10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2017; 13(11):1409-1419. doi:10.7150/ijbs.21916 This issue Cite
Review
Altered Neuroendocrine Immune Responses, a Two-Sword Weapon against Traumatic Inflammation
State Key Laboratory of Trauma, Burns and Combined Injury, Institute of Surgery Research, Daping Hospital, Third Military Medical University, Chongqing, 400042, China
Received 2017-7-14; Accepted 2017-9-23; Published 2017-11-1
Abstract
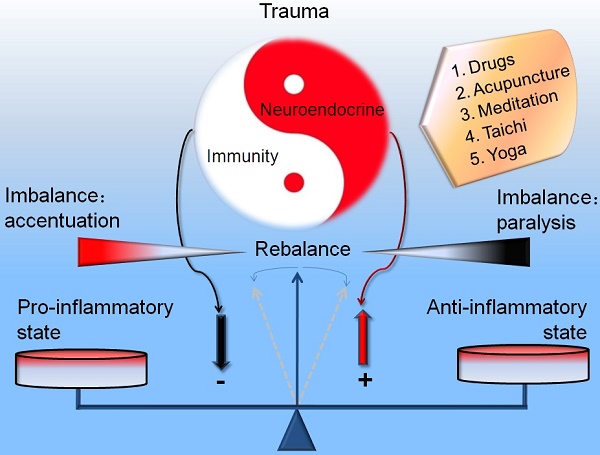
During the occurrence and development of injury (trauma, hemorrhagic shock, ischemia and hypoxia), the neuroendocrine and immune system act as a prominent navigation leader and possess an inter-system crosstalk between the reciprocal information dissemination. The fundamental reason that neuroendocrinology and immunology could mix each other and permeate toward the field of traumatology is owing to their same biological languages or chemical information molecules (hormones, neurotransmitters, neuropeptides, cytokines and their corresponding receptors) shared by the neuroendocrine and immune systems. The immune system is not only modulated by the neuroendocrine system, but also can modulate the biological functions of the neuroendocrine system. The interactive linkage of these three systems precipitates the complicated space-time patterns for the courses of traumatic inflammation. Recently, compelling evidence indicates that the network linkage pattern that initiating agents of neuroendocrine responses, regulatory elements of immune cells and effecter targets for immune regulatory molecules arouse the resistance mechanism disorders, which supplies the beneficial enlightenment for the diagnosis and therapy of traumatic complications from the view of translational medicine. Here we review the alternative protective and detrimental roles as well as possible mechanisms of the neuroendocrine immune responses in traumatic inflammation.
Keywords: trauma and injury, stress, infection, hormones, neuroendocrine system, immunity, translational medicine.
Introduction
It is a long time since the sage of the past (Galen, a Greek Physician; Bian Que, a Chinese physician) had noticed the inextricably functional linkage between immune and nervous systems. The sophisticated relationship between neuroendocrine and immune responses was initially confirmed by the experiments of Ishigami's phagocytosis in 1919 and Metalnikov's conditioned reflex in 1924. Then Hans Selye, a Hungarian endocrinologist, named the specific phenomenon as stress. "Every stress leaves an indelible scar, and the organism pays for its survival after a stressful situation by becoming a little older." Trauma is just a severe stress that most of the organisms inevitably encounter during their lives.
In the early phase of trauma, the stressful response occurs with the aid of pain, ischemia, hypoxia, etc. [1-4]. Within the immediate neuroendocrine immune reflex, the mediators (hormones, neurotransmitters, neuropeptides, cytokines and inflammatory mediators) are synergistically involved in the aseptic and adaptive inflammation via the regulation of the amount and bioactivity [5-9]. Under the guidance of biological activities of fight or flight, the injured bodies may succumb to successive infections from wound surface or intestinal ducts [10, 11], which may consequently result in the uncontrolled inflammation. During these courses, crosstalk between the neuroendocrine and immune systems, the congenerous captains of their own ship, can result in the production of factors by the nervous and endocrine systems [12], which synergistically alters immune function and subsequent immunomodulation against successive infectious agents and other pathogens in trauma. Thus, during the courses of remedy for trauma patients, the injured bodies showed the varying inflamed state (low, moderate, excessive) owing to the changes of regulatory ability of the neuroendocrine immune network. Studies involving either anti-inflammatory or pro-inflammatory agents further suggest that the local inflammation produced by injury is important for organ regeneration [12]. It significantly determines the dynamic changes of vital organs, which is related to the outcome of trauma patients. Therefore, it is of magnificent values to ideally rein the dynamic equilibrium of neuroendocrine immune responses for the inflammatory response, structural remodeling and functional repair in trauma.
Structural and functional basis for the neuroendocrine immune network
The traumatic inflammation refers to the multiple aspects of neuroendocrine immune network (Figure 1). Brain can play an immunomodulatory role, whose functions are mostly elucidated in homeostasis maintaining of the immune system in response to changes of the environment [13-16] while the immune system possesses the sensing ability [13, 17-19]. The central and peripheral nervous systems linked in countless ways to the immune system. Through anatomical analysis, the encephalic locus involved in the immune regulation at least includes the dorsolateral prefrontal cortex (DLPFC), orbitofrontal cortex (OFC), medial prefrontal cortex (MPFC), hypothalamus, pituitary, locus ceruleus (LC), hippocampus and medulla oblongata in trauma. Hypothalamic-pituitary-adrenal (HPA) axis and autonomic nervous system (ANS) (mainly sympathetic and parasympathetic nerve) play a hinge locus [20-22]. Thus, circulating inflammatory molecules have the ability to target their cognate receptors at the levels of blood-brain barrier (BBB) under the orchestration of integrated responses in trauma. Other links also include the scattered neuroendocrine immune reflex arc in organs (intestine [23], skin [24, 25], adrenal gland [26, 27], bone marrow [28-31], etc.). Neuroendocrine-immune interactions can be conceptualized using a series of feedback loops, which culminate into distinct neuroendocrine-immune phenotypes [32]. Thus, changes in the peripheral nervous system at the site of local inflammation might be hallmarks of traumatic complications.
Schematic drawing of neuroendocrine immune pathways involved in the regulative mechanisms of traumatic inflammation. Hypothalamic-pituitary-adrenal (HPA) axis, sympathetico-adrenomedullary (SAM) axis and cholinergic pathway synergistically involved in the immune-mediated inflammation in trauma. PAF: platelet activating factor. CRH: Corticotropin-releasing hormone, Ach: Acetylcholine, ACTH: adrenocorticotropic hormone.
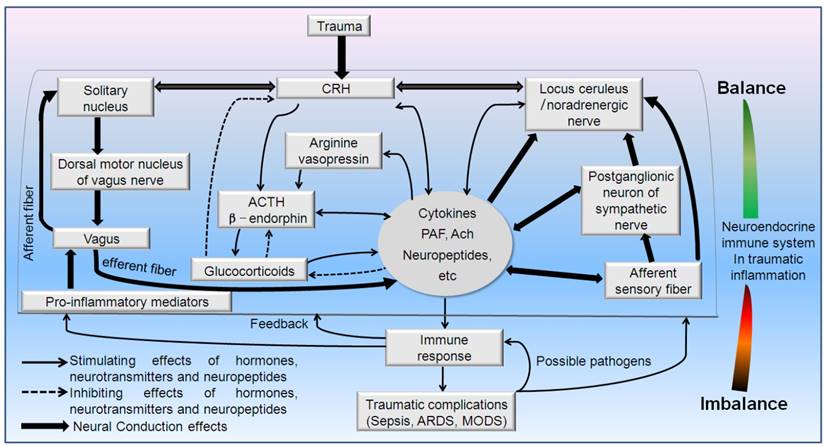
The in vivo hormones, neurotransmitters and neuropeptides possess the robust immunomodulatory capacity [33-35]. In turn, the immune system informs the neuroendocrine system [23, 36]. Meanwhile, many other cells including endothelial cells in brain ventricle, microglia, and atrocities can also release multiple immunomodulatory elements of the central nervous system (CNS). Also, the neuronal and endocrinal cells may receive immune signals via their corresponding immune-related receptors (cytokine receptors, pattern recognition receptors, chemokine receptors, nuclear receptors) [37-43]. Concurrently, the immunocytes release various cytokines (lymphokines, monokines, etc.) to affect the neuroendocrine responses as well as sensing the local or distant stressful signals [43-45] termed “flowing brain”. The sharing of ligands and receptors allows the immune system to serve as the sixth sense notifying the nervous system of the presence of foreign entities [46]. Thus, brain and immune systems may interact reciprocally via the route of nerve and body fluids [4, 23, 28, 44]. The standing and flowing brain act just as a vivid mirror of immune responses.
Among them, one of the most typical findings is Ghrelin, an endogenous ligand for growth hormone (GH) secretagogue receptor (i.e., ghrelin receptor) [47, 48] and one of the first hormones rapidly increasing in the human physiological response to bacterial endotoxic shock [49]. It was demonstrated to mediate the increased vascular sensitivity in the hyperdynamic phase of sepsis [50] in addition to its effects on GH release and energy homeostasis in traumatic infections. Ghrelin could inhibit pro-inflammatory cytokine production, mononuclear cell binding, and Nuclear factor-κB (NF-κB) activation in human endothelial cells in vitro and endotoxin-induced cytokine production in vivo [51]. Conversely, the reduced central (brain) responsiveness to ghrelin due to the decreased GH, plays a major role in producing the hyper-inflammatory state, resulting in severe organ injuries and high mortality after endotoxemia in aged animals [52]. It has sympathoinhibitory properties that are mediated by central ghrelin receptors involving a NPY/Y1 receptor-dependent pathway [50]. Ghrelin's inhibitory effect on TNF-α production in sepsis is partially because of its modulation of the overstimulated sympathetic nerve activation [53]. It also improved the tissue perfusion in severe sepsis, which might be mediated by down-regulation of endothelin-1 (ET-1) involving a NF-κB-dependent pathway [54]. High ghrelin levels have been considered to be a positive predictor of ICU-survival in sepsis patients [55] besides its potential therapeutic use [34]. Collectively, the immune system is regulated via the secretion of neuron hormones and peripheral ANS while the peripheral immune signals are transmitted into the brain via the cytokines and afferent activities of vagus in trauma. The complicated interactions included the stimulating, inhibitory and modulating effects of these common biological stimuli (hormones, neurotransmitters, neuropeptides and inflammatory mediators) [56, 57].
The dynamic regularity of neuroendocrine immune network in traumatic inflammation
HPA axis
In severe traumatic stress, the hypothalamus integrates signals from peripheral systems through afferent sympathetic, parasympathetic, and limbic circuits converging to the paraventricular nucleus (PVN), which translates into neuroendocrine perturbations, altered neuronal signaling [58]. First, the activation of HPA axis resulted in the releasing of corticotropin-releasing hormone (CRH) in the PVN, a central cellular switchboard, into the hypophyseal portal system. CRH could then stimulate the secretion of hypophyseal adrenocorticotropic hormone (ACTH) and the downstream glucocorticoids in adrenal glands [59, 60]. Actually, CRH may modulate the immune responses in trauma via two pathways: an anti-inflammatory one operated by centrally released CRH, most likely through stimulation of glucocorticoid and catecholamine release, and a pro-inflammatory one, through direct action of peripherally released CRH [61-63]. Researchers showed that CRH deficiency disrupted endogenous glucocorticoid production and enhances allergen-induced airway inflammation in mice [64]. However, CRH deficiency impairs but does not block pituitary-adrenal responses to diverse stressors [65], further suggesting that pituitary-adrenal activity is augmented by factors besides CRH in trauma.
Generally, trauma-induced glucocorticoids act as a neuroendocrine alarm signal of danger in a manner of non-physiologically pulsatile fashion. The bidirectional roles of glucocorticoids in modulation of inflammation may change therapeutic strategy at least via the regulation of inflammatory genes for inflammatory diseases [66]. Concurrently, Glucocorticoids also regulate inflammatory responses via non-genomic pathways in cytoplasm and mitochondria. Crosstalk between HPA-axis-increased glucocorticoids and mitochondrial stress determines immune responses and clinical manifestations of patients with sepsis [67]. Previous results further indicated that glucocorticoids bestow its suppressive effects via its low-affinity glucocorticoid receptor (GR) and its permissive effects via the high-affinity mineralocorticoid receptor (MR) in the acute inflammatory response [68]. The neuroendocrine control of the innate immune system by glucocorticoids is critical for the delicate balance between cell survival and damage in the presence of inflammatory mediators [69]. Actually, glucocorticoids produce a persisting sensitization of CNS innate immune effectors so that they will generate a potentiated pro-inflammatory response after the glucocorticoid rise has dissipated, thereby enhancing the sickness response to trauma or succeeding infections and maximizing the animal's ability to neutralize danger [70]. The emerging evidence highlights both pro-inflammatory and anti-inflammatory actions of glucocorticoids on both the innate and adaptive immune systems. In this framework, they are ready to reinforce the innate immune system, and repress the adaptive immune system [71], to help to resolve inflammation and restore homeostasis in injured lungs [72], the most common target organ in trauma. Thus, HPA axis is the principal anti-inflammatory pathway in traumatic inflammation. The destruction or disabling of HPA axis may promote the development of traumatic complications [73, 74]. Growing evidence has proved that the ex vivo usage of endotoxin and IL-1 activated the HPA axis via the body fluid route or vagus [75, 76], which synergistically propel the pathogenesis of traumatic inflammation and sepsis. Additionally, the melatonin released from hypophyseal portal system could maintain the pro- and anti-inflammatory balance by influencing leukocyte migration and apoptosis in carp [77]. Also, the adrenal medulla may directly integrate neuronal, hormonal, and immune signaling during inflammation, through induction of paracrine factors that signal to both adrenal cortex and sensory afferents of the adrenal gland, and autocrine factors [78], which determine the duration and type of paracrine secretory signaling in traumatic inflammatory conditions, both suggesting the provincial complicated modulating effects for the axis. Thus, studies of HPA axis activation on the outcome of trauma patients remain needed.
Sympathetico-adrenomedullary (SAM) axis
Concurrently, the sympathetic nervous system is initiated via PVN and LC once trauma occurs, inducing the releasing of catecholamine (CA) through the ending of autonomic nerves and adrenal medulla [79, 80]. The ANS belongs to the neuromodulatory route secondary to the HPA axis in traumatic inflammation. The norepinephrine releasing from the sympathetic nervous ending could affect the immune system [81]. It was reported that a suppressive role for noradrenergic innervation on the hemorrhage-induced increase in lung TNF-α content in vivo [82]. The effects of norepinephrine are protective from lung injury but maybe contribute to the generalized immunosuppression in severe trauma. Meanwhile, Beta-blockade of propranolol can protect against the detrimental effects of trauma on lungs by blunting the exaggerated sympathetic response after shock and injury [83]. Also, the activation of adrenergic receptors and releasing of CA play an important role in traumatic infections [84, 85]. Under physiological conditions, the activation of adrenergic receptors could alleviate the levels of pro-inflammatory mediators via the enhancement of IL-10 secretion. In the cecal ligation and puncture model of B6D2F1 male mice, the secretion of TNF-α and IL-6 in spleen macrophages obviously decreased after the 2 h treatment of epinephrine or IL-10 [86], while the treatment of ICI-118551, an antagonist of β2 adrenergic receptors resulted in the increase in pro-inflammatory mediators, demonstrating the expression of pro-inflammatory mediators in the peripheral tissues via epinephrine in the early phase of trauma. On the other hand, the sympathetic nervous system and gut-derived norepinephrine mediated the pro-inflammatory responses in kupffer cells in livers via the activation of α2A adrenergic receptors [87]. Also, beta-adrenergic receptor activation by catecholamine of macrophages mediates the hemorrhagic shock / resuscitation (HS/R)-induced release of HMGB1, a late inflammatory mediator [29, 88]. Blocking this novel signalling axis may present a novel therapeutic target for traumatic inflammation. Thus, what effect the SAM plays for the inflammation outcome is closely related to their microenvironment and modulating measures.
Regarding the key role of activation of adrenergic receptors and excessive releasing of CA, Wang et al postulated the theory of sympathetic excitotoxicity in sepsis [89]. It was viewed that the paradigm shift of sympathetic nervous system activation determined the progression and outcome in inflammatory responses. Presently, results indicated that the activation of sympathetic nervous system could play anti-inflammatory effects, and even synergistic role with HPA axis and parasympathetic nerve system. But it generally occurs in mild injury or local infections. Once the severe traumatic infections happened, such modulating paradigm will be transformed from anti-inflammatory into pro-inflammatory pattern in sympathetic nerve system. Therefore, it is of great necessity to efficiently control the intensities and durations of traumatic inflammation for avoiding or containing the vicious cycle of sympathetic excitotoxicity, which is valuable for reversing the harmful outcome for infectious inflammation in trauma patients.
Cholinergic pathway
The role of cholinergic pathway in injury has been investigated extensively owing to the anti-inflammatory capacity of vagus, the tenth cranial nerve stimulation as well as nicotinic acetylcholine receptor activation [90-94]. The increase of vagal tone is the significant representation in encephalon immune modulation. The efferent limb of vagus could alleviate the morality of septic shock by inhibiting the TNF-α secretion. Acetylcholine (ACh) is the principal neurotransmitters of vagus. Tracey et al named the anti-inflammatory mechanism as cholinergic anti-inflammatory pathway [95, 96]. The vagus expresses IL-1 receptor, converting the immune signalling to neuronal signaling through the ascending route of cholinergic signals reaching to the brain stem. Conversely, the descending route of vagus could modulate the peripheral leukocyte activity and inflammatory response via HPA axis and neuronal route inhibiting the secretion of macrophage cytokines [97]. In addition, acetylcholine is also synthesized by lymphoid cells and suppresses macrophage activation in injury. Researchers showed that the electric stimulation of efferent limb and administration of a7 nAChR agonists [98, 99], which significantly inhibited the TNF-α production in livers, hearts and spleens as well as the reduction in the levels of serum TNF-α, alleviating the incidence of septic shock. Vagotomy and deficiency of a7 nAChR could obviously elevate the synthesis and releasing of TNF-α in inflammatory status, and enhance the animals' lethality in lipopolysaccharide challenge [100]. Especially, the existence of pulmonary parasympathetic inflammatory reflex was also postulated concerning an acute lung injury model after local but not systemic challenge [101]. In recent years, many anti-inflammatory drugs (aspirin, indomethacin, ibuprofen, CNI-1493, α-MSH, etc.) were found to excite vagus. The possible anti-inflammatory mechanism refers to the involvement of cholinergic anti-inflammatory pathway, which further supplies the robust evidence for the regulatory effects of neuroendocrine axes on inflammation [102, 103]. These findings deeply demonstrated that cholinergic anti-inflammatory pathway is the key defensive pathway for the specific inhibition of local excessive inflammation in trauma.
Immune receptors, mediators and organs in the neuroendocrine responses in trauma
Meanwhile, immune receptors and mediators, especially pathogen-associated molecular patterns (PAMP) and pattern recognition receptors (PRRs) have been involved in the regulation of neuroendocrine responses in trauma. Among them, CD14, scavenger receptors, toll-like receptor 4 (TLR4) and HMGB1 have been investigated extensively [11, 104]. The adrenal deficiency could significantly blunt the mRNA expression of SR-A, CD14, TLR4 and MD2 in injured lung tissues. Adrenalectomised animals showed enhancement of inflammatory responses and severe tissue injuries in trauma. The increase of CD14 after the pretreatment of corticosterone could improve the sensitivity of LPS stimulation. Also, the role of TLR4 acts as a crucial receptor in the innate immune system and their role in inflammation, stress, and tissue injury, including injury to the lung and brain have been clearly mentioned [104]. TLR4 is involved in neuroinflammation due to the lung-brain interaction. TLR4 knockout and administration of a TLR4 antagonist (100 μg/mice) to WT mice ameliorate neuroinflammation due to lung-brain interaction after prolonged mechanical ventilation [105]. Also, HMGB1-alarmin can be released from activated immune cells and from stressed and / or necrotic cells in response to tissue injury. It exerts its influence by interacting with several receptors, such as RAGE and some TLRs. RAGE and TLR4 transmembrane receptors are highly expressed in the lung and play an important role in innate immune inflammatory responses. The HMGB1-RAGE axis mediates traumatic brain injury-induced pulmonary dysfunction in lung transplantation [106]. Notably, there have been at least 37 formally recognized cytokines and their receptors, and 60 classical neurotransmitters plus over 50 neuroactive peptides [57]. So the complex modulation loops between neuroendocrine and immune responses in traumatic inflammation may be far beyond our research expectation.
Additionally, another most fascinating aspect in lung inflammation is the crosstalk between the lung and other vital organs (brain, adrenal glands, intestinal ducts, etc.) through neural, cellular or humoral pathways. In that way, the work of Tracey KJ and the new findings of Gonzalez-Lopez on mechanical ventilation that trigger hippocampal apoptosis by vagal and dopaminergic pathways has been found [107]. In other words, not only trauma or sepsis might cause distant inflammation. An impaired adrenocorticotropic hormone response as well as a significant increase for lung interleukin-6 were found, particularly in nonsurvivors compared with survivors among cecal ligation and puncture-induced mice [108]. Therefore, the traumatic pulmonary inflammation should be further considered in uncontrolled neuroendocrine responses. Taken together, all of these modulatory circuits might integrate the lungs, immune and nervous systems and play a key role in regulating lung inflammation and immunity through the neural innervations in trauma.
Philosophical thinking on the rebalance of neuroendocrine immune responses in trauma
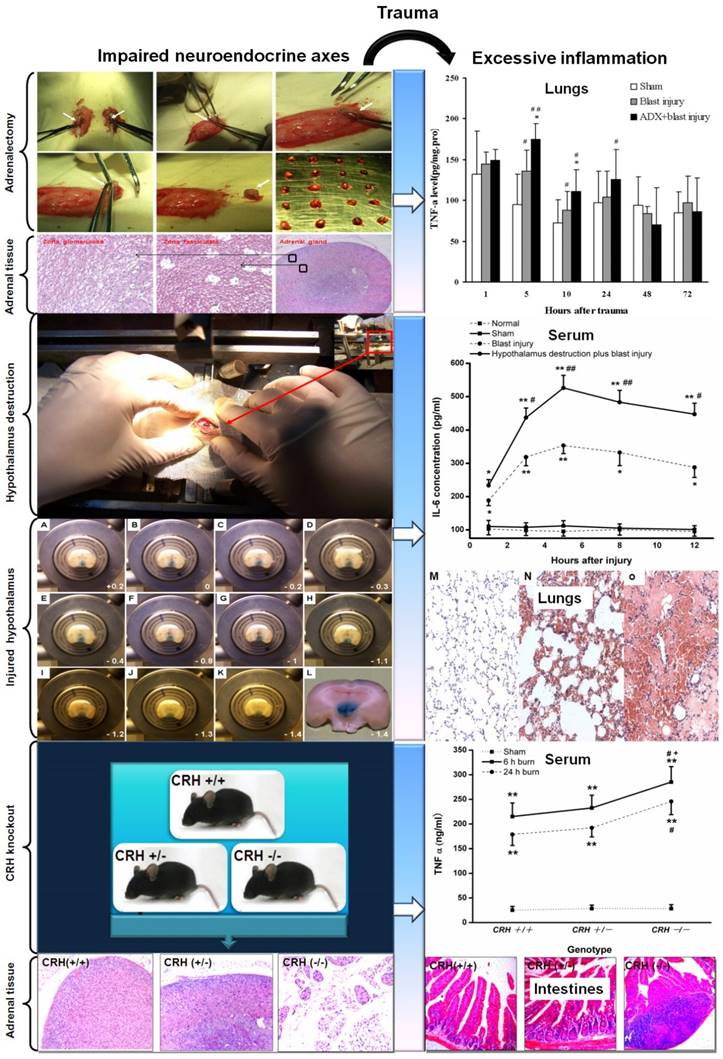
Actually, trauma belongs to a severe injury pattern followed with the immediate releasing of effecter molecules (hormones, neurotransmitters, neuropeptides, cytokines, complement, etc.). The aim of these extensive responses is to confront the potential danger in internal environment. The immunocytes (neutrophils, mononuclear macrophage, lymphocytes) may change their biological activities in response to these stressful hormones. Primarily, the releasing cytokines and inflammatory mediators could be controlled within a reasonable range in number and category occasionally through "neuroendocrine" G-Protein-Coupled Receptors (GPCRs) [6, 109]. Concerning the complicated neuroendocrine network as well as multiple hormones, neurotransmitters, neuropeptides and their targets, we could view that a certain magnitude of neuroendocrine responses may promote the relieving of the intensities and durations of traumatic stress from the point of biological evolution, which had been negatively confirmed by enhancing the risk of infections in injured animals with impaired neuroendocrine axes (adrenalectomy, CRH knockout, hypothalamus destruction) [11, 73] (Figure 2), relative glucocorticoids deficiency, glucocorticoids resistance, or circadian disruption of hormone release [110-112]. Moreover, once the intensity, frequency and duration reached above the threshold of auto-regulation, HPA axis and ANS will loss the coordinate regulatory capacity in an uncoupling state. Consequently, the regulatory effects of neuroendocrine responses may transform from the defensive state to the flight outcome [113-115]. The optimal regulatory response will finally disintegrate into the vicious or even paralysis outcome. The whole body responses will develop towards the uncontrolled directions. These trauma patients will undoubtedly succumb to the successive infectious complication. So, it is necessary to insist on the strategy of integrity, balance and space-time consonance for the treatment of traumatic inflammation on the basis of neuroendocrine immune network. Also, any efficient medical measures for the traumatic inflammation may help the rebalancing of neuroendocrine immune response. Only the stringent modulation of uncontrolled inflammation could avoid the traumatic complications and result in a favorable outcome for the trauma patients.
Enlightenment of the balancing role in neuroendocrine immune response for the traumatic inflammation
On the basis of neuroendocrine immune network, we could easily found that the potential limitations existed in the therapeutic strategy of traumatic inflammation to some extent. First, within the experiencing treatment scope, doctors occasionally focus their procedure on the controlling of the source of injury or infection, rectification of the disequilibrium of water-electrolyte and the nutritional support. The severe stress owing to the somato- and psycho-trauma remains needed to attract the extensive attention. Results showed that appropriate neuroendocrine modulation may regulate inflammation to reach an optimum defense while preventing excessive host cell damage [116]. However, the deleterious or malignant neuroendocrine cascade should be paid more attention especially for the trauma patients with potential infectious complications and exposure to some wretched circumstances [4]. Second, growing evidence have showed that we have tried to control the inflammatory cascade via the re-balancing of pro- and anti-inflammatory responses as well as the recovery of immune state. However, it remains lack of insightful assurance on the intrinsic relations between the neuroendocrine and immune systems. Third, regarding the judgment of the pathogenetic condition and outcome for the trauma patients, most of the neuroendocrine measures depend on the level and reactivity of serum cortisol as well as dehydroepiandrosterone and its sulfate. Presently, it is in great need to form the evaluation system or personalized prewarning formula for the traumatic infections depending on the key parameters of neuroendocrine immune network. Fourth, we have paid a great enthusiasm on the west medicine while some Chinese traditional medical measures (acupuncture [117-120], Chinese patent medicine [121, 122], meditation [123-125], Taichi [126], etc.) in the neuroendocrine immune regulation have been attracted little attention [127]. There are still many controversies that need to be resolved in order to use integrated traditional Chinese medicine-west medicine (tcm-wm) rigorously as therapy for trauma.
The impairment of neuroendocrine immune balance deteriorates the traumatic inflammation. Adrenalectomy, hypothalamus destruction and CRH knockout promoted the excessive inflammation and tissue (lungs and intestines) injury. A-L: representative brain tissues between Bregma +0.2 and -1.4 in SD rats. Lesions in the hypothalamus perfused and stained with ferrocyanatum kalium were seen. M: Sham, N: 5h after blast injury, 0: 5h after hypothalamus destruction plus blast injury (stained with hematoxylin and eosin, lower power lens). # P < 0.05 and # # P < 0.01 compared with sham group, * P < 0.05, * * P < 0.01 compared with corresponding blast injury group. (Yang C, et al. Injury. 2011, 42(9):905-912; Yang C, et al. Cytokine. 2011, 54(1):29-35; Yang C, et al. Surgery.2015, 158(1):255-265.)
Graphical abstract. Potential modulatory strategy for the rebalancing of neuroendocrine immune responses in traumatic inflammation. The ideal modulating measures emphasize the dynamic regulation between the imbalance and rebalance of neuroendocrine immune responses in a nonlinear manner in trauma. The drug and nondrug (acupuncture, meditation, taichi, yoga) treatment are recommended.
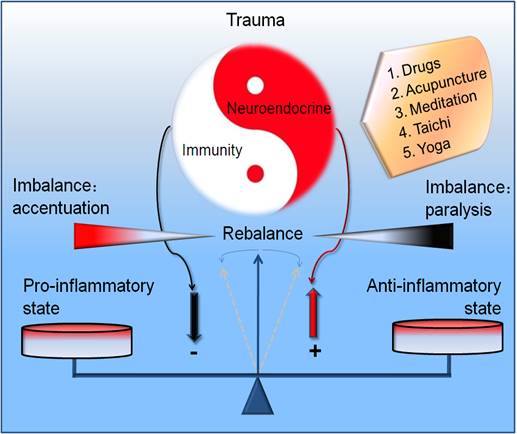
Nevertheless, there has been steady progress in application of these methods of evidence-based medicine and modern neuroscience to these ancient practices. Since there remains lack of the stringent discrimination and rational regression for the modulation of traumatic inflammation, we need the global and dialectical idea from this ancient traditional medicine on the basis of neuroendocrine immune network, reconstructing the regulating network which was in chaos in traumatic infections, and recovering the physiological resonance. Such measures may be more efficient compared with the isolated organic function support and rigid structure repair. Collectively, it is of great importance to grasp the integrity of neuroendocrine immune network. The simple modulation of part of the network despite of the personalized occasion could result in the new inflammatory injury. Only the concordant and adaptive rhythm as well as their complexity and nonlinear regularity of these three systems [128] was realized can we grasp the ideal balancing point in traumatic inflammation (Figure 3/Graphical abstract). Undoubtedly, it is the key point for the remedy of traumatic infections.
Perspective and potential challenges
The insightful disclosure of neuroendocrine immune network has remarkably improved our understanding of how the excessive inflammation loses resonance in trauma. Through the dynamic and exquisite feedback loops and the circadian rhythm of key neuroendocrine-immune system, the uncontrolled inflammation may be pulled back into a standardized route. The inappropriate feedbacks of mediators may be wisely controlled to avoid a sustained inflammatory cascade that may have profound detrimental consequences depending on the tissues and the severity of trauma. The cross-regulation of neuroendocrine and immune system further endows them with the ability to stringently respond to various endogenous and endogenous stressful signals in trauma. The tightly regulated network comprising endoplasmic reticulum stress [81, 129], apoptosis and autophagy [130, 131], microenvironment regulation, post-transcriptional splicing [132], post-translational modifications and metabolic regulations [133, 134] is essential for the appropriate orchestration of traumatic inflammation and for the prevention of harmful traumatic complications (acute respiratory distress syndrome (ARDS), multiple organ dysfunction syndrome, (MODS)).
Conclusions
Our knowledge of the regulatory mechanisms of neuroendocrine immune network will shed new light on the pathogenesis of traumatic inflammatory diseases and will further provide important clues for their diagnostic and therapeutic approaches. Several intriguing and important aspects of the balancing of neuroendocrine immune cycles are revealing the complexity of the links between the mind and the body although it remains elusive, and therefore present advantageous challenges for future trauma research.
Abbreviations
Adrenocorticotropic hormone: ACTH
Autonomic nervous system: ANS
Acetylcholine: ACh
Acute respiratory distress syndrome: ARDS
Blood-brain barrier: BBB
Central nervous system: CNS
Corticotropin-releasing hormone: CRH
Dorsolateral prefrontal cortex: DLPFC
Endothelin-1: ET-1
Glucocorticoid receptor: GR
Growth hormone: GH
Hypothalamic-pituitary-adrenal axis: HPA axis
Locus coeruleus: LC
Medial prefrontal cortex: MPFC
Mineralocorticoid receptor: MR
Multiple organ dysfunction syndrome: MODS
Nuclear factor-κB: NF-κB
Orbitofrontal cortex: OFC
Paraventricular nucleus: PVN
Pathogen-associated molecular patterns: PAMP
Pattern recognition receptors: PRRs
Sympathetico-adrenomedullary axis: SAM axis
Toll-like receptor 4: TLR4
Acknowledgements
The authors thank Professor Min Zhao (University of California, Davis) for their critical reading of this manuscript. We own our best thanks for the devotion of Ader R, Blalock JE, Dinarello CA, Sharp T, Besedovsky HO, Kelley KW, Tracey KJ, Fontana S and Berczi I to neuroimmunoendocrinology. We also apologize for the omission of any references due to the space constraints of this review and wish to thank members of their laboratories for helpful criticism.
Funding
This work was partly supported by the grants from Natural Science Foundation of China (81372105, 31271242, 81530063), the Special Funds for Major State Basic Research Projects (613307), and Medical Research Funding of PLA (AWS14C003).
Authors' contributions
CY, XY, JG and JD drafted the manuscript; CY and JJ critically reviewed the manuscript. All authors read and approved the final manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Kuhlman KR, Vargas I, Geiss EG, Lopez-Duran NL. Age of Trauma Onset and HPA Axis Dysregulation Among Trauma-Exposed Youth. J Trauma Stress. 2015;28:572-9
2. Prasanna KL, Mittal RS, Gandhi A. Neuroendocrine dysfunction in acute phase of moderate-to-severe traumatic brain injury: a prospective study. Brain Inj. 2015;29:336-42
3. Bobadilla L, Asberg K, Johnson M, Shirtcliff EA. Experiences in the military may impact dual-axis neuroendocrine processes in veterans. Developmental psychobiology. 2015;57:719-30
4. Santarsieri M, Kumar RG, Kochanek PM, Berga S, Wagner AK. Variable neuroendocrine-immune dysfunction in individuals with unfavorable outcome after severe traumatic brain injury. Brain Behav Immun. 2015;45:15-27
5. Pratap UP, Sharma HR, Mohanty A, Kale P, Gopinath S, Hima L. et al. Estrogen upregulates inflammatory signals through NF-kappaB, IFN-gamma, and nitric oxide via Akt/mTOR pathway in the lymph node lymphocytes of middle-aged female rats. Int Immunopharmacol. 2015;29:591-8
6. Jiang JX. Posttraumatic stress and immune dissonance. Chin J Traumatol. 2008;11:203-8
7. Lotti T, D'Erme AM, Hercogova J. The role of neuropeptides in the control of regional immunity. Clinics in dermatology. 2014;32:633-45
8. Padro CJ, Sanders VM. Neuroendocrine regulation of inflammation. Seminars in immunology. 2014;26:357-68
9. West TA, Sharp S. Neuroendocrine dysfunction following mild TBI: when to screen for it. The Journal of family practice. 2014;63:11-6
10. Mulak A, Bonaz B. Brain-gut-microbiota axis in Parkinson's disease. World J Gastroenterol. 2015;21:10609-20
11. Yang C, Yan J, Wang HY, Zhou LL, Zhou JY, Wang ZG. et al. Effects of bilateral adrenalectomy on the innate immune responses following trauma in rats. Injury. 2011;42:905-12
12. Mescher AL, Neff AW, King MW. Inflammation and immunity in organ regeneration. Dev Comp Immunol. 2017;66:98-110
13. Zakharova LA. [Cross-regulation in development of neuroendocrine and immune systems]. Ontogenez. 2010;41:414-24
14. Sumner RC, Parton A, Nowicky AV, Kishore U, Gidron Y. Hemispheric lateralisation and immune function: a systematic review of human research. J Neuroimmunol. 2011;240-241:1-12
15. Taub DD. Neuroendocrine interactions in the immune system. Cell Immunol. 2008;252:1-6
16. Rivest S. Interactions between the immune and neuroendocrine systems. Prog Brain Res. 2010;181:43-53
17. Sternberg EM. Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nat Rev Immunol. 2006;6:318-28
18. Haddad JJ. On the mechanisms and putative pathways involving neuroimmune interactions. Biochem Biophys Res Commun. 2008;370:531-5
19. Zhong LY, Yang ZH, Li XR, Wang H, Li L. Protective effects of melatonin against the damages of neuroendocrine-immune induced by lipopolysaccharide in diabetic rats. Exp Clin Endocrinol Diabetes. 2009;117:463-9
20. De la Fuente M. Crosstalk between the nervous and the immune systems in health and sickness. Curr Pharm Des. 2014;20:4605-7
21. Wrona D. Neural-immune interactions: an integrative view of the bidirectional relationship between the brain and immune systems. J Neuroimmunol. 2006;172:38-58
22. Savino W, Silva PO, Besedovsky H. Network of bidirectional interactions between the neuroendocrine and immune systems. Preface. Ann N Y Acad Sci. 2009;1153:xi
23. Di Comite G, Grazia Sabbadini M, Corti A, Rovere-Querini P, Manfredi AA. Conversation galante: how the immune and the neuroendocrine systems talk to each other. Autoimmun Rev. 2007;7:23-9
24. Arck P, Paus R. From the brain-skin connection: the neuroendocrine-immune misalliance of stress and itch. Neuroimmunomodulation. 2006;13:347-56
25. Mazon AF, Verburg-van Kemenade BM, Flik G, Huising MO. Corticotropin-releasing hormone-receptor 1 (CRH-R1) and CRH-binding protein (CRH-BP) are expressed in the gills and skin of common carp Cyprinus carpio L. and respond to acute stress and infection. J Exp Biol. 2006;209:510-7
26. Yang J, Wu R, Qiang X, Zhou M, Dong W, Ji Y. et al. Human adrenomedullin and its binding protein attenuate organ injury and reduce mortality after hepatic ischemia-reperfusion. Ann Surg. 2009;249:310-7
27. Kanczkowski W, Alexaki VI, Tran N, Grossklaus S, Zacharowski K, Martinez A. et al. Hypothalamo-pituitary and immune-dependent adrenal regulation during systemic inflammation. Proc Natl Acad Sci U S A. 2013;110:14801-6
28. ThyagaRajan S, Priyanka HP. Bidirectional communication between the neuroendocrine system and the immune system: relevance to health and diseases. Ann Neurosci. 2012;19:40-6
29. Liu Y, Yuan Y, Li Y, Zhang J, Xiao G, Vodovotz Y. et al. Interacting neuroendocrine and innate and acquired immune pathways regulate neutrophil mobilization from bone marrow following hemorrhagic shock. J Immunol. 2009;182:572-80
30. Whetsell M, Bagriacik EU, Seetharamaiah GS, Prabhakar BS, Klein JR. Neuroendocrine-induced synthesis of bone marrow-derived cytokines with inflammatory immunomodulating properties. Cell Immunol. 1999;192:159-66
31. Yang C, Jiang J, Yang X, Wang H, Du J. Stem/progenitor cells in endogenous repairing responses: new toolbox for the treatment of acute lung injury. J Transl Med. 2016;14:47
32. Ashley NT, Demas GE. Neuroendocrine-immune circuits, phenotypes, and interactions. Horm Behav. 2017;87:25-34
33. Wieck A, Grassi-Oliveira R, do Prado CH, Rizzo LB, de Oliveira AS, Kommers-Molina J. et al. Differential neuroendocrine and immune responses to acute psychosocial stress in women with type 1 bipolar disorder. Brain Behav Immun. 2013;34:47-55
34. Taub DD. Novel connections between the neuroendocrine and immune systems: the ghrelin immunoregulatory network. Vitam Horm. 2008;77:325-46
35. Kamimura D, Yamada M, Harada M, Sabharwal L, Meng J, Bando H. et al. The gateway theory: bridging neural and immune interactions in the CNS. Front Neurosci. 2013;7:204
36. Bonneau RH. Controlling immunopathology at the expense of critical immune functions-a tipping of the balance by neuroendocrine-immune interactions. Brain Behav Immun. 2007;21:888-9
37. Garate I, Garcia-Bueno B, Madrigal JL, Caso JR, Alou L, Gomez-Lus ML. et al. Stress-induced neuroinflammation: role of the Toll-like receptor-4 pathway. Biol Psychiatry. 2013;73:32-43
38. Heijnen CJ. Receptor regulation in neuroendocrine-immune communication: current knowledge and future perspectives. Brain Behav Immun. 2007;21:1-8
39. De Kloet ER, Oitzl MS, Schobitz B. Cytokines and the brain corticosteroid receptor balance: relevance to pathophysiology of neuroendocrine-immune communication. Psychoneuroendocrinology. 1994;19:121-34
40. Correa SG, Maccioni M, Rivero VE, Iribarren P, Sotomayor CE, Riera CM. Cytokines and the immune-neuroendocrine network: What did we learn from infection and autoimmunity? Cytokine Growth Factor Rev. 2007;18:125-34
41. Dhawan L, Liu B, Blaxall BC, Taubman MB. A novel role for the glucocorticoid receptor in the regulation of monocyte chemoattractant protein-1 mRNA stability. J Biol Chem. 2007;282:10146-52
42. Breuel KF, Kougias P, Rice PJ, Wei D, De Ponti K, Wang J. et al. Anterior pituitary cells express pattern recognition receptors for fungal glucans: implications for neuroendocrine immune involvement in response to fungal infections. Neuroimmunomodulation. 2004;11:1-9
43. Liezmann C, Stock D, Peters EM. Stress induced neuroendocrine-immune plasticity: A role for the spleen in peripheral inflammatory disease and inflammaging? Dermatoendocrinol. 2012;4:271-9
44. Maddali S, Stapleton PP, Freeman TA, Smyth GP, Duff M, Yan Z. et al. Neuroendocrine responses mediate macrophage function after trauma. Surgery. 2004;136:1038-46
45. Lansac G, Dong W, Dubois CM, Benlarbi N, Afonso C, Fournier I. et al. Lipopolysaccharide mediated regulation of neuroendocrine associated proprotein convertases and neuropeptide precursor processing in the rat spleen. J Neuroimmunol. 2006;171:57-71
46. Weigent DA. Lymphocyte GH-axis hormones in immunity. Cell Immunol. 2013;285:118-32
47. Miyake S, Yamamura T. Ghrelin: friend or foe for neuroinflammation. Discov Med. 2009;8:64-7
48. Wu JT, Kral JG. Ghrelin: integrative neuroendocrine peptide in health and disease. Ann Surg. 2004;239:464-74
49. Vila G, Maier C, Riedl M, Nowotny P, Ludvik B, Luger A. et al. Bacterial endotoxin induces biphasic changes in plasma ghrelin in healthy humans. J Clin Endocrinol Metab. 2007;92:3930-4
50. Wu R, Zhou M, Cui X, Simms HH, Wang P. Upregulation of cardiovascular ghrelin receptor occurs in the hyperdynamic phase of sepsis. Am J Physiol Heart Circ Physiol. 2004;287:H1296-302
51. Li WG, Gavrila D, Liu X, Wang L, Gunnlaugsson S, Stoll LL. et al. Ghrelin inhibits proinflammatory responses and nuclear factor-kappaB activation in human endothelial cells. Circulation. 2004;109:2221-6
52. Wu R, Zhou M, Dong W, Ji Y, Miksa M, Marini CP. et al. Ghrelin hyporesponsiveness contributes to age-related hyperinflammation in septic shock. Ann Surg. 2009;250:126-33
53. Wu R, Zhou M, Das P, Dong W, Ji Y, Yang D. et al. Ghrelin inhibits sympathetic nervous activity in sepsis. Am J Physiol Endocrinol Metab. 2007;293:E1697-702
54. Wu R, Dong W, Zhou M, Cui X, Hank Simms H, Wang P. Ghrelin improves tissue perfusion in severe sepsis via downregulation of endothelin-1. Cardiovasc Res. 2005;68:318-26
55. Koch A, Sanson E, Helm A, Voigt S, Trautwein C, Tacke F. Regulation and prognostic relevance of serum ghrelin concentrations in critical illness and sepsis. Crit Care. 2010;14:R94
56. Barnard A, Layton D, Hince M, Sakkal S, Bernard C, Chidgey A. et al. Impact of the neuroendocrine system on thymus and bone marrow function. Neuroimmunomodulation. 2008;15:7-18
57. Kelley KW, McCusker RH. Getting nervous about immunity. Seminars in immunology. 2014;26:389-93
58. Burfeind KG, Michaelis KA, Marks DL. The central role of hypothalamic inflammation in the acute illness response and cachexia. Semin Cell Dev Biol. 2014;54:42-52
59. Mracsko E, Liesz A, Karcher S, Zorn M, Bari F, Veltkamp R. Differential effects of sympathetic nervous system and hypothalamic-pituitary-adrenal axis on systemic immune cells after severe experimental stroke. Brain Behav Immun. 2014;41:200-9
60. Yang C, Jiang JX. Bilateral regulatory action of corticotropin-releasing hormone on immune-mediated inflammation. Chin J Traumatol. 2009;12:350-4
61. Karalis K, Muglia LJ, Bae D, Hilderbrand H, Majzoub JA. CRH and the immune system. J Neuroimmunol. 1997;72:131-6
62. Overman EL, Rivier JE, Moeser AJ. CRF induces intestinal epithelial barrier injury via the release of mast cell proteases and TNF-alpha. PLoS One. 2012;7:e39935
63. Kong L, Wu J, Lin Y, Wang G, Wang J, Liu J. et al. BuShenYiQi granule inhibits atopic dermatitis via improving central and skin Hypothalamic-Pituitary-Adrenal axis function. PLoS One. 2015;10:e0116427
64. Silverman ES, Breault DT, Vallone J, Subramanian S, Yilmaz AD, Mathew S. et al. Corticotropin-releasing hormone deficiency increases allergen-induced airway inflammation in a mouse model of asthma. J Allergy Clin Immunol. 2004;114:747-54
65. Jacobson L, Muglia LJ, Weninger SC, Pacak K, Majzoub JA. CRH deficiency impairs but does not block pituitary-adrenal responses to diverse stressors. Neuroendocrinology. 2000;71:79-87
66. Zhu XY, Liu YJ, Diao F, Fan J, Lu J, Xu RB. Role of glucocorticoids and glucocorticoid receptor in priming of macrophages caused by glucocorticoid receptor blockade. Endocrine. 2007;31:130-7
67. Kasahara E, Inoue M. Cross-talk between HPA-axis-increased glucocorticoids and mitochondrial stress determines immune responses and clinical manifestations of patients with sepsis. Redox report: communications in free radical research. 2015;20:1-10
68. Mavroudis PD, Corbett SA, Calvano SE, Androulakis IP. Circadian characteristics of permissive and suppressive effects of cortisol and their role in homeostasis and the acute inflammatory response. Mathematical biosciences. 2015;260:54-64
69. Bellavance MA, Rivest S. The neuroendocrine control of the innate immune system in health and brain diseases. Immunol Rev. 2012;248:36-55
70. Frank MG, Watkins LR, Maier SF. Stress-induced glucocorticoids as a neuroendocrine alarm signal of danger. Brain Behav Immun. 2013;33:1-6
71. Busillo JM, Cidlowski JA. The five Rs of glucocorticoid action during inflammation: ready, reinforce, repress, resolve, and restore. Trends Endocrinol Metab. 2013;24:109-19
72. Thompson BT. Glucocorticoids and acute lung injury. Crit Care Med. 2003;31:S253-7
73. Yang C, Gao W, Yang X, Wang H, Du J, Zhong H. et al. CRH knockout inhibits the murine innate immune responses in association with endoplasmic reticulum stress after thermal injury. Surgery. 2015;158:255-65
74. Yang C, Gao J, Wang HY, Liu Q, Xu MH, Wang ZG. et al. Effects of hypothalamus destruction on the level of plasma corticosterone after blast injury and its relation to interleukin-6 in rats. Cytokine. 2011;54:29-35
75. Gadek-Michalska A, Tadeusz J, Rachwalska P, Bugajski J. Cytokines, prostaglandins and nitric oxide in the regulation of stress-response systems. Pharmacol Rep. 2013;65:1655-62
76. Ma XC, Chen LT, Oliver J, Horvath E, Phelps CP. Cytokine and adrenal axis responses to endotoxin. Brain Res. 2000;861:135-42
77. Kepka M, Szwejser E, Pijanowski L, Verburg-van Kemenade BM, Chadzinska M. A role for melatonin in maintaining the pro- and anti-inflammatory balance by influencing leukocyte migration and apoptosis in carp. Dev Comp Immunol. 2015;53:179-90
78. Bunn SJ, Ait-Ali D, Eiden LE. Immune-neuroendocrine integration at the adrenal gland: cytokine control of the adrenomedullary transcriptome. J Mol Neurosci. 2012;48:413-9
79. Klatt S, Stangl H, Kunath J, Lowin T, Pongratz G, Straub RH. Peripheral elimination of the sympathetic nervous system stimulates immunocyte retention in lymph nodes and ameliorates collagen type II arthritis. Brain Behav Immun. 2016;54:201-10
80. Karrow NA. Activation of the hypothalamic-pituitary-adrenal axis and autonomic nervous system during inflammation and altered programming of the neuroendocrine-immune axis during fetal and neonatal development: lessons learned from the model inflammagen, lipopolysaccharide. Brain Behav Immun. 2006;20:144-58
81. Yang C, Zhou JY, Zhong HJ, Wang HY, Yan J, Liu Q. et al. Exogenous norepinephrine correlates with macrophage endoplasmic reticulum stress response in association with XBP-1. J Surg Res. 2011;168:262-71
82. Molina PE. Noradrenergic inhibition of TNF upregulation in hemorrhagic shock. Neuroimmunomodulation. 2001;9:125-33
83. Baranski GM, Sifri ZC, Cook KM, Alzate WD, Livingston DH, Mohr AM. Is the sympathetic system involved in shock-induced gut and lung injury? J Trauma Acute Care Surg. 2012;73:343-50 discussion 50
84. Ji MH, Zhu XL, Liu FF, Li GM, Tian M, Wu J. et al. Alpha 2A-adrenoreceptor blockade improves sepsis-induced acute lung injury accompanied with depressed high mobility group box-1 levels in rats. Cytokine. 2012;60:639-45
85. Kandasamy K, Prawez S, Choudhury S, More AS, Ahanger AA, Singh TU. et al. Atorvastatin prevents vascular hyporeactivity to norepinephrine in sepsis: role of nitric oxide and alpha(1)-adrenoceptor mRNA expression. Shock. 2011;36:76-82
86. Zhou M, Das P, Simms HH, Wang P. Gut-derived norepinephrine plays an important role in up-regulating IL-1beta and IL-10. Biochim Biophys Acta. 2005;1740:446-52
87. Zhang F, Wu R, Qiang X, Zhou M, Wang P. Antagonism of alpha2A-adrenoceptor: a novel approach to inhibit inflammatory responses in sepsis. J Mol Med. 2010;88:289-96
88. Yang H, Wang H, Tracey KJ. HMG-1 rediscovered as a cytokine. Shock. 2001;15:247-53
89. Miksa M, Wu R, Zhou M, Wang P. Sympathetic excitotoxicity in sepsis: pro-inflammatory priming of macrophages by norepinephrine. Front Biosci. 2005;10:2217-29
90. He Y, Ye ZQ, Li X, Zhu GS, Liu Y, Yao WF. et al. Alpha7 nicotinic acetylcholine receptor activation attenuated intestine-derived acute lung injury. J Surg Res. 2016;201:258-65
91. Ma P, Yu K, Yu J, Wang W, Ding Y, Chen C. et al. Effects of Nicotine and Vagus Nerve in Severe Acute Pancreatitis-Associated Lung Injury in Rats. Pancreas. 2016;45:552-60
92. Wu H, Li L, Su X. Vagus nerve through alpha7 nAChR modulates lung infection and inflammation: models, cells, and signals. Biomed Res Int. 2014;2014:283525
93. Tarras SL, Diebel LN, Liberati DM, Ginnebaugh K. Pharmacologic stimulation of the nicotinic anti-inflammatory pathway modulates gut and lung injury after hypoxia-reoxygenation injury. Surgery. 2013;154:841-7 discussion 7-8
94. dos Santos CC, Shan Y, Akram A, Slutsky AS, Haitsma JJ. Neuroimmune regulation of ventilator-induced lung injury. Am J Respir Crit Care Med. 2011;183:471-82
95. Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR. et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458-62
96. Tracey KJ. Reflex control of immunity. Nat Rev Immunol. 2009;9:418-28
97. Pavlov VA, Parrish WR, Rosas-Ballina M, Ochani M, Puerta M, Ochani K. et al. Brain acetylcholinesterase activity controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Brain Behav Immun. 2009;23:41-5
98. Boland C, Collet V, Laterre E, Lecuivre C, Wittebole X, Laterre PF. Electrical vagus nerve stimulation and nicotine effects in peritonitis-induced acute lung injury in rats. Inflammation. 2011;34:29-35
99. Kox M, Vaneker M, van der Hoeven JG, Scheffer GJ, Hoedemaekers CW, Pickkers P. Effects of vagus nerve stimulation and vagotomy on systemic and pulmonary inflammation in a two-hit model in rats. PLoS One. 2012;7:e34431
100. Altavilla D, Guarini S, Bitto A, Mioni C, Giuliani D, Bigiani A. et al. Activation of the cholinergic anti-inflammatory pathway reduces NF-kappab activation, blunts TNF-alpha production, and protects againts splanchic artery occlusion shock. Shock. 2006;25:500-6
101. Yang X, Zhao C, Gao Z, Su X. A novel regulator of lung inflammation and immunity: pulmonary parasympathetic inflammatory reflex. QJM. 2014;107:789-92
102. Mozsik G, Domotor A, Abdel-Salam OM. Molecular pharmacological approach to drug actions on the afferent and efferent fibres of the vagal nerve involved in gastric mucosal protection in rats. Inflammopharmacology. 2006;14:243-9
103. Bernik TR, Friedman SG, Ochani M, DiRaimo R, Ulloa L, Yang H. et al. Pharmacological stimulation of the cholinergic antiinflammatory pathway. J Exp Med. 2002;195:781-8
104. Chen T, Chen C, Zhang Z, Zou Y, Peng M, Wang Y. Toll-like receptor 4 knockout ameliorates neuroinflammation due to lung-brain interaction in mechanically ventilated mice. Brain Behav Immun. 2016;56:42-55
105. Dezfulian C, Trzeciak S, Girard TD. Lung-Brain Interaction after Cardiac Arrest? Am J Respir Crit Care Med. 2017;195:1127-8
106. Weber DJ, Gracon AS, Ripsch MS, Fisher AJ, Cheon BM, Pandya PH. et al. The HMGB1-RAGE axis mediates traumatic brain injury-induced pulmonary dysfunction in lung transplantation. Sci Transl Med. 2014;6:252ra124
107. Gonzalez-Lopez A, Lopez-Alonso I, Aguirre A, Amado-Rodriguez L, Batalla-Solis E, Astudillo A. et al. Mechanical ventilation triggers hippocampal apoptosis by vagal and dopaminergic pathways. Am J Respir Crit Care Med. 2013;188:693-702
108. Jennewein C, Tran N, Kanczkowski W, Heerdegen L, Kantharajah A, Drose S. et al. Mortality of Septic Mice Strongly Correlates With Adrenal Gland Inflammation. Crit Care Med. 2016;44:e190-9
109. Verburg-van Kemenade BM, Van der Aa LM, Chadzinska M. Neuroendocrine-immune interaction: regulation of inflammation via G-protein coupled receptors. Gen Comp Endocrinol. 2013;188:94-101
110. Straub RH. Rheumatoid arthritis-a neuroendocrine immune disorder: glucocorticoid resistance, relative glucocorticoid deficiency, low-dose glucocorticoid therapy, and insulin resistance. Arthritis Res Ther. 2014;16(Suppl 2):I1
111. Silverman MN, Sternberg EM. Neuroendocrine-immune interactions in rheumatoid arthritis: mechanisms of glucocorticoid resistance. Neuroimmunomodulation. 2008;15:19-28
112. Esquifino AI, Cano P, Jimenez-Ortega V, Fernandez-Mateos P, Cardinali DP. Neuroendocrine-immune correlates of circadian physiology: studies in experimental models of arthritis, ethanol feeding, aging, social isolation, and calorie restriction. Endocrine. 2007;32:1-19
113. Straub RH, Schradin C. Chronic inflammatory systemic diseases: An evolutionary trade-off between acutely beneficial but chronically harmful programs. Evol Med Public Health. 2016;2016:37-51
114. Straub RH, Fassold A. [Neuroendocrine immune interactions in rheumatic diseases]. Z Rheumatol. 2010;69:340-8
115. Straub RH, Cutolo M, Buttgereit F, Pongratz G. Energy regulation and neuroendocrine-immune control in chronic inflammatory diseases. J Intern Med. 2010;267:543-60
116. Verburg-van Kemenade BM, Ribeiro CM, Chadzinska M. Neuroendocrine-immune interaction in fish: differential regulation of phagocyte activity by neuroendocrine factors. Gen Comp Endocrinol. 2011;172:31-8
117. Kou W, Bell JD, Gareus I, Pacheco-Lopez G, Goebel MU, Spahn G. et al. Repeated acupuncture treatment affects leukocyte circulation in healthy young male subjects: a randomized single-blind two-period crossover study. Brain Behav Immun. 2005;19:318-24
118. Hahm ET, Lee JJ, Lee WK, Bae HS, Min BI, Cho YW. Electroacupuncture enhancement of natural killer cell activity suppressed by anterior hypothalamic lesions in rats. Neuroimmunomodulation. 2004;11:268-72
119. Lee SW, Liong ML, Yuen KH, Krieger JN. Acupuncture and immune function in chronic prostatitis/chronic pelvic pain syndrome: a randomized, controlled study. Complementary therapies in medicine. 2014;22:965-9
120. Briggs JP, Shurtleff D. Acupuncture and the Complex Connections Between the Mind and the Body. JAMA. 2017;317:2489-90
121. Gao YL, Chai YF, Yao YM. [Advancement in the research of mechanism of immune dysfunction in sepsis and the regulatory effects of Xuebijing injection]. Zhonghua Shao Shang Za Zhi. 2013;29:162-5
122. Chen Y, Tong H, Zhang W, Zhang X, Pan Z, Qiu J. et al. Curative effect of Xuebijing injection on severe pulmonary contusion. Journal of traditional Chinese medicine = Chung i tsa chih ying wen pan / sponsored by All-China Association of Traditional Chinese Medicine, Academy of Traditional Chinese Medicine. 2013;33:743-51
123. Arora S, Bhattacharjee J. Modulation of immune responses in stress by Yoga. International journal of yoga. 2008;1:45-55
124. Pace TW, Negi LT, Sivilli TI, Issa MJ, Cole SP, Adame DD. et al. Innate immune, neuroendocrine and behavioral responses to psychosocial stress do not predict subsequent compassion meditation practice time. Psychoneuroendocrinology. 2010;35:310-5
125. Infante JR, Peran F, Rayo JI, Serrano J, Dominguez ML, Garcia L. et al. Levels of immune cells in transcendental meditation practitioners. International journal of yoga. 2014;7:147-51
126. Fleshner M. Exercise and neuroendocrine regulation of antibody production: protective effect of physical activity on stress-induced suppression of the specific antibody response. Int J Sports Med. 2000;21(Suppl 1):S14-9
127. Bonaz BL, Bernstein CN. Brain-gut interactions in inflammatory bowel disease. Gastroenterology. 2013;144:36-49
128. Meyer-Hermann M, Figge MT, Straub RH. Mathematical modeling of the circadian rhythm of key neuroendocrine-immune system players in rheumatoid arthritis: a systems biology approach. Arthritis Rheum. 2009;60:2585-94
129. Zhou JY, Zhong HJ, Yang C, Yan J, Wang HY, Jiang JX. Corticosterone exerts immunostimulatory effects on macrophages via endoplasmic reticulum stress. Br J Surg. 2010;97:281-93
130. Gao J, Wang H, Liu Y, Li YY, Chen C, Liu LM. et al. Glutamate and GABA imbalance promotes neuronal apoptosis in hippocampus after stress. Med Sci Monit. 2014;20:499-512
131. Gao J, Chen C, Liu Y, Li Y, Long Z, Wang H. et al. Lycium barbarum polysaccharide improves traumatic cognition via reversing imbalance of apoptosis/regeneration in hippocampal neurons after stress. Life Sci. 2015;121:124-34
132. Shakola F, Suri P, Ruggiu M. Splicing Regulation of Pro-Inflammatory Cytokines and Chemokines: At the Interface of the Neuroendocrine and Immune Systems. Biomolecules. 2015;5:2073-100
133. Demas GE. The energetics of immunity: a neuroendocrine link between energy balance and immune function. Horm Behav. 2004;45:173-80
134. Berendzen KM, Durieux J, Shao LW, Tian Y, Kim HE, Wolff S. et al. Neuroendocrine Coordination of Mitochondrial Stress Signaling and Proteostasis. Cell. 2016;166:1553-63 e10
Author contact
![]() Corresponding authors: Ce Yang, MD, Research Institute of Surgery, Daping Hospital, Third Military Medical University, Changjiang Zhilu, Daping, Chongqing 400042, China Email: sepsismdedu.cn; sepsismdcom; Jianxin Jiang, MD, Research Institute of Surgery, Daping Hospital, Third Military Medical University, Daping, Chongqing 400042, China Email: hellojjxcom
Corresponding authors: Ce Yang, MD, Research Institute of Surgery, Daping Hospital, Third Military Medical University, Changjiang Zhilu, Daping, Chongqing 400042, China Email: sepsismdedu.cn; sepsismdcom; Jianxin Jiang, MD, Research Institute of Surgery, Daping Hospital, Third Military Medical University, Daping, Chongqing 400042, China Email: hellojjxcom

 Global reach, higher impact
Global reach, higher impact