10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2018; 14(3):321-330. doi:10.7150/ijbs.24360 This issue Cite
Research Paper
Overexpressed circPVT1, a potential new circular RNA biomarker, contributes to doxorubicin and cisplatin resistance of osteosarcoma cells by regulating ABCB1
1. Department of Orthopedics, Shanghai Tenth People's Hospital, Tongji University, School of Medicine, Shanghai 200072, PR China
2. Institute of Bone Tumor Affiliated to Tongji University, School of Medicine, Shanghai 200072, PR China
* Contributed equally to this study and share first authorship.
Received 2017-12-14; Accepted 2018-1-24; Published 2018-2-12
Abstract
Circular RNAs (circRNAs) represent a widespread class of non-coding RNAs generated from back-splicing, with a circular loop structure. Many circRNAs have been reported to play essential roles in cancer development and have the potential to serve as a novel class of biomarkers for clinical diagnosis. However, the role of circRNA in osteosarcoma (OS) remains largely unknown. In the current study, we examined the expression level of circular RNA PVT1 (circPVT1), previously screened and identified the oncogenic role in gastric cancer, in OS and found that circPVT1 was significantly up-regulated in the OS tissues, serums and chemoresistant cell lines, correlated with poor prognosis of OS patients. Besides, ROC curve demonstrated that circPVT1 may be a better diagnostic biomarker than alkaline phosphatase (ALP) in OS with more sensitivity and specificity. In addition, functional assays revealed that circPVT1 knockdown by siRNA could weaken the resistance to doxorubicin and cisplatin of OS cells through decreasing the expression of classical drug resistance-related gene ABCB1. These findings may provide a new insight into the role of circPVT1 as a biomarker for the diagnosis and treatment target of OS.
Keywords: circular RNA, circPVT1, osteosarcoma, biomarker, chemoresistance.
Introduction
Osteosarcoma (OS) is regarded as the most common malignant bone tumor among children and adolescents[1]. With the great improvement in surgical technique and neoadjuvant chemotherapy, there has been a huge decline of mortality rate and a significantly improved prognosis of OS [2]. However, the 5-year survival rate of those patients who suffered from tumor recurrence, lung metastasis or multi-drug resistance is still below 20%[3]. The underlying mechanism of OS progression behind these clinical problems was still not completely clear and needed to be further clarified to develop more effective therapeutic strategies[4].
With the rapid development in the RNA-seq technology, more and more non-coding RNAs previously termed as “junk molecule” were screened and identified, such as long non-coding RNA (lncRNA) and circular RNA (circRNA)[5]. Of them, circRNAs are characterized by a covalently closed loop structure with neither a 5'cap nor a 3' polyadenylated tail[6]. They are stable and more expressed compared with their linear counterparts in the cells with the possibility to be biomarkers for disease [7-9]. In addition, circRNAs could widely regulate gene expression in different levels through interacting with DNA, miRNA, lncRNA or protein to participate in the regulation of various cell physiological and pathological processes [10, 11]. A growing number of studies have demonstrated the critical role of circRNAs in tumorigenesis. Zhang et al[12] found that circRNA_100269 is down-regulated in gastric cancer and suppresses tumor cell growth by targeting miR-630. Hsiao KY et al [13] showed that circular RNA CCDC66 promotes colon cancer growth and metastasis via regulation of a subset of oncogenes including DNMT3B, EZH2, MYC, and YAP1. Chen et al[14] reported that circRNA_100290 plays a role in oral cancer by functioning as a sponge of the miR-29 family.
CircPVT1 is derived from a long noncoding RNA region within the oncogene PVT1 locus, which is located on chromosome 8q24, a cancer susceptibility locus [15]. Several reports have shown the oncogenic role of lncRNA PVT1 homologous to PVT1 in the OS progression. Song et al [16] found that lncRNA PVT1 could promote glycolysis and tumor progression by regulating miR-497/HK2 axis in osteosarcoma. Zhou et al [17] showed that lncRNA PVT1 promotes osteosarcoma development by acting as a molecular sponge to regulate miR-195. More importantly, a previous study by Chen et al [15] has shown that circPVT1 could promote cell proliferation by sponging members of miR-125 family and serve as a prognostic marker in gastric cancer. However, the role and function of circPVT1 in OS remains largely unknown.
In the current study, we aimed to identify the relationship between circPVT1 and OS. We found that circPVT1 was significantly up-regulated in the OS tissues, serums and chemoresistant cell lines, correlated with poor prognosis and may be a potential biomarker for the diagnosis and treatment target of OS. In addition, we demonstrated that down-regulation of circPVT1 by siRNA could weaken the resistance to doxorubicin and cisplatin of OS cells through decreasing the expression of ABCB1.
Materials and Methods
Human samples preparation
A total of 80 primary osteosarcoma patients who received the same chemotherapy regimen (methotrexate (MTX), doxorubicin (DXR), cisplatin (DDP) and ifosfamide (IFO) for two cycles) before surgery and underwent complete resection surgery at Shanghai Tenth People's Hospital between 2006 and 2016 were included in this study. The study was approved by the Ethics Committee of Shanghai Tenth People's Hospital, and written informed consent was obtained from all the patients. All patients' slides were reviewed to confirm the diagnosis and to classify the tumor according to Enneking Stage. Besides, we collected blood samples from 50 OS patients before surgery, other 20 patients with benign bone tumor (eight cases of osteoclastoma, twelve cases of fibrous dysplasia) and 20 age- and sex-matched healthy individuals as the control group. All the resected specimens were placed immediately into liquid nitrogen and stored at - 80 ℃. The serum extracted from the blood samples were collected using standard procedures. According to the Huvos scoring system [18], the patients were classified as chemoresistant and chemosensitive groups. The clinical parameters of osteosarcoma patients in this study are shown in Table 1.
Clinical parameters of osteosarcoma patients enrolled in this study
| Pathological characteristics | Cases (n) | circPVT1 expression | P value | |
|---|---|---|---|---|
| High(30) | Low(50) | |||
| Gender | ||||
| Male | 47(58.8%) | 18(60%) | 29(58%) | 0.84 |
| Female | 33(41.2%) | 12(40%) | 21(42%) | |
| Age | ||||
| ≥25 | 24(30%) | 11(36.7%) | 13(26%) | 0.63 |
| <25 | 56(70%) | 19(63.3%) | 37(74%) | |
| Location | 0.45 | |||
| Distal of Femur | 37(46.3%) | 15(50%) | 22(44%) | |
| Proximal of Tibia | 27(33.8%) | 10(33.3%) | 17(34%) | |
| Other | 16(19.9%) | 5(16.7%) | 11(22%) | |
| Enneking stage | 0.044 | |||
| I+IIA | 23(28.8%) | 3(10%) | 20(40%) | |
| IIB/III | 57(71.2%) | 27(90%) | 30(60%) | |
| Lung Metastasis | 0.038 | |||
| Yes | 25(31.3%) | 21(70%) | 4(8%) | |
| No | 55(68.7%) | 9(30%) | 46(92%) | |
| Chemoresistant | 0.025 | |||
| Yes | 32(40%) | 22(73.3%) | 10(20%) | |
| No | 48(60%) | 8(26.7%) | 40(80%) | |
Cell culture
Four human osteosarcoma cell lines (SaoS2, KHOS, U2OS, MG63) were purchased from American Type Culture Collection and cultured in DMEM supplemented with 10% FBS (Gibco, Gran Island, NY, USA), 100 U/mL of penicillin and 100 mg/mL of streptomycin (Invitrogen) at 37°C in a humidified CO2 (5%) atmosphere. The doxorubicin-resistant osteosarcoma cell line MG63R, which was kindly provided by Dr.Yoshio Oda[19] (Kyushu University, Fukuoka, Japan), was generated in a stepwise manner by exposing drug-sensitive MG63 cells to increasing doses of doxorubicin (DXR). The paired U2OS and U2OSR were kindly donated by Dr. Gonos ES[20] (National Hellenic research Foundation, Athens, Greece) using the same selection method. The paired KHOS and KHOSR were kindly donated by Dr. Duan ZF[21] (Massachusetts General Hospital, Boston, USA) and generated by the same method. The surviving cells were subsequently maintained in the conditioned medium with 1 μg/mL DXR (Sigma) to retain its drug-resistant phenotype. Normal osteoblast cells (hFOB1.19) obtained from the Chinese Cell Bank of the Chinese Academy of Sciences (Shanghai, China) were cultured in Ham's F12/ DMEM supplemented with 10% FBS, 100 U/mL penicillin, 100 mg/mL streptomycin and 0.3mg/mL G418. Cultures were maintained at 33.5°C in a humidified CO2 (5%) atmosphere. The established drug-resistant OS cell lines were also across resistant to cisplatin (DDP), which was described in our previous study.
Quantitative real-time PCR (qRT-PCR) analysis
Total RNA was isolated from cells, tissues or serums using the TRIzol kit (Invitrogen, Carlsbad, CA, USA) following to the manufacture's guide. Quantitative real-time PCR (qRT-PCR) analysis was performed to detect the circPVT1 expression using SYBR green kit (TaKaRa, Dalian, China) on the Light Cycler 480 (Roche, Switzerland) in accordance with the instructions. The expression of circPVT1 was normalized to GAPDH. The PCR primers were shown as follows: circPVT1 forward primers:5′-GGTTCCACCAGCGTTATTC-3′,reverseprimers:5′-CAACTTCCTTTGGGTCTCC-3′;ABCB1forwardprimers:5′-TTGGACACAGAAAGCGAAGCAG-3′,reverseprimers:5′-TCAGCATTACGAACTGTAGACAAACG-3′;GAPDHforwardprimers:5′-AATGGGCAGCCGTTAGGAAA-3′,reverseprimers:5′-TGAAGGGGTCATTGATGGCA-3′.
Cell transfection
si-circPVT1 and si-NC were purchased from Genepharm (Shanghai, China) and transfected into MG63R (or U2OSR) cells using Lipofectamine 2000 according to the manufacture's guide. Cells were collected 48h after transfection. CircPVT1 expression levels were determined by qRT-PCR. Three siRNA sequences were designed for the circPVT1. The sequence of si-circPVT1-1 was 5′-CUGUCAGCUGCAUGGAGCUUCGU-3′, si-circPVT1-2 was 5′-GCATTTACAGGCCAGCCTA-3′, si-circPVT1-3 was 5′-GCACTGAGCCATTGTGAAT-3′ (si-circPVT1-1 has the highest inhibition efficiency and si-circPVT1 mentioned in the article refers to si-circPVT1-1) and the relative si-NC sequence was 5′-AAUUCUCCGAACGUGUCACGU-3′.
CCK8 assay
After 48h of transfection in 96-well plates, freshly prepared medium containing several final concentrations of doxorubicin (0, 2, 4, 8, 16, 32 and 64 μg/mL) or cisplatin (0, 1, 2, 4, 8, 16μg/mL) was added to the wells, with three replicate wells for each concentration. After incubating for another 48h, cell viability was measured using Cell Counting Kit-8 (CCK-8, Dojindo, Japan), according to the manufacturer's instructions. The absorbance at 450 nM was measured using Spectra Max 250 spectrophotometer (Molecular Devices, USA).
Colony formation assay
Six-well plates were covered with a layer of 0.6% agar in medium supplemented with 20% fetal bovine serum. A total of 1000 cells were prepared in 0.3% agar and cultured with 8μg/mL doxorubicin or 3μg/mL cisplatin for 2 weeks at 37 °C. The numbers of colonies per well were fixed and stained with crystal violet, imaged and counted. Triplicate independent experiments were performed.
Western blot analysis
Western blot analysis was performed using standard procedures. Briefly, total proteins were extracted and separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto a polyvinylidene difluoride (PVDF) membrane (Millipore, USA). To block nonspecific binding, the membranes were incubated with 5% skim milk powder at room temperature for one hour. The membrane was then incubated with primary antibody against ABCB1 (1:1000, Abcam, UK), followed by horseradish peroxidase (HRP)-labeled secondary antibody (Santa Cruz, USA) and detected by chemiluminescence. An anti-GAPDH antibody (1:1000, Abcam, UK) was used as a protein loading control.
Statistical analysis
All statistical analyses were performed using SPSS 22.0 software (IBM) and Graph pad Prism 5.0. Differences between OS tissues and paired adjacent nontumorous tissues were analyzed using the Student's t test. The correlations between circRNA expression levels and clinicopathological factors were further analyzed by one-way analysis of variance (ANOVA). A receiver operating characteristic (ROC) curve was established to evaluate its diagnostic value. The cutoff value of circRNA was analyzed by SigmaPlot 12.3. Overall survival were calculated by Kaplan-Meier survival analysis and compared using the log-rank test. p values<0.05 were considered statistically significant.
Results
CircPVT1 is up-regulated and correlated with poor clinical outcomes in OS
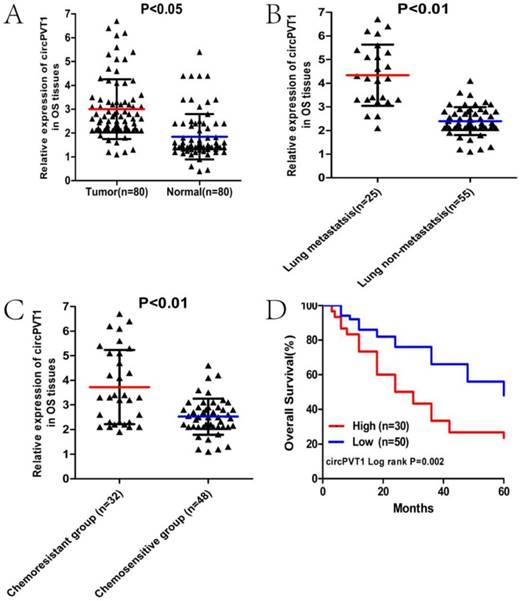
We first used qRT-PCR to measure the circPVT1 expression level in 80 paired OS and paracancerous tissues. As was shown in Figure 1A, circPVT1 was markedly up-regulated in OS tissues compared with the control (P<0.01). Then we classified the 80 patients into lung metastasis and non-lung metastasis groups or chemoresistant and chemosensitive groups according to the clinical information. We found that the expression of circPVT1 was conspicuously higher in the group of lung metastasis or chemoresistance than that of the controlled group (Fig.1B and C). In addition, we divided the 80 patients into two groups with high or low circPVT1 expression based on the average circPVT1 expression level and further K-M survival analysis demonstrated that patients with high circPVT1 expression have shorter overall survival lifetime than those with low circPVT1 expression (Fig.1D). Besides, we also analyzed the correlation between circPVT1 expression level and other clinicopathological parameters in these OS patients and found that increased expression of circPVT1 was obviously related to advanced Enneking stage, chemoresistance and lung metastasis (Table 1). These results indicated the potential oncogenic role of circPVT1 in OS.
CircPVT1 is up-regulated and correlated with poor clinical outcomes in OS. (A) Expression level of circPVT1 in 80 paired OS and paracancerous tissues. (B) Expression level of circPVT1 in OS tissues of lung metastasis and lung non-metastasis group. (C) Expression level of circPVT1 in OS tissues of chemoresistant and chemosensitive group. (D) Patients with increased circPVT1 expression had a reduced overall survival time than those with low level of circPVT1 expression.
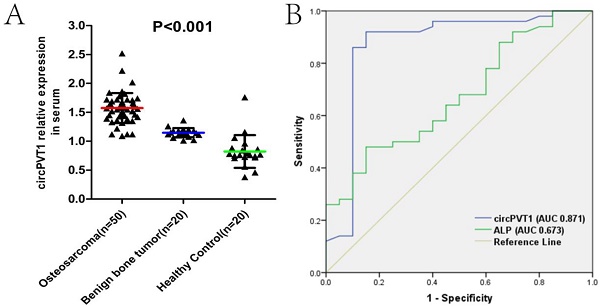
Serum circPVT1 may be a better diagnostic biomarker than ALP in OS
We then examined the expression level of circPVT1 in serum samples from 50 OS patients, 20 benign bone tumor patients and 20 age- and sex-matched healthy controlled individuals. As was shown in the Fig.2A, circPVT1 expression was gradually increased in the benign bone tumor and OS compared with the controlled (Fig.2A). To investigate the diagnostic value of circPVT1 in distinguishing patients from OS and the controlled healthy individuals, ROC curve was used and the results showed that the area under the ROC curve (AUC) was 0.871. In addition, we simultaneously compared the effectiveness of circPVT1, alkaline phosphatase (ALP), and lactate dehydrogenase (LDH), which are two biomarkers commonly used in clinical as diagnostic biomarker in OS. Obviously, circPVT1 was more reliable to separate OS from healthy individuals (AUC 0.871, P<0.001) than ALP (AUC 0.673, P=0.026, Fig.2B). However, there was no significant difference between the AUC of circPVT1 (0.871) and LDH (0.852) (data not shown in the figure). The above results demonstrated the possible clinical significance of circPVT1 as a diagnostic biomarker in OS, comparable to LDH and better than ALP.
CircPVT1 knockdown partly reverses the doxorubicin and cisplatin resistance of OS cells in vitro
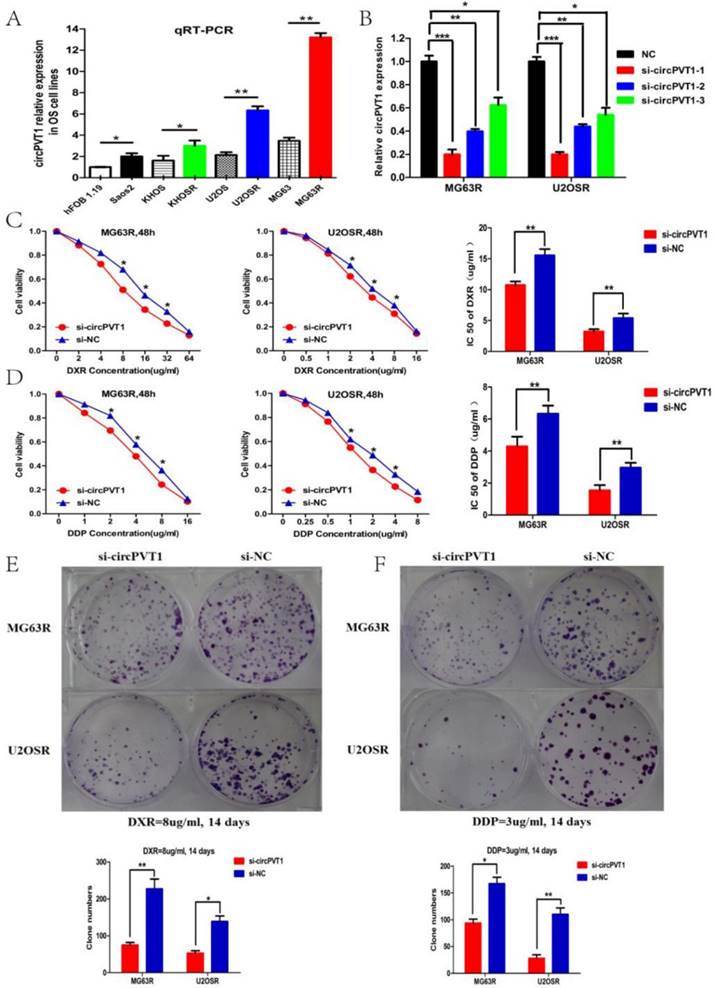
To further identify the function of circPVT1 in OS progression, we further detected the circPVT1 expression level in the seven OS cell lines, including three paired chemoresistant and chemosensitive cell lines(MG63R vs MG63, U2OSR vs U2OS, KHOSR vs KHOS), and the controlled normal osteoblast line hFOB 1.19. The results in the figure 3A illustrated that circPVT1 was up-regulated in the OS cell lines relative to the hFOB 1.19 and significantly elevated in the chemoresistant OS cell lines compared with the corresponding chemosensitive cell lines, which may suggest the potential role of circPVT1 in OS progression, especially for OS chemoresistance.
Serum circPVT1 may be a better diagnostic biomarker than ALP in OS. (A) Expression level of circPVT1 in serum from 50 OS patients, 20 benign bone tumor patients and 20 age- and sex-matched healthy individuals. (B) ROC curves of the serum circPVT1 and ALP in 50 newly diagnosed patients and 20 healthy donors.
To further investigate the role of circPVT1 in OS chemoresistance, siRNAs specifically targeted at circPVT1 junction site were constructed and transfected into the MG63R and U2OSR cell lines. qPCR was conducted to examine the transfection effect and si-circPVT1-1(termed as si-circPVT1) with highest inhibition efficacy was chosen for further assays(Fig.3B). CCK-8 and clone formation assays were performed to explore the function of circPVT1 in cell proliferation and resistance to doxorubicin (DXR) and cisplatin (DDP). IC50 values of MG63R (or U2OSR) cells for doxorubicin and cisplatin in response to circPVT1 knockdown were measured. As was shown in the Fig.3C, compared with MG63R (or U2OSR) cells transfected with si-NC, the IC50 values of doxorubicin in MG63R (or U2OSR) cells transfected with si-circPVT1 were reduced by 28.9% (or 30.8%) (P < 0.01, P < 0.01). The IC50 values of cisplatin in MG63R (or U2OSR) cells were decreased by 36.4% (or 46.7%) (P < 0.01, P < 0.01, Fig.3D), respectively. Similarly, colony formation assays revealed that cell proliferation rate was significantly suppressed in si-circPVT1 MG63R (or U2OSR) cells compared with si-NC transfected cells exposed to 8 μg/mL doxorubicin or 3μg/mL cisplatin for two weeks (P < 0.01,P < 0.01, Fig.3E-F). Taken together, these data suggested that circPVT1 knockdown by siRNA might partly reverse OS cells resistance to doxorubicin and cisplatin in vitro.
CircPVT1 knockdown reduces the expression of classical multidrug resistance related gene-ABCB1 in OS cells
Considering the well-known role of ABCB1 in the multi-drug resistance, we conjectured whether circPVT1 could regulate OS chemoresistance via ABCB1. Then PCR and WB assays were performed to examine the mRNA and protein expression level of ABCB1 in the si-circPVT1 and si-NC transfected MG63R (or U2OSR) cells. And the results revealed that ABCB1 expression was distinctly decreased in the si-circPVT1 cells relative to the si-NC, and comparable to the chemosensitive MG63 (or U2OS) cells, which may suggest that circPVT1 resensitizes OS cells to chemotherapy drugs through reducing the expression of classical multidrug resistance related gene-ABCB1(Fig.4A and B).
CircPVT1 knockdown partly reverses the doxorubicin and cisplatin resistance of OS cells in vitro. (A) Expression level of circPVT1 in seven human OS cell lines and normal osteoblast cell line hFOB1.19, including three paired chemoresistant and chemosensitive OS cell lines. (B) RT-qPCR analysis of the effect on knockdown of circPVT1 expression by siRNA in the MG63R and U2OSR cell lines. si-circPVT1-1 was chosen for the siRNA used in the study owing to the highest knockdown efficiency. (C-D) CCK-8 assay showed that the viability and IC50 value of MG63R (U2OSR) cells in the si-circPVT1 group when exposed to doxorubicin or cisplatin were reduced compared with the control groups. (E-F) Cell colony formation assay showed that the clone numbers of MG63R (U2OSR) cells in the si-circPVT1 group were reduced compared with the si-NC group when exposed to 8ug/ml doxorubicin or 3ug/ml cisplatin for 14 days. *P < 0.05, **P < 0.01.
CircPVT1 knockdown reduces the expression of classical multidrug resistance related gene-ABCB1 in OS cells. (A) The mRNA level of ABCB1 was detected by qPCR in the MG63R (or U2OSR) transfected with si-circPVT1 or si-NC and the controlled MG63 (or U2OS) cells. (B) The protein level of ABCB1 was detected by WB in the MG63R (or U2OSR) transfected with si-circPVT1 or si-NC and the controlled MG63 (or U2OS) cells. *P < 0.05, **P < 0.01.

Discussion
Tumor recurrence, lung metastasis and multi-drug resistance have been three huge obstacles in the clinical treatment of OS[22]. Biomarkers and related targeted molecules are urgently needed to dynamically monitor the changing condition of OS and to further timely intervene[23]. Owing to its naturally closed loop structure, circRNA could be stable in the tissues and serum regardless of RNase digestion. Besides, its relatively higher expression level also makes it an ideal biomarker candidate for cancer diagnosis and treatment [24-27]. Actually, there have been many circRNAs reported to be biomarkers for diagnosis and therapy in different cancers. For example, hsa_circ_0000520, hsa_circ_0001895, hsa_circ_0003159, hsa_circ_0000190 and hsa_circ_0000745 have been respectively reported to involve in gastric cancer development and might serve as novel biomarkers [28-32]. Zhu et al[33] reported that hsa_circ_0013958 could be used as a potential non-invasive biomarker for the early detection and screening of lung adenocarcinoma. Yao et al [34] also reported that circRNA_100876 is closely related to the carcinogenesis of non-small cell lung cancer (NSCLC) and it might be served as a potential prognostic biomarker and therapeutic target for NSCLC. Lu et al[35] showed that hsa_circ_006054, hsa_circ_100219 and hsa_circ_406697 in combination were a promising new class of diagnostic biomarkers for human breast cancer. Besides, hsa_circ_0005075 and hsa_circ_0001649 have been reported to participate in cell adhesion, tumorigenesis and metastasis of hepatocellular carcinoma and may be potential biomarkers [36, 37].
There have been several reports about circRNA and OS progression. Liu et al screened the circular RNA expression profile in OS cell lines by microarray analysis and found that hsa_circRNA_103801 and hsa_circRNA_104980, may be involved in the initiation and progression of OS[38]. Besides, several circular RNAs, such as UBAP2, GLI2, hsa_circ_0009910 and hsa-circ-0016347 have been respectively shown to promote proliferation, invasion and metastasis of OS cells as sponges of miRNAs[39-42]. However, there has been seldom study about circRNA and multi-drug resistance in OS and still lack of a reliable circRNA biomarker for OS diagnosis and treatment.
CircPVT1 (hsa_circ_0001821), located on chromosome 8q24 (chr8:128902834-128903244), is derived from exon 3 of the PVT1 gene, a cancer susceptibility locus and flanks two long introns (35269 bp and 41466 bp), which harbor many Alu repeats [15]. Chen et al first screened and identified the circular RNA, circPVT1, in the gastric cancer. They found that circPVT1 could promote cell proliferation by sponging members of miR-125 family and serve as a prognostic marker in gastric cancer [15]. Amaresh C et al [43] also found that circPVT1 is related to cell senescence and reveals senescence suppressor by sequestering let-7 to enable a proliferative phenotype. Besides, lncRNA PVT1 transcribed from the same gene locus has been demonstrated to promote osteosarcoma development by sponging miR-195 and miR-497[16, 17]. Owing to the sequence homology and molecular function of lncRNA PVT1 and circPVT1, we attempted to clarify the expression and function of circPVT1 in OS. In the present study, we first examined the expression of circPVT1 in OS tissues by qRT-PCR and found that circPVT1 was distinctly up-regulated in the OS tissues compared with the panacancerous tissues. Then, we found that circPVT1 expression in the groups of lung metastasis or chemoresistance was higher that of non-lung metastasis or chemosensitivity. Multivariable analysis also showed increased expression of circPVT1 was obviously related to advanced Enneking stage, chemoresistance and lung metastasis. In addition, K-M survival analysis demonstrated that patients with high circPVT1 expression have shorter overall survival lifetime than those with low circPVT1 expression.
Then, we further detected the circPVT1 expression level in plasma from patients with OS or benign bone tumor and the controlled healthy individuals to explore its possibility to be a biomarker of OS. The results indicated that circPVT1 expression was gradually increased in the benign bone tumor and OS compared with the controlled. ROC curve analysis further demonstrated that circPVT1 may be a better diagnostic biomarker than alkaline phosphatase (ALP), a biomarker commonly used in clinical[44], with more sensitivity and specificity. In addition, we found that circPVT1 was up-regulated in the OS cell lines relative to the hFOB 1.19, especially significantly elevated in the multi-drug resistant OS cell lines. Further function assays demonstrated that knockdown the expression of circPVT1 by siRNA could partly reverse the resistance of OS cells to doxorubicin and cisplatin.
It is well known that classical multi-drug resistance related gene ABCB1 (MDR1) is highly expressed in the drug resistant cell lines and could promote the chemoresistance by the P-gp protein to pump out the intracellular drugs[45-47]. Many non-coding RNAs, like miRNA and lncRNA, have been identified to involve in the process of drug resistance of cancer cells through regulating the expression of ABCB1. For example, Shang et al [48] found that miR-508-5p regulates multidrug resistance of gastric cancer by targeting ABCB1 and ZNRD1. Sun et al [49] showed that miR-186 induces sensitivity of ovarian cancer cells to paclitaxel and cisplatin by targeting ABCB1. Zhou et al [50] reported that sirolimus increases the sensitivity of human OS cells to anticancer drugs in vitro by up-regulating miR-34b interacting with PAK1 and ABCB1. Besides, Zhu et al[51] showed that lncRNA H19 is a major mediator of doxorubicin chemoresistance in breast cancer cells through a cullin4A-ABCB1 pathway. Wang et al [52] found that lncRNA MRUL promotes ABCB1 expression in multidrug-resistant gastric cancer cell sublines. Han et al[53] reported that lncRNA LUCAT1 modulates methotrexate resistance in osteosarcoma via miR-200c/ABCB1 axis.
Considering the role of ABCB1 in chemoresistance, we speculated whether the non-coding RNA, circPVT1, could regulate the multi-drug resistance of OS cells by affecting the expression of ABCB1. Then PCR and WB assays revealed that circPVT1 knockdown conspicuously decreases the mRNA and protein expression of ABCB1. However, the specific regulatory mechanism of circPVT1 and ABCB1 is still needed to be determined in the further study. In consideration of the co-overexpressed relationship of them, circPVT1 may act as related miRNA sponges to facilitate the expression of ABCB1 and bioinformatics analysis combined with dual luciferase reporter system are needed to demonstrate the hypothesis.
In conclusion, the current study demonstrated that circPVT1 is up-regulated in tissues and serums, correlated with poor prognosis and may be a better diagnostic biomarker than ALP in OS. In addition, circPVT1 knockdown could partly reverse the doxorubicin and cisplatin resistance of OS cells in vitro by decreasing the expression of ABCB1. Our results provide new insights into the role of circPVT1 as a biomarker for the diagnosis and treatment target of OS.
Abbreviations
OS: Osteosarcoma; LncRNA: Long non-coding RNA; qRT-PCR: quantitative real-time polymerase chain reaction; WB: Western blotting; NC: Negative control; si-: siRNA; PVT1: Pvt1 oncogene; circPVT1: circular RNA PVT1; ALP: alkaline phosphatase; LDH: lactate dehydrogenase; MRUL: multi-drug resistance related up-regulated lncRNA.
Acknowledgements
This project was supported by a Grant from the National Natural Science Foundation of China (NO.81572630), the Fundamental Research Funds for the Central Universities (NO.22120170216), Shanghai Pujiang Program of Shanghai Science and Technology Commission (NO.13PJD023) and Shanghai Jiaotong University Medical-Engineering Cross Research Fund (NO.YG2012MS49).
Conflicts of Interests
We declare that we have no conflicts of interest.
References
1. Moore DD, Luu HH. Osteosarcoma. Cancer treatment and research. 2014;162:65-92
2. Isakoff MS, Bielack SS, Meltzer P, Gorlick R. Osteosarcoma: Current Treatment and a Collaborative Pathway to Success. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2015;33:3029-35
3. Luetke A, Meyers PA, Lewis I, Juergens H. Osteosarcoma treatment - where do we stand? A state of the art review. Cancer treatment reviews. 2014;40:523-32
4. Morrow JJ, Khanna C. Osteosarcoma Genetics and Epigenetics: Emerging Biology and Candidate Therapies. Critical reviews in oncogenesis. 2015;20:173-97
5. Qu S, Yang X, Li X, Wang J, Gao Y, Shang R. et al. Circular RNA: A new star of noncoding RNAs. Cancer letters. 2015;365:141-8
6. Chen LL, Yang L. Regulation of circRNA biogenesis. RNA biology. 2015;12:381-8
7. Salzman J. Circular RNA Expression: Its Potential Regulation and Function. Trends in genetics. 2016;32:309-16
8. Barrett SP, Salzman J. Circular RNAs: analysis, expression and potential functions. Development (Cambridge, England). 2016;143:1838-47
9. Kulcheski FR, Christoff AP, Margis R. Circular RNAs are miRNA sponges and can be used as a new class of biomarker. Journal of biotechnology. 2016;238:42-51
10. Li J, Yang J, Zhou P, Le Y, Zhou C, Wang S. et al. Circular RNAs in cancer: novel insights into origins, properties, functions and implications. American journal of cancer research. 2015;5:472-80
11. Ebbesen KK, Kjems J, Hansen TB. Circular RNAs: Identification, biogenesis and function. Biochimica et biophysica acta. 2016;1859:163-8
12. Zhang Y, Liu H, Li W, Yu J, Li J, Shen Z. et al. CircRNA_100269 is downregulated in gastric cancer and suppresses tumor cell growth by targeting miR-630. Aging. 2017;9:1585-94
13. Hsiao KY, Lin YC, Gupta SK, Chang N, Yen L, Sun HS. et al. Noncoding Effects of Circular RNA CCDC66 Promote Colon Cancer Growth and Metastasis. Cancer research. 2017;77:2339-50
14. Chen L, Zhang S, Wu J, Cui J, Zhong L, Zeng L. et al. circRNA_100290 plays a role in oral cancer by functioning as a sponge of the miR-29 family. Oncogene. 2017;36:4551-61
15. Chen J, Li Y, Zheng Q, Bao C, He J, Chen B. et al. Circular RNA profile identifies circPVT1 as a proliferative factor and prognostic marker in gastric cancer. Cancer letters. 2017;388:208-19
16. Song J, Wu X, Liu F, Li M, Sun Y, Wang Y. et al. Long non-coding RNA PVT1 promotes glycolysis and tumor progression by regulating miR-497/HK2 axis in osteosarcoma. Biochemical and biophysical research communications. 2017;490:217-24
17. Zhou Q, Chen F, Zhao J, Li B, Liang Y, Pan W. et al. Long non-coding RNA PVT1 promotes osteosarcoma development by acting as a molecular sponge to regulate miR-195. Oncotarget. 2016;7:82620-33
18. Xu M, Jin H, Xu CX, Bi WZ, Wang Y. MiR-34c inhibits osteosarcoma metastasis and chemoresistance. Medical oncology (Northwood, London, England). 2014;31:972
19. Zhao Y, Zhang CL, Zeng BF, Wu XS, Gao TT, Oda Y. Enhanced chemosensitivity of drug-resistant osteosarcoma cells by lentivirus-mediated Bcl-2 silencing. Biochemical and biophysical research communications. 2009;390:642-7
20. Lourda M, Trougakos IP, Gonos ES. Development of resistance to chemotherapeutic drugs in human osteosarcoma cell lines largely depends on up-regulation of Clusterin/Apolipoprotein J. International journal of cancer. 2007;120:611-22
21. Susa M, Iyer AK, Ryu K, Hornicek FJ, Mankin H, Amiji MM. et al. Doxorubicin loaded Polymeric Nanoparticulate Delivery System to overcome drug resistance in osteosarcoma. BMC cancer. 2009;9:399
22. Geller DS, Gorlick R. Osteosarcoma: a review of diagnosis, management, and treatment strategies. Clinical advances in hematology & oncology: H&O. 2010;8:705-18
23. Zhou W, Hao M, Du X, Chen K, Wang G, Yang J. Advances in targeted therapy for osteosarcoma. Discovery medicine. 2014;17:301-7
24. Zhang Y, Liang W, Zhang P, Chen J, Qian H, Zhang X. et al. Circular RNAs: emerging cancer biomarkers and targets. Journal of experimental & clinical cancer research: CR. 2017;36:152
25. Zhang HD, Jiang LH, Sun DW, Hou JC, Ji ZL. CircRNA: a novel type of biomarker for cancer. Breast cancer (Tokyo, Japan). 2017 Jul 18. doi: 10.1007/s12282-017-0793-9
26. Meng S, Zhou H, Feng Z, Xu Z, Tang Y, Li P. et al. CircRNA: functions and properties of a novel potential biomarker for cancer. Molecular cancer. 2017;16(1):94
27. Han YN, Xia SQ, Zhang YY, Zheng JH, Li W. Circular RNAs: A novel type of biomarker and genetic tools in cancer. Oncotarget. 2017;8:64551-63
28. Tian M, Chen R, Li T, Xiao B. Reduced expression of circRNA hsa_circ_0003159 in gastric cancer and its clinical significance. 2017. Jun 15. doi: 10.1002/jcla.22281.
29. Shao Y, Chen L, Lu R, Zhang X, Xiao B, Ye G. et al. Decreased expression of hsa_circ_0001895 in human gastric cancer and its clinical significances. Journal of clinical laboratory analysis. 2017;39:1010428317699125
30. Huang M, He YR, Liang LC, Huang Q, Zhu ZQ. Circular RNA hsa_circ_0000745 may serve as a diagnostic marker for gastric cancer. World journal of gastroenterology. 2017;23:6330-8
31. Chen S, Li T, Zhao Q, Xiao B, Guo J. Using circular RNA hsa_circ_0000190 as a new biomarker in the diagnosis of gastric cancer. Clinica chimica acta; international journal of clinical chemistry. 2017;466:167-71
32. Sun H, Tang W, Rong D, Jin H, Fu K, Zhang W. et al. Hsa_circ_0000520, a potential new circular RNA biomarker, is involved in gastric carcinoma. Cancer biomarkers: section A of Disease markers. 2017 Oct 18. doi: 10.3233/CBM-170379
33. Zhu X, Wang X, Wei S, Chen Y, Chen Y, Fan X. et al. hsa_circ_0013958: a circular RNA and potential novel biomarker for lung adenocarcinoma. The FEBS journal. 2017;284:2170-82
34. Yao JT, Zhao SH, Liu QP, Lv MQ, Zhou DX, Liao ZJ. et al. Over-expression of CircRNA_100876 in non-small cell lung cancer and its prognostic value. Pathology, research and practice. 2017;213:453-6
35. Lu L, Sun J, Shi P, Kong W, Xu K, He B. et al. Identification of circular RNAs as a promising new class of diagnostic biomarkers for human breast cancer. Oncotarget. 2017;8:44096-107
36. Shang X, Li G, Liu H, Li T, Liu J, Zhao Q. et al. Comprehensive Circular RNA Profiling Reveals That hsa_circ_0005075, a New Circular RNA Biomarker, Is Involved in Hepatocellular Crcinoma Development. Medicine. 2016;95:e3811
37. Qin M, Liu G, Huo X, Tao X, Sun X, Ge Z. et al. Hsa_circ_0001649: A circular RNA and potential novel biomarker for hepatocellular carcinoma. Cancer biomarkers: section A of Disease markers. 2016;16:161-9
38. Liu W, Zhang J, Zou C, Xie X, Wang Y, Wang B. et al. Microarray Expression Profile and Functional Analysis of Circular RNAs in Osteosarcoma. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology. 2017;43:969-85
39. Zhang H, Wang G, Ding C, Liu P, Wang R, Ding W. et al. Increased circular RNA UBAP2 acts as a sponge of miR-143 to promote osteosarcoma progression. Oncotarget. 2017;8:61687-97
40. Li JF, Song YZ. Circular RNA GLI2 promotes osteosarcoma cell proliferation, migration, and invasion by targeting miR-125b-5p. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2017;39:1010428317709991
41. Jin H, Jin X, Zhang H, Wang W. Circular RNA hsa-circ-0016347 promotes proliferation, invasion and metastasis of osteosarcoma cells. Oncotarget. 2017;8:25571-81
42. Deng N, Li L, Gao J, Zhou J, Wang Y, Wang C. et al. Hsa_circ_0009910 promotes carcinogenesis by promoting the expression of miR-449a target IL6R in osteosarcoma. Biochemical and biophysical research communications. 2017 Nov 5. pii: S0006-291X(17)32205-2
43. Amaresh C. Panda, Ioannis Grammatikakis, Kyoung Mi Kim, Supriyo De, Jennifer L. Martindale, Rachel Munk, et al. Identification of senescence-associated circular RNAs (SAC-RNAs) reveals senescence suppressor CircPVT1. Nucleic Acids Res. 2017;45:4021-4035
44. Ren HY, Sun LL, Li HY, Ye ZM. Prognostic Significance of Serum Alkaline Phosphatase Level in Osteosarcoma: A Meta-Analysis of Published Data. BioMed research international. 2015;2015:160835
45. Sui H, Fan ZZ, Li Q. Signal transduction pathways and transcriptional mechanisms of ABCB1/Pgp-mediated multiple drug resistance in human cancer cells. The Journal of international medical research. 2012;40:426-35
46. Choi YH, Yu AM. ABC transporters in multidrug resistance and pharmacokinetics, and strategies for drug development. Current pharmaceutical design. 2014;20:793-807
47. Bruhn O, Cascorbi I. Polymorphisms of the drug transporters ABCB1, ABCG2, ABCC2 and ABCC3 and their impact on drug bioavailability and clinical relevance. Expert opinion on drug metabolism & toxicology. 2014;10:1337-54
48. Shang Y, Zhang Z, Liu Z, Feng B, Ren G, Li K. et al. miR-508-5p regulates multidrug resistance of gastric cancer by targeting ABCB1 and ZNRD1. Oncogene. 2014;33:3267-76
49. Sun KX, Jiao JW, Chen S, Liu BL, Zhao Y. MicroRNA-186 induces sensitivity of ovarian cancer cells to paclitaxel and cisplatin by targeting ABCB1. Journal of ovarian research. 2015;8:80
50. Zhou Y, Zhao RH, Tseng KF, Li KP, Lu ZG, Liu Y. et al. Sirolimus induces apoptosis and reverses multidrug resistance in human osteosarcoma cells in vitro via increasing microRNA-34b expression. Acta pharmacologica Sinica. 2016;37:519-29
51. Zhu QN, Wang G, Guo Y, Peng Y, Zhang R, Deng JL. et al. LncRNA H19 is a major mediator of doxorubicin chemoresistance in breast cancer cells through a cullin4A-MDR1 pathway. Oncotarget. 2017;8:91990-2003
52. Wang Y, Zhang D, Wu K, Zhao Q, Nie Y, Fan D. Long noncoding RNA MRUL promotes ABCB1 expression in multidrug-resistant gastric cancer cell sublines. Molecular and cellular biology. 2014;34:3182-93
53. Han Z, Shi L. Long non-coding RNA LUCAT1 modulates methotrexate resistance in osteosarcoma via miR-200c/ABCB1 axis. Biochemical and biophysical research communications. 2017 Nov 21. pii: S0006-291X(17)32300-8
Author contact
![]() Corresponding author: Zhang Chun-Lin, MD, Department of Orthopedics, Shanghai Tenth People's Hospital, Tongji University, School of Medicine, 301, Yan-chang Middle Road, Shanghai 200072, China. E-mail: shzhangchunlin123com. Fax: +86 13761904091.
Corresponding author: Zhang Chun-Lin, MD, Department of Orthopedics, Shanghai Tenth People's Hospital, Tongji University, School of Medicine, 301, Yan-chang Middle Road, Shanghai 200072, China. E-mail: shzhangchunlin123com. Fax: +86 13761904091.

 Global reach, higher impact
Global reach, higher impact