Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2015; 11(10):1150-1159. doi:10.7150/ijbs.12044 This issue Cite
Research Paper
Beneficial Effects of Highly Palatable Food on the Behavioral and Neural Adversities induced by Early Life Stress Experience in Female Rats
1. Department of Oral and Maxillofacial Surgery, Dental Research Institute, Seoul National University School of Dentistry, Seoul, 110-768, Korea
2. Department of Brain Science, Daegu Gyeongbuk Institute of Science & Technology, Dae Gu, 711-873, Korea
Received 2015-3-4; Accepted 2015-7-13; Published 2015-8-1
Abstract
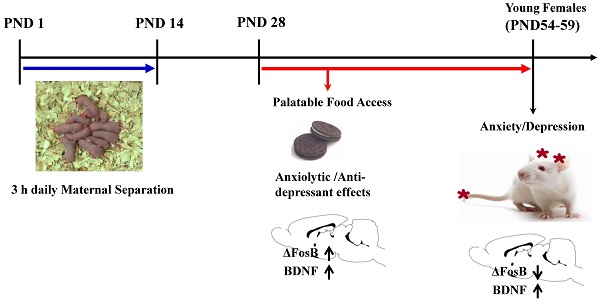
This study examined the effects of highly palatable food during adolescence on the psycho-emotional and neural disturbances caused by early life stress experience in female rats. Female Sprague-Dawley pups were separated from dam for 3 h daily during the first two weeks of birth (MS) or left undisturbed (NH). Half of MS females received free access to chocolate cookies in addition to ad libitum chow from postnatal day 28. Pups were subjected to the behavioral tests during young adulthood. The plasma corticosterone response to acute stress, ΔFosB and brain-derived neurotrophic factor (BDNF) levels in the brain regions were analyzed. Total caloric intake and body weight gain during the whole experimental period did not differ among the experimental groups. Cookie access during adolescence and youth improved anxiety-/depression-like behaviors by MS experience. ΔFosB expression was decreased, but BDNF was increased in the nucleus accumbens of MS females, and ΔFosB expression was normalized and BDNF was further increased following cookie access. Corticosterone response to acute stress was blunted by MS experience and cookie access did not improve it. Results suggest that cookie access during adolescence improves the psycho-emotional disturbances of MS females, and ΔFosB and/or BDNF expression in the nucleus accumbens may play a role in its underlying neural mechanisms.
Keywords: Early life stress, Highly palatable food, Nucleus accumbens, Female
Introduction
There is a growing body of evidence that identical dietary manipulations can have divergent responses between the sexes. At the molecular level, it has been demonstrated that there are sexually dimorphic responses of the hippocampal transcriptome between male and female rats exposed to the same diet [1]. At the metabolic/neuroendocrine level, female rats exhibit different hypothalamic neuropeptide responses to a prolonged high fat diet [2] and higher capacity than males to compensate a high lipid influx [3]. Short term high fat-fed adult females have decreased glucocorticoid receptor mRNA levels in the hippocampus and their hypothalamic-pituitary-adrenal (HPA) axis responds differently from males to a subsequent stress [4, 5]. At the behavioral level, short term exposure of adult rats to fat diet reduces anxiety and increases exploration in males, while it has the opposite effect in females [6]. Puberty is a crucial developmental period characterized by increased endocrine plasticity and changes in stress responsiveness [7]. Studies have suggested that post-weaning high fat diet can modify the basal HPA axis activity and the endocrine responses to an acute stress by affecting both stress and metabolic mediators in a sexually dimorphic manner [8, 9]. We have previously found that prolonged consumption of highly palatable food during adolescence increases anxiety- and depression-like behaviors in male rats, but not in female rats [10]. Prolonged consumption of cafeteria diet high in fat (32% fat content) improved behavioral adversities both in male and female rats that subjected to a similar maternal separation (MS) protocol used in this study, with greater beneficial effects in males [11]. The behavioral and neuroendocrine adversities observed in our female MS rats [12] appeared to differ from ones in male MS rats [13, 14].
In our previous study, prolonged access to highly palatable food, a moderate fat diet (~21% fat) [6, 15], during adolescence and youth improved some anxiety-related symptoms and the HPA axis dysfunction in male MS rats [14]. Studies have suggested that modulation of the stress axis function is implicated in the positive emotional behaviors by highly palatable diet. That is; exposure to a highly preferred diet high in fat was suggested to reduce stress sensitivity [16]; individuals offered with highly palatable food had more pleasant emotions such as satisfaction, enjoyment, and desire [17] and consumption of palatable food decreased sympathetic responses following psychological and immunological stresses [18], stress hormone levels following restraint [19] and anxiety-like behaviors during the elevated plus maze test in rats [20]. However, in this study, the moderate fat diet (~21% fat) during adolescence and youth did not improve the HPA axis dysfunction of MS females, although it improved not only anxiety- but also depression-like behaviors.
In order to investigate the neural mechanisms underlying the psycho-emotional effect of highly palatable diet access in our MS females, we have examined brain-derived neurotrophic factor (BDNF) and ΔFosB levels in the nucleus accumbens (NAc). The NAc, a basal forebrain structure constituting a mesolimbic dopaminergic pathway, has a role in reward, motivation, and reinforcement [21]. Development of anhedonia, a core symptom of major depressive disorder, has been ascribed to dysfunction of the reward pathway, in which the NAc plays a pivotal role [22, 23]. The NAc neurons are activated responding to behavioral stress paradigm [24, 25], and have been implicated in anxiety disorders [26, 27]. The mesolimbic dopaminergic activity and the stress-induced activation of the NAc neurons were blunted in our male MS rats that showed anxiety and depression-like behaviors [13, 28]. BDNF was suggested to be involved in hedonic feeding via modulation of the mesolimbic dopamine system [29, 30], and exposure to a palatable diet increased BDNF and ΔFosB levels and dopamine receptor D1 binding in the NAc [16, 31, 32].
Materials and Methods
Animals
Sprague-Dawley rats were purchased (Samtako Bio, Osan, Korea), and cared in a specific-pathogen-free barrier area with constant control of temperature (22 ±1℃), humidity (55%), and a 12/12 hr light/dark cycle (lights-on at 07:00 AM). Standard laboratory food (Purina Rodent Chow, Purina Co., Seoul, Korea) and membrane filtered purified water were available ad libitum. Animals were cared according to the Guideline for Animal Experiments, 2000, edited by the Korean Academy of Medical Sciences, which is consistent with the NIH Guidelines for the Care and Use of Laboratory Animals, revised 1996. All animal experiments were approved by the Committee for the Care and Use of Laboratory Animals at Seoul National University.
Experimental protocol
Nulliparous females and proven breeder males were used for breeding in the laboratory of the animal facility, and the pups were reared in a controlled manner to minimize and standardize unwanted environmental stimulation from in utero life. Twelve hours after confirming delivery [postnatal day (PND) 1], pups were manipulated as we previously described [13, 14, 33 - 35]. Each litter was assigned either for the maternal separation (MS) group or for the non-handled (NH) group. MS pups were removed from their dam and home cage and placed closely together in a new cage bedded with woodchips (Aspen shaving, Animal JS Bedding, Cheongyang, Korea) during 9:00 h - 12:00 h, and then returned to their home cage and dam. No additional treatment to keep the pups warm during the separation period was offered. MS was performed daily from PND 1 through 14, and then the pups were left with their dam undisturbed until weaning on PND 22. The NH group remained undisturbed until weaning except for routine cage cleaning performed twice a week. On the weaning day, 2 NH and 4 MS female pups were randomly selected from each NH or MS litter, respectively, and placed 2 NH or 2 MS pups together in each cage. Two female MS pups housed together received free access to highly palatable food (HPF) (Oreo cookie, Kraft Foods Global, Inc., East Hanover, NJ, USA), in addition to ad libitum chow from PND 28 (MS+HPF group), and the rest 2 female MS pups in each litter (MS group) and NH pups (NH group) received standard chow only. The nutrient composition formulae of standard chow and Oreo cookie is shown in Table 1. Daily food intake and weight gain were recorded from PND 29. For the evaluation of 24 h food intake, premeasured amount of chow and cookies was provided, and on the next day, left amount of chow and cookies was weighed and subtracted from the value provided on the previous day. Special care was taken to include spillage. Caloric intake was calculated according to the nutrient composition formulae of chow and cookies. Total amounts of food consumed by the pups in each cage were divided by the number of pups in each cage and the each calculated value was considered as n=1. Water was freely available to all experimental groups, and the food conditions were continued throughout the whole experimental period. The schematic diagram of experimental protocol is provided in Figure 1.
Nutrient contents (%) in standard chow and Oreo cookie
| Nutrient | Chow | Cookie |
|---|---|---|
| Protein | 28.507 | 4.25 |
| Fat | 13.496 | 21.26 |
| Carbohydrate | 57.996 | 36.15 |
| Sugar | 0 | 38.28 |
| Vitamine | 0.001 | 0 |
| Sodium | 0 | 0.05 |
Ambulatory Activity
NH, MS and MS+HPF females (n=8 from 4 different litters in each group; total 24 pups from 8 different litters) were subjected to the ambulatory test on PND 54. On each trial, the rat was placed in the center of the activity chamber (43.2 cm in length, 42.2 cm in width, and 30.5 cm in height, MED Associates, VT, USA), a transparent acryl chamber equipped with two horizontal planes of 16 infrared photocell-detector pairs placed in x, y dimension, spaced 2.5 cm apart, and its ambulatory activity was monitored by the computerized system for 30 min. Light condition of the test room was maintained at the same intensity with animal rooms under day-light condition. Ambulatory activity was measured as the total counts of beam interruptions in the horizontal sensor during each consecutive 5 min session. Defecation activity, weight of fecal boli, during the ambulation test of each rat was scored as well. Grooming activity was further analyzed; i.e., forepaw and head grooming was considered as rostral grooming, and body, legs, and tail/genital grooming as caudal grooming [36]. The activity chamber was cleaned with 70% ethanol after each use to eliminate any olfactory cues of the previously tested rat.
Elevated Plus Maze
Two days after the ambulatory activity test (PND 56), rats were subjected to the behavioral assessment in an elevated plus maze, a plus shaped acryl maze with two opposite open arms (50 cm in length and 10 cm in width) and two opposite closed arms (50 cm in length, 10 cm in width, and 31 cm in height), extending out from a central platform (10 cm x 10 cm). The whole apparatus was elevated 50 cm above the floor. The test procedure was followed as previously described [37]. Each rat was placed in the center of the maze facing one of the open arms, and then allowed to explore the open or closed arms of the maze for 5 min. The time spent in the different arms was recorded. Four paws had to be inside the entrance line to each arm, which signaled the start of the time spent in the specific arm, and then the end time was recorded when all four paws were outside the line again. The maze was cleaned with 70% ethanol after each test to prevent influences of the previously tested rat.
Forced Swim Test
Three days after the elevated plus maze test (PND 59), rats were subjected to the forced swim test, according to the method previously described [38]. Each rat was allowed to swim in a glass cylinder (54 cm in height and 24 cm in diameter) filled with water in 40 cm of depth (23-25 ℃) for 5 min, and the test sessions were recorded by a video camera from the side of the cylinder. Duration of rat's immobility in the water was scored from videotapes using a stopwatch. Immobility was defined as the state in which rats were judged to be making only the movements necessary to keep their head above the surface of the water.
Experimental protocol.
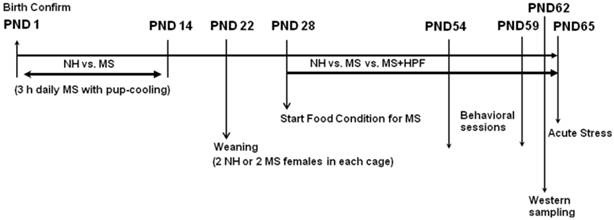
Rats were placed in the test room at least 2 h prior to each test to minimize unwanted stress effects, and all behavioral assessments were performed between 9:00 AM and 12:00 PM of the day to avoid the influences of circadian variances. Behavioral scoring was done with the observer blind of the treatment of the rats.
Plasma corticosterone assay
A week after the end of behavioral sessions, rats were placed in a restraint box for 2 h, in which rats were able to move their four limbs but not to change their body orientation. Tail blood was collected at 0, 30, 60 and 120 min time points during the restraint period, and centrifuged at 2,000 rpm for 20 min. The plasma samples were frozen in liquid nitrogen, and stored at - 80 °C until used for the assay. Plasma levels of corticosterone were determined by radioimmunoassay using 125I-labelled Coat-A-Count kit (Siemens, CA, USA). The sensitivity of the assay was 5.7ng/ml. The intra-assay coefficient of variation was 4-12.2 %.
Western blot analysis
Rats that are naïve from the behavioral tests (n=6 from 3 different litters in each group; total 18 pups from 6 different litters) were sacrificed on PND 62 for the western blot analysis of ΔFosB and BDNF levels in the brain regions. Retroperitoneal fat pads were collected at the time of sacrifice and the brains were removed immediately after decapitation. Tissue samples of nucleus accumbens (NAc) and hippocampus were rapidly dissected on ice, frozen in liquid nitrogen and stored at - 80 °C until used. The NAc tissue dissection was performed using a fine blade according to the method used in our previous studies [28, 39]; however, a possible inclusion of nearby ventro-medial striatum cannot be avoided. The tissues were homogenized in a single detergent lysis buffer (50 mM Tris, pH 8.0; 150 mM NaCl; 1% Triton X-100; protease and phosphatase inhibitor cocktail 0.5%) and then centrifuged at 13,000 g for 20 min at 4oC. The supernatants transferred into new tubes were measured for protein content using a protein assay kit (Biorad DC, Biorad, Inc., Hercules, CA), aliquoted at an 80 μg/20 μl concentration in lysis buffer, and stored at - 80 °C, otherwise used in the same day. The samples were mixed with loading buffer (100 mM Tris, pH 6.8; 200 mM dithiothreitol; 4% SDS; 20% glycerol; 0.2% bromophenol blue) at 1:1 dilution, boiled for 5 min, quickly chilled on ice, and then electrophorazed on 12% SDS-polyacrylamide Tris-glycine gels. The proteins transferred onto nitrocellulose membranes (Hybond-C, Amersham, Bucks, UK) were treated with 5% nonfat dry milk in 1X Tris-buffered saline-Tween (10 mM C4H11NO3; 0.145 M NaCl; 0.2 % SDS; 0.1 % Tween 20) overnight at 40C. The membranes were reacted with polyclonal rabbit anti-∆FosB (1:1000 dilution; Santa Cruz Biotechnology, Dallas, TX, USA) or anti-BDNF (1:500 dilution; Millipore, Temecula, CA, USA), and the bound antibodies were detected with chemiluminescence according to the manufacturer's instructions (Lumi-light western blotting substrate; Roche, Indianapolis, IN, USA), and quantified using a digital image analysis system (LAS-1000, Fuji film, Tokyo, Japan). Digitized values of each sample were normalized to the loading control β-actin, and then all values were converted to relative values to the averaged value of NH group.
Statistical analysis
Data were analyzed by one- or two-way [corticosterone data; treatment (handling or food condition, 2 levels each) X time (4 levels)] analysis of variance (ANOVA), and preplanned comparisons between groups was performed by post hoc Fisher's PLSD test when necessary, using StatView software (Abacus, Berkeley, CA, USA). Body weight and food intake data were further analyzed by repeated measures ANOVA, followed by Bonferroni correction for P value adjustment. The level of significance was set at P < 0.05, and all values were presented as means ± S.E.M.
Results
Food intake and body weight gain
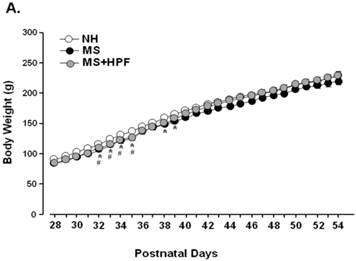
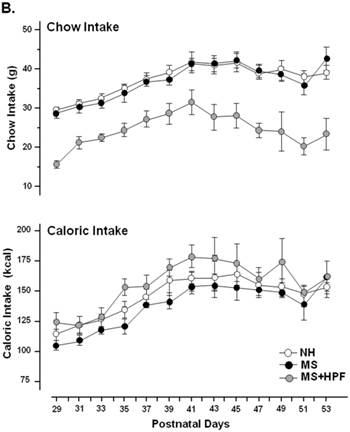
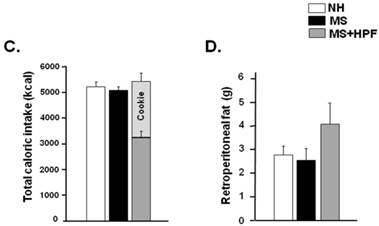
MS females appeared to be lighter than the age-matching NH females until PND 39 and the weight difference was not observed thereafter (Figure 2A). Statistically significant differences (P<0.05) between NH and MS females were observed during PND 32 - 39, except on PND 36 and 37. Palatable food access diminished the weight difference by MS experience, and the statistical significance between NH and MS+HPF disappeared after PND 36. Repeated measures ANOVA revealed that body weight gain over time is different between MS and MS+HPF [F(1,780)=2.146; P=0.0008], but not between NH and MS. Daily chow intake of MS females did not differ from the age-matching NH females (Figure 2B). Cookie access suppressed daily chow intake of MS females, but daily caloric intake tended to be increased by cookie access without statistical significances. Analysis of caloric intake with repeated measures ANOVA revealed no effects of maternal separation and food condition. Total caloric intake during the whole experimental period (PND 28 - 62) did not differ among the experimental groups (Figure 2C). About 40 % of the total calorie consumed by MS+HPF females originated from the cookies (3259.921 ± 211.657 kcal from chow, 2184.641 ± 186.077 kcal from cookies). Retroperitoneal fat pad of MS females on PND 62 did not differ from the age-matching NH females, and tended to be increased with cookie access without statistical significance (P=0.0833, MS vs. MS+HPF) (Figure 2D).
Body weight gain (A), daily intake of chow and calorie (B), total caloric intake (C), and weight of retroperitoneal fat pad (D). NH; non-handled fed with chow only, MS; maternal separation fed with chow only, MS+HPF; maternal separation fed with chow and cookie, HPF; highly palatable food, *P<0.05; NH vs. MS, #P<0.05; NH vs. MS+HPF, n=14 in each group, Data are presented by means ± S.E.M.
Behavioral assessments
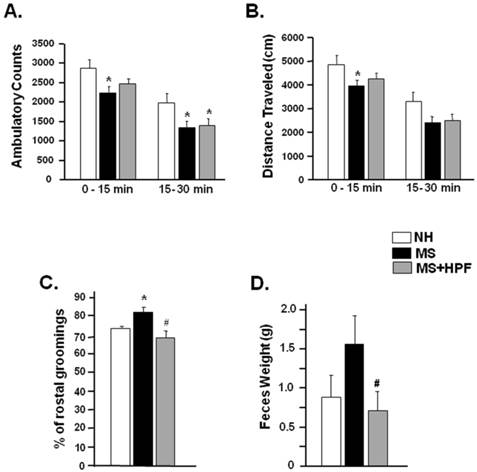
Ambulatory activities of NH, MS and MS+HPF females were measured in a computerized activity chamber on PND 54. Ambulatory counts of MS females during the first (0 - 15 min) and the later session (15 - 30 min) were decreased significantly compared with NH females; however, a significant decrease (P<0.05) relative to NH was observed only during the later session in MS+HPF group (Figure 3A). Total distance traveled during the first 15 min session was significantly decreased in MS (P<0.05), but not in MS+HPF, compared to NH (Figure 3B). Grooming behavior and defecation activity were scored during the ambulatory activity test (Figure 3C & D). MS experience significantly increased rostral grooming (P<0.05, NH vs. MS) while cookie access reduced it (P<0.05, MS vs. MS+HPF) (Figure 3C). Defecation activity of MS females tended to be increased relative to NH without statistical significance, and cookie access significantly reduced it (P<0.05, MS vs. MS+HPF) (Figure 3D).
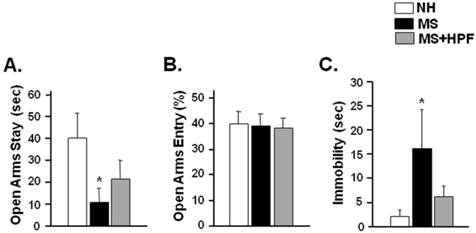
In order to further assess the anxiety-like behaviors, rats were subjected to an elevated plus maze test 2 days after the ambulatory activity test (PND 56). Time spent in the open arms was significantly decreased in MS females (P < 0.05), but not in MS+HPF, compared with NH (Figure 4A). Percent open arm entry did not differ among the experimental groups (Figure 4B). To assess depression-like behaviors, rats were subjected to forced swimming test 3 days after the elevated plus maze test (PND 59). Immobility duration during the 5 min of forced swimming test session was significantly increased in MS females (P < 0.05) compared with NH, and the immobility score of MS+HPF females did not differ from NH (Figure 4C).
Plasma corticosterone levels
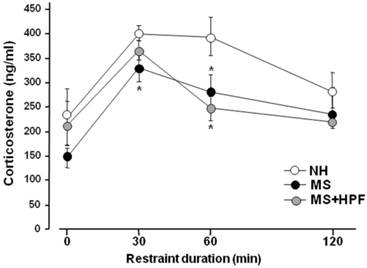
A week after the swim test, rats received restraint stress and the tail bloods were collected at 0, 30, 60 and 120 min time points during 2 h of restraint session, and was used for the plasma corticosterone assay (Figure 5). The basal corticosterone levels (0 time point) did not differ among the groups; however, the stress-induced increases of corticosterone level were lower in MS females than in NH at 30 and 60 min time points after the onset of stress (P<0.05, NH vs. MS at each time point). The plasma corticosterone levels of MS+HPF did not differ from NH at 30 min after the onset of stress, but was lower than NH at 60 min time point (P < 0.05; 394.29 ± 38.35 ng/ml in NH vs. 247.48 ± 24.57 ng/ml in MS+HPF). Analysis of the stress-induced corticosterone levels with 2-way ANOVA revealed main effects of maternal separation [F(1,56)=8.814, P=0.0045] and time [F(3,56)=9.335, P<0.0001], and no effect of food condition. Significant interactions between maternal separation and time or between food condition and time were not found.
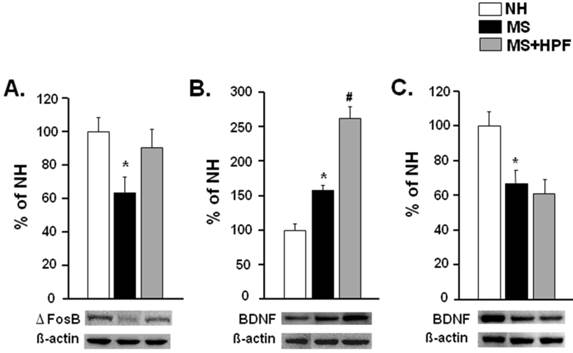
ΔFosB and BDNF western blots
ΔFosB and BDNF levels in the NAc were examined with western blot analysis (Figure 6). ΔFosB was significantly reduced, but BDNF was increased in the NAc of MS females (P < 0.05) compared with NH (Figure 6A & B). ΔFosB level in the NAc of MS females was normalized by cookie access; i.e. no difference between NH and MS+HPF, and BDNF level was further increased (P < 0.05, MS vs. MS+HPF). BDNF levels in the hippocampus of MS females were markedly decreased relative to NH (P < 0.05) and it was not recovered by cookie access (Figure 6C).
Ambulatory activity test performed on PND 54. Ambulatory counts scored consecutively at every 5 min session. Grooming behaviors and defecation activity during 30 min of the ambulatory activity test were scored. Total ambulatory counts (A) and traveled distance (B), Rostral grooming (C) and Feces weight (D). NH; non-handled fed with chow only, MS; maternal separation fed with chow only, MS+HPF; maternal separation fed with chow and cookie, HPF; highly palatable food, *P<0.05 vs. NH, #P<0.05 vs. MS, n=8 in each group, Data are presented by means ± S.E.M.
Time spent in and entry to open arms during elevated plus maze test (A, B) and immobility during forced swim test (C). Rats were subjected to the elevated plus maze test on PND 56 and the forced swim test on PND 59. NH; non-handled fed with chow only, MS; maternal separation fed with chow only, MS+HPF; maternal separation fed with chow and cookie, HPF; highly palatable food, *P<0.05 vs. NH, n=8 in each group, Data are presented by means ± S.E.M.
Plasma corticosterone levels during 2 h of restraint session. Rats were subjected to restraint stress following a week of recovery from the forced swim test. Feeding condition continued during the recovery period. Rats were placed in the restraint box and tail blood was collected at each time point. NH; non-handled fed with chow only, MS; maternal separation fed with chow only, MS+HPF; maternal separation fed with chow and cookie, HPF; highly palatable food, *P<0.05 vs. NH at each time point, n=8 in each group, Data are presented by means ± S.E.M.
Western blot analyses of ΔFosB and BDNF levels in the NAc (A, B) and BDNF level in the hippocampus (C). Rats that are naïve from the behavioral tests were sacrificed on PND 62 to collect the tissue samples for western blot analysis. NH; non-handled fed with chow only, MS; maternal separation fed with chow only, MS+HPF; maternal separation fed with chow and cookie, HPF; highly palatable food, *P<0.05 vs. NH, #P<0.05 vs. MS, n=6 in each group, Data are presented by means ± S.E.M.
Discussion
Palatable food access improved the psycho-emotional behaviors of MS females
In this study, the behavioral scores representing anxiety and depression, such as ambulatory activity, rostral grooming and defecation activity during activity test; open arms stay during elevated plus maze test; immobility during forced swim test, were improved in MS females with free access to Oreo cookies during adolescence and youth. The corticosterone response to acute stress was blunted in MS females, as reported in MS males [14]. The blunted corticosterone response seems to be a consequence of MS experience; i.e. experience of repeated stress, since the HPA axis responses to acute stress challenge appeared to be blunted following experiences of chronic repeated stress [40, 41]. Studies suggested that exposure to highly preferred diet high in fat can modify the basal HPA axis activity and the endocrine responses to an acute stress [9], improve stress responses [17, 19] and decrease anxiety-like behaviors [18, 20]. Also, free access to Oreo cookies (~21% fat content; a moderate fat diet) during adolescence and youth normalized the blunted HPA axis function and improved anxiety-like behaviors in male MS rats [14]. That is, it is likely that adolescent cookie access may improve the blunted HPA axis function by MS experience, repeated stress in early life, and ameliorate the behavioral adversities. However, in this study, the cookie access during adolescence and youth did not improve the HPA axis activity responding to acute stress in female MS rats. It is suggested that the anxiolytic and/or antidepressant efficacies of adolescence cookie access in female MS rats may not be related with the HPA axis function, though it was in male MS rats. Male and female rats differ in numerous neuroendocrine and behavioral parameters, and vulnerability to stress is gender dependent [42, 43]. Previous study reported that a 7-day moderate fat diet protocol leads to a male-selective exaggerated corticosterone release following an acute stress [6].
Palatable food access and neuronal function in the NAc of female rats
The present study demonstrated that ΔFosB expression is decreased and BDNF increased in the NAc of female rats by MS experience. It has been reported that either psychologic or metabolic stress increases ΔFosB expression in the NAc [44-46]. Studies have suggested that transcription factor ΔFosB is related with BDNF expression in the NAc neurons [32, 47-49]. Taken together, it is suggested that decreased ΔFosB and increased BDNF expression in the NAc may be a long-term consequence of MS stress in early life, possibly modulating the NAc neuronal function. The NAc neuronal function was suggested to be modulated by behavioral stress paradigm [24, 25] and its dysfunction has been implicated in depression and anxiety disorders [22, 23, 26, 27]. Indeed, increased BDNF signaling in the NAc has been reported in stress-induced models of depression [50-52], and stress-induced depressive effects was blunted in mice over-expressing ΔFosB in striatum [53]. Thus, it is likely that depression- and/or anxiety-like behaviors of female MS rats may be related with decreased ΔFosB and increased BDNF expression in the NAc.
In this study, cookie access during adolescent period increased ΔFosB and BDNF expressions in female MS rats. This result concurs with previous reports showing that exposure to a palatable diet results in increased levels of ΔFosB in the NAc [31], and that high fat diet increased BDNF levels in the NAc of ΔFosB over-expressing mice [32]. Considering previous report revealing that increased ΔFosB expression in striatum exerts resilience to stress-induced depressive effects [53], it is concluded that increased ΔFosB in the NAc of our MS females with cookie access; i.e. normalized to its basal level, might have contributed to the antidepressant and/or anxiolytic efficacy of adolescence cookie access. However, it is not clear if increased BDNF level in the NAc of MS females with cookie access is implicated in its antidepressant and anxiolytic effects, since increased BDNF signaling in the NAc was reported mostly in depressive models [50-52], but rarely in antidepressant models. Further studies are warranted.
Effects of MS and HPF on the hippocampal BDNF levels
Decreased BDNF level in the hippocampus has been reported both in male and female rats that were subjected to a similar MS protocol used in this study [54, 55]. Concurrently, BDNF level was decreased in the hippocampus of our female MS rats relative to NH controls in this study. Hippocampal neurogenesis has been implicated in symptoms of anxiety and depression [56, 57], and the hippocampus is well known to be involved in the feedback regulation of the HPA axis activity. Recalling that the HPA activity was blunted in our MS females, it is likely that decreased BDNF levels in the hippocampus may be implicated in anxiety and/or depression disorders by MS experience, possibly in relation with the blunted HPA axis activity. The relation between the hippocampal BDNF level and the HPA axis activity in our MS females was further supported by the fact that cookie access did not improve both of them in this study. Previous study showed that prolonged consumption of high fat diet (32% fat) increases BDNF expression in the hippocampus of male MS rats subjected to a similar MS protocol that was used in this study [58]. The effect of cookie access during adolescence (~21% fat) on the hippocampal BDNF levels of male MS rats is currently under investigation.
Behavioral effects of fat/sugar contents in Oreo cookie
In this study, free access to Oreo cookies improved the psycho-emotional adversities in female rats with early life stressful experience. Oreo cookie is a chocolate cookie, not only high in fat but also high in sugar as shown in Table 1. In human study, eating chocolate reduced negative mood compared to drinking water, whereas no effects were found on neutral and positive moods [59]. And the mood improving effect of chocolate was dependent on the palatability of chocolate (milk chocolate vs. plain chocolate), suggesting that eating sweet palatable food improves an experimentally induced negative mood state. It was reported that sucrose craving is increased in depressed animals with chronic mild stress and palatable milk chocolate craving is increased specifically in subjects with depressed mood [60]. Free choice of sucrose and/or lard in addition to chow all modulated the stress axis responses to acute stress [61]. Also, short-term exposure to a moderate fat diet (20 % corn oil; similar fat content with Oreo cookies) induced neuroendocrine and behavioral alterations in a sexually dimorphic manner [4-6]. Taken together, it is concluded that sugar and fat contents of Oreo cookies might have contributed to improve the neural and behavioral adversities in MS females. Further studies are warranted to examine if the same amount of fat or sugar provision as Oreo cookie access would produce similar improvements in MS females observed in this study.
Lastly, retroperitoneal fat depot tended to be increased in MS females by cookie access in this study. In addition to its modulation effect on the stress axis function, palatable food access markedly increased circulating leptin and insulin levels with increased fat depot [15, 61]. Both leptin and insulin have been suggested to exert a regulatory function in the meso-limbic reward system, and especially insulin increased expression of dopamine transporters in the ventral tegmental area [62, 63]. As described above, the meso-limbic reward system is highly implicated in the psycho-emotional disorders in relation with the stress axis function [22-27]. Thus, a tentative modulation, if any, in the meso-limbic reward system by increased leptin and/or insulin with increased fat depot is suggested to play a role in the mood elevation by palatable food access. Indeed, prolonged consumption of a high fat diet (32% fat content) reduced anxiety-like behaviors and increased plasma leptin and insulin levels with markedly increased fat depot in female rats that subjected to a similar MS protocol used in this study [11]. However, it is not yet clear whether or not the behavioral improvements observed in our MS females with cookie access (a moderate fat diet with ~21% fat content) is related with increased fat depot, because the increase in retroperitoneal fat depot by cookie access did not reach a statistical significance and further, neither circulating leptin nor insulin was measured in the current study.
In conclusion, dysfunctions in the HPA axis, the NAc neurons, and the hippocampus appeared to be implicated in the psycho-emotional adversities of young female MS rats by experience of maternal separation during the first two weeks of birth. Free access to highly palatable food, a moderate fat diet, during adolescence and youth improved anxiety- and depression-like behaviors in MS females without affecting body weight gain, and functional modulation in the NAc neurons may play a role in its underlying neural mechanisms.
Acknowledgements
Authors thank Dr. SB Yoo for help with statistical analysis and Dr. JY Lee with experimental techniques. This study was supported by grants from the National Research Foundation (2013R1A1A3A04-006580) and through the Oromaxillofacial Dysfunction Research Center for the Elderly at Seoul National University (2014050477) funded by the Korea Government (Ministry of Science, ICT and Future Planning).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Martin B, Pearson M, Brenneman R. et al. Conserved and differential effects of dietary energy intake on the hippocampal transcriptomes of females and males. PLOS ONE. 2008;3:e2398
2. Priego T, Sa´nchez J, Pico´ C. et al. Sex-associated differences in the leptin and ghrelin systems related with the induction of hyperphagia under high-fat diet exposure in rats. Horm Behav. 2009;55:33-40
3. Priego T, Sa´nchez J, Pico´ C. et al. Sex-differential expression of metabolism-related genes in response to a high-fat diet. Obesity. 2008;16:819-26
4. Kitraki E, Soulis G, Gerozissis K. Impaired neuroendocrine response to stress following a short term fat-enriched diet. Neuroendocrinology. 2004;79:338-45
5. Soulis G, Kitraki E, Gerozissis K. Early neuroendocrine alterations in female rats following a diet moderately enriched in fat. Cell Mol Neurobiol. 2005;25:869-80
6. Soulis G, Papalexi E, Kittas C. et al. Early impact of a fat-enriched diet on behavioral responses of male and female rats. Behav Neurosci. 2007;121:483-90
7. Romeo RD, McEwen BS. Stress and the adolescent brain. Ann NY Acad Sci. 2006;1094:202-14
8. Boukouvalas G, Antoniou K, Papalexi E. et al. Post weaning high fat feeding affects rats' behavior and hypothalamic pituitary adrenal axis at the onset of puberty in a sexually dimorphic manner. Neuroscience. 2008;153:373-82
9. Boukouvalas G, Gerozissis K, Markaki E. et al. High-fat feeding influences the endocrine responses of pubertal rats to an acute stress. Neuroendocrinology. 2010;92:235-45
10. Jahng JW, Kim JY, Lee JY. et al. Free access to highly palatable food during adolescence increases anxiety- and depression-like behaviors in males, but not in females. AChemS2013 Abstract.
11. Maniam J, Morris MJ. Palatable cafeteria diet ameliorates anxiety and depression-like symptoms following an adverse early environment. Psychoneuroendocrinology. 2010;35:717-28
12. Yoo SB, Kim BT, Kim JY. et al. Adolescence fluoxetine increases serotonergic activity in the raphe-hippocampus axis and improves depression-like behaviors in female rats that experienced neonatal maternal separation. Psychoneuroendocrinology. 2013;38:777-88
13. Lee JH, Kim HJ, Kim JG. et al. Depressive behaviors and decreased expression of serotonin reuptake transporter in rats that experienced neonatal maternal separation. Neurosci Res. 2007;58:32-9
14. Lee JH, Kim JY, Jahng JW. Highly palatable food during adolescence improves anxiety-like behaviors and the HPA axis dysfunction by experience of neonatal maternal separation. Endocrinol Metab (Seoul). 2014;29:169-78
15. le Fleur SE, Houshyar H, Roy M. et al. Choice of lard, but not total lard calories, damps adrenocorticotrophin responses to restraint. Endocrinology. 2005;146:2193-9
16. Teegarden SL, Bale TL. Decreases in dietary preference produce increased emotionality and risk for dietary relapse. Biol Psychiatr. 2007;61:1021-9
17. Desmet PM, Schifferstein HN. Sources of positive and negative emotions in food experience. Appetite. 2008;50:290-301
18. Buwalda B, Blom WA, Koolhaas JM. et al. Behavioral and physiological responses to stress are affected by high-fat feeding in male rats. Physiol Behav. 2001;73:371-7
19. Pecoraro N, Reyes F, Gomez F. et al. Chronic stress promotes palatable feeding, which reduces signs of stress: feedforward and feedback effects of chronic stress. Endocrinology. 2004;145:3754-62
20. Prasad A, Prasad C. Short-term consumption of a diet rich in fat decreases anxiety response in adult male rats. Physiol Behav. 1996;60:1039-142
21. Salamone JD, Correa M. Motivational views of reinforcement: implications for understanding the behavioral functions of nucleus accumbens dopamine. Behav Brain Res. 2002;137:3-25
22. Di Chiara G, Loddo P, Tanda G. Reciprocal changes in prefrontal and limbic dopamine responsiveness to aversive and rewarding stimuli after chronic mild stress: implications for the psychobiology of depression. Biol Psychiatr. 1999;46:1624-33
23. Yadid G, Overstreet DH, Zangen A. Limbic dopaminergic adaptation to a stressful stimulus in a rat model of depression. Brain Res. 2001;896:43-7
24. Imperato A, Angelucci L, Casolini P. et al. Repeated stressful experiences differently affect limbic dopamine release during and following stress. Brain Res. 1992;577:194-9
25. Saal D, Dong Y, Bonci A. et al. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577-82
26. da Cunha IC, Lopes APF, Steffens SM. et al. The microinjection of AMPA receptor antagonist into the accumbens shell, but not into the accumbens core, induces anxiolysis in an animal model of anxiety. Behav Brain Res. 2008;188:91-9
27. Kochenborger I, Zanatta D, Berretta LM. et al. Modulation of fear/anxiety responses, but not food intake, following a-adrenoceptor agonist microinjections in the nucleus accumbens shell of free feeding rats. Neuropharmacology. 2012;62:427-35
28. Jahng JW, Ryu V, Yoo SB. et al. Mesolimbic dopaminergic activity responding to acute stress is blunted in adolescent rats that experienced neonatal maternal separation. Neuroscience. 2010;171:144-52
29. Cordeira JW, Frank L, Sena-Esteves M. et al. Brain-derived neurotrophic factor regulates hedonic feeding by acting on the mesolimbic dopamine system. J Neurosci. 2010;30:2533-41
30. Xu B, Goulding EH, Zang K. et al. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4receptor. Nat Neurosci. 2003;6:736-42
31. Nestler EJ, Barrot M, Self DW. DeltaFosB: A sustained molecular switch for addiction. Proc Natl Acad Sci USA. 2001;98:11042-6
32. Teegarden SL, Nestler EJ, Bale TL. Delta FosB-mediated alterations in dopamine signaling are normalized by a palatable high-fat diet. Biol Psychiatr. 2008;64:941-50
33. Kim HJ, Lee JH, Choi SH. et al. Fasting-induced increases of arcuate NPY mRNA and plasma corticosterone are blunted in the rat experienced neonatal maternal separation. Neuropeptides. 2005;39:587-94
34. Ryu V, Lee JH, Yoo SB. et al. Sustained hyperphagia in adolescent rats that experienced neonatal maternal separation. Int J Obes. 2008;32:1355-62
35. Ryu V, Yoo SB, Kang DW. et al. Post-weaning isolation promotes food intake and body weight gain in rats that experienced neonatal maternal separation. Brain Res. 2009;1295:127-34
36. Kalueff AV, Aldridge JW, LaPorte JL. et al. Analyzing grooming microstructure in neurobehavioral experiments. Nat Protoc. 2007;2:2538-44
37. Daniels WM, Pietersen CY, Carstens ME. et al. Maternal separation in rats lead to anxiety-like behavior and a blunted ACTH response and altered neurotransmitter levels in response to a subsequent stressor. Metab Brain Dis. 2004;19:3-14
38. Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730-2
39. Choi YJ, Kim JY, Jin WP. et al. Disruption of oral sensory relay to brain increased anxiety-and depression-like behaviors in rats. Arch Oral Biol. 2013;58:1652-8
40. Jahng JW, Yoo SB, Ryu V. et al. Hyperphagia and depression-like behavior by adolescence social isolation in female rats. Int J Devl Neurosci. 2012;30:47-53
41. Lee JY, Kim JY, Ryu V. et al. Bicuculline ameliorated chronic, but not acute, stress-induced feeding suppression. Int J Pharmacol. 2015;11:335-42
42. Faraday MM, O'Donoghue VA, Grunberg NE. Effects of nicotine and stress on locomotion in Sprague-Dawley and Long-Evans male and female rats. Pharmacol Biochem Behav. 2003;74:325-33
43. Wigger A, Neumann ID. Periodic maternal deprivation induces gender-dependent alterations in behavioral and neuroendocrine responses to emotional stress in adult rats. Physiol Behav. 1999;66:293-302
44. Perrotti LI, Hadeishi Y, Ulery PG. et al. Induction of deltaFosB in reward-related brain structures after chronic stress. J Neurosci. 2004;24:10594-602
45. Stamp JA, Mashoodh R, van Kampen JM. et al. Food restriction enhances peak corticosterone levels, cocaine-induced locomotor activity, and deltaFosB expression in the nucleus accumbens of the rat. Brain Res. 2008;1204:94-101
46. Vialou V, Cui H, Perello M. et al. A role for ΔFosB in calorie restriction-induced metabolic changes. Biol Psychiatr. 2011;70:204-7
47. Benavides DR, Bibb JA. Role of Cdk5 in drug abuse and plasticity. Ann NY Acad Sci. 2004;1025:335-44
48. Bogush A, Pedrini S, Pelta-Heller J. et al. AKT and CDK5/p35 mediate brain-derived neurotrophic factor induction of DARPP-32 in medium size spiny neurons in vitro. J Biol Chem. 2007;282:7352-9
49. Svenningsson P, Nairn AC, Greengard P. DARPP-32 mediates the actions of multiple drugs of abuse. AAPS J. 2005;7:E353-60
50. Bessa JM, Morais M, Marques F. et al. Stressinduced anhedonia is associated with hypertrophy of medium spiny neurons of the nucleus accumbens. Transl Psychiatry. 2013;3:e266
51. Krishnan V, Han MH, Graham DL. et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391-404
52. Weiss F, Ciccocioppo R, Parsons LH. et al. Compulsive drug-seeking behavior and relapse. Neuroadaptation, stress, and conditioning factors. Ann N Y Acad Sci. 2001;937:1-26
53. Donahue RJ, Muschamp JW, Russo SJ. et al. Effects of striatal ΔFosB overexpression and ketamine on social defeat stress-induced anhedonia in mice. Biol Psychiatry. 2014;76:550-8
54. Hill RA, Klug M, Von Soly SK. et al. Sex-specific disruptions in spatial memory and anhedonia in a “two hit” rat model correspond with alterations in hippocampal brain-derived neurotrophic factor expression and signaling. Hippocampus. 2014;24(10):1197-211
55. Lippmann M, Bress A, Nemeroff CB. et al. Long-term behavioral and molecular alterations associated with maternal separation in rats. Eur J Neurosci. 2007;25:3091-8
56. Hanson ND, Owens MJ, Nemeroff CB. Depression, antidepressants, and neurogenesis: a critical reappraisal. Neuropsychopharmacology. 2011;36:2589-602
57. Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10:1110-5
58. Maniam J, Morris MJ. Voluntary exercise and palatable high-fat diet both improve behavioural profile and stress responses in male rats exposed to early life stress: Role of hippocampus. Psychoneuroendocrinology. 2010;35:1553-64
59. Macht M, Muller J. Immediate effects of chocolate on experimentally induced mood states. Appetite. 2007;49:667-74
60. Willner P, Benton D, Brown E. et al. “Depression“ increases “craving“ for sweet rewards in animal and human models of depression and craving. Psychopharmacology. 1998;136:272-83
61. Foster MT, Warne JP, Ginsberg AB. et al. Palatable foods, stress, and energy stores sculpt corticotropin-releasing factor, adrenocorticotrophin, and corticosterone concentrations after restraint. Neuroendocrinology. 2009;150:2325-33
62. Figlewicz DP, Evans SB, Murphy J. et al. (Expression of receptors for insulin and leptin in the ventral tegmental area/substantia nigra (VTA/SN) of the rat. Brain Res. 2003;964:107-15
63. Figlewicz DP, Szot P, Chavez M. et al. Intraventricular insulin increases dopamine transporter mRNA in rat VTA/substantia nigra. Brain Res. 1994;644:331-4
Author contact
![]() Corresponding authors: Jeong Won Jahng, Email:jwjahngac.kr; Jong-Ho Lee, Email:leejonghac.kr, Dental Research Institute, Department of Oral & Maxillofacial Surgery, Seoul National University School of Dentistry, Seoul, 110-768, Korea, Fax: 82-2-766-4948
Corresponding authors: Jeong Won Jahng, Email:jwjahngac.kr; Jong-Ho Lee, Email:leejonghac.kr, Dental Research Institute, Department of Oral & Maxillofacial Surgery, Seoul National University School of Dentistry, Seoul, 110-768, Korea, Fax: 82-2-766-4948

 Global reach, higher impact
Global reach, higher impact