10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2019; 15(1):229-238. doi:10.7150/ijbs.28830 This issue Cite
Research Paper
Psoralen Protects Chondrocytes, Exhibits Anti-Inflammatory Effects on Synoviocytes, and Attenuates Monosodium Iodoacetate-Induced Osteoarthritis
1. National Innovation and Attracting Talents “111” base, Key Laboratory of Biorheological Science and Technology, Ministry of Education, College of Bioengineering, Chongqing University, Chongqing 400030, P.R. China.
2. Department of Cardiovascular Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430022, China.
3. Tikrit Universtiy, College of medicine, department of microbiology, P.O. Box (45) Salahaddin province, Tikrit, Iraq.
4. Department of Mechanics and Engineering Structure, Hubei Key Laboratory of Theory and Application of Advanced Materials Mechanics, Wuhan University of Technology, China
5. Center for Precision Medicine, School of Medicine and School of Biomedical Sciences, Huaqiao University, Xiamen, 361021, Fujian, China.
Received 2018-7-28; Accepted 2018-10-19; Published 2019-1-1
Abstract
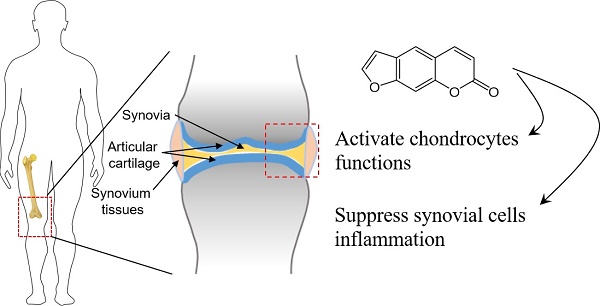
Current study examined whether psoralen (PSO) exhibits anti-inflammatory responses, protection and activation of chondrocytes, and relieve osteoarthritis (OA). Rats chondrocytes and human synoviocytes were cultured in tumor necrosis factor-α (TNF-α) conditioned culture medium with/without PSO to test the cell morphologies and cytotoxicities in vitro. Cartilaginous extracellular matrix (ECM) and proliferative gene/protein expression levels were evaluated in chondrocytes. Meanwhile, matrix metalloproteinases (MMPs) and interleukins (ILs) gene/protein expression were analyzed in synoviocytes. SD rats of monosodium iodoacetate (MIA) induced OA model were used in order to assess the effects of PSO on attenuating degeneration of the articular cartilage in vivo. Results showed TNF-α conditioned culturing with/without PSO (1-100 µM) had no any toxicity on both the cell lines. PSO (10 µM) activated cartilaginous specific ECM expression along with up-regulation of proliferative genes at transcriptional levels. Interestingly, PSO significantly reversed TNF-α induced up-regulation of MMP13 and ILs synoviocytes in a dose-dependent manner (1 to 20 µM), while down-regulated cartilaginous ECM production. Following six weeks of PSO treatments to articular cartilage osteoarthritis, compared to MIA-induced group, the appearance and physiological structure of articular cartilage was more integrated with greatly organized chondrocytes and abundant cartilage matrix. In conclusion, PSO protects and activates chondrocytes, antagonizing the expression of MMPs and ILs secreted by synovial cells, and effectively attenuates MIA-induced OA.
Keywords: anti-inflammation, natural product, degeneration of cartilage, knee articular, synovium, osteoarthritis
Introduction
The important indicator of osteoarthritis (OA) is degeneration of the cartilage. Clinically, OA is usually treated locally with injectable glucocorticoid and non-steroidal anti-inflammatory drugs (NSAIDs), which inhibit the process of inflammatory reactions. Sodium hyaluronate injection is another therapeutical approach to treat OA in order to enhance joint lubrication [1, 2]. But almost of the therapies have limited effects. Knee-joint OA is one of the commonest clinical symptoms characterized by persistent pains in the knee-joint and experience difficulty in walking. These clinical symptoms seriously affect person's normal movements and routine life. Many factors have been reported that affect the OA of the knee-joint, but molecular mechanisms involved in the disease progression is not much clear. Studies stated that the joint synovial fluid present in OA knee-joint contains huge amount of inflammatory mediators such as TNF-α and IL-1β etc. [3-5]. These inflammatory mediators have been demonstrated to disrupt the functions of chondrocytes and stimulates role of synovial tissues to enhanced secretion of inflammatory mediators to promote the OA [6, 7]. Therefore, exploration of effective drugs that potentially lower inflammatory responses such as inhibition of synovium and chondrocytes secreting inflammatory mediators, protection and activation of chondrocyte functions are all important tasks for alleviating OA.
Psoralen (PSO) is a natural compound extracted from Fructus psoraleae, has been proved to possess strong effects for bone protection [8, 9], anti-inflammation [10] and inhibitions of tumor growth [11]. It is further said that PSO can inhibit the degeneration of cartilage in the lumbar intervertebral disc [12]. Moreover, previously we reported and confirmed that PSO has the functions of activating chondrocyte and promoting the secretion of aggrecan and type II Collagen [13]. It is further said that PSO can increase the expression of cyclin D1 in chondrocytes via Wnt/β-catenin signaling pathway, and thus promoted the proliferation of the cells [14]. Furthermore, these studies elaborated that PSO has a certain modulatory effect on chondrocytes, but it failed to clarify whether it can be effective in the treatment of OA, and anti-inflammatory effects of PSO on synovial cells still demands further exploration.
Huge literature extensively highlighted PSO activated chondrocytes, but current study pioneered to investigate the antagonistic effects of PSO on TNF-α induced synovial cells that are secreting inflammatory mediators. Moreover, the MIA-induced knee-joint OA model was established in SD rats, and the effects of PSO in preventing OA by intra-articular local injection was investigated with macro- and micro- scopical evaluation. The results showed that PSO efficiently protected and activated chondrocytes, antagonizing the expression of MMPs and ILs secreted by synovial cells induced by TNF-α treatment, while effectively inhibited the development of knee-joint OA at macro and micro levels. PSO not only can inhibit inflammation of synovium, but also activates cartilaginous ECM synthesis. This makes psoralen as a promising drug for the treatment of OA.
Materials and Methods
Cell culture
Rat chondrocytes were isolated according to a method we adapted in our previous study [13], and cultured with DMEM/F12 (1:1) supplemented with 10% FBS medium. The human materials used for this study were obtained according to ethical principles, while the protocol was reviewed and approved by the Institutional Review Board (IRB) of Chongqing University. The PSO were purchased and prepared based on the previous report [13]. The human synoviocytes were prepared according following various previous reports [4, 15, 16], and cultured with high glucose DMEM supplemented with 10% FBS medium. Chondrocytes (Passage 3) and synoviocytes were used in the experiments.
Cell viability and cytotoxicity assay
Cell viability was performed by the MTS assay (Promega, Madison, WI, G358C) according to the manufacturer's instructions. The cells were seeded at a density of 1×104 cells/well in a 24-well plate. The cells were then treated with TNF-α with or without PSO for 3 days. After that, all the cells were washed with PBS and incubated with serum free medium containing 10% MTS reagent. After 3 hours of incubation at 37°C with 5% CO2, aliquots were pipetted into a 96-well plate and measured at 490 nm using an enzyme labeling instrument (Bio-Rad, Berkeley, CA).
qRT-PCR assay
At 24 and 48 h, the cells with different treatments were directly lysed in the 6-well plates with RNA pure High-purity Total RNA Rapid Extraction Kit (Spi-column) (Bio Teke Corporation, Beijing, China) according to the manufacturer's instruction. The total RNA was quantified spectrophotometrically and reverse-transcribed using RT-First Strand cDNA Synthesis kit (Thermo, K1622) according to the manufacturer's instructions in two steps. The SsoAdvanced SYBR Green PCR supermix (Bio-Rad No.1725264) and CFX96 Real-Time PCR Detection System (Bio-Rad) was used to perform qRT-PCR. All the primers were designed via NCBI primer blast and synthesized by Invitrogen (Table 1 and 2). The data was analyzed following 2-ΔΔct method.
List of Primer Sequences of Rat for Real-Time PCR
| Genes | Primer sequences (forward/reverse) | Product size(bp) |
|---|---|---|
| COL2A1 | 5'- AATTTGGTGTGGACATAGGG -3' | 94 |
| 5'- AAGTATTTGGGTCCTTTGGG -3' | ||
| ACAN | 5'- AACTTCTTCGGAGTGGGTGGT -3' | 166 |
| 5'- CAGGCTCTGAGACAGTGGGG -3' | ||
| MMP13 | 5'- ACCCAGCCCTATCCCTTGAT -3' | 178 |
| 5'- TCTCGGGATGGATGCTCGTA -3' | ||
| MAPK1 | 5'- CCTGGGTATTCTTGGATCTCCA -3' | 106 |
| 5'- CCACGGCACCTTATTTTTGTG -3' | ||
| CDK1 | 5'- TCCTCCAGGGGATTGTGTTTT -3' | 100 |
| 5'- GCCAGTTTGATTGTTCCTTTGTC -3' | ||
| MKI67 | 5'- GCCCCTGGAAGATTATGGTGG -3' | 128 |
| 5'- GGGTTCTGACTGGTTGTGGTTGT -3' | ||
| P21 | 5'- GGATTCCATTCATTGAGACCTCA -3' | 122 |
| 5'- CTTCTTCTTTTTCCCATCTTTGCT -3' | ||
| MYC | 5'- CTCGGTGCAGCCCTATTTCA -3' | 185 |
| 5'- TAGCGACCGCAACATAGGAC -3' | ||
| GAPDH | 5'- CAAGGTCATCCATGACAACT -3' | 253 |
| 5'- CAGATCCACAACGGATACAT -3' |
List of Primer Sequences of Human for Real-Time PCR
| Genes | Primer sequences (forward/reverse) | Product size(bp) |
|---|---|---|
| MMP1 | 5'- GCTGAAAGTGACTGGGAAACC -3' | 169 |
| 5'- TGCTCTTGGCAAATCTGGCGTG -3' | ||
| MMP2 | 5'- GTGACGGAAAGATGTGGTG -3' | 179 |
| 5'- GGTGTAGGTGTAAATGGGTG -3' | ||
| MMP3 | 5'- GACAAAGGATACAACAGGGAC -3' | 122 |
| 5'- TGAGTGAGTGATAGAGTGGG -3' | ||
| MMP9 | 5'- TCTATGGTCCTCGCCCTGAA -3' | 219 |
| 5'- CATCGTCCACCGGACTCAAA -3' | ||
| MMP12 | 5'- AACCAACGCTTGCCAAATCC -3' | 86 |
| 5'- TTTCCCACGGTAGTGACAGC -3' | ||
| MMP13 | 5'- GCCATTACCAGTCTCCGAGG -3' | 127 |
| 5'- TACGGTTGGGAAGTTCTGGC -3' | ||
| IL1B | 5'- CAGAAGTACCTGAGCTCGCC -3' | 153 |
| 5'- AGATTCGTAGCTGGATGCCG -3' | ||
| IL6 | 5'- AGTGAGGAACAAGCCAGAGC -3' | 187 |
| 5'- AGCTGCGCAGAATGAGATGA -3' | ||
| IL12 | 5'- GTCATGGTGGATGCCGTTCA -3' | 162 |
| 5'- ACTCCAGGTGTCAGGGTACT -3' | ||
| GAPDH | 5'- GCACCGTCAAGGCTGAGAAC -3' | 138 |
| 5'- TGGTGAAGACGCCAGTGGA -3' |
Cell immuno-staining assays
The chondrocytes and synoviocytes were cultured with different treatments for 48 h.
The cell immuno-staining was performed according to the previous protocols [17]. The primary antibodies: Collagen II (Santa Cruz, sc-7763), MMP-13 (Santa Cruz, sc-30073) for chondrocytes, and IL-1β (Bioss, bs-20449R), MMP-13 (Santa Cruz, sc-30073) for synoviocytes were used. After different secondary antibodies incubation, the samples were observed and captured by fluorescent or bright field microscopy (Olympus).
Western blot assay
After 48 h with different treatments; the chondrocytes were lysed with lysis buffer plus PMSF (1%). The proteins were prepared according to the previous protocol. Proteins were separated by 8% to 12% SDS-PAGE and subsequently transferred to PVDF (polyvinylidene fluoride) membranes (0.2μm; Bio-Rad). Then the membranes were incubated overnight at 4°C with primary antibodies: collagen II (Santa Cruz, sc-7763), aggrecan (Abcam, ab36861), and MMP13 (Santa Cruz, sc-30073) antibodies. The targeted proteins were detected according to the previous protocols [15, 18].
Animal model and drug administration
Animal experiments were carried out according to the protocols approved by Chongqing University Animal Care and Use Committee. According to the references [19, 20], OA was induced by a single intra-articular knee injection of monosodium iodoacetate (MIA) (Sigma) (6 mg/kg body weight, 50 μL) into the bilateral knee-joints of the Sprague-Dawley (SD) rats (200-250g). Two weeks followed by MIA injection, the rats had their knee-joints treated with 1 mg/kg (body weight) of PSO (MIA+PSO group) and sterile saline solution (MIA group) via intra-articular knee injection daily for 1 week, and once every three days for another 1 week. At the beginning of MIA injection, sterile saline solution was used in the later every injection for control group. At 4 and 8 weeks' post-injection of MIA, articular cartilage samples were harvested for macro- and micro- evaluation.
Histologcial assessment table
| Item | Description | Score |
|---|---|---|
| Organization structure of knee articular cartilage | integrity | 2 |
| minor defect | 1 | |
| structural failure | 0 | |
| Cartilage matrix | compact | 2 |
| loose | 1 | |
| Extremely sparse | 0 | |
| Glycosaminoglycan content | abundant | 2 |
| lacked | 1 | |
| rare | 0 | |
| Organization of chondrocytes | orderly and uniform distribution | 2 |
| partially organized | 1 | |
| arranged disorderly | 0 | |
| Inflammation | none | 2 |
| mild | 1 | |
| severe | 0 | |
| Cartilage superficial layer | Integrity | 2 |
| minor ulceration or erosion | 1 | |
| severe destroy | 0 | |
| Necrosis | none | 2 |
| minor | 1 | |
| large area | 0 |
Histologcial staining
The harvested samples were made into paraffin sections. All the specimens were separately stained with hematoxylin and eosin (H&E), Safranin-O/ green, and Alcian blue. In the microscopic examinations, the histological scores were rated according to the histologcial assessment table (Table 3) followed from our previous study [21, 22], the score of each section come from the sum of each item. The statistical analysis was based on the mean score for each group.
Statistical analysis
All data were performed by SPSS, expressed as means ± standard deviation (SD). Statistical comparisons were made by ANOVA to check differences of groups. A value of p<0.05 was accepted as statistically significant.
Results
The effects of PSO on cytotoxicity of TNF-α induced chondrocytes and synoviocytes
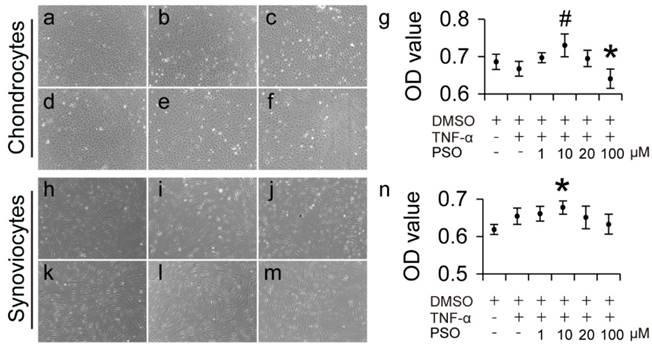
After 3-days culture period, TNF-α induced chondrocytes with conditioned culturing at different concentrations (1 to 100 µM) of PSO grew well and were polygonal, elliptical and spindle-shaped and there were no obvious morphological changes compared to control (Figure 1a-f). In addition, TNF-α induced synoviocytes with conditioned culturing at PSO concentration (1 to 100 µM) exhibited fibroblast, slender-shape (Figure 1: i-m), also there were no obvious morphological changes compared to control (Figure 1h). According to the OD value from MTS assay, 5 ng/ml TNF-α exhibited no toxicity both in chondrocytes and synoviocytes. When different concentration of PSO was added in the TNF-α conditioned culture medium, 100 μM PSO (* p < 0.05) suppressed cell viability in chondrocytes, and 10 μM PSO (* p < 0.05) slightly increased cell viability in synoviocytes versus the control group (0.1% DMSO). In addition, supplementing with 10 μM PSO (# p < 0.05) in TNF-α conditioned culture medium showed promoting effect on cell viability when compared with TNF-α group. These results indicated that TNF-α conditioned culturing with or without PSO showed no toxicity to both chondrocytes and synoviocytes, and 10 μM concentration of PSO was the optimal dosage for further studies.
The effects of psoralen on morphology and cytotoxicity of TNF-α induced chondrocytes and synoviocytes for 3 days. (a-f): Cell morphology of chondrocytes with different conditioned culture: control (a); TNF-α (b); TNF-α+1 µM (c); TNF-α+10 µM (d); TNF-α+20 µM (e); TNF-α+100 µM (f). (h-m): Cell morphology of synoviocytes with different conditioned culture: control (h); TNF-α (i); TNF-α+1 µM (j); TNF-α+10 µM (k); TNF-α+20 µM (l); TNF-α+100 µM (m). (g): Cell viability of chondrocytes (was tested via cck8 assay) with different conditioned culture. (n): Cell viability of synoviocytes (was tested via cck8 assay) with different conditioned culture. The data are presented as mean ± sd (𝑛=6), (*) p< 0.05 (vs. control) and (#) p< 0.05 (vs. TNF-α) were accepted as statistically significant.
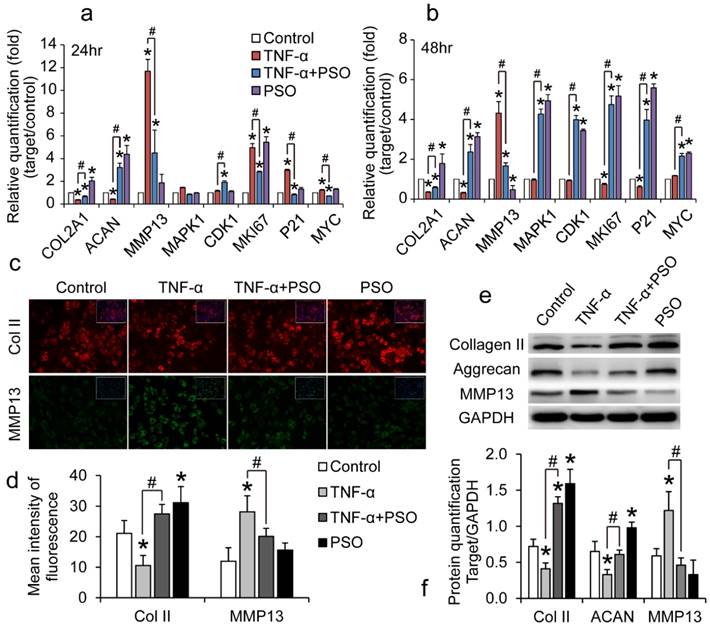
PSO activates and protects chondrocyte against TNF-α induced abnormal expression of genes and proteins
Chondrocytes were treated with TNF-α or TNF-α plus PSO for 24 and 48 h. TNF-α treatment interfere in regular cartilaginous and cell proliferative gene expression and protein synthesis of chondrocytes (Figure 2), however PSO antagonized these effects. Compared to control, TNF-α significantly up-regulated MMP-13 (* p< 0.05) and down-regulated aggrecan (ACAN) (* p< 0.05) and COL2A1 (* p< 0.05) mRNA levels both at 24 and 48 h, while PSO recovered these gene levels (# p< 0.05; Psoralen + TNF-α vs TNF-α) (Figure 2: a and b). Similar to the previous gene expression results, PSO not only activated cartilaginous matrix synthesis, but also restored TNF-α induced down-regulation of COL2A1, ACAN and up-regulating of MMP-13 at translation level. According to the immunofluorescent staining (Figure 2: c and d) and western blotting analyses (Figure 2: e and f), TNF-α stimulated MMP-13 protein synthesis (* p< 0.05), meanwhile restrained type II collagen (* p< 0.05) and aggrecan expression (* p< 0.05). With PSO treatment in TNF-α induced chondrocytes, type II collagen (# p< 0.05), aggrecan (# p< 0.05) and MMP-13 (# p< 0.05) protein levels returned to relative normal condition based on control group. Furthermore, PSO alone promoted proliferative specific gene MKI67 (* p< 0.05) expression both at 24 and 48 h versus control, and promoted other proliferative markers such as MAPK1 (* p< 0.05), CDK1 (* p< 0.05), P21 (* p< 0.05), and MYC (* p< 0.05) expression at 48 h compared with respective controls. Besides for previously mentioned proliferative genes, PSO recovered the TNF-α induced abnormal expression, and exhibited significant differences (# p< 0.05). All these results indicated that PSO activates cartilaginous ECM synthesis, stimulated cell proliferation genes at transcriptional level, and finally protected chondrocytes against TNF-α induced abnormal genes expression.
The effects of psoralen on TNF-α-induced chondrocytes gene and protein expression. (a and b): chondrocytes were treated with TNF-α or TNF-α plus PSO for 24 and 48 hours. (c and d): immunofluorescent staining of COL2A1 and MMP13 of chondrocytes for 48 hours treatments. (e and f): western blotting for type II collagen (Collagen II), Aggrecan, MMP13 of chondrocytes for 48 hours treatments. (*) p< 0.05 (vs. control) and (#) p< 0.05 (vs. TNF-α) were accepted as statistically significant, n=3.
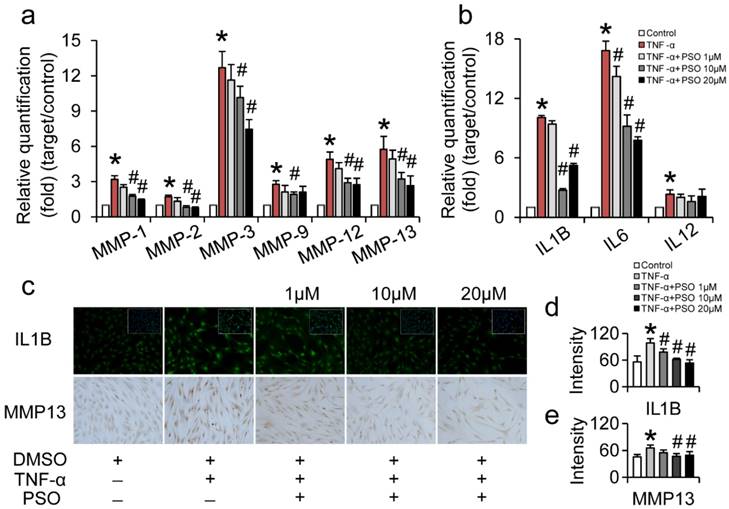
PSO inhibits TNF-α induced MMPs and interleukins production of synoviocytes
After 24 h treatment with TNF-α or TNF-α plus PSO, TNF-α significantly up-regulated MMP-1, -2, -3, -9, -12, -13 and IL-1B, -6, -12 expression in synoviocytes. However, PSO (1 to 20 µM) reversed these expressions of MMPs and ILs induced by TNF-α in a dose-dependent manner (Figure 3: a and b). Compared with control, the mRNA levels of MMP-1, -2, -3, -9, -12, -13 and IL-1β, -6, -12 in TNF-α group showed significant difference (* p< 0.05). Specifically, combined TNF-α plus PSO (10 and 20 µM, # p< 0.05) significantly down-regulated those genes expression (except IL-12) when compared to TNF-α group. Besides, TNF-α induced MMP-13 and IL-1B protein synthesis (Figure 3 c) was promoted by 0.44-fold (MMP-13, * p< 0.05) and 0.76-fold (IL-1B, * p< 0.05) respectively, compared to their respective controls. Whereas, PSO significantly (# p< 0.05) suppressed the MMP-13 and IL-1β synthesis versus TNF-α group in a dose-dependent manner (Figure 3: d and e). These results indicated that PSO prevented TNF-α induced inflammation in synoviocytes via down-regulating MMP-1, -2, -3, -9, -12, -13, and IL-1β, -6, -12 genes expression, along with inhibition of MMP-13 and IL-1β protein synthesis.
PSO prevents degradation of articular cartilage by MIA-induced OA
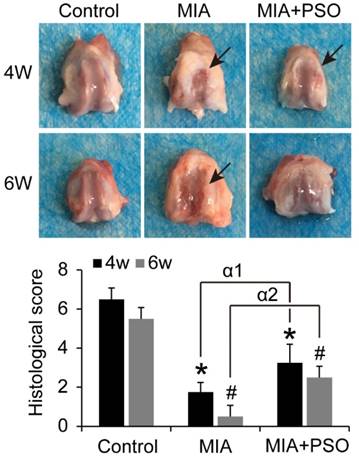
Under macroscopic observations, the articular cartilage presented obvious cartilage degradation in MIA group, and tiny visible lesion in MIA plus PSO treatment group (Figure 4). The histological score of MIA and MIA+PSO group exhibited significant difference versus control. For the MIA group, the scores were 1.75 ± 0.50 (* p< 0.05) at week 4 and 0.50 ± 0.56 (# p< 0.05) at week 6. For the MIA+PSO treatment group, the scores were 3.25 ± 0.96 (* p< 0.05) at week 4 and 2.50 ± 0.58 (# p< 0.05) at week 6. In addition, the appearance and physiological structure of articular cartilage were more integrated in MIA+PSO compared to MIA group. The histological scores of MIA+PSO were higher than MIA group, and showed significant difference both during week 4 (α1 p< 0.05) and week 6 (α2 p< 0.05).
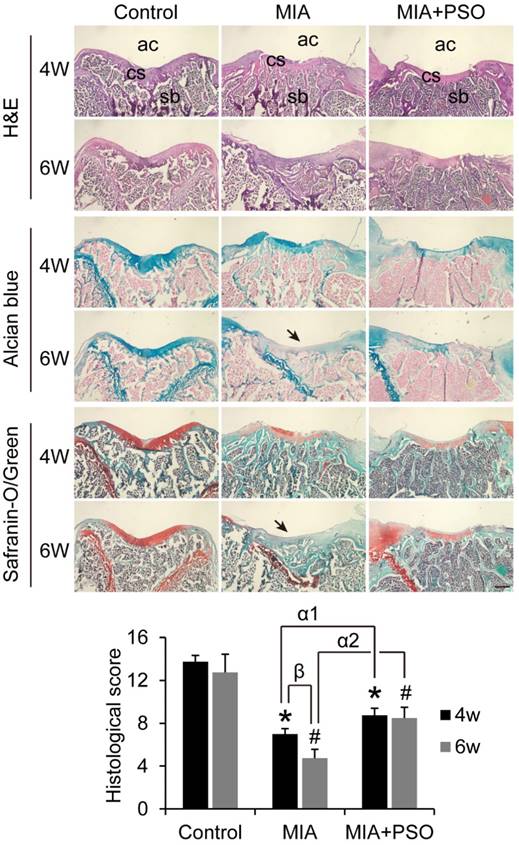
According to the H&E histological staining, alcian blue and safranin-o/green, the control group exhibited organized chondrocytes and abundant cartilage matrix, the subchondral bone was covered by integrated cartilage surface both in week 4 and 6 (Figure 5). By contrast, MIA and MIA+PSO groups showed various degrees of cartilaginous degradation. Especially, after 6 weeks with MIA treatments, there were no more visible cartilaginous matrix (Figure 5: indicated by arrow), and the subchondral bone was exposed to articular cavity. Comparing the controls during week 4 and 6, the histological scores were 7.00 ± 0.50 (* p< 0.05) and 4.75 ± 0.81 (# p< 0.05) in MIA group with a significant difference, respectively. Additionally, the histological scores were 8.75 ± 0.65 (* p< 0.05 and α1 p< 0.05) and 8.50 ± 1.00 (# p< 0.05 and α2 p< 0.05) in MIA+PSO group, it is worth noted that not only the results exhibited significant difference versus control, but also were obviously higher than MIA group. Besides, in MIA group, the histological score of 4 weeks (β p< 0.05) was higher than that of 6 weeks. These results indicated that PSO suppressed cartilage matrix degradation, protected in situ chondrocytes viability, delays cartilage surface erosion, and finally prevented degradation of articular cartilage by MIA-induced OA.
The effects of psoralen on TNF-α-induced synoviocytes gene and protein expression. (a and b): synoviocytes were treated with TNF-α or TNF-α plus different concentration of PSO for 24 hours. (c): immuno-staining of IL1B and MMP13 of synoviocytes for 24 hours treatments. (d and e): fluorescent intensity of IL1B and MMP13. (*) p< 0.05 (vs. control) and (#) p< 0.05 (vs. TNF-α) were accepted as statistically significant, n=3.
Macroscopically evaluation of articular cartilage for 4 and 6 weeks with MIA and PSO treatments. Arrows indicate necrosis. For the histological score, (*) p< 0.05 and (#) p< 0.05 were accepted as statistically significant when compared to Control. (α1) p< 0.05 and (α2) p< 0.05 were accepted as statistically significant when compared to MIA group at week 4 and 6 respectively.
Discussion
Inflammatory responses manifest as secreting MMPs or inflammatory mediators and cartilage degradation are the typical biological characteristics of OA. These drugs having anti-inflammatory, protective and activating chondrocytes properties provide a hope to relieve the process of OA. Here we found PSO not only exhibited anti-inflammatory effects, but could also activate and protect chondrocytes and cellular physiology, and alleviates degeneration of the articular cartilage in OA.
According to the MTS assay, TNF-α conditioned culturing with/without PSO had no any toxicity in both chondrocytes and synoviocytes. While 10 µM PSO in the TNF-α conditioned culture medium slightly promoted cell viability in chondrocytes, meanwhile 100 µM PSO suppressed cell growth compared with control. These results verified the previous conclusion from another point of view: high concentration of PSO stagnated cell proliferation, whereas PSO 10 µM concentration set as the optimal dosage.
PSO activated and stimulated cartilaginous ECM synthesis and transcription of cell proliferative genes, protected chondrocytes against TNF-α induced abnormal genes expression including COL2A1, ACAN, MMP-13, MAPK1, CDK1, MKI67, P21 and MYC. Among them, MAPK1, P21, MYC, especially CDK1 and MKI67 are the common markers for cell proliferation. It has been proved that CDK1 is a highly conserved protein that functions as a serine/ threonine kinase, and is a key player in cell cycle regulation. While MKI67 is a cellular marker for cell proliferation, which is present during all active phases of the cell cycle (G1, S, G2, and mitosis), but is absent in quiescent cells (G0). Recently it has been verified that PSO promotes chondrocytes proliferation, up-regulates the expression of cyclin D1 via the Wnt/β-catenin signaling pathway [14]. Current findings are in accordance with previous findings, and stimulatory effects of PSO on cell growth of chondrocytes were proven by the activation of cell proliferative genes (CDK1, MKI67).
MMPs, especially MMP-13 maintained the ECM metabolic balance in cartilage [23]. Notably results showed that PSO alone have some inhibitory effects on the expression of MMP-13, which is specific for cartilage degeneration. Some studies demonstrated that MMP-13 over-expression can induce the onset of OA through excessive ECM degradation [24, 25]. It is further said that MMP-13 levels correlate with the presence of pathological chondrocytes that undergo hypertrophic differentiation in the early stage of OA development [23]. Thus, the selective and targeted increase concentrations of PSO on cartilaginous ECM and proliferative gene/protein expressions, while excluding MMPs expression is a prerequisite for being a candidate drug for alleviation OA.
Current study demonstrated that PSO strongly suppressed TNF-α induced up-regulation of MMP-1, -2, -3, -9, -12, -13 and IL-1β, -6, and -12 expressions in synoviocytes. It has been reported that the high expression of MMP-1, -2 and -3 could activate other members of the MMPs family. MMP-1 can activate latent MMP-2, the later can can activate latent MMP-13 and MMP-3 can activate MMP-1, 9 and 13 [26]. Previously it is reported that the ability of MMPs to activate each other creates a complex network of proteases in the synovial fluids which may compromise the regeneration of injured tissues like ligaments and cartilage [4]. Inflammatory mediators especailly IL-1β deteriorate arthritis directly is well documented. IL-1β has an important role in the promotion of the growth of OA lesions, while IL-6 work as a pro-inflammatory cytokine to stimulate immune response that have a significant role in initiating joint OA pathology [27].
So far we have proved that PSO not only activates chondrocytes cellular functions including cartilaginous ECM synthesis, cell proliferation and cytoprotection against TNF-α induced injury, but also it prevents TNF-α induced inflammation in synoviocytes via down-regulating MMPs and ILs expression. This dual function of PSO on activation and anti-inflammation provides an alternative drug for OA treatment.
Based on the in vitro studies, we locally injected 1 mg/kg (body weight) of PSO into the articular cavity in MIA-induced OA rats. Histological observation showed protective effect of PSO on cartilage surface erosion and matrix degradation. We speculate these positive effects on OA treatment by PSO is primarily due to the MMP-13 inhibition and cartilaginous ECM promotion of synoviocytes and chondrocytes. Although those anti-inflammatory drugs such as glucocorticoids (e.g. dexamethasone) or NSAIDs were used for treating OA clinically, they lacked the characteristics for stimulating cartilaginous ECM recovery so that would compromise the therapeutically effects on alleviating OA. Therefore, the versatile PSO as inflammation and MMPs inhibitor, and also cartilaginous activator satisfied the need of OA treatment requirements.
Histological staining of H&E, alcian blue and safranin-o/green for 4 and 6 weeks after MIA and PSO treatments, ac: articular cavity; cs: cartilage surface, sb: subchondral bone. (*) p< 0.05 and (#) p< 0.05 were accepted as statistically significant when compared to Control. (α1) p< 0.05 and (α2) p< 0.05 were accepted as statistically significant when compared to MIA group at week 4 and 6 respectively. (β) p< 0.05 were accepted as statistically significant in MIA group. Bar: 500 µm.
Conclusions
PSO have the potential to stimulate chondrocytes proliferation and up-regulate the expressions of cartilage-specific genes. In addition, PSO shows anti-inflammatory effects on synoviocytes via inhibiting TNF-α induced abnormal up-regulation of MMPs and ILs. Finally, PSO can increase the synthesis of cartilage matrix, maintain the formation of chondroid tissue, protect in situ chondrocytes viabilities, delay cartilage surface erosion, finally prevents degradation of articular cartilage by MIA-induced OA. These findings indicated that PSO may be a potential promoting compound for preventing OA.
Acknowledgements
This work was supported by the Visiting Scholar Foundation of Key Laboratory of Biorheological Science and Technology (Chongqing University), Ministry of Education (Grant Number: CQKLBST-2018-009) and (Grant Number: CQKLBST-2018-006), the China Postdoctoral Science Foundation (Grant Number: 2018M630867), the Fundamental Research Funds for the Central Universities (WUT: 2016IVB063, 2018IB005), National Key R&D Plan (2016YFA0101100), National Natural Science Foundation of China (11532004, 11602181), and the Innovation and Attracting Talents Program for College and University (''111'' Project) (B06023).
Author Contributions
Kang Xu, Chunli Wang, and Yongqiang Sha designed the project, performed the experiments, collected the data and wrote the manuscript. Mohanad Kh Al-ani and Qingjia Chi analyzed the data, wrote and revised the manuscript. Nianguo Dong and Li Yang designed the project, gave financial support, wrote and revised the manuscript. All authors read and approved the final manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Mauro GL, Sanfilippo A, Scaturro D. The effectiveness of intra-articular injections of Hyalubrix((R)) combined with exercise therapy in the treatment of hip osteoarthritis. Clinical cases in mineral and bone metabolism: the official journal of the Italian Society of Osteoporosis, Mineral Metabolism, and Skeletal Diseases. 2017;14:146-52
2. Takahashi K, Goomer RS, Harwood F, Kubo T, Hirasawa Y, Amiel D. The effects of hyaluronan on matrix metalloproteinase-3 (MMP-3), interleukin-1beta(IL-1beta), and tissue inhibitor of metalloproteinase-1 (TIMP-1) gene expression during the development of osteoarthritis. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 1999;7:182-90
3. Tang Z, Yang L, Zhang J, Xue R, Wang Y, Chen PC. et al. Coordinated expression of MMPs and TIMPs in rat knee intra-articular tissues after ACL injury. Connective tissue research. 2009;50:315-22
4. Zhang Y, Huang W, Jiang J, Xie J, Xu C, Wang C. et al. Influence of TNF-alpha and biomechanical stress on matrix metalloproteinases and lysyl oxidases expressions in human knee synovial fibroblasts. Knee surgery, sports traumatology, arthroscopy: official journal of the ESSKA. 2014;22:1997-2006
5. Zhang Y, Jiang J, Xie J, Xu C, Wang C, Yin L. et al. Combined effects of tumor necrosis factor-alpha and interleukin-1beta on lysyl oxidase and matrix metalloproteinase expression in human knee synovial fibroblasts in vitro. Experimental and therapeutic medicine. 2017;14:5258-66
6. Yuan PW, Liu DY, Chu XD, Hao YQ, Zhu C, Qu Q. Effects of preventive administration of juanbi capsules on TNF-alpha, IL-1 and IL-6 contents of joint fluid in the rabbit with knee osteoarthritis. Journal of traditional Chinese medicine = Chung i tsa chih ying wen pan. 2010;30:254-8
7. Kammermann JR, Kincaid SA, Rumph PF, Baird DK, Visco DM. Tumor necrosis factor-alpha (TNF-alpha) in canine osteoarthritis: Immunolocalization of TNF-alpha, stromelysin and TNF receptors in canine osteoarthritic cartilage. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 1996;4:23-34
8. Tang DZ, Yang F, Yang Z, Huang J, Shi Q, Chen D. et al. Psoralen stimulates osteoblast differentiation through activation of BMP signaling. Biochemical and biophysical research communications. 2011;405:256-61
9. Wong RW, Rabie AB. Effect of psoralen on bone formation. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2011;29:158-64
10. Chen CH, Hwang TL, Chen LC, Chang TH, Wei CS, Chen JJ. Isoflavones and anti-inflammatory constituents from the fruits of Psoralea corylifolia. Phytochemistry. 2017;143:186-93
11. Wang X, Cheng K, Han Y, Zhang G, Dong J, Cui Y. et al. Effects of Psoralen as an Anti-tumor Agent in Human Breast Cancer MCF-7/ADR Cells. Biological & pharmaceutical bulletin. 2016;39:815-22
12. Yang L, Sun X, Geng X. Effects of psoralen on chondrocyte degeneration in lumbar intervertebral disc of rats. Pakistan journal of pharmaceutical sciences. 2015;28:667-70
13. Xu K, Pan X, Sun Y, Xu W, Njunge L, Yang L. Psoralen activates cartilaginous cellular functions of rat chondrocytes in vitro. Pharmaceutical biology. 2015;53:1010-5
14. Zheng W, Lin P, Ma Y, Shao X, Chen H, Chen D. et al. Psoralen promotes the expression of cyclin D1 in chondrocytes via the Wnt/beta-catenin signaling pathway. International journal of molecular medicine. 2017;40:1377-84
15. Wang C, Xie J, Jiang J, Huang W, Chen R, Xu C. et al. Differential expressions of the lysyl oxidase family and matrix metalloproteinases-1, 2, 3 in posterior cruciate ligament fibroblasts after being co-cultured with synovial cells. International orthopaedics. 2015;39:183-91
16. Fu C, Xie J, Hu N, Liang X, Chen R, Wang C. et al. Titanium particles up-regulate the activity of matrix metalloproteinase-2 in human synovial cells. International orthopaedics. 2014;38:1091-8
17. Xu K, Pan X, Qiu X, Wang D, Dong N, Yang L. et al. Neural crest-derived cells migrate from nerve to participate in Achilles tendon remodeling. Wound repair and regeneration: official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2018;26:54-63
18. Wang C, Xu C, Chen R, Yang L, Sung KP. Different expression profiles of the lysyl oxidases and matrix metalloproteinases in human ACL fibroblasts after co-culture with synovial cells. Connective tissue research. 2018;59:369-80
19. Morais SV, Czeczko NG, Malafaia O, Ribas JMF, Garcia JB, Miguel MT. et al. Osteoarthritis model induced by intra-articular monosodium iodoacetate in rats knee. Acta cirurgica brasileira. 2016;31:765-73
20. Gong K, Shao W, Chen H, Wang Z, Luo ZJ. Rat model of lumbar facet joint osteoarthritis associated with facet-mediated mechanical hyperalgesia induced by intra-articular injection of monosodium iodoacetate. Journal of the Formosan Medical Association = Taiwan yi zhi. 2011;110:145-52
21. Song Y, Xu K, Yu C, Dong L, Chen P, Lv Y. et al. The use of mechano growth factor to prevent cartilage degeneration in knee osteoarthritis. Journal of tissue engineering and regenerative medicine. 2017
22. Xu K, Al-Ani MK, Sun Y, Xu W, Pan L, Song Y. et al. Platelet-rich plasma activates tendon-derived stem cells to promote regeneration of Achilles tendon rupture in rats. Journal of tissue engineering and regenerative medicine. 2017;11:1173-84
23. Li NG, Shi ZH, Tang YP, Wang ZJ, Song SL, Qian LH. et al. New hope for the treatment of osteoarthritis through selective inhibition of MMP-13. Current medicinal chemistry. 2011;18:977-1001
24. Li H, Wang D, Yuan Y, Min J. New insights on the MMP-13 regulatory network in the pathogenesis of early osteoarthritis. Arthritis research & therapy. 2017;19:248
25. Perotto JH, Camejo FA, Doetzer AD, Almeida LE, Azevedo M, Olandoski M. et al. Expression of MMP-13 in human temporomandibular joint disc derangement and osteoarthritis. Cranio: the journal of craniomandibular practice. 2017:1-6
26. Kerrigan JJ, Mansell JP, Sandy JR. Matrix turnover. Journal of orthodontics. 2000;27:227-33
27. Cai H, Sun HJ, Wang YH, Zhang Z. Relationships of common polymorphisms in IL-6, IL-1A, and IL-1B genes with susceptibility to osteoarthritis: a meta-analysis. Clinical rheumatology. 2015;34:1443-53
Author contact
![]() Corresponding authors: Dr. Kang Xu, Department of Cardiovascular Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430022, China. Email: kangxuedu.cn and Prof. Li Yang, National Innovation and Attracting Talents “111” base, Key Laboratory of Biorheological Science and Technology, Ministry of Education, College of Bioengineering, Chongqing University, Chongqing 400030, China. Email: yanglibmeedu.cn
Corresponding authors: Dr. Kang Xu, Department of Cardiovascular Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430022, China. Email: kangxuedu.cn and Prof. Li Yang, National Innovation and Attracting Talents “111” base, Key Laboratory of Biorheological Science and Technology, Ministry of Education, College of Bioengineering, Chongqing University, Chongqing 400030, China. Email: yanglibmeedu.cn

 Global reach, higher impact
Global reach, higher impact