10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2018; 14(10):1163-1174. doi:10.7150/ijbs.25920 This issue Cite
Research Paper
Topical Application of Tat-Rac1 Promotes Cutaneous Wound Healing in Normal and Diabetic Mice
1. Department of Pathology, University of Colorado Denver Anschutz Medical Campus, Aurora, CO, USA.
2. Department of Biomedical Research, National Jewish Health, CO, USA.
3. Department of Ophthalmology, University of Colorado Denver Anschutz Medical Campus, Aurora, CO, USA.
4. Molecular Throughput Inc., Las Vegas, NV89118, USA.
5. Current address: Department of Dermatology, Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, China
6. Current address: Institute of Combined Injury, State Key Laboratory of Trauma, Burn and Combined Injury, Chongqing Engineering Research Center for Nanomedicine, College of Preventive Medicine, Third Military Medical University, Chongqing, China
7. Department of Pathology, the First Affiliated Hospital of Kunming Medical University, Kunming, P.R. China
8. Current address: Department of Dermatology, Xijing Hospital, Fourth Military Medical University, Xi'an, Shanxi, China
9. Current address: Department of Dermatology, Peking University International Hospital, Beijing 102206, China
10. Veterans Affairs Medical Center, VA Eastern Colorado Health Care System, Aurora, CO, USA.
*The authors contributed equally to this work.
Abstract
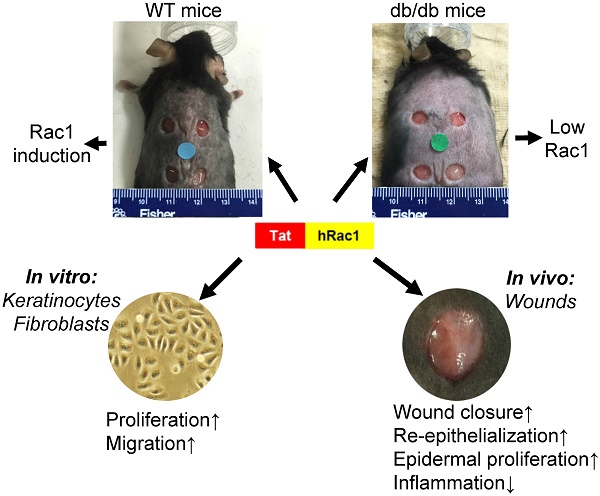
The endogenous small GTPase, Rac1, plays a critical role during normal skin wound healing. It remains to be determined whether endogenous Rac1 can be appropriately activated in chronic wounds; if not, whether exogenous Rac1 has therapeutic effects on wound healing. Here we show that Rac1 protein levels were lower in wounds of db/db diabetic mice than wounds in wild type mice during the healing process. To assess the therapeutic potential of exogenous Rac1 in wound healing, we produced a Tat-Rac1 fusion protein that enters into cells through protein transduction. Tat-Rac1 increased proliferation and migration of keratinocytes and dermal fibroblasts in vitro. Topical application of Tat-Rac1 accelerated cutaneous wound closure in vivo in db/db mice as well as wild type mice. Further analyses revealed that Tat-Rac1 had faster re-epithelialization, higher keratinocyte proliferation and migration without an earlier onset of myofibroblast activation than vehicle treated wounds. Tat-Rac1 also reduced inflammation in wounds. Our findings revealed the failure of diabetic wounds to elevate Rac1 expression and suggested a therapeutic strategy utilizing a Rac1-based biologic to compensate for this defect thereby promoting wound healing.
Keywords: Rac1, Tat-Rac1, wound healing, diabetic wounds

 Global reach, higher impact
Global reach, higher impact