996;45:1644-54
123. Zhidan W, Xie Y, Morrison RF, Bucher NL, Farmer SR. PPARgamma induces the insulin-dependent glucose transporter GLUT4 in the absence of C/EBPalpha during the conversion of 3T3 fibroblasts into adipocytes. The Journal of clinical investigation. 1998;101:22-32
124. Jing D, Xian Z, Lei Z, Hui-Xi B, Xu N, Bin B. et al. Quercetin reduces obesity-associated ATM infiltration and inflammation in mice: amechanismincluding AMPKα1/SIRT1. Journal of Lipid Research. 2014;55:363-74
125. Leto D, Saltiel AR. Regulation of glucose transport by insulin: traffic control of GLUT4. Nature reviews Molecular cell biology. 2012;13:383-96
126. Alam MM, Meerza D, Naseem I. Protective effect of quercetin on hyperglycemia, oxidative stress and DNA damage in alloxan induced type 2 diabetic mice. Life sciences. 2014;109:8-14
127. Yamashita Y, Okabe M, Natsume M, Ashida H. Comparison of Anti-Hyperglycemic Activities Between Low- and High-Degree of Polymerization Procyanidin Fractions from Cacao Liquor Extract. Journal of Food and Drug Analysis. 2012;20:283-7
128. Yamashita Y, Okabe M, Natsume M, Ashida H. Cacao liquor procyanidin extract improves glucose tolerance by enhancing GLUT4 translocation and glucose uptake in skeletal muscle. Journal of nutritional science. 2012;1:e2
129. Matsuda H, Kogami Y, Nakamura S, Sugiyama T, Ueno T, Yoshikawa M. Structural requirements of flavonoids for the adipogenesis of 3T3-L1 cells. Bioorganic & medicinal chemistry. 2011;19:2835-41
130. Vishnu Prasad CN, Anjana T, Banerji A, Gopalakrishnapillai A. Gallic acid induces GLUT4 translocation and glucose uptake activity in 3T3-L1 cells. FEBS letters. 2010;584:531-6
131. Ueda M, Nishiumi S, Nagayasu H, Fukuda I, Yoshida K, Ashida H. Epigallocatechin gallate promotes GLUT4 translocation in skeletal muscle. Biochemical and biophysical research communications. 2008;377:286-90
132. Zygmunt K, Faubert B, MacNeil J, Tsiani E. Naringenin, a citrus flavonoid, increases muscle cell glucose uptake via AMPK. Biochemical and biophysical research communications. 2010;398:178-83
133. Klover PJ, Mooney RA. Hepatocytes: critical for glucose homeostasis. The international journal of biochemistry & cell biology. 2004;36:753-8
134. Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13:1016-23
135. Hardie DG. AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes & development. 2011;25:1895-908
136. Rutter GA. Nutrient-secretion coupling in the pancreatic islet β-cell: recent advances. Molecular Aspects of Medicine. 2001;22:247-84
137. Rutter GA. Visualising insulin secretion. The Minkowski Lecture 2004. Diabetologia. 2004;47:1861-72
138. Schnell S, Schaefer M, Schofl C. Free fatty acids increase cytosolic free calcium and stimulate insulin secretion from beta-cells through activation of GPR40. Molecular and cellular endocrinology. 2007;263:173-80
139. Lin CL, Lin JK. Epigallocatechin gallate (EGCG) attenuates high glucose-induced insulin signaling blockade in human hepG2 hepatoma cells. Molecular nutrition & food research. 2008;52:930-9
140. Li Y, Zhao S, Zhang W, Zhao P, He B, Wu N. et al. Epigallocatechin-3-O-gallate (EGCG) attenuates FFAs-induced peripheral insulin resistance through AMPK pathway and insulin signaling pathway in vivo. Diabetes research and clinical practice. 2011;93:205-14
141. Zhang ZF, Li Q, Liang J, Dai XQ, Ding Y, Wang JB. et al. Epigallocatechin-3-O-gallate (EGCG) protects the insulin sensitivity in rat L6 muscle cells exposed to dexamethasone condition. Phytomedicine: international journal of phytotherapy and phytopharmacology. 2010;17:14-8
142. Lou T, Zhang Z, Xi Z, Liu K, Li L, Liu B. et al. Berberine inhibits inflammatory response and ameliorates insulin resistance in hepatocytes. Inflammation. 2011;34:659-67
143. Kappel VD, Cazarolli LH, Pereira DF, Postal BG, Zamoner A, Reginatto FH. et al. Involvement of GLUT-4 in the stimulatory effect of rutin on glucose uptake in rat soleus muscle. The Journal of pharmacy and pharmacology. 2013;65:1179-86
144. Kappel VD, Zanatta L, Postal BG, Silva FR. Rutin potentiates calcium uptake via voltage-dependent calcium channel associated with stimulation of glucose uptake in skeletal muscle. Archives of biochemistry and biophysics. 2013;532:55-60
145. Tzeng TF, Liou SS, Liu IM. Myricetin Ameliorates Defective Post-Receptor Insulin Signaling via beta-Endorphin Signaling in the Skeletal Muscles of Fructose-Fed Rats. Evidence-based complementary and alternative medicine: eCAM. 2011;2011:150752
146. Liu IM, Tzeng TF, Liou SS, Lan TW. Myricetin, a naturally occurring flavonol, ameliorates insulin resistance induced by a high-fructose diet in rats. Life sciences. 2007;81:1479-88
147. Pinent M, Blay M, Bladé MC, Salvadó MJ, Arola L, Ardévol A. Grape Seed-Derived Procyanidins Have an Antihyperglycemic Effect in Streptozotocin-Induced Diabetic Rats and Insulinomimetic Activity in Insulin-Sensitive Cell Lines. Endocrinology. 2004;145:4985-90
148. Park CE, Kim MJ, Lee JH, Min BI, Bae H, Choe W. et al. Resveratrol stimulates glucose transport in C2C12 myotubes by activating AMP-activated protein kinase. Experimental & molecular medicine. 2007;39:222-9
149. Asano K, Takagi K, Haneishi A, Nakamura S, Yamada K. (-)-Epigallocatechin-3-gallate stimulates both AMP-activated protein kinase and nuclear factor-kappa B signaling pathways. Food chemistry. 2012;134:783-8
150. Li Y, Ding Y. Minireview: Therapeutic potential of myricetin in diabetes mellitus. Food Science and Human Wellness. 2012;1:19-25
151. Murase T, Misawa K, Haramizu S, Hase T. Catechin-induced activation of the LKB1/AMP-activated protein kinase pathway. Biochemical pharmacology. 2009;78:78-84
152. Pu P, Gao DM, Mohamed S, Chen J, Zhang J, Zhou XY. et al. Naringin ameliorates metabolic syndrome by activating AMP-activated protein kinase in mice fed a high-fat diet. Archives of biochemistry and biophysics. 2012;518:61-70
153. Brusq JM, Ancellin N, Grondin P, Guillard R, Martin S, Saintillan Y. et al. Inhibition of lipid synthesis through activation of AMP kinase: an additional mechanism for the hypolipidemic effects of berberine. J Lipid Res. 2006;47:1281-8
154. Cheng Z, Pang T, Gu M, Gao AH, Xie CM, Li JY. et al. Berberine-stimulated glucose uptake in L6 myotubes involves both AMPK and p38 MAPK. Biochimica et biophysica acta. 2006;1760:1682-9
155. Lee YS, Kim WS, Kim KH, Yoon MJ, Cho HJ, Shen Y. et al. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes. 2006;55:2256-64
156. Yin J, Gao Z, Liu D, Liu Z, Ye J. Berberine improves glucose metabolism through induction of glycolysis. American journal of physiology Endocrinology and metabolism. 2008;294:E148-56
157. Ding Y, Dai X-q, Zhang Z-f, Li Y. Myricetin attenuates hyperinsulinemia-induced insulin resistance in skeletal muscle cells. European Food Research and Technology. 2012;234:873-81
158. Cazarolli LH, Folador P, Moresco HH, Brighente IM, Pizzolatti MG, Silva FR. Stimulatory effect of apigenin-6-C-beta-L-fucopyranoside on insulin secretion and glycogen synthesis. European journal of medicinal chemistry. 2009;44:4668-73
159. Cazarolli LH, Kappel VD, Pereira DF, Moresco HH, Brighente IM, Pizzolatti MG. et al. Anti-hyperglycemic action of apigenin-6-C-beta-fucopyranoside from Averrhoa carambola. Fitoterapia. 2012;83:1176-83
160. Vessal M, Hemmati M, Vasei M. Antidiabetic effects of quercetin in streptozocin-induced diabetic rats. Comparative biochemistry and physiology Toxicology & pharmacology: CBP. 2003;135C:357-64
161. Scazzocchio B, Vari R, Filesi C, D'Archivio M, Santangelo C, Giovannini C. et al. Cyanidin-3-O-beta-glucoside and protocatechuic acid exert insulin-like effects by upregulating PPARgamma activity in human omental adipocytes. Diabetes. 2011;60:2234-44
162. Adisakwattana S, Charoenlertkul P, Yibchok-Anun S. alpha-Glucosidase inhibitory activity of cyanidin-3-galactoside and synergistic effect with acarbose. Journal of enzyme inhibition and medicinal chemistry. 2009;24:65-9
163. Nizamutdinova IT, Jin YC, Chung JI, Shin SC, Lee SJ, Seo HG. et al. The anti-diabetic effect of anthocyanins in streptozotocin-induced diabetic rats through glucose transporter 4 regulation and prevention of insulin resistance and pancreatic apoptosis. Molecular nutrition & food research. 2009;53:1419-29
164. Takikawa M, Inoue S, Horio F, Tsuda T. Dietary anthocyanin-rich bilberry extract ameliorates hyperglycemia and insulin sensitivity via activation of AMP-activated protein kinase in diabetic mice. The Journal of nutrition. 2010;140:527-33
165. Zhang ZF, Lu J, Zheng Y-l, Hu B, Fan S-h, Wu D-m. et al. Purple sweet potato color protects mouse liver against d-galactose-induced apoptosis via inhibiting caspase-3 activation and enhancing PI3K/Akt pathway. Food and Chemical Toxicology. 2010;48:2500-7
166. Zhang ZF, Fan SH, Zheng YL, Lu J, Wu DM, Shan Q. et al. Purple sweet potato color attenuates oxidative stress and inflammatory response induced by d-galactose in mouse liver. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association. 2009;47:496-501
167. Mahmoud AM, Ashour MB, Abdel-Moneim A, Ahmed OM. Hesperidin and naringin attenuate hyperglycemia-mediated oxidative stress and proinflammatory cytokine production in high fat fed/streptozotocin-induced type 2 diabetic rats. Journal of diabetes and its complications. 2012;26:483-90
168. Ahmed OM, Mahmoud AM, Abdel-Moneim A, Ashour MB. Antihyperglycemic and Antihyperlipidemic Effects of Hesperidin and Naringin in High Fat Diet/Streptozotocin Type 2 Diabetic Rats. Life Science Journal. 2011;8:91-101
169. Ghosh D, Konishi T. Anthocyanins and anthocyanin-rich extracts: role in diabetes and eye function. Asia Pacific journal of clinical nutrition. 2007;16:200-8
170. Stewart AJ, Bozonnet S, Mullen W, Jenkins GI, Lean ME, Crozier A. Occurrence of flavonols in tomatoes and tomato-based products. Journal of agricultural and food chemistry. 2000;48:2663-9
171. Hertog MGL, Hollman PCH, Katan MB. Content of potentially anticarcinogenic flavonoids of 28 vegetables and 9 fruits commonly consumed in the Netherlands. Journal of agricultural and food chemistry. 1992;40:2379-83
172. Erdman JW Jr, Balentine D, Arab L, Beecher G, Dwyer JT, Folts J. et al. Flavonoids and heart health: proceedings of the ILSI North America Flavonoids Workshop, May 31-June 1, 2005, Washington, DC. The Journal of nutrition. 2007;137:718S-37S
173. López M, Martı́nez F, Del Valle C, Orte C, Miró M. Analysis of phenolic constituents of biological interest in red wines by high-performance liquid chromatography. Journal of Chromatography A. 2001;922:359-63
174. Jeon SM, Park YB, Choi MS. Antihypercholesterolemic property of naringin alters plasma and tissue lipids, cholesterol-regulating enzymes, fecal sterol and tissue morphology in rabbits. Clinical nutrition (Edinburgh, Scotland). 2004;23:1025-34
175. Kim HJ, Oh GT, Park YB, Lee MK, Seo HJ, Choi MS. Naringin alters the cholesterol biosynthesis and antioxidant enzyme activities in LDL receptor-knockout mice under cholesterol fed condition. Life sciences. 2004;74:1621-34
176. Miyake Y, Shimoi K, Kumazawa S, Yamamoto K, Kinae N, Osawa T. Identification and antioxidant activity of flavonoid metabolites in plasma and urine of eriocitrin-treated rats. Journal of agricultural and food chemistry. 2000;48:3217-24
177. He J, Giusti MM. Anthocyanins: natural colorants with health-promoting properties. Annual review of food science and technology. 2010;1:163-87
178. Kreft S, Knapp M, Kreft I. Extraction of rutin from buckwheat (Fagopyrum esculentumMoench) seeds and determination by capillary electrophoresis. Journal of agricultural and food chemistry. 1999;47:4649-52
179. Crozier A, Jaganath IB, Clifford MN. Dietary phenolics: chemistry, bioavailability and effects on health. Natural product reports. 2009;26:1001-43
180. Reinli K, Block G. Phytoestrogen content of foods--a compendium of literature values. Nutrition and cancer. 1996;26:123-48
181. Veitch NC. Isoflavonoids of the leguminosae. Natural product reports. 2013;30:988-1027
182. Martineau LC, Couture A, Spoor D, Benhaddou-Andaloussi A, Harris C, Meddah B. et al. Anti-diabetic properties of the Canadian lowbush blueberry Vaccinium angustifolium Ait. Phytomedicine: international journal of phytotherapy and phytopharmacology. 2006;13:612-23
183. Sasaki R, Nishimura N, Hoshino H, Isa Y, Kadowaki M, Ichi T. et al. Cyanidin 3-glucoside ameliorates hyperglycemia and insulin sensitivity due to downregulation of retinol binding protein 4 expression in diabetic mice. Biochemical pharmacology. 2007;74:1619-27
184. Guo H, Xia M, Zou T, Ling W, Zhong R, Zhang W. Cyanidin 3-glucoside attenuates obesity-associated insulin resistance and hepatic steatosis in high-fat diet-fed and db/db mice via the transcription factor FoxO1. The Journal of nutritional biochemistry. 2012;23:349-60
185. Inaguma T, Han J, Isoda H. Improvement of insulin resistance by Cyanidin 3-glucoside, anthocyanin from black beans through the up-regulation of GLUT4 gene expression. BMC Proceedings. 2011;5:P21
186. Daisy P, Balasubramanian K, Rajalakshmi M, Eliza J, Selvaraj J. Insulin mimetic impact of Catechin isolated from Cassia fistula on the glucose oxidation and molecular mechanisms of glucose uptake on Streptozotocin-induced diabetic Wistar rats. Phytomedicine: international journal of phytotherapy and phytopharmacology. 2010;17:28-36
187. Ueda M, Furuyashiki T, Yamada K, Aoki Y, Sakane I, Fukuda I. et al. Tea catechins modulate the glucose transport system in 3T3-L1 adipocytes. Food & function. 2010;1:167-73
188. Yan J, Zhao Y, Suo S, Liu Y, Zhao B. Green tea catechins ameliorate adipose insulin resistance by improving oxidative stress. Free radical biology & medicine. 2012;52:1648-57
189. Bao S, Cao Y, Fan C, Fan Y, Bai S, Teng W. et al. Epigallocatechin gallate improves insulin signaling by decreasing toll-like receptor 4 (TLR4) activity in adipose tissues of high-fat diet rats. Molecular nutrition & food research. 2014;58:677-86
190. Hossain. Apigenin Causes Biochemical Modulation, Glut4 and Cd38 Alterations to Improve Diabetes and to Protect Damages of Some Vital Organs in Experimental Diabetes. American Journal of Pharmacology and Toxicology. 2014;9:39-52
191. Ong KW, Hsu A, Song L, Huang D, Tan BK. Polyphenols-rich Vernonia amygdalina shows anti-diabetic effects in streptozotocin-induced diabetic rats. Journal of ethnopharmacology. 2011;133:598-607
192. Kim MS, Hur HJ, Kwon DY, Hwang J-T. Tangeretin stimulates glucose uptake via regulation of AMPK signaling pathways in C2C12 myotubes and improves glucose tolerance in high-fat diet-induced obese mice. Molecular and cellular endocrinology. 2012;358:127-34
193. Onda K, Horike N, Suzuki T, Hirano T. Polymethoxyflavonoids tangeretin and nobiletin increase glucose uptake in murine adipocytes. Phytotherapy research: PTR. 2013;27:312-6
194. Prasath GS, Pillai SI, Subramanian SP. Fisetin improves glucose homeostasis through the inhibition of gluconeogenic enzymes in hepatic tissues of streptozotocin induced diabetic rats. European journal of pharmacology. 2014;740:248-54
195. Fang XK, Gao J, Zhu DN. Kaempferol and quercetin isolated from Euonymus alatus improve glucose uptake of 3T3-L1 cells without adipogenesis activity. Life sciences. 2008;82:615-22
196. Cazarolli LH, Folador P, Pizzolatti MG, Mena Barreto Silva FR. Signaling pathways of kaempferol-3-neohesperidoside in glycogen synthesis in rat soleus muscle. Biochimie. 2009;91:843-9
197. Paoli P, Cirri P, Caselli A, Ranaldi F, Bruschi G, Santi A. et al. The insulin-mimetic effect of Morin: a promising molecule in diabetes treatment. Biochimica et biophysica acta. 2013;1830:3102-11
198. Kandasamy N, Ashokkumar N. Protective effect of bioflavonoid myricetin enhances carbohydrate metabolic enzymes and insulin signaling molecules in streptozotocin-cadmium induced diabetic nephrotoxic rats. Toxicology and applied pharmacology. 2014;279:173-85
Author contact
![]() Corresponding authors: Aditya Arya, PhD. Department of Pharmacy, Faculty of Medicine, University of Malaya, 50603 Kuala Lumpur, Malaysia. Tel (Office): +603-7967 5749 Fax: +603-7967 4964 Email: adityaedu.my, adityaarya18com. Fatemeh Hajiaghaalipour, Department of Pharmacy, Faculty of Medicine, University of Malaya, 50603 Kuala Lumpur, Malaysia. Email: sara.alipour22com
Corresponding authors: Aditya Arya, PhD. Department of Pharmacy, Faculty of Medicine, University of Malaya, 50603 Kuala Lumpur, Malaysia. Tel (Office): +603-7967 5749 Fax: +603-7967 4964 Email: adityaedu.my, adityaarya18com. Fatemeh Hajiaghaalipour, Department of Pharmacy, Faculty of Medicine, University of Malaya, 50603 Kuala Lumpur, Malaysia. Email: sara.alipour22com

 Global reach, higher impact
Global reach, higher impact