10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2018; 14(11):1504-1512. doi:10.7150/ijbs.25001 This issue Cite
Research Paper
Depressive symptoms in patients with irritable bowel syndrome: a meta-analysis of comparative studies
1. The National Clinical Research Center for Mental Disorders & Beijing Key Laboratory of Mental Disorders, Beijing Anding Hospital, Capital Medical University, Beijing, China;
2. Guangdong Mental Health Center, Guangdong General Hospital & Guangdong Academy of Medical Sciences, Guangdong Province, China;
3. Unit of Psychiatry, Faculty of Health Sciences, University of Macau, Macao SAR, China;
4. Department of Gastroenterology, China-Japan Friendship Hospital, Beijing, China;
5. The Affiliated Brain Hospital of Guangzhou Medical University (Guangzhou Huiai Hospital), Guangzhou, China;
6. Department of Psychiatry, University of Melbourne, Melbourne, Victoria, Australia;
7. University of Notre Dame Australia, Perth, Australia;
8. Division of Psychiatry, University of Western Australia Medical School, Perth, Australia;
9. School of Public Health, Jilin University, Jilin province, China
# These authors contributed equally to the work.
Received 2018-1-17; Accepted 2018-5-13; Published 2018-8-15
Abstract
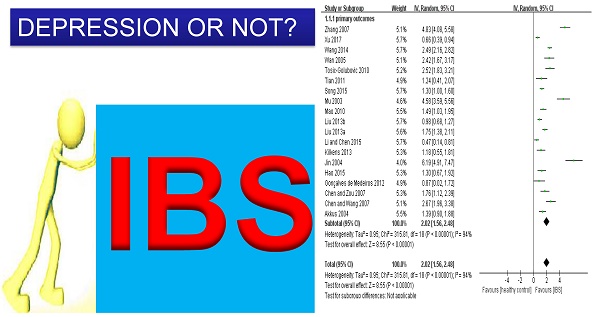
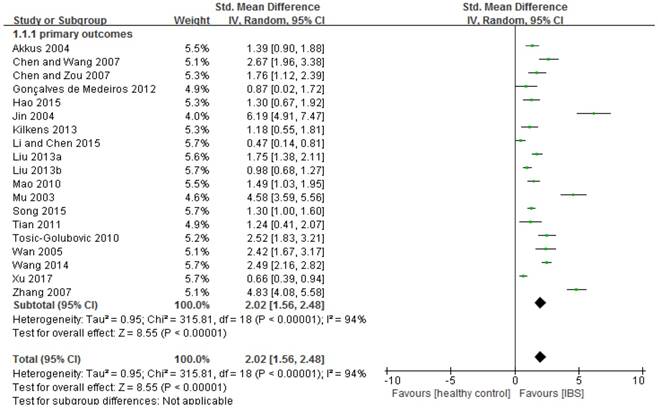
Depression is common in patients with irritable bowel syndrome (IBS), but the reported prevalence across different studies is inconsistent. This meta-analysis systematically examined the presence and severity of depressive symptoms in patients with IBS. Two investigators independently performed a literature search. The pooled depressive symptom severity was calculated using a random effects model. Subgroup, sensitivity and meta-regression analyses were conducted to examine the moderating factors of the development of depressive symptoms. Twenty four studies (n=2,837) comparing depressive symptoms between IBS patients (n=1,775) and healthy controls (n=1,062) were identified; 14 (58.3%) studies were rated as high quality. Compared to healthy controls, IBS patients had more frequent (OR=9.21, 95%CI: 4.56-18.57, P<0.001; I2=76%) and more severe depressive symptoms (n=1,480, SMD=2.02, 95%CI: 1.56-2.48, P<0.001; I2=94%). Subgroup analyses revealed that patients with all IBS subtypes had more severe depressive symptoms than controls. In addition, versions of the Hamilton Depression Rating Scale (HAM-D) and IBS diagnostic criteria were significantly associated with depressive symptom severity. Meta-regression analyses revealed that female gender, younger age and small sample size were significantly associated with more severe depressive symptoms. In conclusion, meta-analytic data showed that IBS patients had more frequent and severe depressive symptoms than healthy controls. Adequate screening and treatment for depression should be developed and implemented in this patient population.
Keywords: IBS, depressive symptoms, controlled studies, meta-analysis
Introduction
Irritable bowel syndrome (IBS) is one of the most common functional gastrointestinal (GI) diseases with the prevalence of around 20% in the general population [1-3]. The Rome diagnostic criteria are the diagnostic standard for research and clinical care of IBS [4, 5]. According to the predominant stool pattern, IBS is traditionally classified into four subtypes; IBS with constipation (IBS-C), IBS with diarrhea (IBS-D), mixed IBS (IBS-M) and non-subtyped IBS (IBS-U) [6]. Typical symptoms of IBS include recurrent abdominal pain, bloating, change in bowel habits without detectable structural or biochemical abnormalities [7]. IBS is associated with poor quality of life, impaired social functions [8, 9] and psychological-psychiatric conditions, such as depression [10]; approximately 20-40% of IBS patients present with depressive symptoms [11, 12].
The close association between IBS and depression is supported by psychophysiological and neuro-imaging studies [13, 14], and this association might be related to the 'brain-gut axis' that is defined as the bidirectional connecting system through neural, neuroimmune and neuroendocrine pathways between the digestive system and the brain [15]. Psychosocial factors can affect the gut physiology via the brain-gut axis in IBS [10, 16]. Antidepressants are effective to some extent in treating IBS directly through the brain-gut axis independent of changes in depressive symptoms [17, 18].
The relationship between IBS and depression has not been consistent across studies. IBS has been associated with more severe depressive symptoms compared to healthy controls in some [19-22], but not all [23, 24] studies. Additionally, the association between IBS subtype and depressive symptoms is also uncertain with studies finding either an association with IBS-C [25] or IBS-D [26, 27] but not with other subtypes [25], or not at all[28, 29].
Two meta-analyses [27, 30] concluded that IBS patients had more severe depressive symptoms than healthy controls, but the association between IBS subtypes and depressive symptoms was inconsistent. These studies only covered English databases and the included studies employed self-reported scales on depressive symptoms, such as the Hospitalization Anxiety and Depression Scale (HADS) and the Beck Depression Inventory (BDI). As the reliability of self-reported scales are affected by impaired insight and cognitive functions that are common in depression, investigator-rated tools, such as the Hamilton Depression Rating Scale (HAMD) [31, 32] are generally thought to be more objective and suitable for research purposes.
To the best of our knowledge, no meta-analysis or systemic review on depressive symptoms in IBS using interviewer-rated tools have been published. Thus, the aim of this study was to conduct a systematic meta-analysis to compare objectively rated depressive symptoms between IBS patients and healthy controls, and examine the association between IBS subtypes and depressive symptoms.
Methods
Search strategy
The meta-analysis was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [33]. Two authors (ZQE and WF) independently performed a literature search using both English (PubMed, PsycINFO, Embase, Cochrane Library) and Chinese (Wan Fang, SinoMed and Chinese National Knowledge Infrastructure (CNKI)) databases, from their inception to September 12, 2017 with the following search terms: ("irritable bowel syndrome" OR "colon diseases, functional" OR "functional bowel diseases" OR "IBS") AND ("depressive" OR "depression" OR "melancholia"). Furthermore, the references of included studies, meta-analyses and review papers were manually searched [27, 30, 34] to identify additional relevant studies.
Selection criteria
The search results were imported into the EndNote X7 software (Thomson Reuters, Philadelphia, PA, USA). The inclusion criteria were based on the PICOS acronym: Participants (P): Patients with IBS according to any diagnostic criteria. Intervention (I): not applicable. Comparison (C): healthy controls. Outcomes (O): depressive symptoms assessed with validated interviewer-rated scales. To assess depressive symptoms more objectively, only studies using investigator-rated scales were included. Study design (S): published case-control, cohort (only baseline data were included) and cross-sectional studies. Studies were excluded if they (1) made no comparisons between patients with IBS/IBS subtype and healthy controls; (2) did not provide meta-analyzable data on depressive symptoms. If more than one publication were published based on the same dataset, only publications with complete data were included.
Data extraction
Two reviewers (ZQE and WF) independently checked and extracted data from the studies using a pre-defined electronic Excel form: first author, year of publication, country, study design, IBS diagnostic criteria, and the assessment tools and means and standard deviations (SDs) of depressive symptoms. The first or corresponding authors were contacted for more information if relevant data were incomplete. Extracted data were analyzed independently by two reviewers (ZQE and QG). Any controversy was resolved by consensus or with the involvement of a third reviewer (WZ).
Statistical analysis
Data analyses were performed using the Review Manager Version 5.3 software (http://www.cochrane.org) and the Comprehensive Meta Analysis, Version 2.2.064 (http://www.Meta-Analysis.com), according to the recommendation of the Cochrane Handbook for Systematic Reviews [35]. Due to the unavoidable heterogeneity in study characteristics, the random effects model was used to synthesize the data. Heterogeneity was examined by the I2 and Q statistics. Significant heterogeneity was considered when I2 values were of >50% or P<0.1 in the Q statistics [36, 37]. For continuous and dichotomous outcomes SMDs and odds ratios (OR), respectively were calculated to evaluate the results' effect size (ES). ES values over 0.8, 0.5-0.8 and 0.2-0.5 constituted large, medium and small effect sizes, respectively [38]. Subgroup and sensitivity analyses were conducted to explore potential sources of heterogeneity. Subgroup analyses were performed according to the following variables: 1) subtypes: IBS-C vs. IBS-D vs. IBS-M vs. IBS-U; 2) Chinese studies vs. non-Chinese studies; 3) IBS diagnostic criteria: Rome I vs. Rome II vs. Rome III; 4) treatment settings: inpatients vs. outpatients vs. mixed; 5) HAMD versions: HAMD-17 vs. HAMD-24 vs. HAMD-not reported (NR); 6) refractory vs. non-refractory IBS. Random effects meta-regression was used to evaluate the impact of continuous moderating variables, such as age, proportion of females, sample size and the Newcastle-Ottawa Scale (NOS) scores with the primary outcome [39]. Potential publication bias was assessed with the funnel plots and Egger's regression test [40, 41].
Assessment of study quality
Two reviewers (ZQE and WF) independently evaluated the methodological quality of each study using the NOS [42, 43], which has a score ranging from 0 to 9 points. The total NOS score of ≥7 points were rated as high quality [44, 45].
Results
Literature search
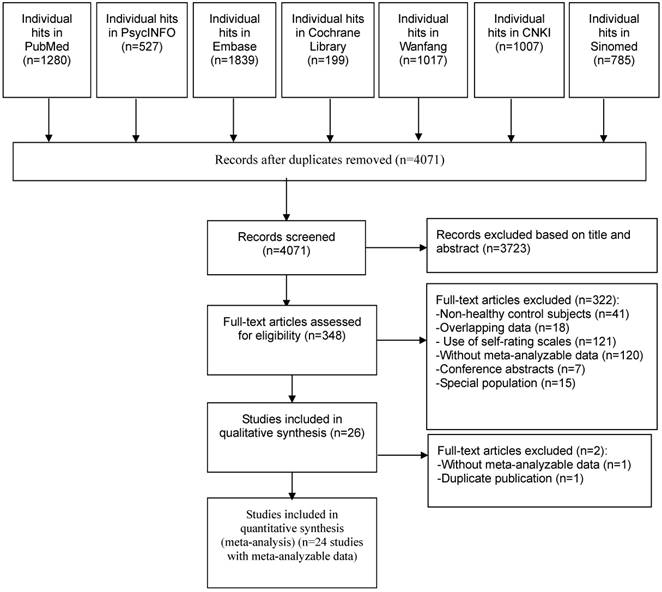
Out of 6,654 studies, 4,071 were identified after duplicate publications were removed. Eventually, 24 studies met full criteria and were included in the meta-analysis. The screening process according to the PRISMA flow diagram is presented in Figure 1. One study [46] reported data on both refractory and non-refractory IBS patients separately, therefore the data were extracted and analyzed as two separate arms. In order to avoid inflating the sample size in the control group, half numbers of healthy controls were assigned to each arm in the analyses.
PRISMA flow diagram
Characteristics of the studies included in the meta-analysis
| Authors | Country | N | Design, n (IBS/control) | Assessment scales on depressive symptoms | Patients with IBS | Healthy controls | NOS scores | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IBS diagnostic criteria | Subject type | Mean age (yrs) | Male (%) | Subject type | Mean age (yrs) | Male (%) | ||||||
| Akkus et al, 2004 | Turkey | 82 | Case control, 32/50 | HAMD-17 | Rome I | Outpatients | 44.8 | 59.4 | Hospital staff; Healthy volunteers | 47.6 | 44.0 | 7 |
| Chen and Wang et al, 2007 | China | 60 | Case control, 30/30 | HAMD | Rome II | Inpatients; Outpatients | NR | 40.0 | Health examination population; Healthy volunteers | NR | 43.3 | 8 |
| Chen and Zou et al, 2007 | China | 54 | Case control, 27/27 | HAMD-24 | Rome II | Outpatients | 40.3 | 44.4 | Health examination population | 37 | 48.2 | 6 |
| Gonçalves de Medeiros et al, 2012 | United Kingdom | 27 | RCT, 21/8 | HAMD | Rome II | Outpatients | 39.9 | 23.8 | Healthy volunteers | 32.3 | 75.0 | 6 |
| Hao et al, 2015 | China | 50 | Case control, 30/20 | HAMD | Rome III | Inpatients | 38C | 53.3 | Health examination population | 42C | 55.0 | 8 |
| Jin et al, 2004 | China | 58 | RCT, 36/22 | HAMD-17 | Rome II | Inpatients; Outpatients | 42.7 | 44.4 | Health examination population | 41.1 | 59.1 | 5 |
| Kilkens et al, 2013 | Netherlands | 46 | Case control, 23/23 | HAMD-17 | Rome II | Outpatients | 32.9 | 39.1 | Healthy volunteers | 28.6 | 39.1 | 8 |
| Li and Chen et al, 2015 | China | 140 | Case control, 70/70 | HAMD-24 | Rome III | Outpatients | 51.0 | 48.6 | Health examination population | 49 | 50.0 | 7 |
| Li et al, 2015 | China | 64 | Case control, 32/32 | HAMD-24 | Rome III | Inpatients; Outpatients | 40.8 | 34.4 | Health examination population; Healthy volunteers | 39 | 31.3 | 8 |
| Liu et al, 2013 | China | 801 | Cross-sectional, 601/100 | HAMD-17 | Rome III | Outpatient | 38.3 | 49.5 | Health examination population | 39.7 | 45.0 | 7 |
| Mao et al, 2010 | China | 96 | Case control, 56/40 | HAMD | Rome III | Inpatients | 35.2 | 32.1 | Health examination population | 32.2 | 35.0 | 6 |
| Mu et al, 2003 | China | 60 | Case control, 30/30 | HAMD | Rome II | Outpatients | NR | 33.3 | Hospital staff | NR | 40.0 | 5 |
| Shi et al, 2012 | China | 90 | Case control, 60/30 | HAMD-24 | Rome III | Inpatients; Outpatients | NR | 30.0 | Health examination population | NR | 66.7 | 8 |
| Shi and Zhang et al, 2012 | China | 57 | Case control, 32/25 | HAMD-17 | Rome III | Outpatients | 40.0 | 40.6 | NR | 39.2 | 56.0 | 6 |
| Song et al, 2015 | China | 204 | Case control, 102/102 | HAMD-24 | Rome III | Outpatients | 48.1 | 38.2 | Health examination population | 42.3 | 42.2 | 7 |
| Tian et al, 2011 | China | 30 | Case control, 20/10 | HAMD | Rome III | NR | 45C | 55.0 | Health examination population | 42C | 60.0 | 7 |
| Tosic-Golubovic et al, 2010 | Serbia | 60 | Case control, 30/30 | HAMD | Rome II | Outpatients | 43.9 | 50.0 | Community | 41.6 | 50.0 | 8 |
| Wan et al, 2005 | China | 50 | Case control, 30/20 | HAMD | Rome II | Outpatients | 37.0 | 0 | Hospital staff; Patient's relative; Students | 38 | 0 | 6 |
| Wang et al, 2012 | China | 116 | Case control, 56/60 | HAMD-24 | Rome II | Inpatients; Outpatients | NR | NR | Health examination population; Patient's relative | NR | NR | 6 |
| Wang et al, 2014 | China | 260 | Case control, 150/110 | HAMD | Rome III | NR | NR | 41.3 | Health examination population; Patient's relative | NR | NR | 8 |
| Xu et al, 2012 | China | 134 | Case control, 69/65 | HAMD-24 | Rome III | Inpatients; Outpatients | NR | 42.0 | Hospital staff; Patient's relative | NR | 50.8 | 8 |
| Xu et al, 2014 | China | 215 | Case control, 112/103 | HAMD-24 | Rome III | Outpatients | 63.7 | 69.6 | Healthy volunteers | 56.3 | 57.3 | 5 |
| Xu et al, 2017 | China | 66 | Cross-sectional, 46/20 | HAMD-24 | Rome III | Outpatients | 34.2 | 49.1 | Healthy volunteers | 29.5 | 60.0 | 8 |
| Zhang et al, 2007 | China | 115 | Case control, 80/35 | HAMD | Rome II | NR | 20-73 | 55.0 | NR | 20-60 | 45.7 | 5 |
a Only data from IBS subjects and healthy control groups were extracted if there were multiple study arms.
b Rome I/II/III are standard criteria for diagnosis of IBS.
c median age.
HAMD=Hamilton Depression Rating Scale; IBS= irritable bowel syndrome; NR=not reported; NOS=Newcastle-Ottawa Scale; yrs=years; RCT=randomized controlled trial.
Study characteristics
There were 2,837 subjects (1,775 IBS patients and 1,062 healthy controls) in the 24 studies (Table 1). Twenty studies were conducted in China (n=2,620) [46-65], and one each in Serbia (n=60) [66], The Netherlands (n=46) [67], United Kingdom (n=29) [68] and Turkey (n=76) [69]. IBS was diagnosed using the Rome I criteria in one [69], Rome II criteria in 10 [50, 56, 58-60, 62, 65-68], and Rome III criteria in 13 studies [46-49, 51-55, 57, 61, 63, 64]. The severity of depressive symptoms was assessed using the Hamilton Depression Rating Scale (HAMD-17) in 5 studies [46, 61, 65, 67, 69] and HAMD-24 in 9 studies [50-53, 55, 57, 60, 63, 64], but HAMD versions that were not reported (HAMD-NR) in 10 studies [47-49, 54, 56, 58, 59, 62, 66, 68]. One study included female subjects only [56]. The mean age ranged from 32.9 to 63.7 years, and the proportion of males ranged from 0% to 69.6% in the patient group.
Depressive symptoms in IBS: forest plot of HAMD total scores
Quality assessment
The NOS score assessing quality of the studies ranged from 4 to 8 points (Supplemental Table 1); 58.3% of studies (n=14) [46, 48-50, 52-55, 57, 63, 64, 66, 67, 69] were assessed as “high quality” (NOS≥7).
Primary outcome
Compared to healthy controls, IBS patients had more severe depressive symptoms (n=1,480, SMD=2.02, 95%CI: 1.56-2.48, P<0.001; Figure 2). The funnel plot showed asymmetry, while Egger's regression test showed publication bias (P=0.005). Sensitivity analysis revealed that the significance in bias remained (n=1,334, SMD=1.51, 95%CI: 1.16-1.86, P<0.001) after excluding three outlying studies (i.e., SMD>3) [59, 62, 65]. Subgroup analyses further revealed that the significance remained in all of the 18 subgroup analyses (Table 2; Supplemental figure 3). In addition, IBS diagnostic criteria (p=0.01) and HAMD versions (p=0.002) were significantly associated with more severe depressive symptoms compared with the control group (Table 2). In meta-regression analyses younger age (slope=-0.033, p<0.001), proportion of female gender (slope=0.021, p<0.001) and small sample size (slope=-0.002, p<0.001) were significantly associated with more severe depressive symptoms. NOS scores did not have significant impact on the primary outcome (slope =0.095, P=0.08).
Secondary outcomes
The Hamilton Depression Rating Scale (HAM-D) contains six separate factors, namely factor I (anxiety/somatization), factor II (weight), factor III (cognitive disturbance), factor IV (diurnal variation), factor V (psychomotor retardation) and factor VI (sleep disturbances) [70-73]. Compared to healthy controls, IBS patients had significantly higher scores in most HAM-D factors (SMD=4.03 to 13.54, 95%CI: 1.28-22.45, P<0.001; I2=41% to 99%, Supplemental Figure 2): anxiety/somatization (SMD=4.03), weight (SMD=4.78), psychomotor retardation (SMD=4.23) and sleep disturbances (SMD=13.54).
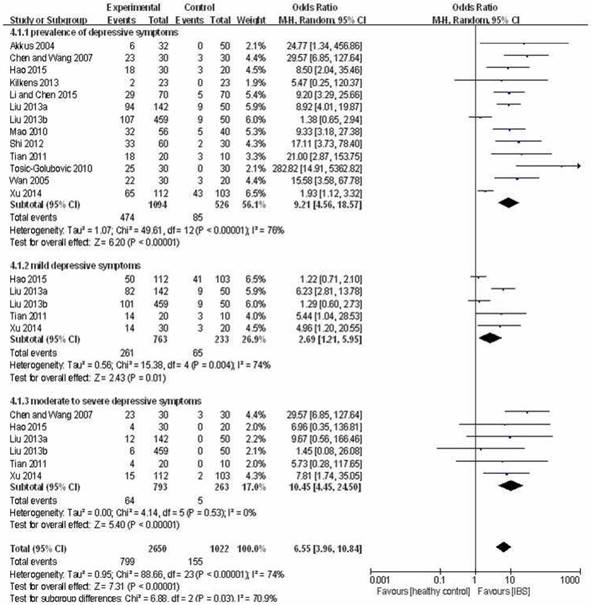
Prevalence of depressive symptoms (HAM-D total score > 7) in IBS patients was higher than in healthy controls (OR=9.21, 95%CI: 4.56-18.57, P<0.001; I2=76%, Figure 3). In addition, there was higher prevalence of mild depressive symptoms (HAM-D total score: 8-19) (OR=2.69, 95%CI: 1.21-5.95, P=0.01; I2=74%, Figure 3) as well as moderate to severe depressive symptoms (HAM-D total score ≥ 20) (OR=10.45, 95%CI: 4.45-24.50, P<0.001; I2=0%, Figure 3) in the IBS group than in the control group.
Discussion
This was the first meta-analysis on the frequency and severity of depressive symptoms measured by rater-administered scales in IBS. In psychiatric research the HAM-D is the most widely used scale of depressive symptoms with good psychometric properties [74, 75]. All studies in this meta-analysis measure the presence and severity of depressive symptoms using HAM-D scales, which significantly decreases the heterogeneity attributed to different assessment instruments.
Subgroup analyses of moderating variables of the primary outcome
| subgroups | Study arms (subjects) | SMDs (95%CI) | I2 (%) | Pha | P-value for each subgroup | P-value across subgroups |
|---|---|---|---|---|---|---|
| Overall | 18 (1480) | 2.02 (1.56, 2.48) | 94 | <0.001 | <0.001 | NA |
| Subtypes | ||||||
| IBS-C | 6 (145) | 2.38 (1.10, 3.67) | 95 | <0.001 | 0.0003 | 0.81 |
| IBS-D | 7(253) | 2.08 (1.46, 2.70) | 86 | <0.001 | <0.001 | |
| IBS-M | 3(27) | 2.50 (1.86, 3.14) | 26 | 0.26 | <0.001 | |
| IBS-U | 2(33) | 2.21 (1.69, 2.72) | 0 | 0.66 | <0.001 | |
| Study setting | 0.12 | |||||
| China | 14 (1374) | 2.17 (1.62, 2.71) | 95 | <0.001 | <0.001 | |
| Other countriesb | 4 (106) | 1.50 (0.86, 2.15) | 74 | 0.008 | <0.001 | |
| IBS diagnosis criteria | 0.01 | |||||
| Rome I | 1 (32) | 1.39 (0.90, 1.88) | NA | NA | <0.001 | |
| Rome II | 9 (307) | 2.95 (1.95, 3.95) | 93 | <0.001 | <0.001 | |
| Rome III | 8 (1141) | 1.30 (0.85, 1.74) | 92 | <0.001 | <0.001 | |
| Patients | 0.15 | |||||
| Inpatients | 2 (86) | 1.42 (1.05, 1.79) | 0 | 0.62 | <0.001 | |
| Outpatients | 11 (1078) | 1.57 (1.14, 1.99) | 91 | <0.001 | <0.001 | |
| Mixed | 2 (66) | 4.39 (0.94, 7.84) | 95 | <0.001 | 0.01 | |
| NR | 3 (250) | 2.85 (1.16, 4.54) | 95 | <0.001 | 0.0009 | |
| HAM-D version | 0.002 | |||||
| HAMD-17 | 4 (692) | 2.06 (1.19, 2.94) | 94 | <0.001 | <0.001 | |
| HAMD-24 | 4 (311) | 1.00 (0.51, 1.49) | 87 | <0.001 | <0.001 | |
| NR | 10 (477) | 2.42 (1.74, 3.10) | 92 | <0.001 | <0.001 | |
| IBS severity | 0.11 | |||||
| Refractory IBS | 3 (208) | 3.43 (1.50, 5.36) | 96 | <0.001 | 0.0005 | |
| Non-refractory IBS | 16 (1272) | 1.80 (1.32, 2.27) | 94 | <0.001 | <0.001 |
a P-value of heterogeneity analysis.
b One study each in Turkey, Serbia, Netherlands, and in the United Kingdom.
NA=Not applicable; NR=Not reported; SMDs=Standard mean differences; IBS-C=Constipation-predominant Irritable Bowel Syndrome; IBS-D=Diarrhea-predominant irritable bowel syndrome; IBS-M=Mixed Irritable Bowel Syndrome; IBS-U=Un-subtyped Irritable Bowel Syndrome; Rome I/II/III=A standardize criteria for diagnosis of IBS; HAM-D=Hamilton Depression Rating Scale.
Forest plot of the prevalence of depressive symptoms in IBS patients versus healthy controls
In this study, compared to healthy controls IBS patients had more severe depressive symptoms overall (SMD=2.02) and also in specific domains, namely anxiety/somatization (SMD=4.03), weight (SMD=4.78), psychomotor retardation (SMD=4.23) and sleep disturbances (SMD=13.54). Further, depressive symptoms were more frequent in IBS patients (OR=9.21), particularly moderate to severe depressive symptoms (OR=10.45). Both frequency and severity of depressive symptoms were higher in this study than in other meta-analyses [27, 30], which may be due to several reasons. First, this study focused more broadly on depressive symptoms rather than major depressive disorder. Second, studies of this meta-analysis only used the HAMD scales, which maintained the homogeneity of assessment compared to other meta-analyses that covered studies employing different self-reported tools to evaluate depressive symptoms [27, 30]. It is likely that patients who had severe to very severe depressive symptoms were unable to complete self-reported scales and were therefore excluded from studies included in previous meta-analyses. Using rater-administered scales is more likely to include patients with wider range of severity which could lead to a larger effect size in this study.
In this study all IBS subtypes were associated with increased risk of the development of depressive symptoms. Patients with IBS-M showed the largest effect size (SMD = 2.50; 95% CI, 1.86-3.14), which is different from studies that found IBS-C/IBS-D with the largest effect size [18, 25, 26]. Possible factors for this discrepancy may relate to the different number of included studies and measures of depressive symptoms across meta-analyses.
Subgroup and meta-regression analyses found that HAM-D versions (HAMD-17, HAMD-24 and HAMD-NR), IBS diagnostic criteria (ROME I, ROME II and ROME III), younger age, female gender and small sample size were significantly associated with more severe depressive symptoms. In terms of gender differences, earlier studies [76] found that women with IBS had more severe IBS symptoms and lower quality of life than men, regardless of diagnostic criteria used. In addition, the prevalence of IBS in women is approximately 1.5 to 3 fold higher than in men [77-80]. Further, in IBS patients with severe symptoms (>3 Manning criteria), 80% are women. The consensus in the literature is that women have more anxiety and depressive symptoms than men with IBS [81], which is supported by the current study, but not others [30].
IBS occurs in all age groups [82] although around half of those with IBS develop initial symptoms before age of 35 years [83]. As the prevalence of IBS and severity of pain usually decrease after the age of 50 years [84], there may be less depressive symptoms in older patients, which is consistent with our findings. Different sample sizes could influence the power to detect significant results [38], which could account for the association between sample size and the prevalence of depressive symptoms. The findings of clinical trials with small sample size are usually not stable, thus results of small studies should be interpreted with caution [85].
A previous study [30] showed no significant association between IBS diagnostic criteria and severity of depressive symptoms. However, in this study patients diagnosed according to Rome II or Rome III criteria had more severe depressive symptoms than healthy controls, while no significant difference was found between those diagnosed with Rome I criteria and controls. Reasons for the discrepancy may include the different depression scales used (self-reported scales vs. interviewer-rated scales) and the differences between the three diagnostic criteria in terms of the frequency and severity of IBS symptoms. For example, more IBS symptoms and stringent severity were adopted in Rome II than Rome III criteria, while Rome III criteria contain more items on the socioeconomic burden of IBS than Rome II [86]. Further, only one study using Rome I was included in this meta-analysis.
The results of this meta-analysis should be interpreted with caution due to several methodological limitations. First, studies included in the meta-analysis focused on depressive symptoms, but not major depressive disorder. The prevalence studies of major depressive disorder in IBS needs more sophisticated methodology. Second, there was publication bias in the meta-analysis. Third, high heterogeneity remained in some subgroup analyses. Fourth, relevant variables related to IBS, such as pharmacotherapy, were not examined due to incomplete information. Finally, most studies were conducted in China, which may lead to selection bias.
In conclusion, patients with IBS of all subtypes had more frequent and severe depressive symptoms than healthy controls, particularly female and younger patients. Regular screening on depressive symptoms and effective interventions should be developed for this patient population.
Acknowledgements
Funding
The study was supported in part by grants from the Beijing Municipal Administration of Hospitals Incubating Program (code: PX2016016), the Capital City Clinical Practice and Research Funding of Beijing Municipal Science & Technology Commission (Z141107002514033; Z151100004015042), the Clinical Medicine Development Funding of Beijing Municipal Administration of Hospitals (ZYLX201403; ZYLX201607), and the Beijing Municipal Administration of Hospital's Ascent Plan (DFL20151801).
Review registration: CRD42017076997.
Supplementary Material
Supplementary figures and table.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Soares RL. Irritable bowel syndrome: a clinical review. World J Gastroenterol. 2014;20:12144-60
2. Gwee K-A. Irritable bowel syndrome in developing countries-a disorder of civilization or colonization? Neurogastroenterol Motil. 2005;17:317-24
3. Drossman DA, Camilleri M, Mayer EA. et al. AGA technical review on irritable bowel syndrome. Gastroenterology. 2002;123:2108-31
4. Mearin F, Lacy BE. Diagnostic criteria in IBS: useful or not? Neurogastroenterol Motil. 2012;24:791-801
5. Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology. 2006;130:1377-90
6. Longstreth GF, Thompson WG, Chey WD. et al. Functional bowel disorders. Gastroenterology. 2006;130:1480-91
7. Saha L. Irritable bowel syndrome: Pathogenesis, diagnosis, treatment, and evidence-based medicine. World J Gastroenterol. 2014;20:6759-73
8. Occhipinti K, Smith JW. Irritable bowel syndrome: a review and update. Clin Colon Rectal Surg. 2012;25:46-52
9. Mönnikes H. Quality of life in patients with irritable bowel syndrome. J Clin Gastroenterol. 2011;45:S98-S101
10. Van Oudenhove L, Levy RL, Crowell MD. et al. Biopsychosocial aspects of functional gastrointestinal disorders: how central and environmental processes contribute to the development and expression of functional gastrointestinal disorders. Gastroenterology. 2016;150:1355-67
11. Talley NJ, Howell S, Poulton R. The irritable bowel syndrome and psychiatric disorders in the community: is there a link? Am J Gastroenterol. 2001;96:1072-9
12. Kabra N, Nadkarni A. Prevalence of depression and anxiety in irritable bowel syndrome: A clinic based study from India. Indian J Psychiatry. 2013;55:77-80
13. Jones MP, Crowell MD, Olden KW. et al. Functional gastrointestinal disorders: an update for the psychiatrist. Psychosomatics. 2007;48:93-102
14. Van Oudenhove L, Coen SJ, Aziz Q. Functional brain imaging of gastrointestinal sensation in health and disease. World J Gastroenterol. 2007;13:3438-45
15. Van Oudenhove L, Vandenberghe J, Demyttenaere K. et al. Psychosocial factors, psychiatric illness and functional gastrointestinal disorders: a historical perspective. Digestion. 2010;82:201-10
16. Weaver KR, Sherwin LB, Walitt B. et al. Neuroimaging the brain-gut axis in patients with irritable bowel syndrome. World J Gastrointest Pharmacol Ther. 2016;7:320-33
17. ACOGI Force, Brandt LJ, Chey WD. et al. An evidence-based position statement on the management of irritable bowel syndrome. Am J Gastroenterol. 2008;104(suppl 1):S1-35
18. Ford AC, Quigley EM, Lacy BE. et al. Effect of antidepressants and psychological therapies, including hypnotherapy, in irritable bowel syndrome: systematic review and meta-analysis. Am J Gastroenterol. 2014;109:1350-65
19. Seminowicz DA, Labus JS, Bueller JA. et al. Regional gray matter density changes in brains of patients with irritable bowel syndrome. Gastroenterology. 2010;139:48-57
20. Thijssen AY, Mujagic Z, Jonkers DM. et al. Alterations in serotonin metabolism in the irritable bowel syndrome. Alimentary Pharmacol Ther. 2016;43:272-82
21. Cho HS, Park JM, Lim CH. et al. Anxiety, depression and quality of life in patients with irritable bowel syndrome. Gut Liver. 2011;5:29-36
22. Sugaya N, Nomura S, Shimada H. Relationship between cognitive factors and anxiety in individuals with irritable bowel syndrome. Int J Behav Med. 2012;19:308-15
23. Ålander T, Heimer G, Svärdsudd K. et al. Abuse in women and men with and without functional gastrointestinal disorders. Dig Dis Sci. 2008;53:1856-64
24. Berman S, Suyenobu B, Naliboff BD. et al. Evidence for alterations in central noradrenergic signaling in irritable bowel syndrome. Neuroimage. 2012;63:1854-63
25. Muscatello MR, Bruno A, Pandolfo G. et al. Depression, anxiety and anger in subtypes of irritable bowel syndrome patients. J Clin Psychol Med Settings. 2010;17:64-70
26. Guthrie E, Creed F, Fernandes L. et al. Cluster analysis of symptoms and health seeking behaviour differentiates subgroups of patients with severe irritable bowel syndrome. Gut. 2003;52:1616-22
27. Fond G, Loundou A, Hamdani N. et al. Anxiety and depression comorbidities in irritable bowel syndrome (IBS): a systematic review and meta-analysis. Eur Arch Psychiatry Clin Neurosci. 2014;264:651-60
28. Camilleri M, McKinzie S, Busciglio I. et al. Prospective study of motor, sensory, psychologic, and autonomic functions in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2008;6:772-81
29. Farzaneh N, Ghobakhlou M, Moghimi-Dehkordi B. et al. Evaluation of psychological aspects among subtypes of irritable bowel syndrome. Indian J Psychol Med. 2012;34:144-8
30. Lee C, Doo E, Choi JM. et al. The increased level of depression and anxiety in irritable bowel syndrome patients compared with healthy controls: systematic review and meta-analysis. J Neurogastroenterol Motil. 2017;23:349-62
31. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56-62
32. Hedlund JL, Vieweg BW. The Hamilton rating scale for depression: a comprehensive review. Journal of Operational Psychiatry. 1979;10:149-65
33. Moher D, Liberati A, Tetzlaff J. et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535
34. Sibelli A, Chalder T, Everitt H. et al. A systematic review with meta-analysis of the role of anxiety and depression in irritable bowel syndrome onset. Psychol Med. 2016;46:3065-80
35. Higgins J E. Cochrane Handbook for Systematic Reviews of Interventions[J]. Naunyn-Schmiedebergs Archiv für experimentelle Pathologie und Pharmakologie. 2011;5(2):S38
36. Higgins JP, Thompson SG, Deeks JJ. et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-60
37. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539-58
38. Cohen DJ. Statistical power analysis for the behavioral sciences. Technometrics. 1988;31(4):499-500
39. Thompson SG, Sharp SJ. Explaining heterogeneity in meta-analysis: a comparison of methods. Stat Med. 1999;18:2693-708
40. Egger M, Davey Smith G, Schneider M. et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-34
41. Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046-1055
42. Zeng X, Zhang Y, Kwong J S. et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med. 2015;8:2-10
43. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603-5
44. Zhou FC, Wang YY, Zheng W. et al. Prospective memory deficits in patients with depression: A meta-analysis. J Affect disord. 2017;220:79-85
45. Asbridge M, Hayden JA, Cartwright JL. Acute cannabis consumption and motor vehicle collision risk: systematic review of observational studies and meta-analysis. BMJ. 2012;344:e536
46. Liu BB, Jia L, Jiang SM. et al. A large sample and multi-center survey of the depression and anxiety status of patients with refractory irritable bowel syndrome in Guangdong province (in Chinese). Chin J Behav Med Brain Sci. 2013;22:140-3
47. Mao HT, Tang YL. Analysis on mental status of the patients with irritable bowel syndrome (in Chinese). Clin Med Eng. 2010;17:1-2
48. Hao XL, Huang XL, Gao F. Analysis on patients of ulcerative colitis with anxiety and depression (in Chinese). Xinjiang Med J. 2015:885-6
49. Tian ZY, Feng LY. Anxiety and depression analysis on ulcerative colitis patients (in Chinese). Chin J Clin Ration Drug Use. 2011;04:11-2
50. Chen WK, Zou YY, Li FJ. et al. Changes of psychosocial factors, enteric mucosal mast celles and 5-hydroxytryptamine in irntabIe bowel syndrome (in Chinese). World Chin J Digestol. 2007;15:46-50
51. Xu YX. The clinical analysis about the relationship between IBS and psychogeny (in Chinese). Fudan Univ. 2014
52. Li S. Clinical studies on the expression of IL-4 and IL-6 in intestinal mucosa and its relation with anxiety-depression of IBS-D patients (in Chinese). North China Univ Sci Tech. 2015
53. Li XM, Chen P, Gao RP. et al. Comparison of the differences of anxiety and depression in the patients with various functional gastrointestinal disorders (in Chinese). Ningxia Med J. 2015;37:995-6
54. Wang GZ. The Correlation Analysis of Irritable Bowel Syndrome and Depression (in Chinese). World Latest Med Inform. 2014:24-5
55. Shi DW. The correlations about clinical symptoms with intestinal flora and the change of HPT in irritable bowel syndrome (in Chinese). Zhengzhou Univ. 2012
56. Wan HY, Chen YL. MentaI status and dysautonomia in patients with irritable boweI syndrome (in Chinese). Chin J Clin Rehabil. 2005;9:72-3
57. Xu F, Liu DS. Observation of the emotional condition in patients with irritable bowel syndrome (in Chinese). J Bengbu Med Coll. 2012;37:561-3
58. Chen CY, Wang Y, Lin Q. et al. The psychological factors of patients with refractory irritable bowel syndrome (in Chinese). J Clin Psychosom Dis. 2007;13:525-6
59. Zhang YJ, Wang P, Jin JJ. et al. The relationship between gastric motility, gastrointestinal hormones and psychological factors in patients with irritable bowel syndrome (in Chinese). J Clin Internal Med. 2007;24(1):54-6
60. Wang XP, Liu YT, Ren QT. Relationship of CRP with the state of anxiety and depression in irritable bowel syndrome (in Chinese). China Modern Doctor. 2012;50:146-7
61. Shi QL, Zhang CC. The relationship of negative emotions with 5-HT and 5-HIAA in peripheral blood of patients with constipation-predominant irritable bowel syndrome (in Chinese). Med Frontier. 2012:10-11
62. Mu B, Wang BM, Huang NX. et al. A study on psychological factor in the pat ients with IBS (in Chinese). J Tianjin Med Univ. 2003;9:543-4
63. Song JZ, Wang QM, Wang C. Symptom features of irritable bowel syndrome complicated with depression (in Chinese). Chin J Digestion. 2015:590-4
64. Xu XJ, Liu L, Yao SK. et al. Visceral sensitivity, gut barrier function and autonomic nerve function in patients with diarrhea-predominant irritable bowel syndrome (in Chinese). J Central South Univ (Med Sci). 2017;42:522-8
65. Jin DD, Xu M, Chen Z. et al. Emotional Disorders and Treatment in Patients with Refractory Irritable Bowel Syndrome (in Chinese). Chin J Gastroenterol. 2004;9:90-3
66. Tosic-Golubovic S, Miljkovic S, Nagorni A. et al. Irritable bowel syndrome, anxiety, depression and personality characteristics. Psychiatr Danub. 2010;22:418-24
67. Kilkens TO, Honig A, Maes M. et al. Fatty acid profile and affective dysregulation in irritable bowel syndrome. Lipids. 2004;39:425-31
68. Goncalves de Medeiros MT, de Oliveira RB, dos Santos AA. et al. The effects of sildenafil on rectal sensitivity and tone in patients with the irritable bowel syndrome. Aliment Pharmacol Ther. 2012;35:577-86
69. Akkus S, Senol A, Ayvacioglu NB. et al. Is female predominance in irritable bowel syndrome related to fibromyalgia? Rheumatol Int. 2004;24:106-9
70. Cleary P, Guy W. Factor analysis of the Hamilton depression scale. Drugs Exp Clin Res. 1977;1:115-20
71. Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278-96
72. Baumann U. Methodologic studies of the Hamilton Rating Scale for Depression. Arch Psychiatr Nervenkr. 1976;222:359-75
73. Zimmerman M, Martinez J H, Young D. et al. Severity classification on the Hamilton depression rating scale. J Affect Disord. 2013;150:384-388
74. Carneiro AM, Fernandes F, Moreno RA. Hamilton depression rating scale and montgomery-asberg depression rating scale in depressed and bipolar I patients: psychometric properties in a Brazilian sample. Health Qual Life Outcomes. 2015;13:42
75. López-Pina JA, Sánhez-Meca J, Rosa-Alcázar AI. The Hamilton Rating Scale for Depression: a meta analytic reliability generalization study. Int J Clin Health Psychol. 2009:9
76. Quigley EM, Bytzer P, Jones R. et al. Irritable bowel syndrome: the burden and unmet needs in Europe. Dig Liver Dis. 2006;38:717-23
77. Manning AP, Thompson WG, Heaton KW. et al. Towards positive diagnosis of the irritable bowel. Br Med J. 1978;2:653-4
78. Kennedy TM, Jones RH, Hungin AP. et al. Irritable bowel syndrome, gastro-oesophageal reflux, and bronchial hyper-responsiveness in the general population. Gut. 1998;43:770-4
79. Drossman DA, Thompson WG, Talley NJ. et al. Identification of sub-groups of functional gastrointestinal disorders. Gastroenterol Int. 1990;3:159-72
80. Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clin Epidemiol. 2014;6:71-80
81. Longstreth GF, Wolde-Tsadik G. Irritable bowel-type symptoms in HMO examinees. Dig Dis Sci. 1993;38:1581-9
82. Tang YR, Yang WW, Liang ML. et al. Age-related symptom and life quality changes in women with irritable bowel syndrome. World J Gastroenterol. 2012;18:7175-83
83. Maxwell PR, Mendall MA, Kumar D. Irritable bowel syndrome. Lancet. 1997;350:1691-5
84. Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:712-21
85. Zhang Z, Xu X, Ni H. Small studies may overestimate the effect sizes in critical care meta-analyses: a meta-epidemiological study. Crit Care. 2013;17:R2
86. Sperber AD, Shvartzman P, Friger M. et al. A comparative reappraisal of the Rome II and Rome III diagnostic criteria: are we getting closer to the 'true' prevalence of irritable bowel syndrome? Eur J Gastroenterol Hepatol. 2007;19:441-7
Author contact
![]() Corresponding authors: Yu-Tao Xiang, 3/F, Building E12, Faculty of Health Sciences, University of Macau, Avenida da Universidade, Taipa, Macau SAR, China. Fax: +853-2288-2314; Phone: +853-8822-4223; E-mail: xyutlycom; or Dr. Gang Wang, Mood Disorders Center, Beijing Anding Hospital, Beijing, China. E-mail: gangwangdoccom.
Corresponding authors: Yu-Tao Xiang, 3/F, Building E12, Faculty of Health Sciences, University of Macau, Avenida da Universidade, Taipa, Macau SAR, China. Fax: +853-2288-2314; Phone: +853-8822-4223; E-mail: xyutlycom; or Dr. Gang Wang, Mood Disorders Center, Beijing Anding Hospital, Beijing, China. E-mail: gangwangdoccom.

 Global reach, higher impact
Global reach, higher impact