10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2019; 15(12):2538-2547. doi:10.7150/ijbs.35357 This issue Cite
Research Paper
microRNA-1 Regulates NCC Migration and Differentiation by Targeting sec63
1. Jiangsu Key Laboratory of Oral Diseases, Nanjing Medical University, Nanjing 210019, China
2. MOE Key Laboratory of Model Animal for Disease Study, Model Animal Research Center, Nanjing University, Nanjing 210029, China
3. Department of Pathophysiology, Key laboratory of Cardiovascular Disease and Molecular Intervention, Nanjing Medical University, Nanjing 210029, China
* These authors contributed equally to this work.
Abstract
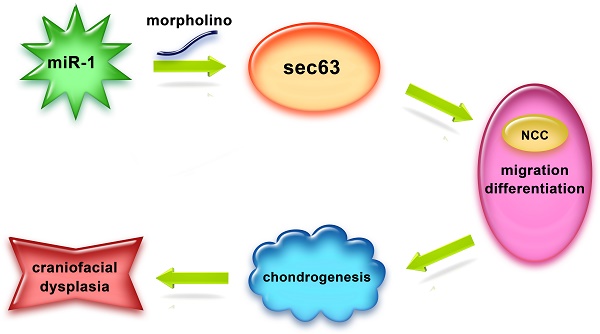
Background/Aims: Neural crest cells play a vital role in craniofacial development, microRNA-1 (miR-1) is essential in development and disease of the cardiac and skeletal muscle, the objective of our study is to investigate effects of miR-1 on neural crest cell in the craniofacial development and its molecular mechanism.
Methods: We knocked down miR-1 in zebrafish by miR-1 morpholino (MO) microinjection and observed phenotype of neural crest derivatives. We detected neural crest cell migration by time-lapse. Whole-mount in situ hybridization was used to monitor the expressions of genes involved in neural crest cell induction, specification, migration and differentiation. We performed a quantitative proteomics study (iTRAQ) and bioinformatics prediction to identify the targets of miR-1 and validate the relationship between miR-1 and its target gene sec63.
Results: We found defects in the tissues derived from neural crest cells: a severely reduced lower jaw and delayed appearance of pigment cells. miR-1 MO injection also disrupted neural crest cell migration. At 24 hours post fertilization (hpf), reduced expression of tfap2a, dlx2, dlx3b, ngn1 and crestin indicated that miR-1 deficiency affected neural crest cell differentiation. iTRAQ and luciferase reporter assay identified SEC63 as a direct target gene of miR-1. The defects of miR-1 deficiency could be reversed, at least in part, by specific suppression of sec63 expression.
Conclusion: miR-1 is involved in the regulation of neural crest cell development, and that it acts, at least partially, by targeting sec63 expression.
Keywords: Cranial Defect, Neural Crest Cells, microRNA, Sec63, iTRAQ

 Global reach, higher impact
Global reach, higher impact