10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2016; 12(4):347-358. doi:10.7150/ijbs.13269 This issue Cite
Research Paper
Vitamin K2 Prevents Glucocorticoid-induced Osteonecrosis of the Femoral Head in Rats
Department of Orthopedic Surgery, Shanghai Jiao Tong University Affiliated Sixth People's Hospital, Shanghai, China
Received 2015-7-17; Accepted 2015-11-21; Published 2016-1-28
Abstract
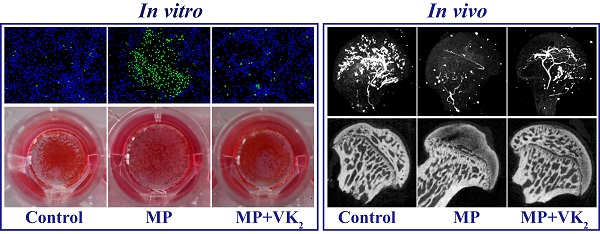
Glucocorticoid medication is one of the most common causes of atraumatic osteonecrosis of the femoral head (ONFH), and vitamin K2 (VK2) has been shown to play an important and beneficial role in bone metabolism. In this study, we hypothesized that VK2 could decrease the incidence of glucocorticoid-induced ONFH in a rat model. Using in vitro studies, we investigated how bone marrow-derived stem cells in the presence of methylprednisolone proliferate and differentiate, specifically examining osteogenic-related proteins, including Runx2, alkaline phosphatase and osteocalcin. Using in vivo studies, we established glucocorticoid-induced ONFH in rats and investigated the preventive effect of VK2. We employed micro-CT scanning, angiography of the femoral head, and histological and immunohistochemical analyses, which demonstrated that VK2 yielded beneficial effects for subchondral bone trabecula. In conclusion, VK2 is an effective antagonist for glucocorticoid on osteogenic progenitors. The underlying mechanisms include acceleration of BMSC propagation and promotion of bone formation-associated protein expression, which combine and contribute to the prevention of glucocorticoid-induced ONFH in rats.
Keywords: Vitamin K2, Osteonecrosis of the femoral head, Glucocorticoid, Osteocalcin, Runx2
Introduction
Glucocorticoid (GC) has been widely used for the treatment of rheumatic, auto-immune and hematopoietic system diseases. However, its abuse is one of the most common causes of atraumatic osteonecrosis of the femoral head (ONFH), contributing to 5%~40% of the cases in which patients develop ONFH [1-3], and the risk increases with higher doses and prolonged treatment. With the development of ONFH, the femoral head finally collapses, causing pain and dysfunction in patients. Several mechanisms have been found in glucocorticoid-induced ONFH, although they are not yet fully understood.
The primary adverse effect of GC is the inhibition of bone formation by affecting bone cell lines. GC has been found to downregulate telomerase activity and the self-renewal ability of mesenchymal stem cells (MSCs) to inhibit MSC proliferation [4]. Extraction of bone marrow stem cells (BMSCs) from steroid-induced ONFH also indicated that GC damaged the proliferation ability of MSCs[5]. Furthermore, studies have indicated that GC inhibited osteogenic differentiation of BMSCs, resulting in a decrease of mature osteoblasts[2, 6]. Among the differentiation effects of GC, downregulation of runt-elated transcription factor Runx2/Cbfa1 is considered to be very important. Runx2 is a key transcriptional modulator of osteoblast formation and regulates the differentiation of BMSCs in many ways. Studies have shown that GC antagonizes Runx2 and, subsequently, ALP expression mediated by Runx2 in cultures of mesenchymal cells[7]. GC was also proven to directly increase apoptosis of osteoblasts and osteocytes, ultimately decreasing bone formation[6, 8]. Recent studies revealed that GC can directly injure endothelial cells and decrease the level of VEGF protein, which is the most important angiogenic factor acting directly on endothelial cells and inducing angiogenesis. Furthermore, GC also suppresses collagen synthesis of myofibroblastic cells, resulting in capillary rarefaction and destruction of the repair process after necrosis[2, 9-12], while fat infiltration and the subsequent increased intraosseous pressure induced by GC aggravate bone ischemia and finally osteonecrosis in femoral heads [2, 13]. Several methods have been put forward to prevent glucocorticoid-induced osteonecrosis, such as pravastatin[14], erythropoietin[15], and G-CSF/SCF[16]; however, no method that was widely used to prevent its incidence was clinically significant.
Vitamin K (VK) has been shown to play an important and beneficial role in bone metabolism, especially in the prevention of osteoporosis and fragility fractures. Osteocalcin (OC) is the primary non-collagenous, Runx2-regulated protein in bone that is produced by osteoblasts during bone formation, consisting of undercarboxylated-OC, referred to as Glu-OC, and carboxylated OC, referred to as Gla-OC, while only Gla-OC confers the greater affinity for calcium to promote bone mineralization[17, 18]. Currently, serum OC has been used to evaluate bone metabolism as a late bone formation marker, and Glu-OC/Gla-OC is a marker of both bone turnover and VK status[19]. VK is the cofactor of carboxylase that is essential for γ-carboxylation of osteocalcin (OC), and studies have shown that VK significantly upregulates OC expression of MSCs in vitro[20] and increases the serum levels of γ-carboxylated OC in vivo[21]. Furthermore, studies have indicated that VK could stimulate osteoblastic cell proliferation[22], promote osteogenesis and increase ALP expression in human BMSCs[20, 23]. Other studies have revealed that VK stimulates bone formation both in sciatic-neurectomized and GC-treated models [24, 25]. Clinically, VKs have been used to increase bone strength and mineral density in osteoporosis[26], except for the classic role in blood coagulation. In addition to bone metabolism effects, VK2 has also been demonstrated to be an anti-calcification component in the vessel wall by activating matrix Gla protein (MGP)[27], protect endothelial cells from serum starvation-induced apoptosis through carboxylated gas6[28] and rescue endothelial cell apoptosis by regulating mitochondrial function[29].
In this study, we hypothesized that VK could prevent the incidence of glucocorticoid-induced ONFH because ONFH incidence is closely related to decreased production of osteoblasts and bone formation-associated proteins[3], while VK has been proven to promote bone remodeling both in vitro and in vivo [25, 30, 31]. Vitamin K2 is a series of vitamers with multiisoprene units at the 3-position of the naphthoquinone, abbreviated as “MK-n”. MK-4, with four isoprene units in its side chain, is the product of tissue-specific conversion directly from dietary phylloquinone and has been shown to be the predominant form of vitamin K in the human body[17, 22, 44]. Studies have indicated that VK2 could enhance bone mineralization and decrease bone resorption more effectively than VK1[17, 32, 33]. Therefore, in this study, we used MK-4 (Sigma, St. Louis, MO, USA) to investigate the effect of VK2 versus steroid-induced ONFH both in vitro and in vivo.
Materials and Methods
Cell culture
Bone marrow stem cells (BMSCs) of 3-week-old Sprague-Dawley (SD) rats (Shanghai Animal Experimental Center, Shanghai, China) were obtained from the femur and tibia according to the method described by Kodama [18] and were cultured with α minimum essential medium (α-MEM; Gibco BRL, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS; Invitrogen, Carlsbad, CA, USA), 100 U/ml penicillin G and 100 μg/ml streptomycin in a humidified atmosphere of 5% CO2 at 37°C. Passage was performed when adherent BMSCs propagated to a density of 80%~90%. BMSCs after three to six passages were used in all experiments, which were carried out at a seeding density of 50,000 cells/cm2. All procedures involving animals were approved by the Institutional Animal Care Committee.
Cell proliferation and viability examination
BMSCs were plated in 96-well plates (four wells in each group) at the same cell density and divided into five groups: (1) the control group; (2) the MP (methylprednisolone, Pfizer) group, which was treated with 5×10-5 M MP; (3) the MP+VK-5 group, which was treated with 5×10-5 M MP and 10-5 M VK2; (4) the MP+VK-6 group, which was treated with 5×10-5 M MP and 10-6 M VK2; and (5) the MP+VK-7 group, which was treated with 5×10-5 M MP and 10-7 M VK2. The MP concentration was determined based on previous studies [2]. We chose three different levels of VK2, to determine its optimal concentration for the following study.
A Cell Counting Kit-8 (CCK-8; Beyotime, Shanghai, China) was used according to the manufacturer's instructions at 0 h, 72 h and 144 h after BMSC adherence. At these time points, 10 μL of CCK-8 reagent was added into 100 μL of culture medium in each well and then incubated for another 3 hours. The absorbance value was measured using a microplate reader (Bio-Rad, Hercules, CA) at 450 nm.
After propagating to a density of more than 90%, BMSCs in the control, MP and MP+VK-6 groups were treated as described above, and the medium was changed every 3 days. The MP+VK-6 group was chosen based on its performance in previous investigations. A ReadyProbes® Cell Viability Imaging Kit (Life Technologies, Gaithersburg, MD, USA) was used to detect cell viability at 144 h after incubation, in which blue dye was used to stain all living cells, and green dye was used to stain the dead cells.
Osteogenic induction
BMSC differentiation was induced 48 h after the cells were plated, with each group's basic medium supplemented with 10-2 M β-sodium glycerophosphate, 50 μg/mL L-ascorbic acid and 10-7 M dexamethasone. The medium was changed every 3 days. For alizarin red staining and immunofluorescence staining, BMSCs were taken from three groups: the control group, the MP group and the MP+VK-6 group. For western blot and qRT-PCR, BMSCs were taken from all five groups.
Alizarin red staining
After osteogenic induction for 21 days, cells in 48-well plates (three wells in each group) were fixed by 4% paraformaldehyde for 20 minutes, then rinsed twice with phosphate-buffered saline (PBS, pH 7.4) and stained with 40 mM alizarin red working solution for 10 minutes. After being rinsed twice with PBS again, these cells were visualized under a light microscope.
Immunofluorescence
BMSCs were imbedded on round cover slips and placed in a 48-well plate. After 3 weeks of osteogenic induction, they were fixed with 4% paraformaldehyde for 20 minutes, treated with 0.1% Triton X-100 for 15 min, and blocked with 10% FBS for 30 min at 37 °C. Then, slips were labeled at 4 °C overnight with rabbit antibodies against rat Runx2 (Millipore, CA, USA), OC (CST, Danvers, MA, USA) and ALP (CST). After being rinsed with PBS three times, these slips were immersed in an Alexa Fluor™488 secondary antibody (Invitrogen) for 1 h at 37 °C. Finally, slips were stained with 4',6-diamidino-2-phenylindole (DAPI) for another 30 seconds, rinsed with PBS and analyzed with a fluorescence microscope.
Alkaline phosphatase activity in BMSCs
The alkaline phosphatase (ALP) activity was determined in cell lysates with a commercial kit (Bioengineering Institute, Nanjing, China) after osteogenic induction for 3 weeks, according to the manufacturer's instructions. Values were measured at 520 nm and normalized to protein concentration.
Western blot
Proteins were extracted with a cell lysis buffer supplemented with proteinase inhibitor, and the total protein concentration was detected with a BCA assay. Then, proteins were denatured at 95 °C for 5 minutes. A 30-µg sample of proteins was subjected to SDS-PAGE and transferred to a PVDF membrane. After being blocked with 5% dried skimmed milk, the membranes were labeled with a primary antibody of Runx2 (Abcam, Cambridge, MA, USA) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH, CST) at a concentration of 1:1000 at 4 °C overnight and then immersed in the secondary antibody working reagent of anti-rabbit IgG (1:1000) at 37 °C for 1 h. After chemiluminescence with a commercial assay, the target bands were detected with a gel image-processing system. The protein levels were normalized against GAPDH.
Quantitative real-time polymerase chain reaction analysis (qRT-PCR)
Total RNA was extracted from osteogenic-induced BMSCs in each group with the TRIzol reagent (Invitrogen). The RT reaction was performed with EasyScript one-step gDNA Removal and cDNA Synthesis Supermix (TransGen Biotech, Beijing, China) from 1 μg of total RNA according to the manufacturer's instructions. qRT-PCR of ALP, OC and Runx2 were performed with TransStart Tip Green qPCR SuperMix (TransGen Biotech). The relative amount of mRNAs was normalized to β-actin. The forward and reverse primers of each cDNA were designed as follows: β-actin, 5′-GTCATCCATGGCGAACTGGT-3′ and 5′-CGTCATCCATGGCGAACTGG-3′; Runx2, 5′-CCGAGACCAACCGAGTCATTTA-3′ and 5′-AAGAGGCTGTTTGACGCCAT-3′; OC, 5′-TCAACAATGGACTTGGAGCCC-3′ and 5′-AGCTCGTCACAATTGGGGTT-3′; ALP, 5′-CAAGGATGCTGGGAAGTCCG-3′ and 5′-CTCTGGGCGCATCTCATTGT-3′. The qRT-PCR reaction system was as follows: cDNA, 1 μL; double-distilled water, 3.4 μL; Tip Green qPCR SuperMix, 5 μl; passive reference dye (50×), 0.2 μL; forward primer one (10 μmol/l), 0.2 μL; reverse primer (10 μmol/l), 0.2 μL. The total volume of the system was 10 μL. The reaction conditions were 95 °C for 30 s first, then 95 °C for 5 s, and finally 60 °C for 30 s, in all 40 cycles. Meanwhile, a 65 °C~95 °C solubility curve was constructed.
Animal model and grouping
A total of 30 SD rats were divided into three groups. Rats in the control group received no treatment. Rats in the MP group were intramuscularly injected with MP 20 mg/kg/d for three continuous days per week, with an injection period of three weeks (total MP: 180 mg/kg). Animals in the MP+VK2 group were first fed VK2 30 mg/kg/d for two weeks and then intramuscularly injected with methylprednisolone as in the MP group, accompanied by consecutive VK2 feed until the animals were sacrificed. All samples were obtained 6 weeks after the first injection of MP.
Serum ALP, Glu-OC and Gla-OC
Blood samples were extracted from SD rats after anesthesia with serum separation tubes and then centrifuged at 3000 rpm for 15 minutes at 4 °C. The serum was separated, and the serum ALP activity was measured using a commercial assay (Beyotime). The absorbance at 405 nm was recorded as relative ALP levels in rats.
Serum Glu-OC, which means undercarboxylated osteocalcin, and Gla-OC, which means carboxylated osteocalcin, were detected with commercially available enzyme immunoassay (EIA) kits (Takara, Shiga, Japan); the absorption of the samples was analyzed at 450 nm, and a standard curve was generated for each protein; absolute concentrations were obtained from the standard curve.
Micro-CT scanning
To evaluate bone morphologic changes in the rats, the right femoral head of each rat was scanned with a micro-CT scanner at a voxel of 9 microns. 2-D images were transferred to CTAn software and the trabecular bone parameters of the upper outer subchondral bone of the femoral head, including bone mineral density (BMD), bone volume (BV), bone volume per tissue volume (BV/TV), trabecular pattern factor (Tb.Pf), trabecular thickness (Tb.Th), and trabecular number (Tb.N), were quantified.
Angiography
After cardiac perfusion with heparinized saline, Microfil (MV-112, Flow Tech, Inc., Carver, MA, USA) was injected through the abdominal aorta until a constant outflow of the compound was observed to exit the abdominal vein. Then, the rats were placed at 4 °C for 1 h to ensure polymerization of the contrast agent. Femoral heads were fixed with 10% formalin and decalcified with a 10% EDTA solution. Finally, the samples were scanned via micro-CT as described above, and the vessels of the femoral head were reconstructed using CTVol software.
Histological and immunohistochemical analyses
After decalcification and paraffin embedding, femoral heads were sectioned at a thickness of 5 µm in the coronal plane. Some of these sections were stained with hematoxylin and eosin (H&E) to evaluate the trabecular structure, while the others were deparaffinized, antigen retrieved, incubated with anti-OC (Abcam), anti-Runx2 (Abcam) and anti-VEGF (Boshide, Wuhan, China) primary antibodies and then incubated with the appropriate biotinylated secondary antibodies. Sections were colored with DAB and counterstained with hematoxylin. Photomicrographs were acquired using a LEICA DM 4000. Then, the images of immunohistochemical staining were analyzed with the software Image-Pro Plus, with which the integrated option density (IOD) of target protein and the total area of trabecular bones were measured, and the mean density (IOD/area) was calculated and counted.
Statistical analysis
SPSS 20.0 (Microsoft, Chicago, IL, USA) was used to analyze the values in each group. All data were expressed as the means and standard deviation (SD). Comparisons of data among the groups were performed using one-way analysis of variance with an SNK post hoc analysis. A P value less than 0.05 was considered statistically significant.
Results
VK2 promoted BMSC proliferation and enhanced cell survival
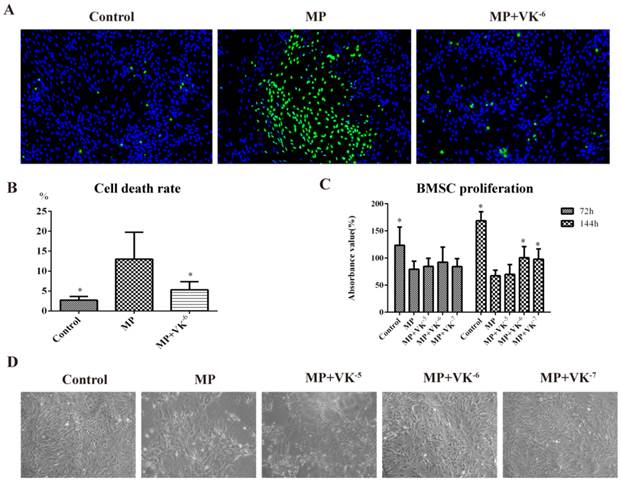
The CCK-8 study showed that BMSC proliferation was significantly suppressed after 72 h and 144 h of incubation of 5×10-5 M MP, while this inhibition was antagonized by VK2, especially at a concentration of 10-6 M (Fig. 1C). Cell viability staining showed a large number of dead cells in the MP group, while fewer were apparent in the MP+VK-6 group—indicating VK2-enhanced cell survival during MP incubation (Fig. 1A-B), which was also observed under the light microscope (Fig. 1D).
VK2 improved osteogenic differentiation of BMSCs
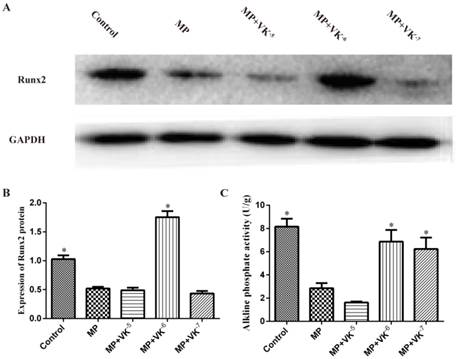
Following treatment with MP and MP supplemented with different concentrations of VK2, the expression of Runx2 was detected by western blotting. MP treatment decreased the level of Runx2, while supplementation of 10-6 M VK2 most clearly enhanced Runx2 expression. ALP activity in BMSCs induced for 3 weeks was also downregulated by MP and upregulated when combined with different concentrations of VK2, except for 10-5 M. (Fig. 2)
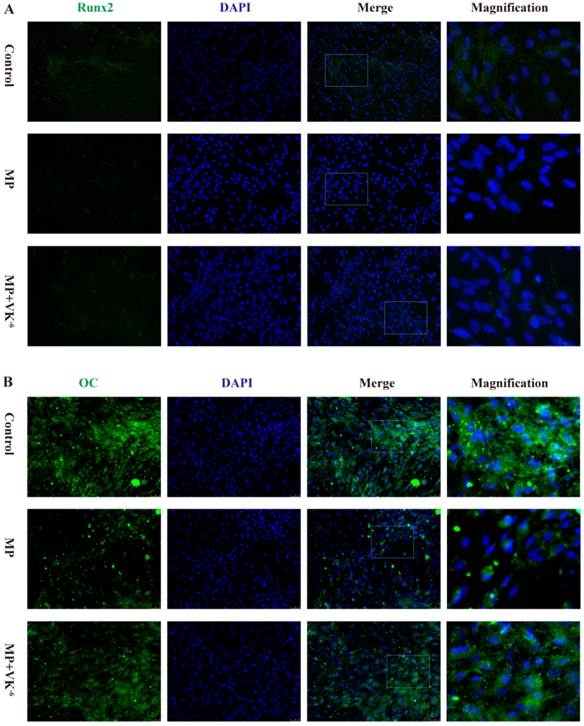
We further detected the expression of these proteins in BMSCs treated with MP and MP supplemented with VK2 by immunofluorescence. The results showed that osteogenic-induced BMSCs exhibited minimal staining of Runx2, OC and ALP in the MP group, while a significant increase of these proteins was detected with supplementation of VK2. (Fig. 3)
To verify whether VK2 promoted osteogenic differentiation of BMSCs treated with MP, the expression of osteogenic-associated genes was detected by qRT-PCR. The mRNA level of Runx2, an early-stage osteogenic marker, was significantly upregulated by 10-5 M VK2 (P < 0.05), although no obvious increase was observed with 10-6 M and 10-7 M VK2 (P > 0.05). The expression of OC and ALP, which were bone mineralization markers, was also significantly upregulated by 10-5 M VK2 (P < 0.05). We observed that 10-6 M and 10-7 M VK2 improved the OC transcription of BMSCs to some degree (P > 0.05) but did not improve ALP transcription. (Fig. 4A-C)
Effects of MP and MP supplemented with VK2 on the proliferation and survival of BMSCs. (A, B) Cell viability staining was performed 144 h after incubation with MP and MP plus 10-6 M VK2, in which all living cells were stained blue and dead cells were stained green. (C) After treatment of MP and MP supplemented with different concentrations of VK2, the proliferation of BMSCs was detected by CCK-8, and the results were expressed as the mean absorbance value (%) ±SD. (D). Images of BMSCs under light microscope at 144 h after cell adherence (*, significant difference versus the MP group, P < 0.05).
Expression of osteogenic proteins in BMSCs treated with MP and MP plus different concentrations of VK2. (A) Western blotting results showed that MP and combined use of VK2 significantly affected the expression of Runx2. (B) Analysis showed that the expression of Runx2 was decreased by MP and the combined use of 10-6 M VK2 significantly enhanced the protein expression. (C) ALP activity in BMSCs was downregulated by MP, while supplementation of VK2 upregulated the ALP level, except for 10-5 M VK2 (*, significant difference versus the MP group, P < 0.05).
Expression of Runx2, OC and ALP of BMSCs cultured in osteogenic medium detected by immunofluorescence; green staining shows expressed proteins and blue staining shows the nucleus. (A) Expression of Runx2 was decreased by MP, and 10-6 M VK2 clearly improved Runx2 expression, even with MP. (B, C) Immunofluorescence staining of OC and ALP showed similar results with Runx2.
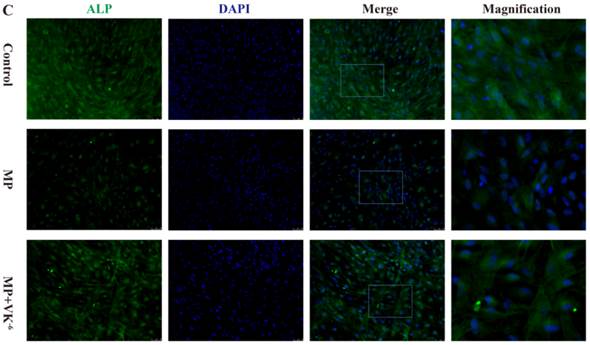
Effects of MP and MP plus VK2 on osteogenic differentiation of BMSCs. (A-C) The mRNA expressions of Runx2, OC and ALP of BMSCs were detected by qRT-PCR after 3 weeks of incubation of the osteogenic medium with MP and MP plus different concentrations of VK2. Values shown are mean ± SD (n = 3). (*, significant difference versus the MP group, P < 0.05). (D) Alizarin red staining of BMSCs exposed to MP and MP plus 10-6 M VK2.
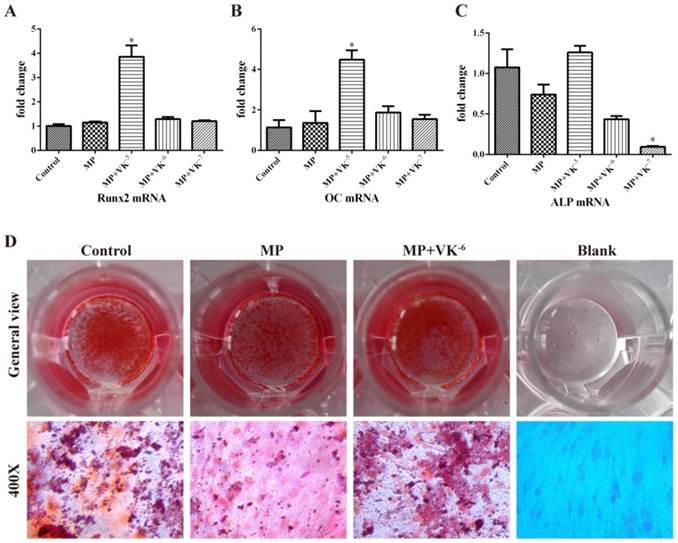
Alizarin red staining showed fewer calcium nodules in the MP group compared with the control group, while the mineralization of BMSCs improved more significantly in those treated with a combination of VK2 than in those treated with MP alone. (Fig. 4D)
VK2 increased serum ALP, Gla-OC and Glu-OC in rats treated with MP
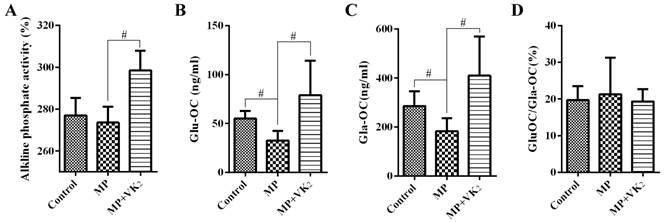
Serum levels of ALP, Glu-OC and Gla-OC were measured 6 weeks after the first injection of MP. The MP group had lower ALP levels than the control group, although we failed to detect a significant difference (P > 0.05). However, we detected a significantly increased ALP level in the rats treated with MP supplemented with VK2. (Fig. 5A) Both Glu-OC and Gla-OC decreased significantly in rats treated with MP compared with the control group, while supplementation of VK2 clearly increased their production. In addition, the ratio of Glu-OC and Gla-OC was decreased by VK2, although this decrease was not statistically significant. (Fig. 5B-D)
VK2 prevented incidence of ONFH in the subchondral area
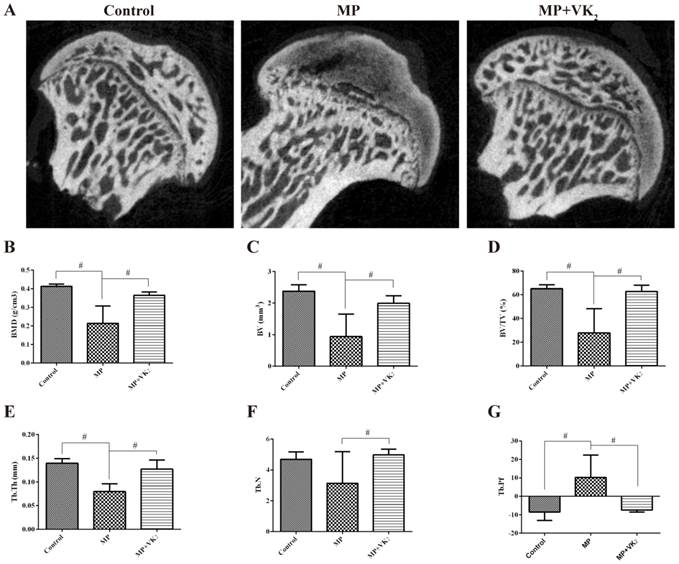
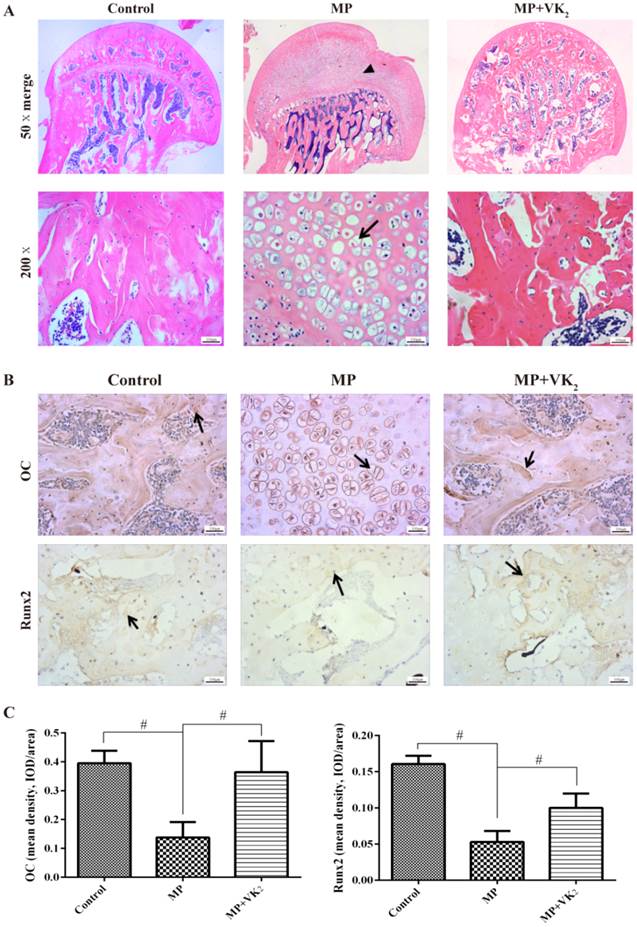
The trabecular changes in the subchondral area of the femoral heads were detected by micro-CT conducted 6 weeks following the first injection of MP. Seven rats in the MP group showed visible osteonecrosis of the femoral head in the microCT images, while only one rat was found with mild osteonecrosis in the MP+VK2 group (Fig. 6A). The BMD of the rats in the MP group was 214.0±29.47 mg/cm3, which was significantly lower than in the control group, while supplementation of VK2 significantly increased the BMD of the area. In addition, the bone parameters had similar performance in rats between the MP+VK2 and control groups. (Fig. 6B-G)
Similar to the microCT results, HE staining showed obvious subchondral necrosis with fat tissue invasion in the subchondral bone trabecula. Meanwhile, a number of hypertrophic fat cells appeared in the trabecular region in the MP group, but hypertrophic fat cells were negative in most femoral heads in the MP+VK2 group (Fig. 7A). We also detected Runx2 and OC expression by immunohistochemical staining. The results showed more positive staining in the MP+VK2 group compared with the MP group (Fig. 7B-C).
VK2 protected the blood supply of the femoral head
To detect the blood supply of the femoral head in this study, microangiography of the femoral head was performed by perfusing the vessels with Microfil and imaging with microCT. The reconstructed 3D micro-CT images showed decreased blood vessels in the femoral head in the MP group, with only trunk vessels left. However, vessels of the femoral head in rats treated with MP+VK2 were significantly denser, although there were fewer than in the control group. (Fig. 8A) Quantification of the blood vessels was performed by morphometric analysis. The total volume of blood vessels of the femoral head in the group treated with MP only was significantly lower than those in the control group, While the total volume of blood vessels in the MP+VK2 were greater than those in the MP group but lower than those in the control group. (Fig. 8C)
Analysis of serum ALP, Glu-OC and Gla-OC in SD rats. (A) The results showed that the rats in the MP group tended to have lower ALP levels than those in the control group, and a significantly increased ALP level was observed in rats treated with MP supplemented with VK2. Values were expressed with the mean absorbance value (%) ± SD (n=10). (B, C) EIA showed significantly decreased Glu-OC and Gla-OC in the MP group and a clear increase in rats of the MP+VK2 group. (D) The ratio of Glu-OC and Gla-OC was increased by MP and decreased with supplementation of VK2 (#, significant difference between the two groups, P<0.05).
MicroCT evaluation of the subchondral region of the femoral heads. (A) 2-D images of the coronal section of the femoral heads. (B-G) Morphometric analysis showed callus parameters of the upper outer subchondral bone of the femoral heads. (#, significant difference between the two groups, P<0.05).
Histological analysis of paraffin sections of the femoral heads. (A) HE staining of coronal sections of representative femoral heads in each group. The triangle indicates osteonecrosis, and the arrow indicates the empty lacunae and hypertrophic fat cells. (B,C) Immunohistochemical staining of Runx2 and OC of the coronal sections of representative femoral heads in each group; more positive brown staining was observed in the MP+VK2 group compared to the MP group. The arrow indicates the target protein staining (#, significant difference between the two groups, P<0.05).
Evaluation of blood supply and revascularization in femoral heads. (A) Representative images of 3-D microangiography of femoral heads in each group evaluated by microCT. (B) Representative images of immunohistochemical staining of VEGF in the femoral heads of each group; arrows indicate VEGF expression. (C) Quantification of total volume of blood vessels in the femoral heads. (D) Mean density of VEGF in the femoral head slices of each group (#, significant difference between the two groups, P<0.05).
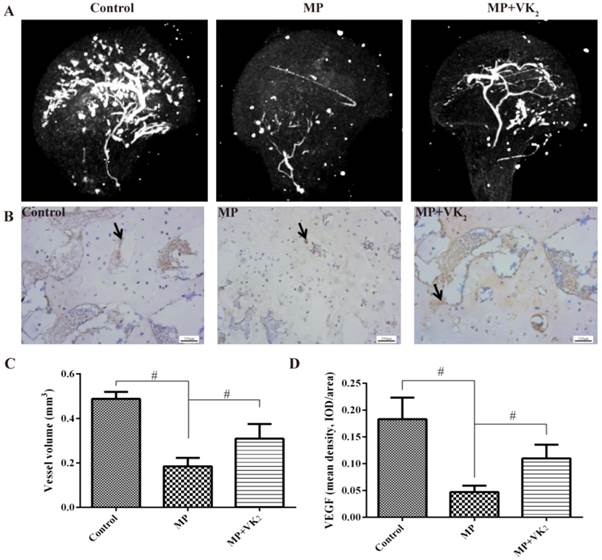
We further detected revascularization of the subchondral area of the femoral head by immunohistochemical analyses of VEGF. Nearly no positive staining was found in the osteonecrosis area. There was significantly less positive staining in surviving trabeculae of the femoral head in the MP group; however, more were observed in the MP+VK2 group. (Fig. 8B,D)
Discussion
Clinically, up to 40% of glucocorticoid users develop some degree of ONFH depending on the steroid's dosage and route of administration[1]. This side effect is believed to occur partly due to suppression of osteogenic differentiation of MSC and apoptosis of osteoblasts and osteocytes [2]. Furthermore, decreased bone volume, osteogenesis-associated proteins and blood vessel volume have been observed in glucocorticoid administered animals[11, 15, 35, 36]. VK2 is widely used for the treatment of osteoporosis, and clinical trials have suggested that VK2 could increase serum OC and prevent the incidence of fracture in osteoporosis[26, 37]. In addition, VK2 increased lumbar BMD in patients given steroid treatment[38, 39]. In vitro and animal studies have provided more evidence for the role of VK2 on bone metabolism. In vitro studies showed that VK2 stimulated osteoblastogenesis in human bone marrow cells[23], stimulated osteocalcin accumulation of human osteoblastic cells[40] and inhibited osteoclast-like cell formation in mouse bone marrow cultures[41]. In animal models, VK2 supplementation stimulated bone formation and suppressed bone resorption both in growing and osteoporosis-model rats[24, 42]. In addition, several articles showed a correlation between VK2 and glucocorticoid. Hara et al.[25] reported inhibition of VK2 on total and trabecular BMD in the proximal metaphysis in rats with prednisolone treatment. Hara et al.[43] also reported that VK2 improved tibial length, dry weight, bone density, femoral length, bone strength, and calcium content in steroid-treated rats. Nobuhiro et al.[39] reported that VK2 prevented lumbar BMD decrease and reduction of osteoprotegerin in patients treated with glucocorticoid. However, these studies focused on VK2's effect on osteoporosis; few studies were found that focused on ONFH.
In our in vitro studies, we observed that MP clearly inhibited BMSC proliferation and induced apoptosis, which is consistent with previous studies[4-6]. Yamaguchi's study had revealed that VK2 promoted osteoblast proliferation[22], and in this study, we discovered VK2 clearly increased BMSC proliferation treated with GC. In addition, VK2 has been revealed to have some biological functions, including preventing oxidative cell death in oligodendrocytes and protecting neurons from methylmercury-induced cell death[44]; these may be related to the discovery that VK2 could regulate mitochondrial function through its ability to transfer electrons in the electron transport chain[29].
One important function of VK2 is the post-translational modification of OC. VK2 is a cofactor of γ-carboxylase, which converts Glu-OC into Gla-OC[40]. Studies have shown that increased serum Glu-OC reflect some degree of VK deficiency and was negatively associated with decreased bone volume[45, 46]. In our study, we observed a decrease in the serum Glu-OC/Gla-OC in the rats with VK2 supplementation and confirmed the γ-carboxylation of VK2 to OC. Interestingly, we observed both increased serum Glu-OC and Gla-OC in rats of the VK2 group. This may be related to the upregulated OC expression by VK2 demonstrated in previous studies[20, 40] and is similar to the results of Shiraki's study, in which a significant rise of serum level of Glu-OC in VK2-treated osteoporotic patients [26]. Consistent with in vivo results, OC expression in BMSC culture was also upregulated by VK2 at the protein and gene levels.
Runx2 is the most important transcription factor to control differentiation in bone marrow stromal cells, and its critical role has been illustrated through manipulating Runx2 in cell cultures[7, 47]. Studies have shown that GC antagonizes Runx2 in mesenchymal cells. We observed similar results in the MP group, and some degree of upregulation in the VK2 groups compared to the MP group, especially in the group containing 10-6 M VK2 with the most obvious promotion. While ALP transcription is mediated by Runx2 and acts as a mature marker of osteogenesis[47, 48], similar results were observed in vitro. These results suggest that VK2 could activate transcription of some osteogenic-related proteins to promote mineralization, except as a cofactor. Other reports have also shown that VK2 could activate the transcription of extracellular matrix-related genes and collagen accumulation through activation of the steroid xenobiotic receptor[49, 50].
GC has been well proven to decrease vessel volume and blood supply of femoral heads[11, 12], and in our in vivo study, we observed that VK2 had some protective effect on the blood supply of the femoral head. Previous studies have shown that vitamin K-dependent matrix Gla protein (MGP) is associated with vascular calcification as an inhibitor, and MK4 act as an anti-calcification component in the vessel wall[27, 51, 52]. Furthermore, protein S, which is also a vitamin K-dependent protein, likely acts as a positive regulator of blood vessel development [53, 54]. VK2 has also been found to be essential for maintaining endothelial cell survival and overall vascular homeostasis[29]. In addition, carboxylated gas6 could protect endothelial cells from serum starvation-induced apoptosis[28]. We postulate that these possibly contributed to VK's protective effect on blood supply.
Moreover, MP administration induced clearly decreased bone volume in the femoral head, while no obvious decrease was observed in rats with VK2 supplementation, and the trabecular parameters in the VK2 group were significantly improved as well. Therefore, we believe that VK2 could prevent ONFH in rats with MP administration.
Conclusion
In conclusion, VK2 is an effective antagonist for glucocorticoid on osteogenic progenitors. The underlying mechanisms include acceleration of BMSC propagation and promotion of bone formation-associated proteins, which combine and contribute to the prevention of glucocorticoid-induced ONFH in rats.
Acknowledgements
The current research was funded by the National Natural Science Foundation of China (No. 81301572) and the SMC-Chen Xing Plan for Splendid Young Teachers of Shanghai Jiao Tong University.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Koo KH, Kim R, Kim YS. et al. Risk period for developing osteonecrosis of the femoral head in patients on steroid treatment. Clin Rheumatol. 2002;21:299-303
2. Kerachian MA, Seguin C, Harvey EJ. Glucocorticoids in osteonecrosis of the femoral head: a new understanding of the mechanisms of action. J Steroid Biochem Mol Biol. 2009;114:121-8
3. Weinstein RS. Glucocorticoid-induced osteonecrosis. Endocrine. 2012;41:183-90
4. Hong L, Wei N, Joshi V. et al. Effects of glucocorticoid receptor small interfering RNA delivered using poly lactic-co-glycolic acid microparticles on proliferation and differentiation capabilities of human mesenchymal stromal cells. Tissue Eng Part A. 2012;18:775-84
5. Wang BL, Sun W, Shi ZC. et al. Decreased proliferation of mesenchymal stem cells in corticosteroid-induced osteonecrosis of femoral head. Orthopedics. 2008;31:444
6. Tan G, Kang PD, Pei FX. Glucocorticoids affect the metabolism of bone marrow stromal cells and lead to osteonecrosis of the femoral head: a review. Chin Med J (Engl). 2012;125:134-9
7. Koromila T, Baniwal SK, Song YS. et al. Glucocorticoids antagonize RUNX2 during osteoblast differentiation in cultures of ST2 pluripotent mesenchymal cells. J Cell Biochem. 2014;115:27-33
8. O'Brien CA, Jia D, Plotkin LI. et al. Glucocorticoids act directly on osteoblasts and osteocytes to induce their apoptosis and reduce bone formation and strength. Endocrinology. 2004;145:1835-41
9. Li X, Jin L, Cui Q. et al. Steroid effects on osteogenesis through mesenchymal cell gene expression. Osteoporos Int. 2005;16:101-8
10. Harada S, Rodan SB, Rodan GA. Expression and regulation of vascular endothelial growth factor in osteoblasts. Clin Orthop Relat Res. 1995:76-80
11. Weinstein RS, Wan C, Liu Q. et al. Endogenous glucocorticoids decrease skeletal angiogenesis, vascularity, hydration, and strength in aged mice. Aging cell. 2010;9:147-61
12. Kerachian MA, Harvey EJ, Cournoyer D. et al. Avascular necrosis of the femoral head: vascular hypotheses. Endothelium. 2006;13:237-44
13. Miyanishi K, Yamamoto T, Irisa T. et al. Bone marrow fat cell enlargement and a rise in intraosseous pressure in steroid-treated rabbits with osteonecrosis. Bone. 2002;30:185-90
14. Jiang Y, Zhang Y, Zhang H. et al. Pravastatin prevents steroid-induced osteonecrosis in rats by suppressing PPARgamma expression and activating Wnt signaling pathway. Exp Biol Med (Maywood). 2014;239:347-55
15. Chen S, Li J, Peng H. et al. Administration of erythropoietin exerts protective effects against glucocorticoid-induced osteonecrosis of the femoral head in rats. Int J Mol Med. 2014;33:840-8
16. Wu X, Yang S, Wang H. et al. G-CSF/SCF exert beneficial effects via anti-apoptosis in rabbits with steroid-associated osteonecrosis. Exp Mol Pathol. 2013;94:247-54
17. Booth SL. Roles for vitamin K beyond coagulation. Annu Rev Nutr. 2009;29:89-110
18. Neve A, Corrado A, Cantatore FP. Osteocalcin: skeletal and extra-skeletal effects. J Cell Physiol. 2013;228:1149-53
19. Aonuma H, Miyakoshi N, Hongo M. et al. Low serum levels of undercarboxylated osteocalcin in postmenopausal osteoporotic women receiving an inhibitor of bone resorption. Tohoku J Exp Med. 2009;218:201-5
20. Takeuchi Y, Suzawa M, Fukumoto S. et al. Vitamin K(2) inhibits adipogenesis, osteoclastogenesis, and ODF/RANK ligand expression in murine bone marrow cell cultures. Bone. 2000;27:769-76
21. Kasukawa Y, Miyakoshi N, Ebina T. et al. Effects of risedronate alone or combined with vitamin K2 on serum undercarboxylated osteocalcin and osteocalcin levels in postmenopausal osteoporosis. J Bone Miner Metab. 2014;32:290-7
22. Yamaguchi M, Sugimoto E, Hachiya S. Stimulatory effect of menaquinone-7 (vitamin K2) on osteoblastic bone formation in vitro. Mol Cell Biochem. 2001;223:131-7
23. Koshihara Y, Hoshi K, Okawara R. et al. Vitamin K stimulates osteoblastogenesis and inhibits osteoclastogenesis in human bone marrow cell culture. J Endocrinol. 2003;176:339-48
24. Iwamoto J, Matsumoto H, Takeda T. et al. Effects of vitamin K2 on cortical and cancellous bone mass, cortical osteocyte and lacunar system, and porosity in sciatic neurectomized rats. Calcif Tissue Int. 2010;87:254-62
25. Hara K, Kobayashi M, Akiyama Y. Vitamin K2 (menatetrenone) inhibits bone loss induced by prednisolone partly through enhancement of bone formation in rats. Bone. 2002;31:575-81
26. Shiraki M, Shiraki Y, Aoki C. et al. Vitamin K2 (menatetrenone) effectively prevents fractures and sustains lumbar bone mineral density in osteoporosis. J Bone Miner Res. 2000;15:515-21
27. Wallin R, Schurgers L, Wajih N. Effects of the blood coagulation vitamin K as an inhibitor of arterial calcification. Thromb Res. 2008;122:411-7
28. Hasanbasic I, Rajotte I, Blostein M. The role of gamma-carboxylation in the anti-apoptotic function of gas6. J Thromb Haemost. 2005;3:2790-7
29. Hegarty JM, Yang H, Chi NC. UBIAD1-mediated vitamin K2 synthesis is required for vascular endothelial cell survival and development. Development. 2013;140:1713-9
30. Koshihara Y, Hoshi K. Vitamin K-2 enhances osteocalcin accumulation in the extracellular matrix of human osteoblasts in vitro. J Bone Miner Res. 1997;12:431-8
31. Atkins GJ, Welldon KJ, Wijenayaka AR. et al. Vitamin K promotes mineralization, osteoblast-to-osteocyte transition, and an anticatabolic phenotype by {gamma}-carboxylation-dependent and -independent mechanisms. Am J Physiol Cell Physiol. 2009;297:C1358-67
32. Hara K, Akiyama Y, Nakamura T. et al. The inhibitory effect of vitamin K2 (menatetrenone) on bone resorption may be related to its side chain. Bone. 1995;16:179-84
33. Kim M, Na W, Sohn C. Vitamin K1 (phylloquinone) and K2 (menaquinone-4) supplementation improves bone formation in a high-fat diet-induced obese mice. J Clin Biochem Nutr. 2013;53:108-13
34. Kodama Y, Takeuchi Y, Suzawa M. et al. Reduced expression of interleukin-11 in bone marrow stromal cells of senescence-accelerated mice (SAMP6): relationship to osteopenia with enhanced adipogenesis. J Bone Miner Res. 1998;13:1370-7
35. Weinstein RS, Nicholas RW, Manolagas SC. Apoptosis of osteocytes in glucocorticoid-induced osteonecrosis of the hip. J Clin Endocrinol Metab. 2000;85:2907-12
36. Ma XL, Liu ZP, Ma JX. et al. Dynamic expression of Runx2, Osterix and AJ18 in the femoral head of steroid-induced osteonecrosis in rats. Orthop Surg. 2010;2:278-84
37. Knapen MH, Schurgers LJ, Vermeer C. Vitamin K2 supplementation improves hip bone geometry and bone strength indices in postmenopausal women. Osteoporos Int. 2007;18:963-72
38. Inoue T, Sugiyama T, Matsubara T. et al. Inverse correlation between the changes of lumbar bone mineral density and serum undercarboxylated osteocalcin after vitamin K2 (menatetrenone) treatment in children treated with glucocorticoid and alfacalcidol. Endocr J. 2001;48:11-8
39. Sasaki N, Kusano E, Takahashi H. et al. Vitamin K2 inhibits glucocorticoid-induced bone loss partly by preventing the reduction of osteoprotegerin (OPG). J Bone Miner Metab. 2005;23:41-7
40. Koshihara Y, Hoshi K. Vitamin K2 enhances osteocalcin accumulation in the extracellular matrix of human osteoblasts in vitro. J Bone Miner Res. 1997;12:431-8
41. Akiyama Y, Hara K, Tajima T. et al. Effect of vitamin K2 (menatetrenone) on osteoclast-like cell formation in mouse bone marrow cultures. Eur J Pharmacol. 1994;263:181-5
42. Matsumoto T, Miyakawa T, Yamamoto D. Effects of vitamin K on the morphometric and material properties of bone in the tibiae of growing rats. Metabolism. 2012;61:407-14
43. Hara K, Akiyama Y, Ohkawa I. et al. Effects of menatetrenone on prednisolone-induced bone loss in rats. Bone. 1993;14:813-8
44. Sakaue M, Mori N, Okazaki M. et al. Vitamin K has the potential to protect neurons from methylmercury-induced cell death in vitro. J Neurosci Res. 2011;89:1052-8
45. Yamauchi M, Yamaguchi T, Nawata K. et al. Relationships between undercarboxylated osteocalcin and vitamin K intakes, bone turnover, and bone mineral density in healthy women. Clin Nutr. 2010;29:761-5
46. Urano A, Hotta M, Ohwada R. et al. Vitamin K deficiency evaluated by serum levels of undercarboxylated osteocalcin in patients with anorexia nervosa with bone loss. Clin Nutr. 2015;34:443-8
47. Baniwal SK, Shah PK, Shi Y. et al. Runx2 promotes both osteoblastogenesis and novel osteoclastogenic signals in ST2 mesenchymal progenitor cells. Osteoporos Int. 2012;23:1399-413
48. Hu N, Feng C, Jiang Y. et al. Regulative Effect of Mir-205 on Osteogenic Differentiation of Bone Mesenchymal Stem Cells (BMSCs): Possible Role of SATB2/Runx2 and ERK/MAPK Pathway. Int J Mol Sci. 2015;16:10491-506
49. Horie-Inoue K, Inoue S. Steroid and xenobiotic receptor mediates a novel vitamin K2 signaling pathway in osteoblastic cells. J Bone Miner Metab. 2008;26:9-12
50. Ichikawa T, Horie-Inoue K, Ikeda K. et al. Steroid and xenobiotic receptor SXR mediates vitamin K2-activated transcription of extracellular matrix-related genes and collagen accumulation in osteoblastic cells. J Biol Chem. 2006;281:16927-34
51. Spronk HM, Soute BA, Schurgers LJ. et al. Matrix Gla protein accumulates at the border of regions of calcification and normal tissue in the media of the arterial vessel wall. Biochem Biophys Res Commun. 2001;289:485-90
52. Shea MK, Holden RM. Vitamin K status and vascular calcification: evidence from observational and clinical studies. Adv Nutr. 2012;3:158-65
53. Suleiman L, Negrier C, Boukerche H. Protein S: A multifunctional anticoagulant vitamin K-dependent protein at the crossroads of coagulation, inflammation, angiogenesis, and cancer. Crit Rev Oncol Hematol. 2013;88:637-54
54. Fraineau S, Monvoisin A, Clarhaut J. et al. The vitamin K-dependent anticoagulant factor, protein S, inhibits multiple VEGF-A-induced angiogenesis events in a Mer- and SHP2-dependent manner. Blood. 2012;120:5073-83
Author contact
![]() Corresponding author: Chang-Qing Zhang (Email: zhangcqedu.cn) and You-Shui Gao (Email: acerzhang5233edu.cn). Address: Department of Orthopedic Surgery, Shanghai Jiao Tong University Affiliated Sixth People's Hospital, 600 Yishan Road, Shanghai 200233, China. Telephone: +86-1891-7233480, FAX: 021-64701361
Corresponding author: Chang-Qing Zhang (Email: zhangcqedu.cn) and You-Shui Gao (Email: acerzhang5233edu.cn). Address: Department of Orthopedic Surgery, Shanghai Jiao Tong University Affiliated Sixth People's Hospital, 600 Yishan Road, Shanghai 200233, China. Telephone: +86-1891-7233480, FAX: 021-64701361

 Global reach, higher impact
Global reach, higher impact