Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2018; 14(9):1081-1089. doi:10.7150/ijbs.24692 This issue Cite
Research Paper
Phosphorylation of SMC1A promotes hepatocellular carcinoma cell proliferation and migration
1. Key Laboratory of Medical Cell Biology, Ministry of Education; Institute of Translational Medicine, China Medical University; Liaoning Province Collaborative Innovation Center of Aging Related Disease Diagnosis and Treatment and Prevention, Shenyang, Liaoning Province, China
2. Department of Pathology, The College of Basic Medical Sciences, China Medical University, Shenyang, Liaoning Province, China
3. Department of Cardiology, The First Hospital of China Medical University, Shenyang, Liaoning Province, China
4. Department of Pathology, School of Medicine, University of Alabama at Birmingham, Birmingham, AL 35249-7331, USA
*These authors contributed equally to this work.
Received 2018-1-2; Accepted 2018-5-5; Published 2018-6-8
Abstract
Structural maintenance of chromosomes protein 1A (SMC1A) has been implicated in the development of a variety of cancer types. However, its role in hepatocellular carcinoma remains unknown. In this study, we found that phosphorylated SMC1A was highly expressed in HepG2 and Bel7402 cells when compared with other cancer cell lines. Furthermore, SMC1A knockdown dramatically reduced HepG2 and Bel7402 cell proliferation and migration. Re-expressing phosphomimetic mutants S957DS966D significantly enhanced the proliferation and migration of SMC1A knockdown HepG2 and Bel7402 cells. In addition, phosphorylated SMC1A promotes hepatocellular carcinoma cells growth in vivo. Importantly, the expression of phosphorylated SMC1A was significantly higher in human hepatocellular carcinomacells when compared to peri-tumor benign hepatocytes, and its overexpression was significantly associated with worse prognostic outcomes. These observations suggest that phosphorylation of SMC1A is a vital event in tumorigenesis and disease progression in hepatocellular carcinoma thus necessitating further investigation.
Keywords: Hepatocellular Carcinoma, SMC1 phosphorylation, Proliferation, Migration, Prognosis
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cancer in the world and the third most frequent cause of cancer mortality in the Asia-Pacific region [1, 2]. Early diagnosis of HCC remains a challenging task as the screening tests in high-risk patients (i.e., cirrhosis) are not feasible in individuals with impaired liver function. Despite the recent progress in early detection and therapeutic modalities such as liver transplantation and chemoembolization, patients with HCC who are symptomatic at presentation still have dismal outcomes, with a survival rate of 0-10% [2]. Therefore, it is essential to further investigate the molecular mechanisms of HCC progression to identify novel therapeutic targets, especially in chemo-resistant HCC.
Structural maintenance of chromosomes protein 1A (SMC1A), member of the SMC superfamily and core component of the cohesin complex essential for sister chromatid cohesion [3-5] , plays an important role in chromosome dynamics [6-8], cell cycle regulation [8, 9], double-strand break (DSB) repair [10-12], genomic stability maintenance [13] and tumorigenesis [14]. There has been growing evidence that SMC1A is up-regulated and/or mutated in various tumor types. SMC1A mutations have been identified in human colorectal cancers and their precursor lesions [15, 16]. Furthermore, SMC1A promotes metastasis of colon cancer [17]; overexpression of the molecule is associated with a worse prognosis in patients with advanced disease [3]. In addition, increased SMC1A protein expression has been found in triple-negative breast cancer cells [18], U-25l human glioblastoma cell line and glioma tissue [19, 20]. In keeping with these observations, down-regulation of SMC1A expression generally inhibits cell proliferation, cell cycle progression and cell migration [4]. Conversely, low expression of SMC1A is reported associated with a poor prognosis in patients with acute myeloid leukemia [21].
Moreover, SMC1A is phosphorylated on Serine 957 and 966 by protein kinase ataxia-telangiectasia mutated (ATM) following ionizing radiation [5, 22], an event thought to be a downstream effector of ATM-NBSI-BRCA pathway that affects cell survival and chromosomal stability maintenance after DNA damage [23]. Thus, SMC1A phosphorylation may play an important role in cancer progression. However, the significance of the molecule in hepatocellular carcinogenesis, remains unclear.
In this study, we sought to explore the potential oncogenic role of SMC1A in HCC. Our findings suggest that phosphorylation of SMC1A plays an important role in carcinogenesis and progression of HCC thus may provide a potential therapeutic target in this highly malignant disease in the pursuit of precision medicine.
Results
SMC1A knockdown inhibits proliferation and migration of hepatocellular carcinoma cells
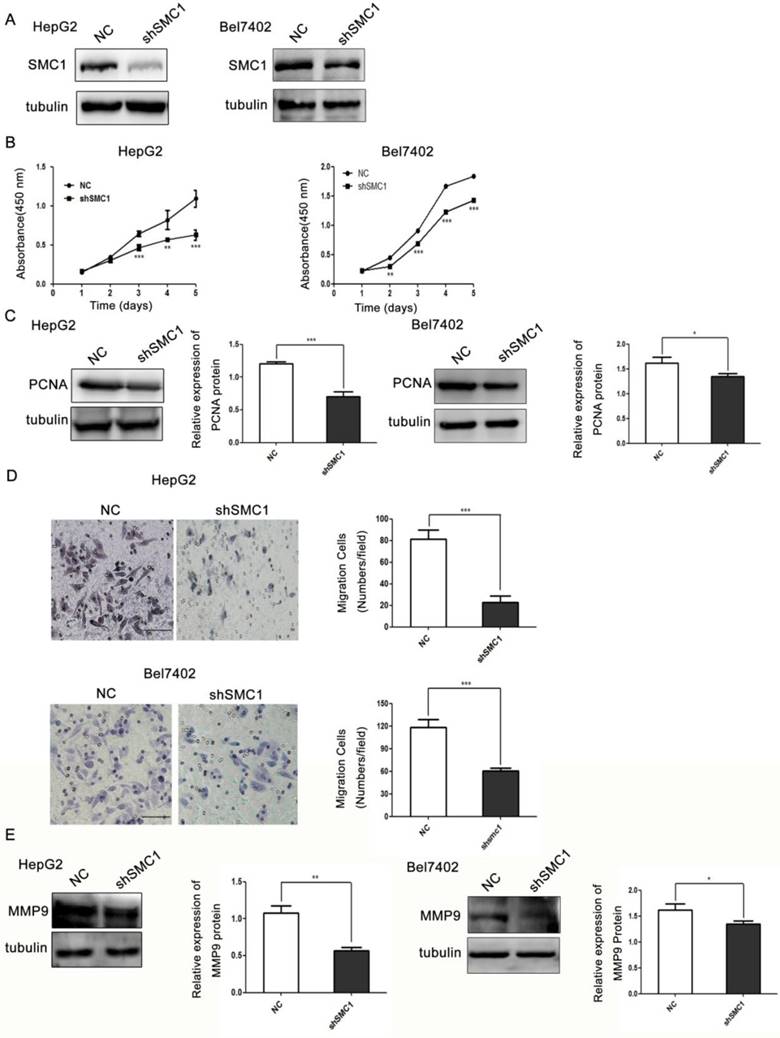
Given that the significance of SMC1A phosphorylation in hepatocellular carcinogenesis remains unclear, we used two cell lines HepG2 and Bel7402 to explore the potential oncogenic role of SMC1A in HCC. And we carried out lentivirus-mediated shRNA knockdown of SMC1A in HepG2 and Bel7402 cells. As shown in Figure 1A, efficient SMC1A knockdown was established successfully (shSMC1) when compared to scrambled shRNA (NC)-transfected cells.
Colorimetric assay for cell viability utilizing Cell Counting Kit-8 (CCK-8) was performed to assess the effect of SMC1A silencing on HepG2 and Bel7402 cell proliferation. As shown in Figure 1B, reduced SMC1A expression markedly inhibited the growth of HepG2 and Bel7402 cells. The proliferation of HepG2 and Bel7402 cells in shSMC1A group was respectively reduced by 42% and 23% when compared to the control group (P<0.001). In keeping with this observation, growth-regulated protein proliferating cell nuclear antigen (PCNA) was used as a marker of proliferating which is involved in DNA replication, DNA repair and cell cycle control [24] . We found that the expression level of proliferating cell nuclear antigen (PCNA) was significantly lower in SMC1A knockdown cells (Figure 1C).
We next examined the role of SMC1A in migration of HepG2 and Bel7402 cells by transwell migration assay. To that end, SMC1A silencing respectively resulted in a 3.5-fold decrease in HepG2 cells and 1.95-fold decrease in Bel7402 cells in migration efficiency (P<0.001, Figure 1D). In line with this observation, the expression of migration-related protein matrix metallo peptidase 9 (MMP9) was significantly lower in SMC1A knockdown cells (Figure 1E). Thus, the findings suggest that SMC1A is essential in HepG2 and Bel7402 cell proliferation and migration.
Phosphorylation of SMC1A promotes proliferation and viability in hepatocellular carcinoma cells
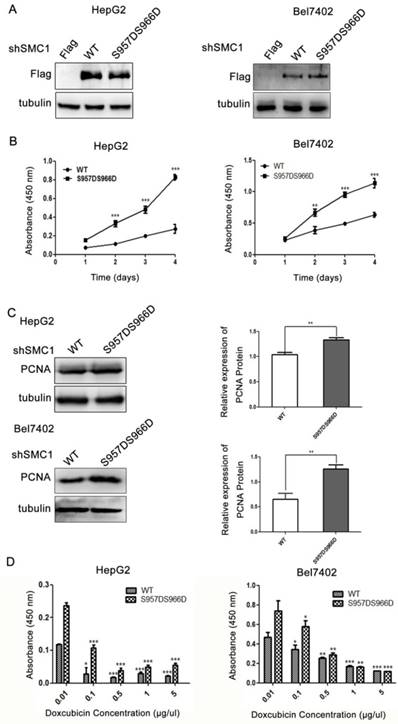
Phosphorylation of SMC1A on serine 957 and/or 966 plays an important role in cell survival and chromosomal stability in the ATM-NBSI-BRCA pathway after DNA damage [23]. However, whether SMC1 phosphorylation has an impact on the proliferation and survival of HCC cells remains unknown. We next mutated serine 957 and 966 phosphorylation sites simultaneously to artificially induce phosphomimetic mutants (S957DS966D) in an effort to elucidate the role of SMC1A phosphorylation in HepG2 and Bel7402 cells. A flag-tagged wild type or S957DS966D mutants SMC1A were transiently re-expressed into HepG2 and Bel7402 cells knocking down endogenous SMC1A (Figure 2A). Semiquantitative analysis of the western blot analysis respectively revealed a 1.3-fold and 1.9-fold increase in PCNA expression in HepG2 and Bel7402 cells expressing S957DS966D mutants when compared to those expressing wild type SMC1A (Figure 2B).
The effect of SMC1A phosphorylation on HepG2 and Bel7402 cell viability was examined by CCK-8 assay. As shown in Figure 2C, cells expressing S957DS966D mutants demonstrated a significantly higher proliferation rate when compared to those expressing wild type SMC1A in HepG2 cell (increased by 67%; P<0.001) and in Bel7402 cell (increased by 44.2%; P<0.001). We next examined the effect of doxorubicin, a cytotoxic agent, on the viability of HepG2 and Bel7402 cells. As illustrated in Figure 2D, cells expressing S957DS966D mutants exhibited significantly higher viability when compared to those expressing wild type SMC1A in all tested concentrations of doxorubicin (P<0.001). Thus, the findings have demonstrated that phosphorylation of SMC1A in serine957 and 966 is essential for proliferation and survival in HepG2 and Bel7402 cells.
Silencing of SMC1A reduces HepG2 and Bel7402 cell proliferation and migration. (A) Interference efficiency of SMC1A by shRNA or negative control (NC; scrambled shRNA) in HepG2 and Bel7402 cells by western blot analysis. The effect of SMC1A knockdown on HepG2 and Bel7402 cell proliferation was performed using Cell Counting Kit-8, ***P< 0.001 (B) and PCNA protein level expression,***P<0.001, *P<0.05 (C). Transwell assay, ***P<0.001 (D) and expression of MMP9 protein level, **P<0.01, *P<0.05 (E) were used to detect the migration of SMC1A knockdown in HepG2 and Bel7402 cells. Scale bar 50 μm. Data were expressed as means ± SD.
SMC1A phosphorylation promotes HepG2 and Bel7402 cell proliferation and viability. (A) The flag-tagged wild-type or S957DS966D mutants SMC1A plasmid was transiently transfected into HepG2 and Bel7402 cells knocking down endogenous SMC1A. Effect of SMC1A phosphorylation on HepG2 and Bel7402 cell proliferation was determined by CCK8 assay, ***P<0.001 (B) and PCNA protein expression, **P<0.01 (C). SMC1A (S957DS966D) mutant promoted HepG2 and Bel7402 cell viability in response to the increased doses of Doxorubicin treatment, *P<0.05, **P<0.01, ***P<0.001 (D). Data were expressed as means ± SD. WT, wild-type.
Phosphorylation of SMC1A induces migration of hepatocellular carcinoma cells
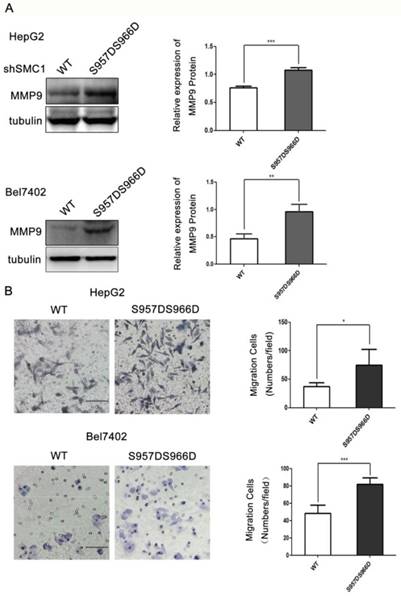
We next asked if SMC1A phosphorylation has a role in cell migration. To that end, expressing S957DS966D dramatically increased the expression of MMP9 by 36% in HepG2 cell and 51.8% in Bel7402 cell when compared to the wild type (Figure 3A). At testing to the function, S957DS966D expressing HepG2 and Bel7402 cells displayed a significantly higher cell migration rate when compared to the wild type SMC1A expressing cells by transwell assay (Figure 3B). Thus, phosphorylation of SMC1A accelerates HepG2 and Bel7402 cell migration.
SMC1A phosphorylation increases HepG2 and Bel7402 cell migration. SMC1A (S957DS966D) mutant significantly promoted HepG2 and Bel7402 cell migration by MMP9 protein level expression, ***P<0.001, **P<0.01 (A) and transwell assay, *P<0.05; ***P<0.001 (B). Scale bar, 20 μm. Data were expressed as means ± SD. WT, wild-type.
Phosphorylated SMC1A promotes hepatocellular carcinoma cells growth in vivo
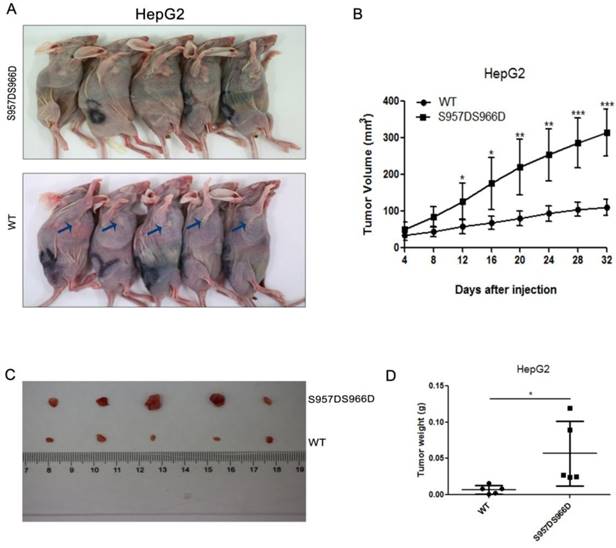
To explore the great significance of phosphorylated SMC1A in HCC in vivo, we injected HepG2 stable cell lines with SMC1 knockdown and reexpression of wild type or S957DS966D mutant SMC1 into nude mice subcutaneously and analyzed the tumor growth. The S957DS966D mutant SMC1 of HepG2 cells demonstrated larger tumor size than the wild type SMC1-expressing cells (Figure 4A). In addition, Figure 4B showed that cells expressing S957DS966D mutant have the greater tumor growth rate than those expressing wild type SMC1A in HepG2 cell. Furthermore, compared with wild type SMC1-expressing cells, the S957DS966D mutant SMC1-expression HepG2 cells also have greater tumor weight (Figure 4C and D). Collectively, our data suggested that phosphorylated SMC1A promoted HCC tumor growth in vivo.
(A) Xenograft was performed using HepG2 stable cell lines with SMC1 knockdown and reexpression of wild type or S957DS966D mutant SMC1. Mice were sacrificed and photographed at day 32. (B) The tumor size was measured every 4 days and tumor volume was analyzed. (C) SMC1 S957DS966D mutant promotes xenograft tumor growth. At 32 days after injection, tumors were removed and photographed. (D) The tumor weight was calculated on day 32. Data are presented as mean ± SD (n=5). *P < 0.05 versus control.
Relationship of SMC1A phosphorylation expression and clinicopathological parameters in liver cancer patients (n = 78)
| Characteristic | SMC1A phosphorylation immunostaining | ||
|---|---|---|---|
| Low (0-4) | High (5-9) | ||
| N (28) | N (50) | P | |
| Gender (N) | 0.477 | ||
| Male | 26 (92.9%) | 44 (88.0%) | |
| Female | 2 (7.1%) | 6 (12.0%) | |
| Age (years) | 51.64 ± 9.56 | 54.76 ± 10.65 | 0.190 |
| Grade | 0.168 | ||
| Ⅰ-Ⅱ | 22 (78.6%) | 32 (64.0%) | |
| Ⅲ | 6 (21.4%) | 18 (36.0%) | |
| Clinical stages | |||
| Ⅰ-Ⅱ | 18 (64.3%) | 20 (40.0%) | 0.040 |
| Ⅲ-Ⅳ | 10 (35.7%) | 30 (60.0%) | |
| Lymph node metasis | 0.322 | ||
| N0 | 28 (100.0%) | 49 (98.0%) | |
| N1 | 0 (0.0%) | 1 (0%) | |
| Distant metasis | 0.322 | ||
| M0 | 28 (100.0%) | 49 (98.0%) | |
| M1 | 0 (0.0%) | 1 (0%) | |
| Cirrhosis | 0.295 | ||
| No | 15 (53.6%) | 33 (66.0%) | |
| Yes | 13 (46.4%) | 17 (34.0%) | |
Phosphorylated SMC1A is highly expressed in human hepatocellular carcinoma tissue and is associated with survival outcomes
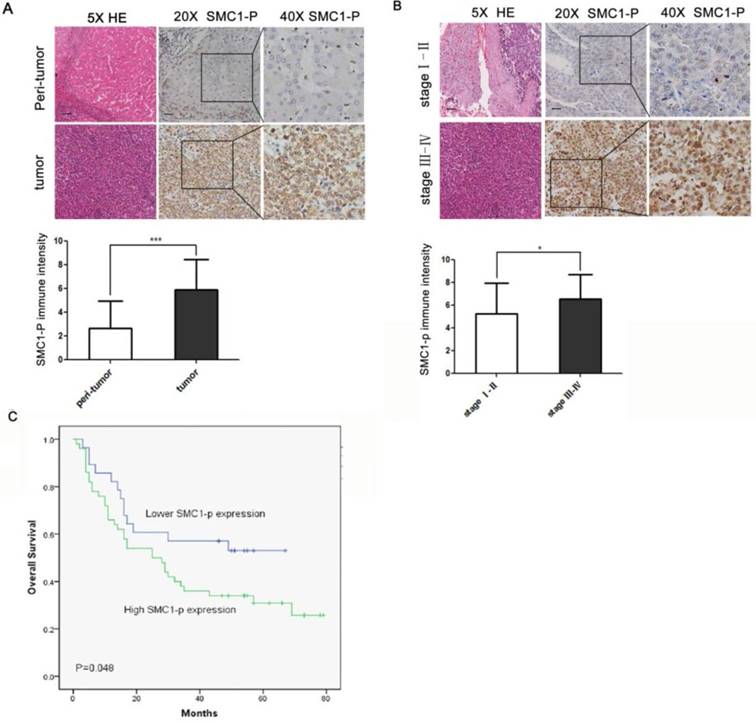
To further elucidate the role of SMC1A phosphorylation in vivo, expression of phosphorylated SMC1A protein was examined in tissue specimens from 78 HCC patients. The relationship between SMC1A phosphorylation expression and clinicpathological parameters of these patients were analysed. Table 1 showed that there was no significant difference between SMC1A phosphorylation expression and gender, age, grade and cirrhosis (P>0.05). However, based on the histopathological TNM staging, patients with higher SMC1A phosphorylation expression were positively relation to those with clinical stage Ⅲ and Ⅳ disease (P<0.05) (Table 1). Semiquantitative immunohistochemical analysis revealed a 2.5-fold higher level of phosphorylated SMC1A in HCC cells when compared to the peri-tumoral benign hepatocytes (Figure 5A; mean score 5.91 vs. 2.65, P<0.001). Furthermore, expression level of phosphorylated SMC1A was significantly higher in advanced HCC (stage Ⅲ-Ⅳ) when compared to early-staged disease (stageⅠ-Ⅱ; Figure 5B). Moreover, a high phosphorylated SMC1A expression (score ≥ 5) was associated with a significantly worse overall survival when compared to those with a low expression level (score < 5; Figure 4C). Thus, the collective findings suggest that phosphorylation of SMC1A is a vital event in tumorigenesis and disease progression in HCC.
Discussion
As a key structural maintenance of chromosomes protein, SMC1 is involved in variety of biological processes, including the regulation of chromosome dynamics [6-8], cell cycle progression [8, 9], DNA double strand break repair [10-12], and genomic stability maintenance [13, 14]. Four core subunits of cohesin are known in budding yeast, two subunits of the structural maintenance of chromosomes (SMC) family, Smc1 and Smc3, and two sister chromatid cohesion (SCC) proteins, Scc1 and Scc3[25, 26]. After S-phase, it is vital that the products of DNA replication, the sister chromatids, are handled as a unit; they must be held together by cohesion. During mitosis, the bipolar attachment of sister chromatids to the mitotic spindle determines their segregation pattern, and correct spindle attachment is, therefore, crucial for equal distribution of the genome.
SMC1 phosphorylation expression is correlated with poor prognosis in human HCC. (A) The expression level of SMC1 phosphorylation protein in HCC (n = 78) and paired peritumoral liver tissue (n = 78) was detected by immunohistochemical staining (top). Scale bars, 20 μm. Scores indicated SMC1 phosphorylation levels by a 20-point quantification scale (bottom), ***P<0.001. (B) Top, immunohistochemical staining of SMC1 phosphorylation was displayed in clinical HCC at different clinical stages. Scale bars, 20 μm. Bottom, column plot analysis of the SMC1 phosphorylation expression in HCC samples at different clinical stages, *P<0.05. (C) Kaplan-Meier survival curve showed that high SMC1 phosphorylation expression (score 5-10, n = 78) was associated with poorer overall survival compared to low SMC1 phosphorylation expression (score 0-4, n = 78), *P<0.05. Data were expressed as means ± SD. SMC1-P, SMC1 phosphorylation; WT, wild-type.
There has been growing evidence that the molecule plays an important role in tumorigenesis. SMC1A mutation and/or up-regulation have been identified in human colorectal carcinoma and several other tumor types [14]. Furthermore, overexpression of the protein has been found in advanced diseases and associated with a poor prognosis [21]. On the contrary, the expression of SMC1A is reversely correlated with the clinical outcomes in acute myeloid leukemia [21]. Thus, the data thus far suggest that SMC1A might play a dual role in tumorigenesis and progression, acting as a promoter in solid tumors and an inhibitor in hematologic malignancies.
The significance of SMC1A in hepatocellular carcinogenesis has not been previously studied. Given that phosphorylation of SMC1A is a crucial event in its regulation of the diverse biological processes, DNA damage repair, and tumorigenesis [14, 23], we first examined the expression of phosphorylated SMC1A in a number of human carcinoma cell lines, including HepG2, Bel7402, A549, Hela and H1299. The observation of the highest expression of phosphorylated SMC1A found in HepG2 and Bel7402, human HCC cell lines, suggests a potential role of this molecule in the initiation and progression in HCC. In keeping with this proposition, SMC1A silencing down-regulated PCNA and MMP9, respectively, and effectively inhibited the proliferation and migration of HepG2 and Bel7402 cells. In addition, our results also showed phosphorylated SMC1A promotes hepatocellular carcinoma cells growth in vivo.
SMC1A is known to exert its biologic function in chromosomal stability after DNA damage via phosphorylation of SMC1A on serine 957 and 966 [14, 23]. Therefore, S957DS966D, simultaneous phosphomimetic mutants, was generated to further explore the carcinogenic role of SMC1A phosphorylation. To that end, re-expression of S957DS966D into the SMC1A knockdown HepG2 and Bel7402 cells resulted in a significantly higher proliferation rate, significantly higher cell viability/cytotoxic drug resistance, and a significantly increased cell migration rate when compared to the cells expressing wild type SMC1A. These findings have further confirmed the essential role of SMC1A phosphorylation on serine 957 and 966 in regulating the proliferation, survival and migration of HCC cells.
The utmost principal observations in this study are the significantly higher expression of phosphorylated SMC1A in human HCC cells when compared to peri-tumoral benign hepatocytes, and the significantly increased expression of the protein in advanced HCC when compared to early-staged diseases. Attesting to its function as a prognostic factor, a higher phosphorylated SMC1A expression was associated with significantly worse survival outcomes when compared to those with a lower expression level.
In summary, these observations indicate that SMC1A is essential in the development and progression of HCC. The mechanisms by which SMC1A promotes HCC cell proliferation, survival and migration may occur, at least in part, via suppression of PCNA and MMP9, proliferation- and migration-related proteins, respectively. The data also suggests SMC1A to be a prognostic marker for HCC. Thus, the collective findings imply that inhibition of SMC1A may serve as a promising therapeutic target, especially for advanced HCC, thus necessitating further investigation in the pursuit of precision medicine.
Materials and methods
Cell culture
Human embryonic kidney (HEK-293T) cell and HepG2 and Bel7402 cells were acquired from the American Type Culture Collection (ATCC, USA). HEK-293T cells were cultured in Dulbecco's modified Eagle's medium (DMEM). HepG2 and Bel7402 cells were maintained in RPMI (Roswell Park Memorial Institute) 1640 medium. All mediums were supplemented with 10% fetal bovine serum (FBS; GE Healthcare HyClone, USA) and streptomycin (100 μg/ml) and penicillin (100 units/ml). All cells were cultured in a humidified incubator at 37 °C with 5% (v/v) CO2.
Plasmid constructions and transfections
SMC1A was cloned to pCDNA3.1-flag/HA vector. The point mutation plasmids of SMC1A were constructed with the Quickchange Site-Directed Mutagenesis Kit (Stratagene, CA, USA). The plasmids were identified by sequencing and transfected into HepG2 and Bel7402 cells using lipofectamine 2000 regent (Thermo Fisher Scientific, USA) according to the instruction. The experiments were carried out 24 h or 48 h after transfection.
Lentiviral Production
The lentiviral vector (GV248) carrying shRNA targeting human SMC1A was obtained from GeneChem (shSMC1A, 5'-CGGGACTGTATTCAGTATA-3'; shCont, 5'-TTCTCCGAACGTGTCACGT-3'). Lentivirus was produced in HEK-293T cells using the shRNA-expression GV248 vector with packaging plasmids pHelper1.0 and pHelper2.0, which were co-transfected into HEK-293T cells by lipofectamine 2000 (Thermo Fisher Scientific, USA) according to the manufacturer's instructions. The mediums with lentiviral particles were separately harvested at 24 h, 48 h and 72 h after transfection. To deposit lentiviral particles, the medium supplemented with 5 × PEG-itTM Solution (System Biosciences, USA) was incubated at 4°C for 24 h. The lentiviral particles were then collected by centrifugation at 1500 × g for 30 min and dissolved in Phosphate Buffer Saline (PBS).
HepG2 and Bel7402 cells were seeded into 6-well plates at the concentration of 5 × 104 cell/well. Lentiviral particles with shSMC1A or Negative Control (NC) were added into HepG2 cells after 12 h of incubation. Then, after 24 h of infection, the medium with lentivirus particles was replaced with refresh growth medium. Stable cell lines were selected out in 5 μg/mL puromycin for 4 days.
In vivo xenograft tumor growth
For the xenograft tumor growth assay, HepG2 stable cell lines with SMC1 knockdown and reexpression of wild type or S957DS966D mutant SMC1 (1×106) were injected subcutaneously into the right flank of 6-week-old male BALB/C nude mice (N=5). Tumors were cultivated for 32 days and the tumor volume was measured every 4 days. All animal experiments were approved by the Committee of China Medical University.
Western Blotting Analysis
Proteins were loaded and separated on 10% Sodium Dodecyl Sulfate (SDS) polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, USA). The membranes were blocked in TBS/T buffer (pH 7.5, 20 mM Tris-HCl, 150 mM Nacl, 0.1% Tween-20) with 5% Bull Serum Albumin (BSA) at room temperature for 1 h. Then the membranes were incubated with rabbit anti-SMC1 (ab21583, Abcam), rabbit anti-SMC1 (phospho S966) (ab1276, Abcam), rabbit anti-PCNA (10205-2-AP, Proteintech), rabbit anti-MMP9 (10375-2-AP, Proteintech) or mouse anti-flag (A00187-100, Genscript) at 4 °C overnight. The secondary antibody with horseradish peroxidase (HRP)-conjugated were incubated for 1 h at room temperature. The signals were developed with (Tanon, China).
Cell proliferation assay
Cell Counting Kit-8 (CCK8) (CK04, Dojindo) assays were performed to evaluate cell proliferation ability. Cells were seeded into 96-well plates at a density of 3 × 103 cells/well. Subsequently, the medium was replaced with 90 μl basic RPIM 1640 medium and 10 μl CCK8. After incubation at 37 °C for 2 h, the absorbance of each well at 450 nm was measured by Absorbance Reader (TECAN, Switzerland).
Cell Migration assay
Cell migration abilities were proceeded by transwell determined by transwell (Corning Life Sciences, MA, USA) migration assay. 3 × 104 cells were seeded into the upper chamber of 24-well plate with 8.0 µm pore polycarbonate filter membrane. The top chamber was added with serum-free RPIM 1640 medium, while the medium of lower chamber was supplemented with 10% FBS in order to produce a chemoattractant effect. After culturing the cells at 37 °C overnight, the non-migrating cells were removed with cotton buds and the lower cells were fixed with ice-methanol and stained with giemsa dye overnight. The number of migrating cells was the average value of total 5 random fields cells.
HCC Specimens
The tissue microarrays of HCC and corresponding adjacent liver tissue were purchased from Shanghai Outdo Biotech Company, China (Cat. No. HLiv-H180Sur-10). SMC1A phosphorylation expression was indentified in 78 cases with detailed patient clinical stage and survival information.
Immunohistochemical (IHC) analysis
The paraffin-embedded sections were deparaffinized with xylene and rehydrated in dH2O. The further immunohistochemistry staining steps were proceeded with UltraSensitiveTM SP (Mouse/ Rabbit) IHC Kit (KIT-9720, MXB Biotechnologies, China). Antigen retrieval was carried out with high pressure heating in citrate buffer (pH 6.0) for 2 min. Sections were incubated with rabbit anti-SMC1 (phospho S966) (ab1276, Abcam) overnight at 4 °C. Finally, the staining results were observed by DAB kit (DAB-0031, MXB Biotechnologies, China). Nuclear immunoreactivity was semiquantitated by evaluating the percentage of positive-staining tumor cells over total tumor cells. The intensity of SMC1 phosphorylation expression was scored by using 5% increments (0%, 5%, 10%, 15%…, 100%; 10% = score of 1). The scores were evaluated by two persons and mean values were analyzed statistically.
Statistical analysis
Descriptive statistics were calculated for all the variables, including continuous variables (reported as mean values and standard deviations) and categorical variables (reported as numbers and percentages). Participators were divide into two different groups according to SMC1A phosphorylation immunostaining as Low (0-4) and High (5-9). The differences between groups were evaluated using t test for continuous data and Chi-square test for categorical data. Kaplan-Meier survival analysis was used to assess the association of SMC1A phosphorylation expression and HCC prognosis. All the statistical analyses were performed using SPSS version 22.0 software (SPSS Inc, Chicago IL, USA) and p-values less than 0.05 were considered statistically significant.
Acknowledgements
This work was supported by National Key R&D Program of China (2016YFC1302400), Natural Science Foundation of China (81502414, 81770001, 81130042, 31171323, 31300963); Ministry of Education Innovation Team Development Plan (IRT13101/17R107); Natural Science Foundation of Liaoning Province of China (LFWK201725, LQNK201747); New Teacher Foundation of China Medical University (XZR20160039).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Siegel RL, KD Miller, A Jemal. Cancer statistics. CA Cancer J Clin. 2015;65(1):p. 5-29
2. Forner A, JM Llovet, J Bruix. Hepatocellular carcinoma. Lancet. 2012;379(9822):p. 1245-55
3. Wang J, Yu S, Cui L. et al. Role of SMC1A overexpression as a predictor of poor prognosis in late stage colorectal cancer. BMC Cancer. 2015;15:p. 90
4. Pan XW, Gan SS, Ye JQ. et al. SMC1A promotes growth and migration of prostate cancer in vitro and in vivo. Int J Oncol. 2016;49(5):p. 1963-1972
5. Kim ST, B Xu, MB Kastan. Involvement of the cohesin protein, Smc1, in Atm-dependent and independent responses to DNA damage. Genes Dev. 2002;16(5):p. 560-70
6. Yan R, BD McKee. The cohesion protein SOLO associates with SMC1 and is required for synapsis, recombination, homolog bias and cohesion and pairing of centromeres in Drosophila Meiosis. PLoS Genet. 2013;9(7):p. e1003637
7. Huber RG, Kulemzina I, Ang K. et al. Impairing Cohesin Smc1/3 Head Engagement Compensates for the Lack of Eco1 Function. Structure. 2016;24(11):p. 1991-1999
8. Luo Y, Deng X, Cheng F. et al. SMC1-mediated intra-S-phase arrest facilitates bocavirus DNA replication. J Virol. 2013;87(7):p. 4017-32
9. Wakeman TP, Kim WJ, Callens S. et al. The ATM-SMC1 pathway is essential for activation of the chromium[VI]-induced S-phase checkpoint. Mutat Res. 2004;554(1-2):p. 241-51
10. Cobbe N, Heck MM. Heck, Review: SMCs in the world of chromosome biology- from prokaryotes to higher eukaryotes. J Struct Biol. 2000;129(2-3):p. 123-43
11. Bauerschmidt C, Woodcock M, Stevens DL. et al. Cohesin phosphorylation and mobility of SMC1 at ionizing radiation-induced DNA double-strand breaks in human cells. Exp Cell Res. 2011;317(3):p. 330-7
12. Schär P, Fäsi M, Jessberger R. SMC1 coordinates DNA double-strand break repair pathways. Nucleic Acids Res. 2004;32(13):p. 3921-9
13. Díaz-Martínez LA, Beauchene NA, Furniss K. et al. Cohesin is needed for bipolar mitosis in human cells. Cell Cycle. 2010;9(9):p. 1764-73
14. Yi F, Wang Z, Liu J. et al. Structural Maintenance of Chromosomes protein 1: Role in Genome Stability and Tumorigenesis. Int J Biol Sci. 2017;13(8):p. 1092-1099
15. Barber TD, McManus K, Yuen KW. et al. Chromatid cohesion defects may underlie chromosome instability in human colorectal cancers. Proc Natl Acad Sci U S A. 2008;105(9):p. 3443-8
16. Cucco F, Servadio A, Gatti V. et al. Mutant cohesin drives chromosomal instability in early colorectal adenomas. Hum Mol Genet. 2014;23(25):p. 6773-8
17. Zhou P, Xiao N, Wang J. et al. SMC1A recruits tumor-associated-fibroblasts (TAFs) and promotes colorectal cancer metastasis. Cancer Lett. 2017;385:p. 39-45
18. Yadav S, Sehrawat A, Eroglu Z. et al. Role of SMC1 in overcoming drug resistance in triple negative breast cancer. PLoS One. 2013;8(5):p. e64338
19. Ma Z, Lin M, Li K. et al. Knocking down SMC1A inhibits growth and leads to G2/M arrest in human glioma cells. Int J Clin Exp Pathol. 2013;6(5):p. 862-9
20. Yang Y, Zhang Z, Wang R. et al. siRNA-mediated knockdown of SMC1A expression suppresses the proliferation of glioblastoma cells. Mol Cell Biochem. 2013;381(1-2):p. 209-15
21. Hömme C, Krug U, Tidow N. et al. Low SMC1A protein expression predicts poor survival in acute myeloid leukemia. Oncol Rep. 2010;24(1):p. 47-56
22. Yazdi PT, Wang Y, Zhao S. et al. SMC1 is a downstream effector in the ATM/NBS1 branch of the human S-phase checkpoint. Genes Dev. 2002;16(5):p. 571-82
23. Kitagawa R, Bakkenist CJ, McKinnon PJ. et al. Phosphorylation of SMC1 is a critical downstream event in the ATM-NBS1-BRCA1 pathway. Genes Dev. 2004;18(12):p. 1423-38
24. Maga G, Hubscher U. Proliferating cell nuclear antigen (PCNA): a dancer with many partners. J Cell Sci. 2003;116(Pt 15):p. 3051-60
25. Guacci V, Koshland D, Strunnikov A. A direct link between sister chromatid cohesion and chromosome condensation revealed through the analysis of MCD1 in S. cerevisiae. Cell. 1997;91(1):p. 47-57
26. Tóth A, Ciosk R, Uhlmann F. et al. Yeast cohesin complex requires a conserved protein, Eco1p(Ctf7), to establish cohesion between sister chromatids during DNA replication. Genes Dev. 1999;13(3):p. 320-33
Author contact
![]() Corresponding authors: lcaoedu.cn; sweiedu
Corresponding authors: lcaoedu.cn; sweiedu

 Global reach, higher impact
Global reach, higher impact