10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2019; 15(3):688-700. doi:10.7150/ijbs.30847 This issue Cite
Research Paper
Andrographolide Enhances TRAIL-Induced Apoptosis via p53-Mediated Death Receptors Up-Regulation and Suppression of the NF-кB Pathway in Bladder Cancer Cells
1. Department of Urology, The First Hospital of Jilin University, Changchun, Jilin Province 130021, China.
2. Institute of Virology and AIDS Research, The First Hospital of Jilin University, Changchun, Jilin Province 130021, China.
3. Department of Nephrology, First Hospital of Jilin University, Changchun, Jilin Province 130021, China.
Abstract
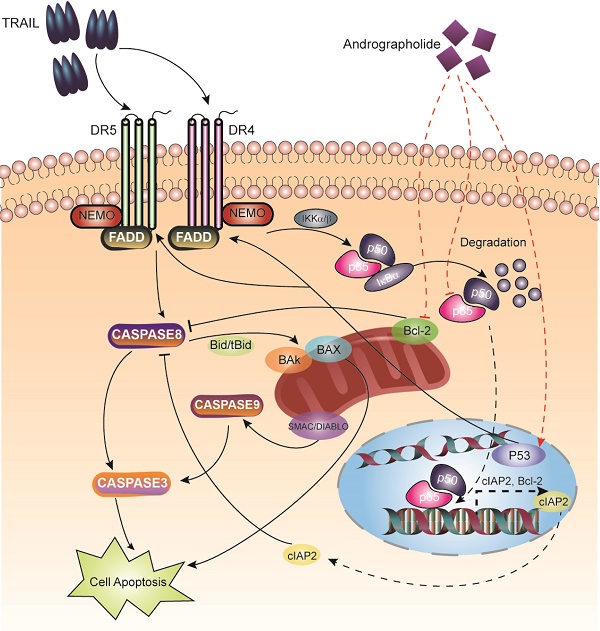
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is an effective chemotherapeutic agent that specifically impairs cancer cells while sparing normal cells; however, some cancer cells develop resistance to TRAIL. Here, we identified Andrographolide, a diterpenoid lactone derived from a traditional herbal medicine Andrographis paniculata, as an ideal sensitizer for TRAIL to overcome bladder cancer. Our results showed that combination treatment of Andro and TRAIL retarded growth, attenuated proliferation, decreased colony formation, inhibited migration and promoted caspases-mediated apoptosis in T24 cells. Additionally, the sensitization by Andro is achieved through up-regulation of death receptors (DR4 and DR5) of TRAIL in a p53-dependent manner. Crucially, Andro is also capable of inactivating NF-κB signaling pathway via transcriptional down-regulation p65/RelA, which is further contributed to enhancement of TRAIL-mediated cytotoxicity. These results indicated that non-toxic doses of Andrographolide sensitized bladder cancer cells to TRAIL-mediated apoptosis, suggesting it as an effective therapeutic agent for TRAIL resistant human bladder cancers.
Keywords: andrographolide, bladder cancer, death receptors, TRAIL, NF-κB

 Global reach, higher impact
Global reach, higher impact