10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2020; 16(6):957-969. doi:10.7150/ijbs.38264 This issue Cite
Review
Innate Lymphoid Cells at the Maternal-Fetal Interface in Human Pregnancy
1. Laboratory for Reproductive Immunology, NHC Key Lab of Reproduction Regulation (Shanghai Institute of Planned Parenthood Research), Hospital of Obstetrics and Gynecology, Fudan University Shanghai Medical College, Shanghai 200082, People's Republic of China.
2. Shanghai Key Laboratory of Female Reproductive Endocrine Related Diseases, Shanghai, 200011, People's Republic of China.
Received 2019-7-8; Accepted 2020-1-6; Published 2020-1-30
Abstract
Pregnancy constitutes a major challenge to the maternal immune system, which must tolerate fetal alloantigen encoded by paternal genes. In addition to their role in inducing maternal-fetal immune tolerance, accumulating evidence indicates that decidual immune cells are involved in several processes required for a successful pregnancy, including trophoblast invasion as well as tissue and spiral artery remodeling. Innate lymphoid cells (ILCs), an important branch of the innate immune system, which has expanded rapidly in recent years, are strong actors in mucosal immunity, tissue homeostasis and metabolism regulation. With the recent identification of ILCs in the human decidua, the role of ILCs at the maternal-fetal interface raises concern. Herein, we review the presence and characterization of ILCs in the human decidua, as well as their function in normal pregnancy and pathological pregnancy, including reproductive failure, preeclampsia and others.
Keywords: innate lymphoid cell, pregnancy, trophoblast invasion, spiral artery remodeling, reproductive failure, preeclampsia.
Introduction
In human pregnancies, the embryo implants into the specialized mucosal (decidua) wall of the uterus. During early pregnancy, maternal-fetal immune tolerance contributes to embryo antigen specific tolerance, trophoblast invasion, spiral artery remodeling and embryonic development and is quite vital for the maintenance of normal pregnancy in mice and humans [1-4]. Once this balance is broken, diseases associated with disturbances of maternal-fetal immune regulation, such as reproductive failure, preeclampsia and intrauterine growth retardation may occur [5-8]. Therefore, the mechanism of maternal-fetal immune regulation is a hotspot worldwide. In human early pregnancy decidua, the main population of lymphocytes is natural killer (NK) cells (accounting for 70%), and the importance of which is self-evident [9]. Innate lymphoid cells (ILCs), as part of the innate immune system, contain NK cells and have recently received attention from researchers. Considering the possibility for fundamental differences in the biology between mouse and human, the evidence mentioned in this article is based on studies in human ILCs at the maternal-fetal interface, unless otherwise specified. However, knockout mice are still powerful tools, especially in the discovery of transcription factors essential for the development and function of ILCs.
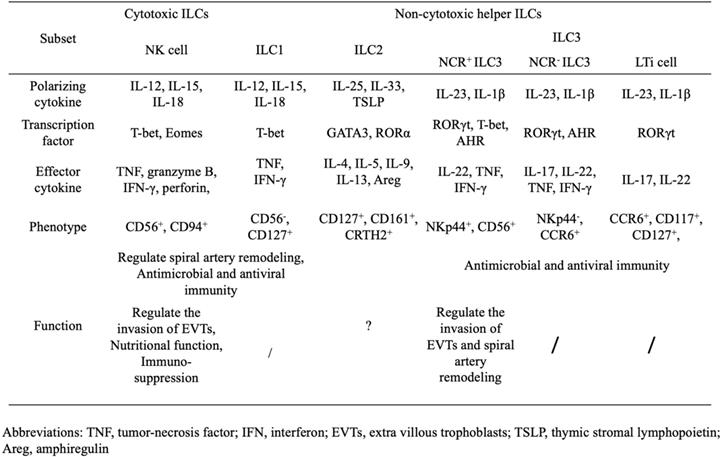
ILCs, as part of the innate immune systems, are characterized by a classic lymphoid cell morphology, dependence on the common cytokine receptor γ-chain and the transcriptional repressor inhibitor of DNA binding 2 (ID2) for their development, and lack of any antigen receptors [10-12]. For decades, only NK cells (which were first discovered in the spleen of mice in 1975 [13]) and lymphoid tissue inducer (LTi) cells (which were first discovered in the lymph node of mice in 1997 [14]) were encompassed in the ILC family. However, quite recently, the ILC family has expanded dramatically and is divided into two distinct lineages, according to the developmental pathways, NK cells and non-cytotoxic helper ILCs [12, 15]. The latter furthermore falls into three categories, including group 1 ILCs (ILC1s), group 2 ILCs (ILC2s) and group 3 ILCs (ILC3s) [15, 16]. This categorization of non-cytotoxic helper ILCs reflects their differential requirement of transcription factors during development and their cytokine expression profiles [12].
As the only ILC subset to exhibit cytotoxicity, NK cells are defined as producing interferon (IFN)-γ and tumor necrosis factor in response to IL-12, IL-15 and IL-18, and expressing CD56 and CD94 in humans (Table 1) [16]. The transcription factor T-bet and Eomesodermin (Eomes) are crucial for their differentiation and acquisition of function [17, 18]. Similar to NK cells, ILC1s share the same pattern of cytokine production and the requirement of T-bet. Considering the resemblances between ILC1s and NK cells, and their inability to produce perforin and granzyme B, ILC1s are also described as non-cytotoxic NK-like cells [19, 20].
Human ILC2s are defined by the expression of CD127, CD161 and the chemo-attractant receptor-homologous molecule expressed on Th2 cells [21]; their production of Th2-cell-associated cytokines (including IL-4, IL-5, IL-9, IL-13, and amphiregulin) in response to IL-25, IL-33 and thymic stromal lymphopoietin[22-24]; and the requirement of the transcription factor GATA-binding protein 3 (GATA3) and retinoic acid receptor-related orphan receptor-α (RORα) for their development [25, 26].
Finally, ILC3s, which contain several cell types, are characterized by their capacity to produce Th17-cell-associated cytokines (IL-17 and/or IL-22), following stimulation by IL-23 and IL-1β, and their dependence on the transcription factor retinoid-related orphan receptor γt (RORγt) during their development process. LTi cells, the prototypical ILC3 population, are vital factors in the formation of secondary lymphoid organs during embryogenesis. The remaining subsets of ILC3s are classified according to their expression of the natural cytotoxicity receptor (NCR), NKp44 in humans or NKp46 in mice. NCR+ILC3s, also known as NK22 cells [27], NCR22 cells [28], NCR-LTi cells [29] and ILC22s [30], produce IL-22 but not IL-17A, and require T-bet and aryl hydrocarbon receptor (AHR), in addition to RORγt, for their differentiation. Notably, CD56, one of the most typical markers of NK cells, is also found on some NCR+ILC3s, while NK cells are unable to produce IL-22 [27, 31]. NCR-ILC3s, which phenotypically mirror LTi cells, express the chemokine receptor CCR6 and are termed LTi-like ILC3s. Unlike LTi cells, AHR is also crucial for the development of NCR-ILC3s [32].
Phenotypical heterogeneity and function of human ILCs.
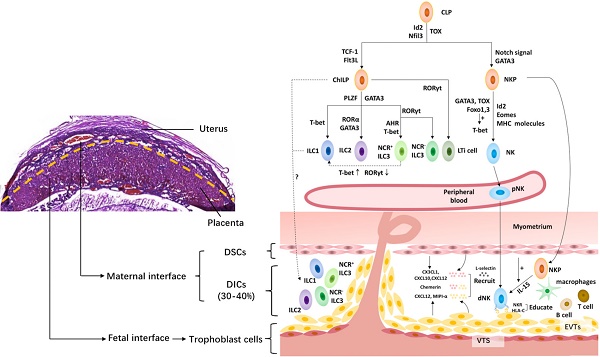
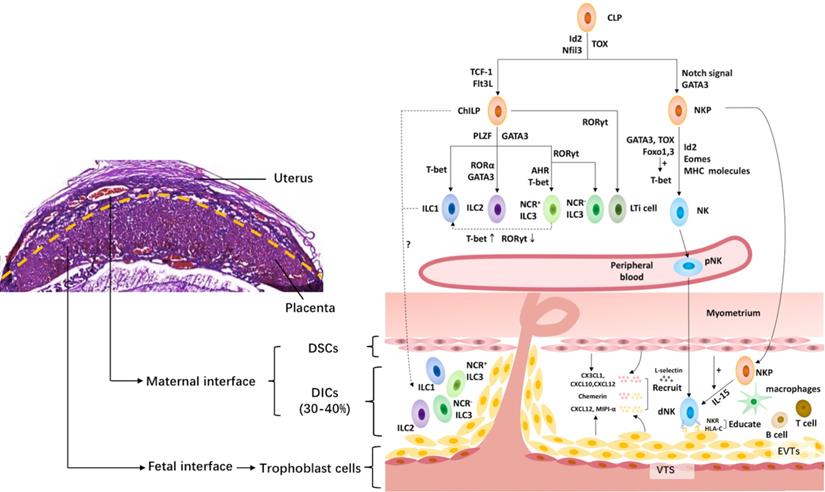
The innate lymphoid cell family at the maternal-fetal interface. The upper left shows the murine maternal-fetal interface stained by hematoxylin-eosin. And the right shows the innate lymphoid cell family at the maternal-fetal interface. All innate lymphoid cells (ILCs) arise from a common lymphoid progenitor (CLP). NK cells develop from a CLP via NK cell precursors (NKP), while the rest of the ILC subsets develop from a CLP via common helper ILC precursors (ChILP). PLZF- ChILP is restricted to lymphoid tissue inducer (LTi) cells, while PLZF+ ChILP are capable of differentiating into all non-cytotoxic helper ILC subsets, except for LTi cells. T-bet, GATA3 and RORγt are required for the lineage maintenance of NK cells, as well as ILC1s, ILC2s and ILC3s. The maternal-fetal interface is the frontier of the direct contact between the embryo and mother. It is mainly composed of fetal trophoblast cells, maternal decidual stromal cells (DSCs) and decidual immune cells (DICs), including ILCs, macrophages, T cells, B cells, etc. At the maternal-fetal interface, all the ILC subsets are identified during the third trimester, while only NK cells, ILC1s and ILC3s are present in early pregnancy. The origin of dNK cells contains in situ progenitors, as well as peripherally derived HPCs and/or pNK homing cells. The migration of NK cells from the periphery to the decidua requires chemokines, including CXCL12 and MIPI-α, secreted by trophoblasts cells, and CX3CL1, CXCL10 and CXCL12 secreted by DSCs, adhesion molecules, such as L-selectin, as well as chemerin expressed in DSC and extravillous trophoblast cells. However, little is known about the origin of the rest of the ILC subsets in the human decidua. Moreover, NK cells acquire functional competence and self-tolerance by NK cell education via constant NK receptor (NKR)-MHC interactions. Id2, inhibitor of DNA binding 2; Flt3L, Flt3 ligand; GATA3, GATA-binding protein 3; PLZF, promyelocytic leukemia zincfinger protein; RORα, retinoic acid receptor-related orphan receptor-α; RORγt, retinoid-related orphan receptor γt; AHR, aryl hydrocarbon receptor; Eomes, Eomesodermin; NCR, natural cytotoxicity receptor; DSC, decidual stromal cells; NKR, natural killer receptors; VTS, villous trophoblasts; EVTs, extravillous trophoblasts.
The Composition of the Maternal-Fetal Interface
The maternal-fetal interface is the frontier of the direct contact between the embryo and mother (Fig. 1). It is mainly composed of fetal trophoblast cells, maternal decidual stromal cells (DSCs) and decidual immune cells (DICs) [33].
Fetal Trophoblast Cell
Human trophoblast cell, the main components of placenta, is divided into two main cell lineages, namely, villous trophoblasts (VTS) and extravillous trophoblasts (EVTs). VTS form chorionic villi, cover the surface of the villi which transports nutrients and oxygen to the fetus, and produce a variety of hormones and pregnancy factors that are required for the development and maintenance of embryos, such as human chorionic gonadotropin (HCG), progesterone and human placental lactogen, neurotransmitters, inhibin and activin. EVTs directly contact with the immune cells of the mother's decidua. They invade the decidua tissue, remodel the spiral artery and intrude into the blood vessels. The invasion of EVT breaks the contractility of spiral arteries for ensuring sufficient blood supply in the placenta [34]. Therefore, the invasion of EVT is an essential process for fetal implantation and placenta formation.
Maternal DSC
DSCs, the main constituent of the decidua, are differentiated from the fibroblast-like precursor cells of nonpregnant endometrium under the induction of estrogen and progesterone. In addition to the nutrient supply in decidua, DSCs also secrete hormones (e.g., prolactin), cytokines, and enzymes; expresses the progesterone receptor; and regulate embryo implantation and placental development. As potential immune cells, DSCs secrete a variety of cytokines and play an important role in immune regulation [35]. By secreting CXCL12, DSCs promote the accumulation of peripheral NK cell in decidua and induce the conversion of pNK to dNK-phenotype [36-39]. Besides, DSCs contribute to Th2 bias at maternal-fetal interface by producing CCL2 and IL-33. DSC-secreted CCL2 also participates in immunosuppression by inhibiting the cytotoxicity of NK cells during pregnancy [40].
Maternal DIC
The composition of DICs is quite special. During early pregnancy, DICs account for 30-40% of the decidual cells. Among them, decidual NK (dNK) cells reach up to 70%, macrophages account for 20%, T cells account for 10%, and dendritic cells and B cells account for a smaller percentage. By interacting with each other and restricting each other, the DICs form a special immune network in the decidual microenvironment. In early pregnancy, to protect the semi-allogeneic fetal placenta from attacking by the maternal immune system, the main role of interactions between DICs is to maintain immune suppression; while, during late pregnancy, that transforms to immune rejection in order to prepare for fetal delivery. Therefore, the number and function of the DICs are changing in different stages of pregnancy [41]. In a normal pregnancy, dynamic changes in the DICs-formed network are required to meet the physiological needs in different periods of pregnancy. Once the balance of the system is broken, it inevitably leads to serious consequences, such as abortion, premature delivery, intrauterine growth retardation and preeclampsia. Therefore, the balance of the DICs-formed network is crucial to the success of pregnancy [42].
ILCs at the Maternal-Fetal Interface
It is well known that NK cells are the main components of the immune system at the maternal-fetal interface. In 1991, the presence of dNK cells was characterized during early placentation [43]. With the increasing focus on the ILCs, other subsets were identified in the human decidua of early pregnancy (Fig. 1) [44-46].
Decidual NK Cells
Similar to other lymphocytes, the ILC family arises from a common lymphoid progenitor (CLP). Notably, NK cells develop from a CLP via NK cell precursors (NKP), while the rest develop from a CLP via common helper ILC precursors (ChILP)[15,47]. Recently, strict NKP, which represent a separate downstream branch of CLPs and only generate cytotoxic NK cells, were defined as a Lin-CD34+CD38+CD123-CD45RA+CD7+CD10+CD127- population and were identified in the human umbilical cord blood, bone marrow, fetal liver, and adult tonsil [48]. Although the complex network that regulates the NK-specific differentiation of CLPs remains unclear, several transcription factors have been revealed. Studies in Nfil3-deficient mice observe a specific lack of NK cells, which demonstrate the necessity of Nfil3 in NK cell lineage differentiation [49, 50], and Nfil3 is required only when the CLP differentiation is toward NKP [51]. Id2, a transcriptional suppressor of E protein activity, is essential for the generation of NK cells at the NKP stage [10, 11] and NK cell maturation [10, 52] in mice. Moreover, the differentiation from CLP to NK cells is promoted by Notch ligands Jagged1 in humans and Jagged2 in mice, respectively [53, 54]. For NK cell maturation, studies in mice reveal that T-bet and some other regulatory factors play a central role in this transition. A deficiency of T-bet results in decreased expression of the transcription factor Blimp1 [55], arrest at the immature stage, and a lack of mature NK cells in the bone marrow and periphery [56]. Some other transcription factors, such as the positive regulator GATA3 [57] and TOX [58, 59], as well as the suppressors Foxo1 and Foxo3 [60], regulate the expression of T-bet and, thus, participate in NK cell maturation in humans. Eomes, another T-box transcription factor family member, is also crucial for NK cell terminal maturation, as murine mature NK cells revert to phenotypic immaturity with the deletion of Eomes [17]. Meanwhile, NK cell maturation is also regulated by MHC molecules [61], which are a combination of polymorphic HLA-C as well as oligomorphic HLA-E and HLA-G molecules expressed by the placental trophoblast cells during human pregnancy [62]. However, despite intense research, how tissue signals control the NK-specific differentiation of CLPs via transcriptional factors is still not fully understood.
During pregnancy, the number of dNK cells accessed peaks in the first trimester (over 70% of decidual leukocytes), then decreases gradually and reaches the bottom at term [43, 63]. Since the concept that dNK cells represent a distinctive, hormonally regulated subset was proposed in 1991, numerous studies have investigated these cells for decades. NK cells in the human decidua are CD56superbrightCD16-, and have a distinct phenotype compared to peripheral blood NK (pNK) cells. CD56superbright dNK cells express CD9 and CD49a, while pNK cells are CD9-CD49a- [8, 64-66]. Except for the widely used classification according to the expression level of CD56, NK cells are also divided into four subsets according to the expression of CD11b and CD27: CD11b+CD27- with high cytotoxicity; CD11b-CD27+ as well as CD11b+CD27+ with best ability to secret cytokines; and CD11b-CD27- with differentiation potential [67-69]. In humans, it is reported that more than 90% of pNK cells are CD11b+CD27-, while for dNK cells, approximately 60% are CD11b-CD27- and over 20% are CD27+ [68]. Moreover, natural killer group 2 (NKG2)A/C/E and killer-cell immunoglobulin-like receptors (KIR) show higher expression levels on dNK cells than those on pNK cells [65, 70, 71], while the T-cell immunoglobulin domain and mucin domain-containing molecule-3 (Tim-3) show lower expression levels on dNK cells than those on pNK cells [72].
As a distinct subset with unique phenotypic and functional features, the origin of dNK cells is not fully understood. One possibility is that dNK cells derive from recruited pNKs with the adaptation of the microenvironment in decidua. The conversion of the pNK to dNK-phenotype can be induced when pNK cells are cultured with DSCs or TGF-β1 [73, 74]. The migration of NK cells from the periphery to the decidua requires chemokines (CXCL12 [39, 75] and MIPI-α [76], secreted by trophoblasts cells, and CX3CL1/fractalkine, CXCL10/IP-10 and CXCL12/SDF-1, secreted by DSCs [77]), adhesion molecules (such as L-selectin [78]), as well as extravillous trophoblast and DSC-derived chemerin [79]. Additionally, the accumulation of NK cells in the decidua is promoted with the increasing level of estradiol (E2), luteinizing hormone and progesterone during pregnancy [80]. Peripherally derived hematopoietic progenitor cells are another possible origin of dNK cells, considering the presence of donor-derived dNK cells in the murine uteri during decidualization following experimental transgenic labeling of transplanted bone marrow cells [81, 82]. Based on the presence of CD34+ hematopoietic precursors with the potential to give rise to functional CD56brightCD16- NK cells in the human decidua, the possibility that dNK cells arise from in situ progenitors is proposed [83]. Additionally, tissue-specific differentiation of resident progenitors occurs in the presence of IL-15 [84] or upon co-culture with DSCs [83]. Thus, the origin of dNK cells is quite complex and is thought to contain in situ progenitors, as well as peripherally derived hematopoietic progenitor cells and/or pNK homing cells [66, 85], and needs more evidence to clarify.
Decidual Non-Cytotoxic Helper ILCs
In addition to NK cells, non-cytotoxic helper ILC subsets are identified in the human decidua, and their phenotypes are quite similar to those previously described. In humans, decidual ILC1s produce IFN-γ, express T-bet, and lacks CD56, Eomes and RORγt. Decidual NCR+ILC3s express CD56, CD117, CD127 and RORγt, produce a high amount of IL-8 and IL-22 but are negative for IFN-γ. Additionally, decidual LTi-like cells are Lin-CD56-NCR-, express IL17A as well as tumor necrosis factor, and express RORγt [8, 44]. In subsequent publications, the presence of LTi-like cells and NCR+ILC3s in the human decidua are confirmed [45, 86, 87]. However, the existence of decidual ILC1s is controversial. In a study by Doisne's group, ILC1s were undetected in the human endometrium or decidua [45]. Moreover, Simoni and colleagues failed to detect any ILC1 population in human tissues and proposed the possibility of contamination by other cells, including T cells, ILC3s, dendritic cells, hematopoietic stem cells and NK cells, based on the t-distribution stochastic neighbor embedding analysis from the mass cytometry data [88]. However, their analysis approach and ILC purification methods were questioned [89, 90]. Thus, more advanced experimental techniques are needed for the precise definition and accurate proof of the existence of ILC1s in the future. Unlike ILC1s and ILC3s, ILC2s are detected as the most abundant ILC subset in the human decidua at the third trimester instead of the first trimester [46]. Taken together, the distribution of decidual ILC subsets changes with the progress of pregnancy, which needs further attention.
Although all groups of non-cytotoxic ILCs are identified in the uterus of pregnant mice, only ILC1 is present in decidua. Other groups, however, can only be found in myometrium and the myometrial lymphoid aggregate of pregnancy. Thus, ILC1 is the only subpopulation of non-cytotoxic ILCs to interact directly with trophoblast in mice. Murine uterine ILC1s express CD49a, lack DX5, CD127 and TRAIL, and share similar pattern of transcription factor with human decidual ILC1s (T-bet+Eomes-RORγt-). Moreover, unlike human, mice lack NCR+ ILC3s in uterus [45].
Non-cytotoxic helper ILCs develop from ChILP, which is defined as expressing of Id2, CD127, and integrin α4β7, as well as lacking common lineage markers and CD25 [91]. Knockout mice have revealed several transcription factors that are essential for non-cytotoxic helper ILC development and function. Transcription factors, such as Nfil3 [92, 93], TCF-1[94], Flt3 ligand [95], Id2 [11, 96] and TOX are necessary for the early development of ChILP [97]. In addition, the transcription factor promyelocytic leukemia zinc finger protein (PLZF) is essential for all non-cytotoxic helper ILC subsets, except for LTi cells, during their development [98, 99], and GATA3 is critical for the generation of PLZF+ ILC progenitors [100]. T-bet, GATA3 and RORγt are the master transcription factors for the maturation of ILC1s [91], ILC2s [25] and LTi-like cells [32, 101, 102], respectively. T-bet, RORγt and AHR are among the factors that then control the final stages of NCR+ILC3 cell maturation [32, 102, 103]. Notably, unlike NK cells, which never express RORγt during their development, there is a rapid increase in T-bet expression and a gradual decrease in RORγt expression during the development of a group of ILC1s [29, 104]. Indeed, this ILC1 population expresses a high level of T-bet and a low level of RORγt [104]. Taken together, the plasticity between ILC1s and ILC3s may exist, and complicate the definition of ILC1s.
Although there are several reports about the origin of NCR+ILC3s, little is known about the origin of non-cytotoxic helper ILCs in the human decidua. Marina et al. found that culturing peripheral or cord blood cells led to the failure of NCR+ILC3 production [27]. In accordance with this, human RORγt+CD34+ cells, the lineage-specified progenitors of NCR+ ILC3s, are located in the tonsil and intestinal lamina propria but not the peripheral blood, thymus, or bone marrow [105], which indicates the possibility that tissue-specific NCR+ILC3s originate in situ.
Functions of Decidual ILCs in Normal Pregnancy
As an arm of the innate immune system, ILCs play an important role in the suppression of fetal rejection, trophoblast invasion (Fig. 2) and spiral artery remodeling (Fig. 3) during pregnancy.
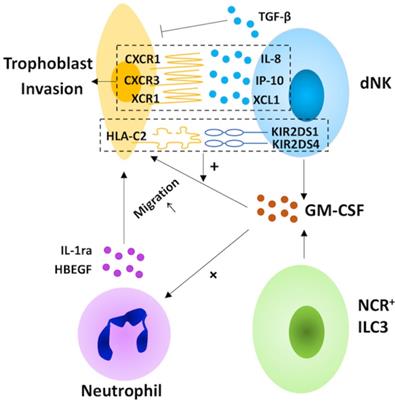
In humans, the invasion of EVTs into the maternal decidualized uterus is vital for the establishment of pregnancy [106]. Decidual NK cells may direct this process by producing chemokines, including IL-8, the ligand for CXCR1 expressed on invasive trophoblasts, IP-10, one of the ligands of the CXCR3 expressed on trophoblast cells, and XCL1, the ligand for XCR1 expressed by EVT [107, 108]. Moreover, dNK cell-derived granulocyte macrophage colony-stimulating factor (GM-CSF), which induces trophoblast migration, increases in response to the ligation of the NK cell-activating receptors KIR2DS1 and 4 [108, 109]. The facilitation of dNK cells in trophoblast invasion is also promoted by the cross-talk between dNK cells and invasive trophoblast cells, such as the binding of maternal KIR2DS1 and fetal HLA-C2 [109, 33]. Paradoxically, dNK cells inhibit EVT invasion via TGF-β secretion [110]. Taken together, dNK cells may play a bi-directional regulatory role in trophoblast invasion (Table 1). Except for dNK cells, trophoblast invasion is also regulated by NCR+ILC3s in the human decidua. NCR+ILC3-derived GM-CSF promotes trophoblast migration directly, as well as by inducing the expression of heparin-binding epidermal growth factor-like growth factor (HBEGF) and IL1ra in neutrophils [108, 111-113]. Thus far, whether decidual LTi-like cells are capable of regulating trophoblast migration is still unknown. However, it is reported that IL-17 promotes trophoblast proliferation and invasion [114]. In light of this, decidual LTi-like cells may promote trophoblast invasion by secreting IL-17, and this warrants further investigation.
The role of decidual innate lymphoid cells in trophoblast invasion. In the human decidua, natural killer (NK) cells and group 3 innate lymphoid cells (ILC3s) participate in the invasion of extravillous trophoblast cells (EVT). Decidual NK (dNK) cells play a bi-directional regulatory role in trophoblast invasion. On the one hand, dNK cells direct this process by secreting chemokines (including interleukin (IL)-8, interferon-inducible protein (IP)-10 and XCL1) and granulocyte macrophage colony-stimulating factor (GM-CSF), which increase in response to the ligation of the NK cell-activating receptors KIR2DS1 and 4. On the other hand, NK cells also inhibit EVT invasion via TGF-β secretion. For NCR+ILC3s, the production of GM-CSF promotes trophoblast migration directly, as well as by inducing the expression of heparin-binding epidermal growth factor-like growth factor (HBEGF) and IL1ra in neutrophils.
Adequate perfusion is the cornerstone of pregnancy maintenance as well as fetal growth. It requires spiral artery remodeling, which is partly regulated by decidual ILCs. Evidence from murine models indicates that the initiation of spiral artery remodeling requires IFN-γ, which is one of the representative cytokines produced by NK cells and ILC1s. Histological observations reveal that IFN-γ promotes the growth of the decidual vascular lumen size and facilitates the separation of vascular smooth muscle cell (VSMC) layers [115, 116]. Quite recently, it was demonstrated that IFN-γ promotes the migration and apoptosis of VSMCs during vascular transformation [117]. In addition to IFN-γ, dNK-derived matrix metalloproteinases (MMP) 2 and 9 also mediate the disruption of the VSMC wall during pregnancy [118]. Meanwhile, human dNK cells are capable of secreting several angiogenic factors, which induce vascular growth and participate in spiral artery remodeling, including vascular endothelial growth factor (VEGF), angiopoietin 1 and 2, placental growth factor and hepatocyte growth factor [107, 119, 120]. In addition, decidual NCR+ILC3s are involved in angiogenesis by secreting cytokines, such as GM-CSF and CXCL8, to upregulate the expression of HBEGF and IL1ra in neutrophils [111-113].
During early pregnancy, the nutritional function of the placenta is not yet perfect. CD49a+Eomes+ NK cells, the dominant population of dNK cells, secrete growth-promoting factors, such as pleiotrophin and osteoglycin, and participate in optimizing the utilization of maternal nutrition in order to meet the needs of fetal growth [8]. Moreover, dNK cells promote trophoblast growth and differentiation by secreting cytokines, including IL-22 [121], GM-CSF and colony-stimulating factor-1 in mice [122-124].
During pregnancy, the survival of semi-allogeneic fetal tissue requires a sophisticated immune regulatory network. It is commonly believed that specialized regulation may occur depending on specific anatomic site during pregnancy. By inducing the generation and differentiation of regulatory T cells via TGF-β, as well as inhibiting Th17-mediated local inflammation via IFN-γ, dNK cells promote maternal tolerance against the semi-allogeneic fetus [119, 125-129]. IL-22 is a key regulator in immunity, inflammation and tissue homeostasis, and is closely related to the occurrence and development of several autoimmune diseases, including inflammatory bowel disease, psoriasis and graft versus host disease, as shown in patient or animal models [130-136]. In view of this, it is possible that decidual NCR+ILC3s exert suppression on fetal rejection by secreting IL-22, but so far direct evidence for this is lacking.
On account of the increased susceptibility and severity to some infections (Among pregnant women, the role of decidual ILCs in defense against infection is quite important [137]. NK- or ILC1-derived IFN-γ, for example, is crucial in antimicrobial and antiviral immunity by activating macrophages, enhancing antigen presentation and promoting Th1 differentiation [138]. Moreover, ILC3s are also an important source of GM-CSF, which recruits inflammatory monocytes and acts to prevent infection. In a mouse model of intestinal inflammation, ILC3 depletion partially impairs the ability to control bacterial infection [139]. Further study is required to determine whether ILC3s have the same anti-infection effect at the maternal-fetal interface.
The role of decidual natural killer cells in spiral artery remodeling. In early pregnancy, decidual natural killer (NK) cells promote the process of spiral artery remodeling by inducing vascular growth via angiogenic factors, including vascular endothelial growth factor (VEGF), angiopoietin (Ang) 1 and 2, placental growth factor (PLGF) and hepatocyte growth factor (HGF), enhancing the migration and apoptosis of vascular smooth muscle cell (VSMC) layers via interferon-γ (IFN-γ) as well as facilitating the disruption of the VSMC wall via matrix metalloproteinase (MMP) 2 and 9.

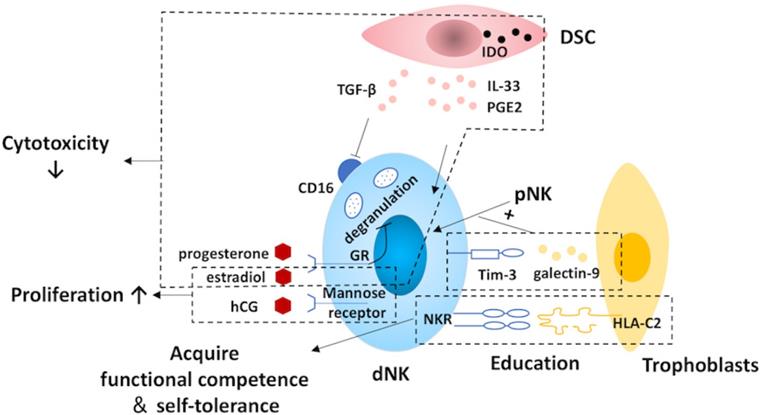
Regulation of decidual natural killer cells. At the maternal-fetal interface, natural killer (NK) cells are regulated by trophoblasts, decidual stromal cells (DSCs) and hormones. NK cells acquire functional competence and self-tolerance by NK cell education via the constant interactions between NK receptors (NKR) and the MHC molecules expressed by the trophoblast cells. Moreover, trophoblasts also promote the conversion of recruited pNK to dNK cells via the galectin-9/Tim-3 pathway. The expression of CD16 on NK cells is suppressed by DSC-derived TGFβ. Additionally, DSCs are capable of inhibiting the cytotoxicity of NK cells by expressing indoleamine 2,3-dioxygenase (IDO) as well as secreting IL-33 and prostaglandin E2 (PGE2). Also, hormones and pregnancy factors produced by trophoblasts, such as human chorionic gonadotropin (hCG), progesterone and estradiol, play a role in dNK regulation. The cytotoxicity of NK cells is suppressed by progesterone via blocking degranulation and estradiol. In addition, the proliferation of dNK cells is promoted by hCG and estradiol. Tim-3, T-cell immunoglobulin domain and mucin domain-containing molecule-3; dNK, decidual natural killer; pNK, peripheral blood natural killer.
Regulation of Decidual ILC Differentiation
The maternal-fetal interface is the core of the mother-fetal dialogic regulatory network, where trophoblast cells, DSCs, DICs, and various cytokines and hormones produced by these cells, with interaction dialogues, jointly construct the special immune tolerance microenvironment, in order to maintain physiological pregnancy. Therefore, trophoblast cells, DSCs and hormones in the microenvironment at the maternal-fetal interface are bound to regulate the differentiation and function of the decidua ILCs. However, research on these regulatory mechanisms is mainly focused on dNK cells (Fig. 4), and little is known about the regulation of non-cytotoxic helper ILC differentiation in the human decidua.
The fetal trophoblasts actively educate the dNK cells. Through constant NK receptor-MHC interactions, NK cells acquire functional competence and self-tolerance, and this process is defined as NK cell education [140, 141]. Herein, the education is much more sophisticated at the maternal-fetal interface both in humans and in mice, as it is an important physiological situation in which allogeneic (paternal) MHC class I molecules are presented [66, 142]. Human genetic epidemiological data and mouse studies suggest that the interactions between the NK receptors and the maternal environment, as well as the paternal MHC molecules expressed by the trophoblast cells regulate NK cell activity and somehow determine pregnancy outcomes [2, 142]. In addition to NK cell education, trophoblasts also promote the conversion of recruited pNK to dNK cells via the galectin-9/Tim-3 pathway at the maternal-fetal interface during early pregnancy [72].
The DSC is another important regulator of dNK differentiation. CD16 is a surface marker that is tightly related to the cytotoxicity of NK cells [143], and its expression on pNK cells is down-regulated by DSC-derived TGF-β in vitro [73]. By secreting IL-33, DSCs suppress the cytotoxicity of NK cells and induce the production of Th2 cytokines, including IL-4, IL-10, and IL-13 [144]. Additionally, DSC can inhibit the cytolytic activity of NK cells by expressing indoleamine 2,3-dioxygenase (IDO) and secreting prostaglandin E2 [145].
During pregnancy, the role of hormones cannot be neglected. Although, dNK cells lack steroid receptors and the classic luteinizing hormone/CG receptor, hormones can still exert their effects through the glucocorticoid receptor or the mannose receptor [146, 147]. Evidence from experiments in mice suggests that E2 not only is favorable for NK cell proliferation but also can reduce their cytotoxicity [80, 148]. Mediated by progesterone induced blocking factor, progesterone contributes to inhibition of dNK cell cytotoxicity via blocking degranulation [149, 150]. Meanwhile, HCG also promotes the proliferation of dNK cells [147].
Role of Decidual ILCs in Pathological Pregnancy
Reproductive Failure
As the only subset of ILCs with cytotoxicity, dNK cells are lowly cytotoxic in adaptation to alloantigen stimulation during normal pregnancy [66, 151]. Dysregulation of cytotoxicity can turn dNK cells into detrimental cells and cause reproductive failure, including spontaneous abortion, unexplained infertility and implantation failure after in vitro fertilization [152-154]. The mechanism behind this connection is wildly discussed. For example, an imbalance between activating and inhibitory receptors on NK cells, specifically, the increased expression of NKG2D, an activating receptor that mediates NK cell cytotoxicity, as well as the lack of KIR, are deciding factors in pregnancy outcomes [155-157]. Furthermore, NK cell impairment to suppress the expansion and activity of Th17 cells, is identified in patients with recurrent spontaneous abortion [129]. Using the lipopolysaccharide-induced mouse abortion model, researchers found that in the uterus, a reduction of a tumor necrosis factor-like weak inducer of apoptosis (TWEAK) in dNK cells and the upregulation of its receptor, may alter the cytotoxicity of dNK cells and, ultimately, result in pregnancy loss [158]. Quite recently, over-activated dNK cells, with an increased expression of angiogenic cytokines, such as VEGF-A and basic fibroblast growth factor, were observed in women with recurrent miscarriage, which suggests the potential relationship between the dysfunction of dNK cells in regulating angiogenesis and recurrent miscarriage [159].
The relationship between NCR+ILC3s and reproductive failure was also investigated. Kamoi et al. found that the percentage of decidual NCR+ILC3s in patients with unexplained recurrent spontaneous abortion was significantly higher than that in those with unexplained infertility [87]. However, the lack of normal pregnant controls in this study requires further investigations to explore the exact relationship between NCR+ILC3s and reproductive failure.
Preeclampsia
Preeclampsia, a serious pregnancy associated disease, is characterized by the insufficient activation of maternal NK cells as well as the excessive activation of decidual LTi-like cells during pregnancy [86, 160, 161]. Many efforts have been made to explain the mechanism of preeclampsia. Several studies suggest that preeclampsia is a result of incomplete trophoblast invasion and spiral artery remodeling [162]. While, some researchers believe that preeclampsia is related to a failure of the maternal vascular system to adapt the necessary volume load that occurs during pregnancy [163, 164]. The ligation of KIR and HLA-C is a pivotal regulator for NK cell activation. A gene linkage analysis shows that the lack of activating KIR (AA genotype) on maternal NK cells, which in turn causes a strong inhibition of NK cells, is a risk factor for preeclampsia [160]. Consistent with this, an additional MHC allele, which prefers binding to inhibitory rather than activating NK cell receptors, causes the suppression of NK cell activity and the incompetence of spiral artery remodeling in mice [142]. Moreover, NCRs, including NKp44 and NKp46, may be potential predictive markers for preeclampsia, in view of the declined expression level of NCRs on pNK cells in women with preeclampsia, which is observed as early as 3-4 months before the onset of preeclampsia [165, 166]. In addition, decidual LTi-like cells are shown as the main source of the increased IL-17 and lead to a higher risk of preeclampsia [86].
Morbidly Adherent Placentation
Morbidly adherent placentation, such as placenta accreta, percreta and increta, is a life-threatening condition. It is reported that the amount of decidual natural killer (dNK) cells in patients with morbidly adherent placentation was significantly lower than normal pregnancy. Moreover, patients with adherent placenta previa showed lower dNK density compare with non-adherent placenta previa [167]. This suggests that the deficiency of NK cells may participate in the process of morbidly adherent placentation. At the maternal-fetal interface, dNK cells inhibit the migration and column formation of extra villous trophoblasts by regulating protease activity and E-cadherin expression [168]. Moreover, this inhibition can be reversed by anti-IFN-γ [168, 169]. Thus, decidual NK cells might protect from morbidly adherent placentation by limiting the over invasion of the extra villous trophoblasts into the uterine wall. However, the relationship between non-cytotoxic helper ILCs and morbidly adherent placentation still remains a mystery and needs further research.
Other Pregnancy-Associated Diseases
In addition to participating in preeclampsia, the ligation of KIR and HLA-C, as the essential regulator for NK cell activation, also affects fetal growth. Pregnancies are at an increased risk of intrauterine growth retardation not only in women with the KIR AA genotype but also in women with the presence of paternal H-2Dd [170]. Additionally, it is reported that decidual LTi-like cells may participate in the development of gestational diabetes and chronic diabetes via secreting IL-17 [86]. Recently, the effect of IL-22 in the prevention of lipopolysaccharide-induced preterm labor in mice was revealed and, thus, presents a potential protective role of decidual NCR+ILC3s in inflammation-induced preterm birth [171]. However, decidual ILC2s may play the opposite role in preterm birth due to the increase in ILC2s in the decidua basalis with spontaneous preterm labor compared to non-labor controls [46].
Concluding Remarks
With a deeper understanding, ILCs in the human decidua are identified and are emerging as indispensable factors in the maintenance of normal pregnancy as well as the development of a wide range of pathological pregnancies (Table 1). Although dNK cells have been studied for decades, research regarding the non-cytotoxic helper ILCs present in the human decidual is still in its infancy. The relatively low number of non-cytotoxic helper ILCs present in the human decidua and the difficulty in the identification, separation and purification of the individual subsets may pose obstacles in further studies. However, given the great deal of similarities between ILCs and T cells in function, especially in the cytokine profile, existing studies from decidual T cells may contribute to shedding light on the exact role of decidual ILCs in human pregnancy.
Acknowledgements
Authors' Contributions
R.Q.C. writing, review, and revision of the manuscript and preparing the figures. W.J.Z revision of the figures. M.Q.L. and D.J.L. writing, review, and revision of the manuscript and revision of the figures.
Funding
This was study supported by the National Key Research and Development Program of China (2017YFC1001404), the National Natural Science Foundation of China (NSFC) (No. 31671200, 31970798, 81901563, 91542108, 81471513 and 81471548), the Innovation-oriented Science and Technology Grant from NPFPC Key Laboratory of Reproduction Regulation (CX2017-2), and the Program for Zhuoxue of Fudan University.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Zhou JZ, Way SS, Chen K. Immunology of the Uterine and Vaginal Mucosae. Trends Immunol. 2018;39:302-14
2. Moffett A, Colucci F. Uterine NK cells: active regulators at the maternal-fetal interface. J Clin Invest. 2014;124:1872-79
3. Erlebacher A. Mechanisms of T cell tolerance towards the allogeneic fetus. Nat Rev Immunol. 2013;13:23-33
4. Guerin LR, Prins JR, Robertson SA, Regulatory T-cells, immune tolerance in pregnancy. a new target for infertility treatment? Hum Reprod Update. 2009;15:517-535
5. Saito S, Shiozaki A, Nakashima A. et al. The role of the immune system in preeclampsia. Mol Aspects Med. 2007;28:192-209
6. Nilsson LL, Djurisic S, Hviid TVF. Controlling the Immunological Crosstalk during Conception and Pregnancy: HLA-G in Reproduction. Front Immunol. 2014;5:198
7. Eide IP, Isaksen CV, Salvesen KA. et al. Fetal growth restriction is associated with reduced FasL expression by decidual cells. J Reprod Immunol. 2007;74:7-14
8. Fu B, Zhou Y, Ni X. et al. Natural Killer Cells Promote Fetal Development through the Secretion of Growth-Promoting Factors. Immunity. 2017;47:1100-13
9. Liu S, Diao L, Huang C. et al. The role of decidual immune cells on human pregnancy. J Reprod Immunol. 2017;124:44-53
10. Boos MD, Yokota Y, Eberl G. et al. Mature natural killer cell and lymphoid tissue-inducing cell development requires Id2-mediated suppression of E protein activity. J Exp Med. 2007;204:1119-30
11. Yokota Y, Mansouri A, Mori S. et al. Development of peripheral lymphoid organs and natural killer cells depends on the helix-loop-helix inhibitor Id2. Nature. 1999;397:702-06
12. Artis D, Spits H. The biology of innate lymphoid cells. Nature. 2015;517:293-301
13. Kiessling R, Klein E, Wigzell H. “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975;5:112-17
14. Mebius RE, Rennert P, Weissman IL. Developing lymph nodes collect CD4+CD3- LTbeta+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity. 1997;7:493-504
15. Eberl G, Colonna M, Di Santo JP. Innate lymphoid cells. Innate lymphoid cells: a new paradigm in immunology. Science. 2015;348:aaa6566
16. Spits H, Artis D, Colonna M. et al. Innate lymphoid cells-a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13:145-49
17. Gordon SM, Chaix J, Rupp LJ. et al. The transcription factors T-bet and Eomes control key checkpoints of natural killer cell maturation. Immunity. 2012;36:55-67
18. Luevano M, Madrigal A, Saudemont A. Transcription factors involved in the regulation of natural killer cell development and function: an update. Front Immunol. 2012;3:319
19. Fuchs A, Vermi W, Lee JS. et al. Intraepithelial type 1 innate lymphoid cells are a unique subset of IL-12- and IL-15-responsive IFN-γ-producing cells. Immunity. 2013;38:769-81
20. Spits H, Bernink JH, Lanier L. NK cells and type 1 innate lymphoid cells: partners in host defense. Nat Immunol. 2016;17:758-64
21. Mjösberg JM, Trifari S, Crellin NK. et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12:1055-62
22. Price AE, Liang HE, Sullivan BM. et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci. 2010;107:11489-94
23. Fallon PG, Ballantyne SJ, Mangan NE. et al. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J Exp Med. 2006;203:1105-16
24. Ebbo M, Crinier A, Vély F. et al. Innate lymphoid cells: major players in inflammatory diseases. Nat Rev Immunol. 2017;17:665-78
25. Hoyler T, Klose CSN, Souabni A. et al. The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity. 2012;37:634-48
26. Halim TYF, MacLaren A, Romanish MT. et al. Retinoic-acid-receptor-related orphan nuclear receptor alpha is required for natural helper cell development and allergic inflammation. Immunity. 2012;37:463-74
27. Cella M, Fuchs A, Vermi W. et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722-25
28. Satoh-Takayama N, Lesjean-Pottier S, Vieira P. et al. IL-7 and IL-15 independently program the differentiation of intestinal CD3-NKp46+ cell subsets from Id2-dependent precursors. J Exp Med. 2010;207:273-80
29. Vonarbourg C, Mortha A, Bui VL. et al. Regulated expression of nuclear receptor RORγt confers distinct functional fates to NK cell receptor-expressing RORγt(+) innate lymphocytes. Immunity. 2010;33:736-51
30. Spits H, Di Santo JP. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat Immunol. 2011;12:21-27
31. Crellin NK, Trifari S, Kaplan CD. et al. Human NKp44+IL-22+ cells and LTi-like cells constitute a stable RORC+ lineage distinct from conventional natural killer cells. J Exp Med. 2010;207(2):281-90
32. F Melo-Gonzalez and M R Hepworth. Functional and phenotypic heterogeneity of group 3 innate lymphoid cells. Immunology. 2017;150:265-75
33. Moffett-King A. Natural killer cells and pregnancy. Nat Rev Immunol. 2002;2:656-63
34. Cui Y, Wang W, Dong N. et al. Role of corin in trophoblast invasion and uterine spiral artery remodelling in pregnancy. Nature. 2012;484:246-50
35. Vinketova K, Mourdjeva M, Oreshkova T. Human Decidual Stromal Cells as a Component of the Implantation Niche and a Modulator of Maternal Immunity. J Pregnancy. 2016;2016:8689436
36. Piao HL, Wang SC, Tao Y. et al. CXCL12/CXCR4 signal involved in the regulation of trophoblasts on peripheral NK cells leading to Th2 bias at the maternal-fetal interface. Eur Rev Med Pharmacol Sci. 2015;19:2153-61
37. Tao Y, Li YH, Piao HL. et al. CD56(bright)CD25+ NK cells are preferentially recruited to the maternal/fetal interface in early human pregnancy. Cell Mol Immunol. 2015;12:77-86
38. Piao HL, Tao Y, Zhu R. et al. The CXCL12/CXCR4 axis is involved in the maintenance of Th2 bias at the maternal/fetal interface in early human pregnancy. Cell Mol Immunol. 2012;9:423-30
39. Hanna J, Wald O, Goldman-Wohl D. et al. CXCL12 expression by invasive trophoblasts induces the specific migration of CD16- human natural killer cells. Blood. 2003;102:1569-77
40. Xu X, Wang Q, Deng B. et al. Monocyte chemoattractant protein-1 secreted by decidual stromal cells inhibits NK cells cytotoxicity by up-regulating expression of SOCS3. PloS One. 2012;7:e41869
41. Erlebacher A. Immunology of the maternal-fetal interface. Annu Rev Immunol. 2013;31:387-411
42. Sargent IL, Borzychowski AM, Redman CWG. NK cells and human pregnancy-an inflammatory view. Trends Immunol. 2006;27:399-404
43. King A, Balendran N, Wooding P. et al. CD3- leukocytes present in the human uterus during early placentation: phenotypic and morphologic characterization of the CD56+ population. Dev Immunol. 1991;1:169-90
44. Vacca P, Montaldo E, Croxatto D. et al. Identification of diverse innate lymphoid cells in human decidua. Mucosal Immunol. 2015;8:254-264
45. Doisne JM, Balmas E, Boulenouar S. et al. Composition, Development, and Function of Uterine Innate Lymphoid Cells. J Immunol. 2015;195:3937-45
46. Xu Y, Romero R, Miller D. et al. Innate lymphoid cells at the human maternal-fetal interface in spontaneous preterm labor. Am J Reprod Immunol. 2018;79:e12820
47. Diefenbach A, Colonna M, Koyasu S. Development, Differentiation, and Diversity of Innate Lymphoid Cells. Immunity. 2014;41:354-65
48. Renoux VM, Zriwil A, Peitzsch C. et al. Identification of a Human Natural Killer Cell Lineage-Restricted Progenitor in Fetal and Adult Tissues. Immunity. 2015;43:394-407
49. Gascoyne DM, Long E, Veiga-Fernandes H. et al. The basic leucine zipper transcription factor E4BP4 is essential for natural killer cell development. Nat Immunol. 2009;10:1118-24
50. Kashiwada M, Levy DM, McKeag L. et al. IL-4-induced transcription factor NFIL3/E4BP4 controls IgE class switching. Proc Natl Acad Sci. 2010;107:821-26
51. Firth MA, Madera S, Beaulieu AM. et al. Nfil3-independent lineage maintenance and antiviral response of natural killer cells. J Exp Med. 2013;210:2981-90
52. Geiger TL, Sun JC. Development and maturation of natural killer cells. Curr Opin Immunol. 2016;39:82-89
53. DeHart SL, Heikens MJ, Tsai S. Jagged2 promotes the development of natural killer cells and the establishment of functional natural killer cell lines. Blood. 2005;105:3521-27
54. Jaleco AC, Neves H, Hooijberg E. et al. Differential effects of Notch ligands Delta-1 and Jagged-1 in human lymphoid differentiation. J Exp Med. 2001;194:991-1002
55. Kallies A, Carotta S, Huntington ND. et al. A role for Blimp1 in the transcriptional network controlling natural killer cell maturation. Blood. 2011;117:1869-79
56. Townsend MJ, Weinmann AS, Matsuda JL. et al. T-bet regulates the terminal maturation and homeostasis of NK and Valpha14i NKT cells. Immunity. 2004;20:477-94
57. Samson SI, Richard O, Tavian M. et al. GATA-3 promotes maturation, IFN-gamma production, and liver-specific homing of NK cells. Immunity. 2003;19:701-11
58. Aliahmad P, de la Torre B, Kaye J. Shared dependence on the DNA-binding factor TOX for the development of lymphoid tissue-inducer cell and NK cell lineages. Nat Immunol. 2010;11:945-52
59. Vong QP, Leung WH, Houston J. et al. TOX2 regulates human natural killer cell development by controlling T-BET expression. Blood. 2014;124:3905-13
60. Deng Y, Kerdiles Y, Chu J. et al. Transcription factor Foxo1 is a negative regulator of natural killer cell maturation and function. Immunity. 2015;42:457-70
61. Cooley S, Xiao F, Pitt M. et al. A subpopulation of human peripheral blood NK cells that lacks inhibitory receptors for self-MHC is developmentally immature. Blood. 2007;110:578-86
62. Apps R, Murphy SP, Fernando R. et al. Human leucocyte antigen (HLA) expression of primary trophoblast cells and placental cell lines, determined using single antigen beads to characterize allotype specificities of anti-HLA antibodies. Immunology. 2009;127:26-39
63. Williams PJ, Searle RF, Robson SC. et al. Decidual leucocyte populations in early to late gestation normal human pregnancy. J Reprod Immunol. 2009;82:24-31
64. Dietl J, Ruck P, Marzusch K. et al. Uterine granular lymphocytes are activated natural killer cells expressing VLA-1. Immunol Today. 1992;13:236
65. Koopman LA, Kopcow HD, Rybalov B. et al. Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential. J Exp Med. 2003;198:1201-12
66. Gaynor LM, Colucci F. Uterine Natural Killer Cells: Functional Distinctions and Influence on Pregnancy in Humans and Mice. Front Immunol. 2017;8:467
67. Vossen MTM, Matmati M, Hertoghs KML. et al. CD27 defines phenotypically and functionally different human NK cell subsets. J Immunol. 2008;180:3739-45
68. Fu B, Tian Z, Wei H. Subsets of human natural killer cells and their regulatory effects. Immunology. 2014;141:483-89
69. Fu B, Wang F, Sun R. et al. CD11b and CD27 reflect distinct population and functional specialization in human natural killer cells. Immunology. 2011;133:350-59
70. Verma S, King A, Loke YW. Expression of killer cell inhibitory receptors on human uterine natural killer cells. Eur J Immunol. 1997;27:979-83
71. Ivarsson MA, Stiglund N, Marquardt N. et al. Composition and dynamics of the uterine NK cell KIR repertoire in menstrual blood. Mucosal Immunol. 2017;10:322-31
72. Li YH, Zhou WH, Tao Y. et al. The Galectin-9/Tim-3 pathway is involved in the regulation of NK cell function at the maternal-fetal interface in early pregnancy. Cell Mol Immunol. 2016;13:73-81
73. Keskin DB, Allan DSJ, Rybalov B. et al. TGFbeta promotes conversion of CD16+ peripheral blood NK cells into CD16- NK cells with similarities to decidual NK cells. Proc Natl Acad Sci. 2007;104:3378-83
74. Cerdeira AS, Rajakumar A, Royle CM. et al. Conversion of peripheral blood NK cells to a decidual NK-like phenotype by a cocktail of defined factors. J Immunol. 2013;190:3939-48
75. Wu X, Jin LP, Yuan MM. et al. Human first-trimester trophoblast cells recruit CD56brightCD16- NK cells into decidua by way of expressing and secreting of CXCL12/stromal cell-derived factor 1. J Immunol. 2005;175:61-68
76. Drake PM, Gunn MD, Charo IF. et al. Human placental cytotrophoblasts attract monocytes and CD56(bright) natural killer cells via the actions of monocyte inflammatory protein 1alpha. J Exp Med. 2001;193:1199-212
77. Carlino C, Stabile H, Morrone S. et al. Recruitment of circulating NK cells through decidual tissues: a possible mechanism controlling NK cell accumulation in the uterus during early pregnancy. Blood. 2008;111:3108-15
78. Frey M, Packianathan NB, Fehniger TA. et al. Differential expression and function of L-selectin on CD56bright and CD56dim natural killer cell subsets. J Immunol. 1998;161:400-08
79. Carlino C, Trotta E, Stabile H. et al. Chemerin regulates NK cell accumulation and endothelial cell morphogenesis in the decidua during early pregnancy. J Clin Endocrinol Metab. 2012;97:3603-12
80. Schumacher A, Costa SD, Zenclussen AC. Endocrine Factors Modulating Immune Responses in Pregnancy. Front Immunol. 2014;5:196
81. Lysiak JJ, Lala PK. In situ localization and characterization of bone marrow-derived cells in the decidua of normal murine pregnancy. Biol Reprod. 1992;47:603-13
82. Chantakru S, Miller C, Roach LE. et al. Contributions from self-renewal and trafficking to the uterine NK cell population of early pregnancy. J Immunol. 2002;168:22-28
83. Vacca P, Vitale C, Montaldo E. et al. CD34+ hematopoietic precursors are present in human decidua and differentiate into natural killer cells upon interaction with stromal cells. Proc Natl Acad Sci. 2011;108:2402-07
84. Verma S, Hiby SE, Loke YW. et al. Human decidual natural killer cells express the receptor for and respond to the cytokine interleukin 15. Biol Reprod. 2000;62:959-68
85. Manaster I, Mandelboim O. The unique properties of uterine NK cells. Am J Reprod Immunol. 2010;63:434-44
86. Barnie PA, Lin X, Liu Y. et al. IL-17 producing innate lymphoid cells 3 (ILC3) but not Th17 cells might be the potential danger factor for preeclampsia and other pregnancy associated diseases. Int J Clin Exp Pathol. 2015;8:11100-07
87. Kamoi M, Fukui A, Kwak-Kim J. et al. NK22 Cells in the Uterine Mid-Secretory Endometrium and Peripheral Blood of Women with Recurrent Pregnancy Loss and Unexplained Infertility. Am J Reprod Immunol. 2015;73:557-67
88. Simoni Y, Fehlings M, Kløverpris HN. et al. Human Innate Lymphoid Cell Subsets Possess Tissue-Type Based Heterogeneity in Phenotype and Frequency. Immunity. 2017;46:148-61
89. Bernink JH, Mjösberg J, Spits H. Human ILC1: To Be or Not to Be. Immunity. 2017;46:756-57
90. Roan F, Ziegler SF. Human Group 1 Innate Lymphocytes Are Negative for Surface CD3ε but Express CD5. Immunity. 2017;46:758-59
91. Klose CSN, Flach M, Möhle L. et al. Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell. 2014;157:340-56
92. Seillet C, Rankin LC, Groom JR. et al. Nfil3 is required for the development of all innate lymphoid cell subsets. J Exp Med. 2014;211:1733-40
93. Geiger TL, Abt MC, Gasteiger G. et al. Nfil3 is crucial for development of innate lymphoid cells and host protection against intestinal pathogens. J Exp Med. 2014;211:1723-31
94. Yang Q, Li F, Harly C. et al. TCF-1 upregulation identifies early innate lymphoid progenitors in the bone marrow. Nat Immunol. 2015;16:1044-50
95. Baerenwaldt A, Burg N, Kreuzaler M. et al. Flt3 Ligand Regulates the Development of Innate Lymphoid Cells in Fetal and Adult Mice. J Immunol. 2016;196:2561-71
96. Nagasawa M, Germar K, Blom B. et al. Human CD5+ Innate Lymphoid Cells Are Functionally Immature and Their Development from CD34+ Progenitor Cells Is Regulated by Id2. Front Immunol. 2017;8:1047
97. Seehus CR, Aliahmad P, Torre B. et al. The development of innate lymphoid cells requires TOX-dependent generation of a common innate lymphoid cell progenitor. Nat Immunol. 2015;16:599-608
98. Constantinides MG, McDonald BD, Verhoef PA. et al. A committed precursor to innate lymphoid cells. Nature. 2014;508(7496):397-401
99. Constantinides MG, Gudjonson H, McDonald BD. et al. PLZF expression maps the early stages of ILC1 lineage development. Proc Natl Acad Sci. 2015;112:5123-28
100. Zhu J. GATA3 Regulates the Development and Functions of Innate Lymphoid Cell Subsets at Multiple Stages. Front Immunol. 2017;8:1571
101. Sawa S, Cherrier M, Lochner M. et al. Lineage relationship analysis of RORgammat+ innate lymphoid cells. Science. 2010;330:665-69
102. Allan DSJ, Kirkham CL, Aguilar OA. et al. An in vitro model of innate lymphoid cell function and differentiation. Mucosal Immunol. 2015;8:340-51
103. Killig M, Glatzer T, Romagnani C. Recognition strategies of group 3 innate lymphoid cells. Front Immunol. 2014;5:142
104. Bernink JH, Peters CP, Munneke M. et al. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat Immunol. 2013;14:221-29
105. Montaldo E, Teixeira-Alves LG, Glatzer T. et al. Human RORγt(+)CD34(+) cells are lineage-specified progenitors of group 3 RORγt(+) innate lymphoid cells. Immunity. 2014;41:988-1000
106. Moffett A, Loke C. Immunology of placentation in eutherian mammals. Nat Rev Immunol. 2006;6:584-94
107. Hanna J, Goldman-Wohl D, Hamani Y. et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med. 2006;12:1065-74
108. Kennedy PR, Chazara O, Gardner L. et al. Activating KIR2DS4 Is Expressed by Uterine NK Cells and Contributes to Successful Pregnancy. J Immunol Author Choice. 2016;197:4292-300
109. Xiong S, Sharkey AM, Kennedy PR. Maternal uterine NK cell-activating receptor KIR2DS1 enhances placentation. J Clin Invest. 2013;123:4264-72
110. Lash GE, Otun HA, Innes BA. et al. Inhibition of trophoblast cell invasion by TGFB1, 2, and 3 is associated with a decrease in active proteases. Biol Reprod. 2005;73:374-81
111. Croxatto D, Micheletti A, Montaldo E. et al. Group 3 innate lymphoid cells regulate neutrophil migration and function in human decidua. Mucosal Immunol. 2016;9:1372-83
112. Jessmon P, Leach RE, Armant DR. Diverse functions of HBEGF during pregnancy. Mol Reprod Dev. 2009;76:1116-27
113. Librach CL, Feigenbaum SL, Bass KE. et al. Interleukin-1 beta regulates human cytotrophoblast metalloproteinase activity and invasion in vitro. J Biol Chem. 1994;269:17125-31
114. Wu HX, Jin LP, Xu B. et al. Decidual stromal cells recruit Th17 cells into decidua to promote proliferation and invasion of human trophoblast cells by secreting IL-17. Cell Mol Immunol. 2014;11:253-62
115. Leonard S, Murrant C, Tayade C. et al. Mechanisms regulating immune cell contributions to spiral artery modification - facts and hypotheses - a review. Placenta. 2006:27 Suppl A:S40-46
116. Robson A, Harris LK, Innes BA. et al. Uterine natural killer cells initiate spiral artery remodeling in human pregnancy. FASEB J. 2012;26:4876-85
117. Liu W, Liu X, Luo M. et al. dNK derived IFN-γ mediates VSMC migration and apoptosis via the induction of LncRNA MEG3: A role in uterovascular transformation. Placenta. 2017;50:32-39
118. Hazan AD, Smith SD, Jones RL. et al. Vascular-leukocyte interactions: mechanisms of human decidual spiral artery remodeling in vitro. Am J Pathol. 2010;177:1017-30
119. Lash GE, Schiessl B, Kirkley M. et al. Expression of angiogenic growth factors by uterine natural killer cells during early pregnancy. J Leukoc Biol. 2006;80:572-80
120. Ma L, Li G, Cao G. et al. dNK cells facilitate the interaction between trophoblastic and endothelial cells via VEGF-C and HGF. Immunol Cell Biol. 2017;95:695-704
121. Wang Y, Xu B, Li MQ. et al. IL-22 secreted by decidual stromal cells and NK cells promotes the survival of human trophoblasts. Int J Clin Exp Pathol. 2013;6:1781-90
122. Dosiou Cand Giudice LC. Natural killer cells in pregnancy and recurrent pregnancy loss: endocrine and immunologic perspectives. Endocr Rev. 2005;26:44-62
123. Drake BL, Head JR. GM-CSF and CSF-1 stimulate DNA synthesis but not cell proliferation in short-term cultures of mid-gestation murine trophoblast. J Reprod Immunol. 1994;26:41-56
124. Athanassakis I, Bleackley RC, Paetkau V. et al. The immunostimulatory effect of T cells and T cell lymphokines on murine fetally derived placental cells. J Immunol. 1987;138:37-44
125. Clark DA, Vince G, Flanders KC. et al. CD56+ lymphoid cells in human first trimester pregnancy decidua as a source of novel transforming growth factor-beta 2-related immunosuppressive factors. Hum Reprod. 1994;9:2270-77
126. Yokota M, Fukui A, Funamizu A. et al. CD56+ lymphoid cells in human first trimester pregnancy decidua as a source of novel transforming growth factor-beta 2-related immunosuppressive factors. Hum Reprod. 1994;9:2270-77
127. Svensson-Arvelund J, Mehta RB, Lindau R. et al. The human fetal placenta promotes tolerance against the semiallogeneic fetus by inducing regulatory T cells and homeostatic M2 macrophages. J Immunol. 2015;194:1534-44
128. Gobert M, Lafaille JJ. Maternal-fetal immune tolerance, block by block. Cell. 2012;150:7-9
129. Fu B, Li X, Sun R. et al. Natural killer cells promote immune tolerance by regulating inflammatory TH17 cells at the human maternal-fetal interface. Proc Natl Acad Sci. 2013;110:E231-40
130. Duffin R, O'Connor RA, Crittenden S. et al. Prostaglandin E₂ constrains systemic inflammation through an innate lymphoid cell-IL-22 axis. Science. 2016;351:1333-38
131. Sonnenberg GF, Fouser LA, Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol. 2011;12(5):383-90
132. Alam MS, Maekawa Y, Kitamura A. et al. Notch signaling drives IL-22 secretion in CD4+ T cells by stimulating the aryl hydrocarbon receptor. Proc Natl Acad Sci. 2010;107:5943-48
133. Monteleone I, Rizzo A, Sarra M. et al. Aryl hydrocarbon receptor-induced signals up-regulate IL-22 production and inhibit inflammation in the gastrointestinal tract. Gastroenterology. 2011;141(e1):237-248
134. Belle AB, Heusch M, Lemaire MM. et al. IL-22 is required for imiquimod-induced psoriasiform skin inflammation in mice. J Immunol. 2012;188:462-69
135. Geboes L, Dumoutier L, Kelchtermans H. et al. Proinflammatory role of the Th17 cytokine interleukin-22 in collagen-induced arthritis in C57BL/6 mice. Arthritis Rheum. 2009;60:390-95
136. Hanash AM, Dudakov JA, Hua G. et al. Interleukin-22 protects intestinal stem cells from immune-mediated tissue damage and regulates sensitivity to graft versus host disease. Immunity. 2012;37:339-50
137. Kourtis AP, Read JS, Jamieson DJ. Pregnancy and Infection. N Engl J Med. 2014;370:2211-18
138. Schroder K, PHertzog PJ, Ravasi T. et al. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163-89
139. Song C, Lee JS, Gilfillan S. et al. Unique and redundant functions of NKp46+ ILC3s in models of intestinal inflammation. J Exp Med. 2015;212:1869-82
140. Kim S, Poursine-Laurent J, Truscott SM. et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436:709-13
141. Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9(5):495-502
142. Kieckbusch J, Gaynor LM, Moffett A. et al. MHC-dependent inhibition of uterine NK cells impedes fetal growth and decidual vascular remodelling. Nat Commun. 2014;5:3359
143. Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633-40
144. Hu WT, Huang LL, Li MQ. et al. Decidual stromal cell-derived IL-33 contributes to Th2 bias and inhibits decidual NK cell cytotoxicity through NF-κB signaling in human early pregnancy. J Reprod Immunol. 2015;109:52-65
145. Croxatto D, Vacca P, Canegallo F. et al. Stromal Cells from Human Decidua Exert a Strong Inhibitory Effect on NK Cell Function and Dendritic Cell Differentiation. PLoS ONE. 2014:9
146. Guo W, Li P, Zhao G. et al. Glucocorticoid receptor mediates the effect of progesterone on uterine natural killer cells. Am J Reprod Immunol. 2012;67:463-73
147. Kane N, Kelly R, P Saunders PTK. et al. Proliferation of uterine natural killer cells is induced by human chorionic gonadotropin and mediated via the mannose receptor. Endocrinology. 2009;150:2882-88
148. Hao S, Zhao J, Zhou J. et al. Modulation of 17beta-estradiol on the number and cytotoxicity of NK cells in vivo related to MCM and activating receptors. Int Immunopharmacol. 2007;7:1765-75
149. Laskarin G, Tokmadzić VS, Strbo N. et al. Progesterone induced blocking factor (PIBF) mediates progesterone induced suppression of decidual lymphocyte cytotoxicity. Am J Reprod Immunol. 2002;48:201-09
150. Faust Z, Laskarin G, Rukavina D. et al. Progesterone-induced blocking factor inhibits degranulation of natural killer cells. Am J Reprod Immunol. 1999;42:71-75
151. Zhang J, Dunk C, Croy AB. et al. To serve and to protect: the role of decidual innate immune cells on human pregnancy. Cell Tissue Res. 2016;363:249-65
152. Somigliana E, Viganò P, Vignali M. Endometriosis and unexplained recurrent spontaneous abortion: pathological states resulting from aberrant modulation of natural killer cell function? Hum Reprod Update. 1999;5:40-51
153. Carolis C, Perricone C, Perricone R. NK cells, autoantibodies, and immunologic infertility: a complex interplay. Clin Rev Allergy Immunol. 2010;39:166-75
154. Miko E, Manfai Z, Meggyes M. et al. Possible role of natural killer and natural killer T-like cells in implantation failure after IVF. Reprod Biomed Online. 2010;21:750-56
155. Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol. 2003;3:781-90
156. Varla-Leftherioti M, Spyropoulou-Vlachou M, Niokou D. et al. Natural killer (NK) cell receptors' repertoire in couples with recurrent spontaneous abortions. Am J Reprod Immunol. 2003;49:183-91
157. Varla-Leftherioti M, Spyropoulou-Vlachou M, Keramitsoglou T. et al. Lack of the appropriate natural killer cell inhibitory receptors in women with spontaneous abortion. Hum Immunol. 2005;66:65-71
158. Qi X, Lei M, Qin L. et al. Endogenous TWEAK is critical for regulating the function of mouse uterine natural killer cells in an immunological model of pregnancy loss. Immunology. 2016;148:70-82
159. Chen X, Liu Y, Cheung WC. et al. Increased expression of angiogenic cytokines in CD56+ uterine natural killer cells from women with recurrent miscarriage. Cytokine. 2018;110:272-276
160. Lyall F, S C Robson, J N Bulmer. Spiral artery remodeling and trophoblast invasion in preeclampsia and fetal growth restriction: relationship to clinical outcome. Hypertens. 2013;62:1046-54
161. Hiby SE, Walker JJ, O'shaughnessy KM. et al. Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success. J Exp Med. 2004;200:957-65
162. Moffett A, Loke YW. The immunological paradox of pregnancy: a reappraisal. Placenta. 2004;25:1-8
163. Bird IM, Boeldt DS, Krupp J. et al. Pregnancy, Programming and Preeclampsia: Gap Junctions at the Nexus of Pregnancy-induced Adaptation of Endothelial Function and Endothelial Adaptive Failure in PE. Curr. Vasc. Pharmacol. 2013;11:712-29
164. Tiralongo GM, Presti DL, I Pisani. et al. Assessment of total vascular resistance and total body water in normotensive women during the first trimester of pregnancy. A key for the prevention of preeclampsia. Pregnancy Hypertens. Int. J. Womens Cardiovasc. Health. 2015;5:193-97
165. Fukui A, Funamizu A, Yokota M. et al. Uterine and circulating natural killer cells and their roles in women with recurrent pregnancy loss, implantation failure and preeclampsia. J Reprod Immunol. 2011;90:105-10
166. Fukui A, Yokota M, A Funamizu. et al. Changes of NK cells in preeclampsia. Am J Reprod Immunol. 2012;67:278-86
167. Laban M, Ibrahim EAS, Elsafty MSE. et al. Placenta accreta is associated with decreased decidual natural killer (dNK) cells population: a comparative pilot study. Eur J Obstet Gynecol Reprod Biol. 2014;181:284-88
168. Hu Y, Dutz JP, MacCalman CD. et al. Decidual NK cells alter in vitro first trimester extravillous cytotrophoblast migration: a role for IFN-gamma. J Immunol. 2006;177:8522-30
169. Bauer S, Pollheimer J, Hartmann J. et al. Tumor necrosis factor-alpha inhibits trophoblast migration through elevation of plasminogen activator inhibitor-1 in first-trimester villous explant cultures. J Clin Endocrinol Metab. 2004;89:812-22
170. Hiby SE, Apps R, Sharkey AM. et al. Maternal activating KIRs protect against human reproductive failure mediated by fetal HLA-C2. J Clin Invest. 2010;120:4102-10
171. Dambaeva S, Schneiderman S, Jaiswal MK. et al. IL22 Prevents Lipopolysaccharide-Induced Preterm Labor in Mice. Biol Reprod. 2018;98:299-308
Author contact
![]() Corresponding authors: Ming-Qing Li, E-mail: mqliedu.cn; or Da-Jin Li, E-mail: djliedu.cn; Laboratory for Reproductive Immunology, NHC Key Lab of Reproduction Regulation (Shanghai Institute of Planned Parenthood Research), Hospital of Obstetrics and Gynecology, Fudan University Shanghai Medical College, Shanghai 200082, People's Republic of China. Tel/Fax: 86-21-33189900.
Corresponding authors: Ming-Qing Li, E-mail: mqliedu.cn; or Da-Jin Li, E-mail: djliedu.cn; Laboratory for Reproductive Immunology, NHC Key Lab of Reproduction Regulation (Shanghai Institute of Planned Parenthood Research), Hospital of Obstetrics and Gynecology, Fudan University Shanghai Medical College, Shanghai 200082, People's Republic of China. Tel/Fax: 86-21-33189900.

 Global reach, higher impact
Global reach, higher impact