10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2019; 15(1):158-168. doi:10.7150/ijbs.28392 This issue Cite
Research Paper
Exosomes Derived from Human Induced Pluripotent Stem Cells-Endothelia Cells Promotes Postnatal Angiogenesis in Mice Bearing Ischemic Limbs
Department of Vascular Surgery, RenJi Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China
*These authors equally contribute to this work.
Abstract
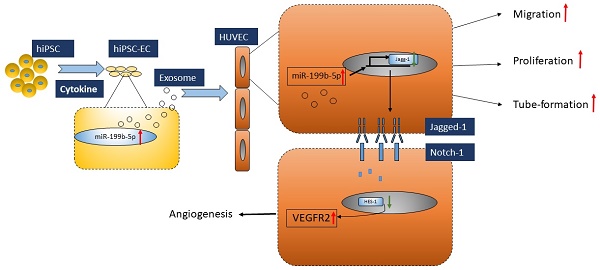
Induced pluripotent stem cell (iPSC) derived endothelial cells (ECs) is a novel therapeutic option for ischemic diseases. Although the detailed mechanism of this novel therapy remains unknown, emerging evidence has demonstrated that exosomes derived from hiPSC-ECs play a critical role in this approach. In this study, we first isolated and characterized the exosomes from iPSCs-ECs (hiPSC-EC-Exo) and determined the functional roles of hiPSC-EC-Exo in neovascularization and the underlying mechanism. Further, we evaluated the effect of exosomes derived from hiPS-ECs on promoting angiogenesis in a mouse model bearing ischemic limbs. Our results showed that miR-199b-5p, an miRNA highly associated with angiogenesis, is significantly upregulated during the differentiation of hiPSC-ECs. Mechanically, our studies found that hiPSC-ECs expressing miR-199b-5p significantly promote cell migration, proliferation and tube formation through Jagged-1-dependent upregulation of VEGFR2 in HUVECs. Similarly, coculture of hiPSC-ECs-Exo with HUVECs also resulted in a significant improvement in HUVEC migration, proliferation, and tube formation, suggesting that exosome-mediated cell-cell communication in a paracrine manner may serve as a fundamental mechanism for iPSC-EC-based treatment. Consequently, we found that the transfer of hiPSC-ECs enriched with miR-199b-5p significantly enhanced micro-vessel density and blood perfusion in ischemic limbs in vivo. Taken together, our studies were the first to demonstrate that transfer of hiPSC-ECs-Exo is a promising approach to treat ischemic injury via the mechanism of promoting neovascularization.
Keywords: hiPSC-EC, exosome, angiogenesis, Jagged-1, miR-199b-5p

 Global reach, higher impact
Global reach, higher impact