10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2016; 12(4):446-453. doi:10.7150/ijbs.12937 This issue Cite
Research Paper
A Phase II Clinical Trial of Concurrent Helical Tomotherapy plus Cetuximab Followed by Adjuvant Chemotherapy with Cisplatin and Docetaxel for Locally Advanced Nasopharyngeal Carcinoma
1. Department of Otolaryngology, Head and Neck Surgery, Chinese People Liberation Army (PLA) General Hospital, Beijing, China.
2. Department of Radiation Oncology, Chinese People Liberation Army (PLA) General Hospital, Beijing, China.
Received 2015-6-11; Accepted 2015-12-17; Published 2016-2-12
Abstract
Purpose: The present clinical trial was designed to evaluate the efficacy and safety of concurrent helical tomotherapy (HT) with cetuximab followed by adjuvant chemotherapy with docetaxel and cisplatin (TP) in the treatment of patients with locoregionally advanced nasopharyngeal carcinoma.
Materials and Methods: This phase II clinical trial included 43 patients with Stage III/IV LANC (33 Stage III and 10 Stage IV). The treatment consisted of concurrent HT with cetuximab (400 mg/m2 loading dose and weekly 250mg/m2), followed by four cycles of chemotherapy [docetaxel (70 mg/m2 on Day 1) and cisplatin (40 mg/m2 on Days 1 and 2 every 3 weeks). Side effects were evaluated with CTCAE criteria (Common Terminology Criteria for Adverse Events 3.0).
Results: The median follow-up duration was 48.0 months [95% confidence interval (CI) 41.7-58.0 months], the 2-year locoregional failure-free rate (LFFR), progression-free survival (PFS), distant failure-free rate (DFFR) and overall survival (OS) were 95.2%, 79.1%, 88.1% and 93.0% respectively; the 3-year LFFR, DFFR, PFS and OS were 92.7%, 85.6%, 72.0% and 85.7% respectively. The most common grade 3 toxicities were oropharyngeal mucositis (81.4%) and RT-related dermatitis (7.0%). No patients had more than grade 3 radiation related toxicities and no patients required nasogastric feeding. One patient experienced grade 3 osteonecrosis at 18 months after treatment.
Conclusions: Concurrent HT with cetuximab followed by adjuvant chemotherapy with TP is an effective strategy for the treatment of LANC with encouraging survival rates and minimal side effects.
Keywords: Locally advanced nasopharyngeal carcinoma, Helical tomotherapy, Cetuximab, Adjuvant chemotherapy, adverse event.
Introduction
Although the worldwide incidence of nasopharyngeal carcinoma (NPC) is relatively low, it is a common head and neck cancer in southern China. Its etiology remains unclear and the prognosis of patients with NPC is still poor since more than 50% of patients present with locally advanced disease (LANC) at time of diagnosis [1]. Among patients with LANC, the non-keratinizing type is common and different from other head and neck cancers in terms of its aggressive behavior and high risk of distant metastasis [2]. The current standard care for LANC patients recommended by the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN) is concurrent chemoradiotherapy (CCRT) with cisplatin (P) followed by adjuvant chemotherapy (ACT) with cisplatin and 5-Fluorouracil (F) based on the results of the randomized inter-group study 0099 [3]. Newly developed radiation technology, effective and less toxic chemotherapy regimens and epidermal growth factor receptor (EGFR) targeted therapy have brought new hope for reducing the rate of distant metastasis and improving overall survival (OS) among these patients.
There is increasing evidence from clinical trials supporting that, compared to cisplatin (P)-5-fluorouracil (5FU) regimen, the taxane-containing chemotherapy improves the response rate and OS in patients with squamous cell carcinoma of the head and neck (SCCHN) and that the EGFR inhibitor cetuximab with concurrent radiotherapy improves the local control and OS when compared to radiotherapy alone [4, 5]. In vitro study has demonstrated that cetuximab is effective in radio-resistant CD133+ cancer stem cells isolated from glioma U87MG cells [6]. Ma et al. have shown that CCRT with cisplatin and cetuximab and IMRT results in a 2-year progression-free survival (PFS) of 86.5% in LANC patients [7]. Helical tomotherapy (HT) can maximize the protection of normal structures and improve locoregional control [8]. Several clinical trials have demonstrated that HT leads to encouraging local control rate for patients with NPC and have minimal acute and late toxicities [9-11]. Leung et al. have reported that patients with NPC treated with HT showed no local recurrence with low late toxicities and a 5-year locoregional control rate of 97% [9]. In our previous study, patients receiving HT had a 1-year relapse-free survival of 95.6% and no grade 2 xerostomia was noted in all patients one year after radiation [11]. In order to minimize treatment related side-effects and to improve efficacy in patients with LANC, in the present study, we designed a treatment strategy that includes concurrent HT plus cetuximab followed by ACT with docetaxel (T) and cisplatin. We evaluated safety and efficacy as measured by locoregional failure-free rate (LFFR), PFS, distant failure-free rate (DFFR), and OS at 2- and 3-year in patients with LANC.
Materials and Methods
Patients
This prospective phase II study (ChiCTR-OCC-15005888) enrolled patients with untreated, histologically proven non-keratinizing type of NPC at stage III-IV (American Joint Committee disease stages: any T N2~N3, or T3~T4N0~3 stage). The sample size (patient number) needed for the present study was calculated with the software NCSS&PASS (optimal two-stage design: a=0.05, β=0.2, P1-P0 =0.15). The sample size was determined to be 43 patients.
Their baseline characteristics are listed in Table 1. The inclusion criteria were as follows: age between 18 and 70 years; ECOG (Eastern Cooperative Oncology Group) performance status of 0 or 1; life expectancy ≥ +6 months; no prior chemotherapy, radiotherapy, or surgery; adequate bone marrow (e.g., white blood cell count ≥4000 cells/μL, platelet counts ≥100 000 cells/mL, hemoglobin >8.0g/dL ), normal renal function ( creatinine clearance >50 ml ) and hepatic function (bilirubin ≤1.5× the upper limit of normal (ULN), alanine aminotransferase ≤3 ×ULN). To rule out synchronous primary cancers and metastasis disease, all patients were fully evaluated with positron emission computed tomography (PET) or magnetic resonance imaging (MRI) of the head and neck, fabric nasopharyngoscopy, chest CT, abdominal ultrasound, as well as bone scans. Patients with synchronous primary cancers or/and metastasis disease were excluded. The present study was approved by the Ethics Review Committee of the Chinese People Liberation Army General Hospital. Informed consent was obtained from all patients prior to participation in the present study.
Characteristics of patients.
| Feature | n (%) |
|---|---|
| Age (years) | |
| Range | 19~67 |
| Median | 44(95%CI 41~47) |
| Sex | |
| Male | 30(70.0) |
| Female | 13(30.0) |
| WHO performance status, n (%) | |
| 0 | 33 (76.7) |
| 1 | 10 (23.3) |
| T classification, n (%) | |
| T1 | 5 (11.6) |
| T2 | 17(39.5) |
| T3 | 14 (32.6) |
| T4 | 7 (16.3) |
| N classification, n (%) | |
| N0 | 2 (4.7) |
| N1 | 6 (14.0) |
| N2 | 32 (74.4) |
| N3 | 3 (7.0) |
| 2002AJCC stage, n (%) | |
| III | 33 (76.7) |
| IV | 10 (23.3) |
AJCC: American Joint Committee on Cancer
Treatments
Chemotherapy and EGFR inhibitor therapy
The therapy regimen was determined by our previous study [12]. Briefly, the patients were treated with concurrent HT with weekly cetuximab, followed by four cycles of ACT with TP one month after radiotherapy. Cetuximab was used at a 250 mg/m2 on a weekly basis (400 mg/m2 initial dose) for 7 times, starting the first day of radiotherapy. The TP regimen consisted of docetaxel (70 mg/m2, Day 1) and cisplatin (40 mg/m2, Days 1 and 2) every 3 weeks.
Radiotherapy
HT was delivered once daily, 5 days per week. The detail treatment plans was the same as previously reported [11]. In brief, the planning dose at D95 (dose received by 95% of the target volume) was prescribed to pGTVnx (the planning gross target volume of primary tumor, pGTVnx) and pGTVnd (the planning gross target volume of metastatic lymph node, pGTVnd) at 70-74Gy, PTV1 (planning target volume, PTV) at 60-62.7 Gy and PTV2 at 52-56 Gy in 33 fractions. No more than 5% of PTV volume received more than 110% of the prescribed dose. Dose-volume constraints for organs at risk (OARs) were utilized: 1) parotid gland V30 < 50% or Dmean < 28Gy; 2) brainstem Dmax <54 Gy; 3) spinal cord Dmax < 45 Gy; 4) optic nerve Dmax <54 Gy; 5) temporo-mandibular joint Dmax <60 Gy; and 6) lens Dmax <5 Gy. Helical tomotherapy plans were developed with a field width of 2.5 cm, a pitch of 0.30-0.38, and a modulation factor equal to 2.0-3.0. During the radiation therapy, the patients underwent megavoltage computed tomography (MVCT) imaging at least once every week to verify patient setup. The imaging frequency was determined by the magnitude of setup errors from initial daily scans.
Dose modifications
The cetuximab dose was reduced if a patient experienced uncontrollable and persistent grade 2 acne-like rash. The chemotherapy doses were adjusted, based on the severity of myelosuppression using the methods reported previously [12]. All patients were given 3-day hydration, and carboplatin was given instead of cisplatin if grade 1 renal toxicity was caused by cisplatin.
Assessment of Outcomes
The toxicities were assessed using the Radiation Therapy Oncology Group (RTOG) toxicity criteria. The assessment of the tumor response was carried out by MRI and flexible fabric nasopharyngoscopy after concurrent HT with cetuximab and 4 weeks after the last cycle of ACT according to the Response Evaluation Criteria in Solid Tumor criteria and reviewed by the committee working in our hospital (otolaryngologist, radiation oncologist, and medical oncologist). Patients were followed up every 3 months in the first year, every 4 months in the second and third year, every 6 months in the fourth and fifth years and then 12 months until progression or death.
Endpoints
The endpoints of the present clinical trial were similar to that of our previous study [12]. The safety of the treatment, including the concurrent HT with cetuximab followed by ACT with TP, was the primary endpoint. The secondary endpoints were the following: the response rate, LFFR, PFS, DFFR, and OS at 2 and 3 years after the treatment. In the present study, LFFR was defined as free from local failure. Locoregional failure was defined as the presence of biopsy-proven residual disease at the nasopharynx and/or regional lymph nodes at the scheduled visit at 3 months after RT or the subsequent development of recurrent disease, whiledistant failure (DF) was defined as the presence of distant metastases. Progression-free survival (PFS) was defined as the duration from the date of enrollment to the date of first occurrence of distant or locoregional recurrence or to the date of last follow-up. Overall survival (OS) was defined as the duration between the date of enrollment and the date of death, due to any cause, or the date of last follow-up. The acute and late toxicities of patients after treatment were graded according to the National Cancer Institute—Common Toxicity Criteria for Adverse Events, version 3.0, and the Radiation Therapy Oncology Group (RTOG) Late Radiation Morbidity Scoring Criteria, respectively.
Statistical Analysis
All the patients have been followed up until recurrence or death and one patient was lost to follow-up. 95% confidence intervals (CIs) have been calculated for means and percentages and the survival curves of LFFR, PFS, DFFR and OS were estimated with the Kaplan-Meier method (R software package Epicalc.StatView 3.0).
Results
Patient characteristics, treatment compliance, and toxicity
In the present study, 46 consecutive patients were screened for the clinical trail from May 2008 to June 2012 and 43 were enrolled. Three patients were excluded because of ineligibility (T2N1 stage). All the patients completed planned HT with 7 cycles of cetuximab, and only one patient received reduced dose because of grade 2 acne-like rash; all patients received ACT with TP. Of these 43 patients, 40 (93.0%) underwent four cycles of treatment at the planned doses. The remaining 2 patients (4.7%) received 3 cycles and 1 patient (2.3%) received reduced doses due to toxicity.
Acute toxicity was observed during concurrent HT with cetuximab and ACT with TP (Table 2). During concurrent HT with cetuximab, the most common grade 2~3 toxicities were oropharyngeal mucositis, radiotherapy (RT)-related dermatitis, cetuximab-related acne-like rash, and weight loss. Three patients needed intravenous (IV) infusion for nutrition due to dysphagia and oropharyngeal pain caused by oropharyngeal mucositis. Grade 3 Oropharyngeal mucositis occurred in 81.4% (35/43) of the patients, but was managed effectively with our previously reported method without radiation break [13]. Briefly, our supportive care regimen for grade 3 oropharyngeal mucositis included Moxifloxacin Hydrochloride and Sodium Chloride Injection 0.4 g IV infusion qd and 15 mL of Sophora flavescens in 250 mL of 0.9% saline IV infusion qd. No patients required nasogastric feeding (NF) and percutaneous endoscopic gastrostomy during and after treatment.
Incidence of acute major toxicities
| Toxicity | No. of patients (%) | ||
|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | |
| Hematologic toxicity | |||
| Leukopenia | 2(4.7) | 13(30.2) | 1(2.3) |
| Neutropenia | 0 | 2(4.7) | 0 |
| Thrombocytopenia | 7(16.2) | 1(2.3) | 0 |
| Anemia | 6(14.0) | 3(7.0) | 1(2.3) |
| Non-hematologic toxicity | |||
| Nausea | 26(60.5) | 3(7.0) | 0 |
| Vomiting | 18(41.9) | 2(4.7) | 0 |
| Oropharyngeal Mucositis | 3(7.0) | 5(11.6) | 35(81.4) |
| Weight loss | 20(46.5) | 10(23.3) | 0 |
| RT-related dermatitis | 28(65.1) | 10(23.3) | 3(7.0) |
| Hypokalaemia | 12(27.9) | 2(4.7) | 1(2.3) |
| Hyponatremia | 1(2.3) | 0 | 0 |
| Creatinine elevation | 1(2.3) | 1(2.3) | 0 |
| Cetuximab-related rash | 16(37.2) | 5(11.6) | 0 |
| Hypomagnesemia | 0 | 3(7.0) | 0 |
| Alanine transferase | 4(9.3) | 0 | 3(7.0) |
During ACT with TP, acute toxic effects were very mild without grade 4 toxicity except for one patient experiencing febrile neutropenia. The rate of grade 3 Leukopenia and anemia were 2.3% (1/43) and 2.3% (1/43), respectively. Grade 3 non-hematologic toxic effects occurring in 7.0% (3/43) patients was abnormal level of Alanine transferase; one (2.3%) patient had a grade 2 increase in creatinine level. His creatinine level returned to normal at 8 months after the treatment. One (2.3%) patient had a grade 3 hypokalaemia.
Regarding late toxic effects (Table 3), grade 2 xerostomia was the most common late toxicity. Hearing impairment was observed in 20 patients (46.5%); 5 (11.6%) experienced sensorineural hearing loss (SNHL); and 15 (34.9%) developed conduction hearing loss, and 10 of the 15 patients needed trans-tympanic ventilation. Two patients (4.7%) had endocrine dysfunction (Thyroid dysfunction) 6 months after HT; one patient (2.3%) experienced osteonecrosis 18 months after treatment. No patients had dysphagia. There were no treatment-related deaths.
Incidence of Major Late Toxicities
| Toxicity | No. of patients (%) | ||
|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | |
| Xerostomia | 21(48.8) | 2(4.7) | 0 |
| Subcutaneous tissue | 2(4.7) | 2(4.7) | 0 |
| Endocrine | 2(4.7) | 0 | 0 |
| Neuropathy | 4(9.3) | 1(2.3) | 0 |
| Osteonecrosis | 0 | 0 | 1(2.3) |
| Sensorineural hearing loss | 3(7.0) | 2(4.7) | 0 |
Efficacy
Treatment response
All the 43 patients underwent MRI assessment one month after concurrent HT with cetuximab, the primary site showed a 95.3% (41/43) complete response (CR) and a 4.7% (2/43) partial response (PR). The neck node sites showed a 97.6% (40/41) CR and a 2.4% (1/41) PR. After 4 cycles of ACT with TP, 4.7% PR at the primary site shifted from PR to CR; only one patient with PR at the neck node sites had biopsy-confirmed residual disease and received salvage neck dissection. No patients progressed during treatment.
Survivals
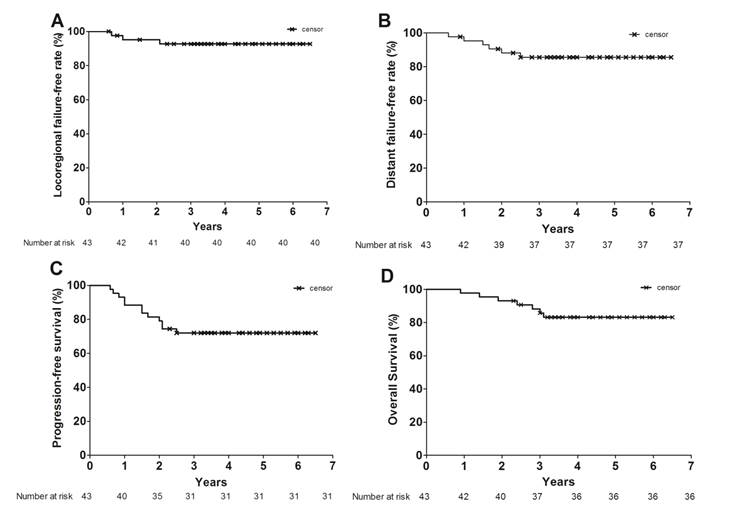
Figure 1 shows the LFFR, DFFR, PFS and OS survival curves. At a median follow-up of 48.0 months [95% CI 41.7-58.0 months], the 2 -year LFFR, DFFR, PFS and OS were 95.2% (95% CI 82.1-98.8), 88.1% (95% CI 78.3-97.9%),79.1% (95% CI 63.6-88.5%) and 93.0% (95% CI 79.9-95.0%), respectively; the 3-year LFFR, DFFR, PFS and OS were 92.7% (95% CI 78.8-97.6%), 85.6% (75.5-96.3%) , 72.0% (56.0-83.0%) and 85.7% (71.0-93.3%), respectively.
Kaplan-Meier estimates of OS (A), PFS (B), LFFR (C), and DFFR (D) among the patients with locally advanced nasopharyngeal carcinoma. Crosses indicate censored patients.
By the end of the study, seven out of the 43 patients had died. One (T4N1 stage) died of cranial recurrent NPC, 2 (1 patient with T3N0 stage and 1 patient with T3N2 stage) died of live metastases, 1 with T3N0 died of lung metastases, 1 with T2N2 died of bone metastases, 1 with T3N1 died of torrential bleeding caused by osteonecrosis, and 1 with T4N0 died of RT-related brain injury. Two patients had second primary cancers, including one meningeal cancer and one kidney cancer found at 18th and 20th months after treatment, respectively; both of them were still alive after surgery.
Discussion
The present study demonstrated that concurrent HT with cetuximab followed by ACT with TP was a feasible strategy in the treatment of patients with LANC. No patients had greater than grade 3 radiation related side-effects. No patient required nasogastric feeding. During concurrent HT with cetuximab, the most common grade 3 toxicities were oropharyngeal mucositis (81.4%) and RT-related dermatitis (7.0%). No hematological toxicity was observed. Previous studies in the literature have reported that the incidence of grade 3~4 oropharyngeal mucositis and radiation dermatitis caused by CCRT with cisplatin in the treatment of NPC ranged from 23.9% to 48% and from 2% to 13.2% respectively [14-17]; the incidences are increased up to 38~87% and 6~24% when cetuximab or bevacizumab is added to the CCRT with cisplatin [7, 18, 19]. The present study found no grade 4 oropharyngeal mucositis or RT dermatitis. The possible reason may be that the patients did not receive the treatment with cytotoxic chemotherapeutic agents during their radiotherapy and therefore, the side effects of cytotoxic reagents such as mucositis did not occur in these patients. In addition, HT appears to have the advantage of reducing the incidence of radiation related toxicity. Leung et al. [9] have shown that acute and late complications after HT for NPC patients are fewer and milder than that of conventional IMRT technique. In their study, 62.5% patients received concurrent HT with chemotherapy: acute grade 3 mucositis occurred in 4% patients (3/72), grade 4 mucositis in 1% patient (1/72) and grade 4 dermatitis in 1% patient (1/72). Shueng et al. [10] have also demonstrated that NPC patients treated with neoadjuvant chemotherapy followed by concurrent HT with chemotherapy show Grade 3 mucositis and dermatitis in 7% (2/28) and 46.4% (13/28) patients respectively. In our previous retrospective NPC study, the incidence of grade 3 mucositis and dermatitis were 5.5% and 6.8 % respectively [11].
Although Bonner et al. have reported that the incidence of acute RT-related toxicity is not significantly increased when HNSCC is treated with RT plus cetuximab [20], our results showed that the incidence of grade 3 oropharyngeal mucositis and RT-related dermatitis were still as high as 81.4 % and 7.0% respectively when cetuximab was added to RT in the treatment of LANC. Giro et al. have also found up to 50% of grade 3-4 of radiation dermatitis during radiotherapy with concurrent cetuximab in head and neck cancer [21]. However, in the present study, grade 3 oropharyngeal mucositis was controlled effectively by using our previously reported method [13]. This result may also be associated with the low toxicity of cetuximab that did not show any significant side effects that are often seen with chemotherapeutic agents, including nausea, vomiting, and mucositis. Only three patients need 7 ~ 12 days IV infusion for nutritional supports due to dysphagia and oropharyngeal pain caused by oropharyngeal mucositis; the remaining patients had a good eating condition; and 23.3 % of patients had only grade 2 weight loss. No patients experienced radiation break and no patients needed NF. Two important phase II clinical trials with novel molecular targeted agents have been reported by Ma et al. and Lee et al. [7, 18]. Ma et al.[7] treated 30 LANC patients with concurrent IMRT and cisplatin plus cetuximab, and reported that 26% patients showed grade 3 weight loss, 33% patients needed NF for a median duration of 41 days (range, 3-200 days), and IMRT was interrupted for 2 days in one patients who experienced a grade 3 oropharyngeal mucositis. Lee et al. [18] treated 44 LANC patients with CCRT with cisplatin-ACT with PF plus six cycles of bevacizumab (anti-vascular endothelial growth factor), and reported that 9% of patients (4/44) needed a feeding tube before therapy and 12% (5/41) of patients and 6% (2/36) of patients needed a NF at 1 year and 2 years after the start of treatment, respectively. Therefore, the incidence of acute grade 3 toxicities observed in this study was significantly less than the aforementioned trials [7, 18].
In the present study, during ACT with TP, no patients had greater than grade 3 hematologic toxicity, except for 1 patient having grade 4 leukopenia with fever; 4.7% patients (2/43) had mildly elevated creatinine. As the patients received ACT one month after concurrent HT with cetuximab, 93.0% (42/43) patients completed 4 cycles of chemotherapy. During HT with cetuximab, 100% of patients received 7 cycles of cetuximab; only one patient received reduced dose of cetuximab.
The compliance of treatment in our study was also better than in previous reports [7, 18]. In Lee et al.'s study [18]; the concurrent and adjuvant phases were both tolerable in 68% (30/44) of patients. In Ma et al.'s study [7], 86% and 50% of patients received +5 and +6 cycles of cisplatin, respectively; and 93% and 73% of patients received +5 and +6 cycles of cetuximab, respectively. However, cisplatin and cetuximab were interrupted in 60% and 33% patients, respectively.
Considering that HT can protect the contralateral parotid gland for preventing late xerostomia and less damage to the cochlea, xerostomia and SNHL caused by HT seemed less common in comparison to IMRT [22, 23]. HT- related grade 2 xerostomia (no Grade 3+ xerostomia) and SNHL are reported to range from 3~14% and 3~3.6% in the treatment of NPC reported by few studies [9, 10, 23]. Our previous report showed that no patient with nasopharyngeal carcinoma treated with HT reported grade 2+ xerostomia one year after radiotherapy [11]. In the present study, with a median follow-up of 48.0 years, only 4.7% patients (2/43) had Grade 2+ xerostomia one year after radiotherapy and recovered at 18 months after treatment. 11.6% patients (5/43) experienced SNHL and 34.9% patients (15/43) developed conduction hearing loss. Other severe late toxicities, including 4.7% (2/43) grade 1 endocrine dysfunction, 4.7% (2/43) grade 2 subcutaneous fibrosis and 2.3% (1/43) osteonecrosis, were found in our study after HT. Dysphagia was not observed in any of the patients.
Regarding the efficacy of our new treatment options, we obtained 79.1% PFS, 93.0% OS, 95.2% LFFR and 88.1% DFFR at 2- year, and 72.0% PFS, 85.7% OS, 92.7% LFFR and 85.6% DFFR at 3-year. Similar survival data are reported from two aforementioned phase II clinical trials. Ma et al. have reported that the 2-year PFS, OS, LFFR, and DFFR are 86.5%, 89.9%, 93%, and 92.8% respectively [7], and Lee et al. have reported 74.7% PFS, 90.9% OS, 83.7% locoregional progression-free interval, and 90.8% distant metastasis-free interval at 2-year [18].
The present study and Ma et al.'s study [7] were both single-center studies with Chinese patients with non-keratinizing type LANC. We employed the treatment strategy of concurrent radiotherapy and targeted agent-ACT, while Ma et al. only treated LANC patients with concurrent phase. The similar survival data might imply that addition of cetuximeb to CCRT without ACT might be an adequate treatment for LANC patients. Chen et al. reported on a phase III clinical study, demonstrating that there is no significant difference between CCRT and CCRT-ACT at 2-year DFFR [24]. However, NPC-9901 and NPC-9902 trials indicate that CCRT could result in significant benefit in both event-free survival and OS [25, 26], but NPC-9902 trial [26] has demonstrated that DFFR is significantly better in patients receiving 3 or more cycles ACT than that in those receiving 0-1 ACT. More recently, Hui et al. [27] showed induction chemotherapy (ICT) is beneficial in LANC patients as measured by 3-year OS. ICT is generally better tolerated than ACT; early use of cytotoxic drugs at full dose would theoretically be more effective in eradicating distant micro-metastasis [2]. Therefore, further studies are needed to validate the optimal timing of chemotherapy for the treatment of LANC.
In addition, 93.0% OS and 95.2% LFFR at 2 year, and 85.7% OS and 92.7% LFFR at 3 year might benefit from HT in the present study. Several retrospective studies have reported the clinical outcomes of HT treatment in NPC patients. Leung et al. [9] have shown that HT has advantages over IMRT for their NPC patients, as shown by better local control and survival, with 5-year locoregional control rate (LCR) and OS being 97% and 90%, respectively. Shueng et al. [10] reported 88.4% LCR and 83.5% OS at 3year, respectively. In our previous retrospective NPC study [11], the 1-year relapse-free survival and OS were 97.2% and 94.8%, respectively.
Summary
The present study demonstrated that the treatment with concurrent HT plus cetuximab followed by ACT with TP LANC patients yielded an acceptable safety profile with minimal side effects and an encouraging survival data. Future randomized phase 3 study is needed to confirm our results.
Abbreviations
LANC: Locoregionally advanced nasopharyngeal carcinoma;
HT: Helical tomotherapy;
EGFR: Epidermal growth factor receptor;
LFFR: Locoregional failure-free rate;
PFS: Progression-free survival;
DFFR: Distant failure-free rate;
OS: overall survival;
LCR: Locoregional control rate;
T: Docetaxel;
P: Cisplatin;
F: 5-FU;
NPC: Nasopharyngeal carcinoma;
NCCN: National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology;
SCCHN: Squamous cell carcinoma of the head and neck;
ACT: Adjuvant chemotherapy;
ECOG: Eastern Cooperative Oncology Group;
RTOG: Radiation Therapy Oncology Group;
AJCC: American Joint Committee on Cancer;
Cis: Confidence intervals;
SNHL: Sensorineural hearing loss;
CR: Complete response;
PR: Partial response;
NF: Nasogastric feeding;
CCRT: Concurrent chemoradiotherapy;
IMRT: Intensity-modulated radiotherapy;
RT: Radiotherapy;
IV: Intravenous;
MRI: Magnetic resonance imaging;
PET: Positron emission computed tomography;
CT: Computed tomography;
ICT: Induction chemotherapy.
Acknowledgements
This work was supported by National Natural Science Foundation of China (Grant No. 81072195) and Beijing Municipal Science and Technology Commission (Grant No. Z121107001012042). The contents of the paper are solely the responsibility of the authors, and do not necessarily represent the official views of these funding agencies. The authors thank Dr. Xu Shuping for his excellent assistance in statistical analyses.
Authors' contributions
ZX and ML designed the study; DL, ZF, and YS collected and assembled the data; ZX and ML wrote the manuscript. All authors read and approved the final manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Lee AW, Sze WM, Au JS. et al. Treatment results for nasopharyngeal carcinoma in the modern era: the Hong Kong experience. Int J Radiat Oncol Biol Phys. 2005;61:1107-16
2. Lee AW, Lin JC, Ng WT. Current management of nasopharyngeal cancer. Semin Radiat Oncol. 2012;22(3):233-44
3. Al-Sarraf M, LeBlanc M, Giri PG. et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized intergroup study 0099. J Clin Oncol. 1998;16:1307-10
4. Hitt R, Lopez-Pousa A, Martinez-Trufero J. et al. Phase III study comparing P plus fluorouracil to paclitxel, P, and fluorouracil induction chemotherapy followed by chemoradiotherapy in locally advanced head and neck cancer. J Clin Oncol. 2005;23:8636-45
5. Posner MR, Hershock DM, Blajman CR. et al. TAX 324 Study Group. P and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357:1705-15
6. Diaz Miqueli A, Rolff J, Lemm M. et al. Radiosensitisation of U87MG brain tumors by anti-epidermal growth factor receptor monoclonal antibodies. Br J Cancer. 2009;100(6):950-8
7. Ma BB, Kam MK, Leung SF. et al. A phase II study of concurrent cetuximab-P and intensity-modulated radiotherapy in locoregionally advanced nasopharyngeal carcinoma. Ann Oncol. 2012;23(5):1287-92
8. Bauman G, Yartsev S, Rodrigues G. et al. A prospective evaluation of helical tomotherapy. Int J Radiat Oncol Biol Phys. 2007;68:632-41
9. Leung SW, Lee TF. Treatment of nasopharyngeal carcinoma by tomotherapy: five-year experience. Radiation Oncology. 2013;8:107-12
10. Shueng PW, Shen BJ, Wu LJ. et al. Concurrent image-guided intensity modulated radiotherapy and chemotherapy following neoadjuvant chemotherapy for locally advanced nasopharyngeal carcinoma. Radiat Oncol. 2011;6:95
11. Ren G, Du L, Ma L. et al. Clinical observation of 73 nasopharyngeal carcinoma patients treated by helical tomotherapy: the China experience. Technol Cancer Res Treat. 2011;10(3):259-66
12. Zhang X, Wang J, Wu W. et al. Efficacy and Safety of Combined Radiotherapy with EGFR Inhibitors and Chemotherapy for Laryngeal Organ Preservation in Patients with Locally Advanced Hypopharyngeal Carcinomas. Curr Cancer Drug Targets. 2014;14(6):589-98
13. Zhang XX, Ma L, Wang JL. et al. Management of oropharyngeal mucositis in patients with head and neck cancer receiving chemoradiotherapy and/ or molecular targeted therapy. Chin J otorhinolaryngol Head and Neck Surg. 2011;46(6):505-88
14. Lee N, Xia P, Poon I. et al. Intensity-modulated radiotherapy in the treatment of nasopharyngeal carcinoma: an update of the UCSF experience. Int J Radiat Oncol Biol Phys. 2002;53:12-22
15. Chan AT, Teo PM, Ngan RK. et al. Concurrent chemotherapy-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: progression-free survival analysis of a phase III randomized trial. J Clin Oncol. 2002;20:2038-44
16. Wang J, Bai S, Chen N Y. et al. The clinical feasibility and effect of online cone beam computer tomography-guided intensity-modulated radiotherapy for nasopharyngeal cancer. Radiother Oncol. 2009;90:221-7
17. Lee N, Harris J, Garden AS. et al. Intensity-modulated radiation therapy with or without chemotherapy for nasopharyngeal carcinoma: radiation therapy oncology group phase II trial 0225. J Clin Oncol. 2009;27:3684-90
18. Lee NY, Zhang Q, Pfister DG. et al. Addition of bevacizumab to standard chemoradiation for locoregionally advanced nasopharyngeal carcinoma (RTOG 0615): a phase 2 multi-institutional trial. Lancet Oncol. 2012;13(2):172-80
19. Pfister D, Su YB, Kraus DH. et al. Concurrent cetuximab, P, and concomitant boost radiotherapy for locoregionally advanced,squamous cell head and neck cancer: a pilot phase II study of a new combined-modality paradigm. J Clin Oncol. 2006;24:1072-8
20. Bonner JA, Harari PM, Giralt J. et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567-78
21. Giro C, Berger B, Bolke E. et al. High rate of severe radiation dermatitis during radiation therapy with concurrent cetuximab in head and neck cancer: results of a survey in EORTC institutes. Radiother Oncol. 2009;90:166-71
22. Nam P. Nguyen, Lexie Smith-Raymond, et al. Feasibility of Tomotherapy to spare the cochlea from excessive radiation in head and neck cancer. Oral Oncol. 2011;47(5):414-9
23. Chen AM, Yang CC, Marsano J, Liu T, Purdy JA. Intensity-modulated radiotherapy for nasopharyngeal carcinoma: improvement of the therapeutic ratio with helical tomotherapy versus segmental multileaf collimator-based techniques. Br J Radiol. 2012;85(1016):e537-43
24. Chen L, Hu CS, Chen XZ. et al. Concurrent chemoradiotherapy plus adjuvant chemotherapy versus concurrent chemoradiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma: a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2012;13(2):163-71
25. Lin JC, Jan JS, Hsu CY. et al. Phase III study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma: Positive effect on overall and progression-free survival. J Clin Oncol. 2003;21:631-7
26. Wee J, Tan EH, Tai BC. et al. Randomized trial of radiotherapy versus concurrent chemoradiotherapy followed by adjuvant chemotherapy in patients with American Joint Committee on Cancer/International Union against cancer stage III and IV nasopharyngeal cancer of the endemic variety. J Clin Oncol. 2005;23:6730-8
27. Hui EP, Ma BB, Leung SF. et al. Randomized phase II trial of concurrent P-radiotherapy with or without neoadjuvant docetaxel and P in advanced nasopharyngeal carcinoma. J Clin Oncol. 2009;27:242-9
Author Biography
Dr. Xinxin Zhang is a professor of the Department of Otolaryngology, Head & Neck Surgery at Chinese PLA General Hospital. She completed her postgraduate training in Experimental Therapeutic Translational Laboratory at Dana-Faber Cancer Institute, Harvard Medical School, USA from 2002~2006. Professor Zhang's main research focus is on the molecular targeted therapy of head and neck cancer and identification of specific biomarkers associated with metastasis and prognosis in clinical and basic research. She has particular experience in the organ preservation of larynx and eye with combined modality treatment. She, now, serves on the editorial board of Journal of Otology and participated in the drafting of NCCN clinical practice guidelines in head and neck cancer (Chinese Version) as a panel member.
Dr. Shiming Yang, professor, Chief physician of the Department of Otolaryngology, Head & Neck Surgery at Chinese PLA General Hospital. His research is centered on deafness. Dr. Yang specializes in the surgical treatment of deafness, especially in the area of auditory implantation.
Dr. Lei Du is a physician with 8 years of research experience in the radiation oncology area. Dr Du has a special interest in precise radiation therapy of head and neck cancer, lung cancer and breast cancer.
Dr. Feifang Zhao obtained his doctor's degree from Chinese PLA General School of Medicine, Chinese Union Medical College in 2012. He has co-authored over 10 publications including studies published in PLoS One and Acta Otolaryngol. His current research focuses on the clinical trials for nasopharyngeal carcinoma.
Dr. Qiuju Wang is the chief physician and professor of the Department of Otolaryngology, Head & Neck Surgery at Chinese PLA General Hospital. Her research area is deafness and tinnitus. Dr Wang specializes in the clinical treatment of hereditary deafness and abrupt deafness.
Dr. Lin Ma is a professor of the Department of Radiation Oncology, Chinese PLA General Hospital. He obtained his doctor's degree from Fundamental Bases of Oncogenesis, Paris VII University (France) in 1998. Professor Ma's main research focus is on the treatment of head and neck cancer with IMRT or Helical Tomotherapy.
![]() Corresponding authors: Dr. Xinxin Zhang, Department of Otolaryngology, Head and Neck Surgery, PLA General Hospital, 28 Fuxing Road, Beijing100853, China. Tel: +86-15910520109; FAX: +86-010-68241383; E-mail: zhangxinxincom.cn; xinxinzhang66com. Or Co- Correspondence: Dr. Lin Ma, Department of Radiation Oncology, Chinese PLA General Hospital, 28 Fuxing Road, Beijing 100853, China. Tel: +86-13911197589; FAX: +86-010-55499243; Email: malinpharmcom.
Corresponding authors: Dr. Xinxin Zhang, Department of Otolaryngology, Head and Neck Surgery, PLA General Hospital, 28 Fuxing Road, Beijing100853, China. Tel: +86-15910520109; FAX: +86-010-68241383; E-mail: zhangxinxincom.cn; xinxinzhang66com. Or Co- Correspondence: Dr. Lin Ma, Department of Radiation Oncology, Chinese PLA General Hospital, 28 Fuxing Road, Beijing 100853, China. Tel: +86-13911197589; FAX: +86-010-55499243; Email: malinpharmcom.

 Global reach, higher impact
Global reach, higher impact