Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2019; 15(2):464-480. doi:10.7150/ijbs.25743 This issue Cite
Research Paper
IL-15 Generates IFN-γ-producing Cells Reciprocally Expressing Lymphoid-Myeloid Markers during Dendritic Cell Differentiation
1. Department of Microbiology, Institute for Immunology and Immunological Disease, Brain Korea 21 PLUS Project for Medical Science, Yonsei University College of Medicine, Seoul, South Korea.
2. The Airway Mucus Institute, and Department of Otorhinolaryngology, Yonsei University College of Medicine, Seoul, South Korea.
3. Department of Biochemistry, College of Life Science & Biotechnology, Yonsei University, Seoul, South Korea.
* These authors contributed equally to the work.
Abstract
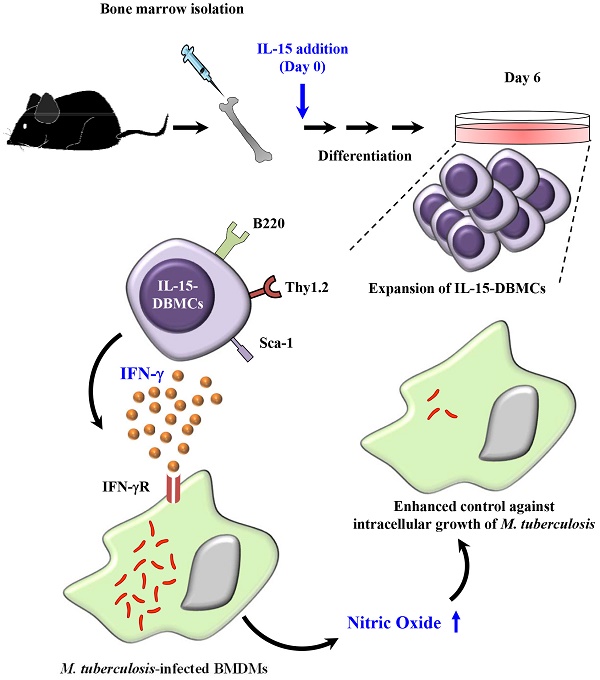
Recently, interest in IL-15-differentiated cells has increased; however, the phenotypic definition of IL-15-differentiated bone marrow-derived cells (IL-15-DBMCs) is still under debate, particularly the generation of IFN-γ-producing innate cells such as premature NK (pre-mNK) cells, natural killer dendritic cells (NKDCs), interferon-producing killer dendritic cells (IKDCs), and type 1 innate lymphoid cells (ILC1s), all of which are IL-15-dependent. Here, we revisited the immunophenotypic characteristics of IFN-γ-producing IL-15-DBMCs and their functional role in the control of intracellular Mycobacterium tuberculosis (Mtb) infection. When comparing the cytokine levels between bone marrow-derived dendritic cells (BMDCs) and IL-15-DBMCs upon stimulation with various TLR agonists, only the CD11cint population of IL-15-DBMCs produced significant levels of IFN-γ, decreased levels of MHC-II, and increased levels of B220. Neither BMDCs nor IL-15-DBMCs were found to express DX5 or NK1.1, which are representative markers for the NK cell lineage and IKDCs. When the CD11cintB220+ population of IL-15-DBMCs was enriched, the Thy1.2+Sca-1+ population showed a marked increase in IFN-γ production. In addition, while depletion of the B220+ and Thy1.2+ populations of IL-15-DBMCs, but not the CD19+ population, inhibited IFN-γ production, enrichment of these cell populations increased IFN-γ. Ultimately, co-culture of sorted IFN-γ-producing B220+Thy1.2+ IL-15-DBMCs with Mtb-infected macrophages resulted in control of the intracellular growth of Mtb via the IFN-γ-nitric oxide axis in a donor cell number-dependent manner. Taken together, the results indicate that IFN-γ-producing IL-15-DBMCs could be redefined as CD11cintB220+Thy1.2+Sca-1+ cells, which phenotypically resemble both IKDCs and ILC1s, and may have therapeutic potential for controlling infectious intracellular bacteria such as Mtb.
Keywords: IL-15, IFN-γ, Dendritic cells, B220, Mycobacterium tuberculosis

 Global reach, higher impact
Global reach, higher impact