10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2021; 17(1):307-327. doi:10.7150/ijbs.53589 This issue Cite
Review
A Perspective Of Intestinal Immune-Microbiome Interactions In Alcohol-Associated Liver Disease
Department of Medicine, University of California San Diego, La Jolla, CA, USA.
Received 2020-9-22; Accepted 2020-11-13; Published 2021-1-1
Abstract
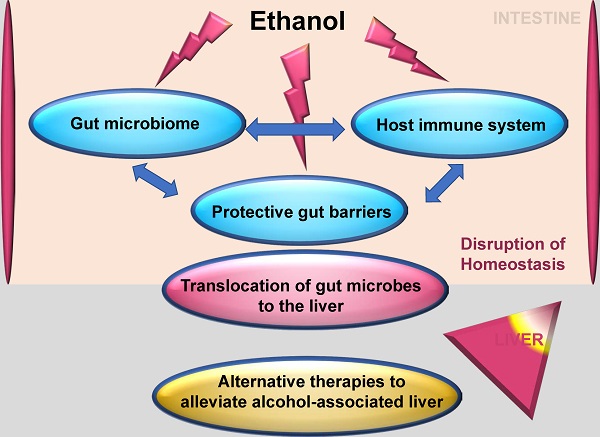
Uncovering the intricacies of the gut microbiome and how it interacts with the host immune system has opened up pathways in the search for the treatment of disease conditions. Alcohol-associated liver disease is a major cause of death worldwide. Research has shed light on the breakdown of the protective gut barriers, translocation of gut microbes to the liver and inflammatory immune response to microbes all contributing to alcohol-associated liver disease. This knowledge has opened up avenues for alternative therapies to alleviate alcohol-associated liver disease based on the interaction of the commensal gut microbiome as a key player in the regulation of the immune response. This review describes the relevance of the intestinal immune system, the gut microbiota, and specialized and non-specialized intestinal cells in the regulation of intestinal homeostasis. It also reflects how these components are altered during alcohol-associated liver disease and discusses new approaches for potential future therapies in alcohol-associated liver disease.
Keywords: Liver, intestine, microbiome, immune system, alcohol-associated liver disease
Introduction
The significance and intricacy of the intestinal immune system cannot be underscored. Specialized cells within the intestine working with lamina propria immune cells are vital for protection against invading pathogens. The gut microbiota, comprising many different bacterial species is a major influencer on the intestinal immune system and is a necessary cog for proper intestinal immune function. Research studies continue to uncover new knowledge about the functionality of specialized cells within the intestine and how they interact with the gut microbiome and the immune system. This has allowed for further understanding to search for potential targets against conditions that are exacerbated due to dysfunctional intestinal homeostasis comprising a disrupted intestinal immune system and an altered intestinal microbiota such as the different stages of alcohol-associated liver disease (ALD). These different stages include steatosis, steatohepatitis, fibrosis, alcoholic hepatitis (AH), alcohol-associated cirrhosis (AC), and hepatocellular carcinoma. Steatosis in ALD is marked by the presence of lipids accumulating in the hepatocytes within the liver due to activation of lipogenic pathways, inhibition of fatty acid oxidation, alteration of very-low-density lipoprotein (VLDL) secretion from the liver, and alteration of triglycerides metabolism which accumulates in the form of lipid droplets [1, 2]. Steatohepatitis also is marked by an increase in lipid content of the liver; however, inflammation is also evident with the increased abundance of inflammatory infiltrates consisting primarily of polymorphonuclear leukocytes (neutrophils) within the liver tissue [1, 2]. Continuous damage to the liver through the fat deposits and inflammation causes the formation of scar tissue known as fibrosis, which over time and continual buildup can lead to cirrhosis of the liver [2]. Long-term damage due to cirrhosis and inflammation of the liver also puts affected individuals at higher risk of developing hepatocellular carcinoma [1, 2]. AH is a clinical diagnosis made due to chronic alcohol abuse paired with clinical findings [1]. Hepatitis C antibodies have been found in a substantial amount of ALD patients with chronic alcohol abuse, and can further exacerbate ALD through inflammation and cirrhosis [3]. In this review, we will discuss the major components that regulate the intestinal immune system. We will also discuss how these components are influenced by the gut microbiome and in particular, the changes that occur during ALD and its numerous stages. Finally, we will look into how the comprehensive understanding of the intestinal immune system has allowed for research into alternative therapies to attenuate ALD.
Interactions of the intestinal epithelium and the intestinal immune system
The physical barriers of the intestine offer several lines of defense from potentially harmful pathogens as well as luminal contents and commensal bacteria from infiltrating the body under normal healthy conditions. Each barrier offers a distinctive structure that collectively functions to maintain a balance for both a healthy host as well as a healthy gut microbiome known as intestinal homeostasis. The intestinal epithelium, the innermost layer of the mucosa, is one of three major physical barriers that include specialized cells such as enterocytes, goblet cells, intestinal microfold (M) cells, enteroendocrine cells, Tuft cells, stem cells, and Paneth cells which are located in the crypts.
Enterocytes are present in large numbers in the small intestine but are also found in the large intestine, providing surveillance for protection [4]. Enterocytes both secrete and respond to cytokines [5, 6]. For instance, cytokines such as the proliferation-inducing ligand (APRIL) and the B cell-activating factor of the tumor necrosis factor family (BAFF) impact B cells and differentiated plasma cells while the thymic stromal lymphopoietin (TSLP) can impact dendritic cells (DCs) responses [7]. In addition to cytokines, enterocytes also secrete antimicrobial peptides (AMPs) such as ß-defensins and regenerating islet-derived 3 (REG3) lectins that destroy bacteria by weakening the cell membrane through the formation of specific pores [8]. Alcohol induces downregulation of the host antimicrobial proteins REG3ß and REG3γ in humans and mice [9-11]. Cathelicidins are another type of AMPs secreted by the enterocytes in the small and large intestine and can disrupt cell membranes of bacteria and fungi in a similarly to REG3 AMPs [12]. The secretion of AMPs is driven by toll-like receptors (TLRs) such as TLR4 and nucleotide-binding oligomerization domain-containing proteins (NODs) such as NOD2 along with the cells of the intestinal epithelial layer [13]. In addition, enterocytes assist with adaptive immune responses by facilitating immunoglobulin A (IgA) transport across the epithelial barrier to the lumen through the transcytosis using the polymeric immunoglobulin receptor (pIgR) [14-16]. IgA is an essential component in preventing microbe invasion across the intestinal barriers from the lumen [17]. These offer a direct response and method of defense to invading microorganisms. Enterocytes also play a large role in controlling inflammatory immune responses as a major barrier from microbial invasions with the help of tight junctions between the epithelial cells and the proteins that it sheds such as Ly6/Plaur domain-containing 8 (Lypd8) [18]. For instance, Lypd8 has been identified as a protein that helps to separate the intestinal microbiota and the epithelial tissue within the large intestine through binding to the flagella of certain bacteria [18]. During ALD the enterocyte barrier of protection and the immune response is highly compromised [19-23]. Ethanol-induced liver disease has been linked to increased levels of nitroxidative stress and altered tight junction proteins in mice, leading to increased intestinal permeability and bacteria crossing past the impaired barrier [24]. Apoptosis of enterocytes has been documented in mice with ethanol-induced liver disease as noted by the increased protein-level expression of apoptosis markers further exacerbating the intestinal permeability condition and liver damage [24]. In addition, fucosylated carbohydrate structures in intestinal epithelial cells play an important role in physiological and pathological processes [25-27]. Among them, intestinal α1-2-fucosylation plays an important role in the regulation of intestinal homeostasis. Certain bacteria possess specific enzymes to hydrolyze α1-2-fucosidic linkages resulting in cleaved L-fucose, which facilitates bacterial colonization [28, 29]. Fut2 is an enzyme that catalyzes the reaction of α1-2-fucosylation and is expressed in epithelial cells of the digestive tract while absent in the liver [30]. Absence of α1-2-fucosylation along the cell surface of enterocytes and mucus results in alterations in intestinal bacteria, barrier function, and pathogen adhesion [25, 31]. In duodenal biopsied of alcohol use disorder patients (AUD), α1-2-fucosylation is down-regulated, and the lack of α1-2-fucosylation in Fut2-/- mice exacerbates ethanol-induced liver injury, steatosis, inflammation, and induces expansion of intestinal Enterococcus faecalis (E. faecalis) that produce a pore-forming toxin called cytolysin [25]. These results suggest that modulation of intestinal α1-2-fucosylation could be used as a therapeutic approach to prevent ALDs.
Intestinal stem cells (ISCs) are an important structural and functional component of the intestinal immune system due to the ability to replenish themselves along with replenishing intestinal epithelial cells which can also differentiate to other specialized intestinal cell types [32]. IL-6 coreceptor glycoprotein 130 (gp130) plays a key regulatory role in the regeneration and tissue growth of the intestine by activation of the gp130-YAP-Notch pathway [33]. YAP expression also has been shown to impact Wnt/ß-catenin signaling and is typically upregulated during times of epithelial regeneration [34]. Regeneration of intestinal epithelial cells is crucial for maintaining the barrier against invading microbes. Ethanol-induced liver disease damages ISCs through dysregulation of the ß-catenin signaling, a regulator of leucine-rich repeat-containing G-protein coupled receptor 5 (Lgr5) and other ISC markers in the small intestine of mice [32]. This effectively stymies proper epithelial regeneration in the intestine during alcohol-induced liver disease, leading to increased bacterial translocation across the compromised intestinal barrier [32].
Paneth cells offer an imposing barrier to microbial products trying to pass the intestinal epithelial layer. Located predominately in the crypts of the small intestine adjacent to ISC in the small intestine, some Paneth cells can also be found in the proximal portion of the large intestine; however, during disease conditions, Paneth cells have been found in the distal portion of the large intestine [35]. Paneth cells hold and secrete large amounts of AMPs to prevent microbial invasion. These AMPs are found throughout the mucus layer and offer an important component of protection from bacteria crossing the intestinal barrier due to their ability to kill microbes [36-39]. Such AMPs include lysozyme C, α-defensins, phospholipases, cryptdins, and lectins. Lysozyme C is a noted AMP for preventing the buildup of peptidoglycans in tissues [40, 41]. α-defensins, found in human Paneth cells, have similar functions to the ß-defensins. Cryptdins, which are found in mice, can also be referenced to α-defensins [42]. Cryptdins can also be referenced to α-defensins, however, cryptdins are found in mice [42]. Phospholipases from Paneth cells, which include secretory group IIA phospholipase A2 target gram-positive bacteria, target specific bacterial membrane components [43]. Lectin AMPs known as REG3 are specifically noted for their ability to target and destroy harmful bacteria by damaging the cell wall [44]. In mice, several different subsets of REG3 AMPs exist, including REG3α, REG3ß, and REG3γ. In humans REG3ß is not found, REG3α shares homology to mouse REG3γ, and REG3γ has considerable homology to mouse REG3ß [45]. While each of these REG3 subsets targets gram-positive bacteria, each has distinct functions in immune responses. REG3α can penetrate gram-positive bacterial cell membranes via a hexameric membrane-penetrating pore formed through the binding of REG3α to a peptidoglycan [8]. REG3ß and REG3γ both increase as a response to immune system activation by infection. REG3ß can also act to destroy gram-negative bacteria through the binding to carbohydrate portions of lipopolysaccharides on gram-negative bacteria [8]. REG3γ helps to prevent bacteria from spreading on the epithelium along with mediating the spatial distance of the host from commensal microbes [8, 46]. During ethanol-induced liver disease, Paneth cell function is altered. Chronic-binge ethanol feeding in mice has been tied to an increase in proximal small intestine Paneth cells along with IL-17A in mice, promoting an innate inflammatory immune system response [47]. On the other hand, AMPs such as α-defensins were found to be reduced in male mice fed for 8 weeks ethanol-containing Lieber-DeCarli liquid diet [48]. REG3 expression levels were found to be reduced in patients with ALD [10] which has been associated with an overall increase of mucosa-associated bacteria in the small intestine compared to the non-ALD controls [11]. Expression of REG3 lectins was also reduced in mice after intragastric ethanol feeding [49, 50] as well as the ten-day feeding plus ethanol binge model [10] leading to a higher incidence of bacterial infiltration and translocation to the liver. In addition, REG3 knockout mice fed a long-term ethanol diet were found to have a higher liver injury due to more pronounced bacterial translocation [11].
Goblet cells also distribute AMPs such as resistin-like molecule ß (RELM-ß) and trefoil factor (TFF). RELM-ß acts to promote inflammation and immune response to invading pathogens through its ability to increase interferon-gamma (IFN-γ) expression in CD4 T cells [51]. TFF2 is crucial due to its ability to help with epithelial repair and replacement in areas subject to mucosal damage [52]. Goblet cells secrete an important component, mucin; that constitutes another major physical barrier known as the mucus layer, offering protection against luminal pathogens [53]. The mucus layer is found throughout the digestive tract including the stomach, the small intestine, and the colon; however, the mucus has distinct properties in each section of the tract [54]. MUC5AC is secreted by goblet cells to form the mucus layer in the stomach, whereas MUC2 is the major secreted component of the mucus layer throughout the intestine [54]. This mucus layer serves as a buffer between gut microbes passing into the intestinal epithelium in both the small and large intestine [54]. The mucus layer comprises an outer layer that has direct contact with the lumen and an inner layer which has direct contact with the intestinal epithelium. While mucin can keep most microbes from penetrating the barrier, other components of the mucus layer helps in the defense against these microbes. Goblet cells release RELM-ß into the mucus layer to destroy microbes that can penetrate the layer [55]. On the inner areas of the mucus layer, AMPs derived from the Paneth cells and IgA, both prevent microbial adherence to the gut epithelium [46]. Another major function of goblet cells includes the creation of goblet cell associated-antigen passages (GAPs) upon mucin secretion. GAPs within the small intestine are open and the GAPs in the colon remain closed to prevent an influx of excessive amounts of bacteria [56, 57]. These GAPs play a crucial role in intestinal immune response as they send soluble luminal antigens to lamina propria dendritic cells (LP-DCs) [56]. Once GAPs transport antigens to the lamina propria, LP-DCs provide an appropriate immune response. Antigen delivery of GAPs favors a certain subset of tolerogenic LP-DCs that are CD103+ [56]. In mice with ethanol-induced liver disease promoted by the continuous intragastric feeding of an ethanol diet, mucin production in the intestinal system increases considerably, seemingly as a positive response to protect the intestine from bacterial invasion from the lumen [49]. However, this thickening of the mucus layer is detrimental as AMPs struggle to control bacterial overgrowth in the lumen due to the wider physical barrier of the mucus layer between the intestinal epithelium and the lumen [49]. Alcohol and its oxidative metabolites, specifically acetaldehyde can cause major changes in the intestinal barrier, increase paracellular permeability, and alter goblet cells [58-62]. Chronic alcohol increases the thickness of the intestinal mucus layer in patients with alcohol use disorder [63].
M cells are associated with Peyer's patches of the small intestine, isolated lymphoid follicles, and colonic patches [64]. M cells offer specialized capabilities over enterocytes in their ability to perform endocytosis and transcytosis, allowing for handling a wide and varied range of microbial products [64, 65]. M cells act to transport antigens to both macrophages and LP-DCs and are consequential in antigen monitoring for the intestinal immune response [64, 66]. M cells have a unique structure in the basolateral pocket which contains a partner B lymphocyte that helps with M cell maturation [64]. M cells can also be induced by cytokines such as the receptor activator of nuclear factor-kappa-Β ligand (RANKL) [65]. In Peyer's patches, antigens transported through M cells are exposed to DCs. Subsequently, DCs present the antigens to T and B cells leading to the determination of the immune response [67, 68]. Tumor necrosis factor receptor 2 (TNFR2) is also required for stimulation of M cells. Stimulation of M cells triggers transcytosis of microbes into the lamina propria further exacerbating inflammation and disease progression [69]. Impacts of ALD on M cells have been documented in mice fed a 45-day ethanol diet. Ethanol abuse induced structural changes in M cells such as mitochondrial swelling and disruption of cell-matrix within the cells [70]. Changes in wild type mice fed a 19-week ethanol Lieber-DeCarli diet found an overall decrease in cells within Peyer patches and particularly fewer T and B cells [71]. Overall functional changes in M cells during ALD are currently understudied. Given the transcytosis functionality of M cells, research into M cell function during ALD could offer further detail into their role in the intestinal immune system and microbiota interaction.
Enteroendocrine cells (EECs) are a very small part of the epithelium, but their function is far-reaching within areas such as the small intestine, colon, and gut microbiota. In response to nutrients, EECs secrete peptide hormones like somatostatin, motilin, cholecystokinin, neurotensin, vasoactive intestinal peptide, enteroglucagon, gastric inhibitory peptide (GIP), glucagon-like peptide-1, peptide YY, oxyntomodulin and glucagon-like peptide-2, histamine, and secretin to control gastrointestinal function and can also release cytokines in response to microbial metabolites and pathogenic molecules [72]. Short-chain fatty acids (SCFAs) are detected through G protein receptors 41 and 43 (GPR41, GPR43) expressed in EEC and promote inflammatory responses in mice [72-74]. These activated cytokines can alter the gut barriers through tight junction alteration [75]. During inflammation, the assistance that EECs offer in the way of intestinal immune system activation comes at a cost as EECs are responsible for appetite regulation and energy metabolism [73-75]. Chronic alcoholics presented a slightly significant increase in the numbers of glucagon and GIP suggesting that chronic alcohol consumption may have an effect on some endocrine cell types in the duodenal mucosa [76]. It has also been reported and impairment of intestinal somatostatin production by chronic ethanol exposure in rats [77].
Tuft cells, similar to EECs constitute a very small number of cells within the epithelium and are also found throughout the colon and small intestine; however, their relative number is higher is the distal portion of the small intestine [78]. Tuft cells function as a communicator amongst the intestinal immune cells. They produce the cytokine interleukin-25 (IL-25) which activates T helper 2 (Th2) cells in type 2 immune system response [79]. Tuft cells proliferate in mice in response to inflammation and immune system activation [78].
Schematic showing molecular level changes between the small intestine in healthy condition versus alcohol-associated liver disease condition. Abbreviations: AMP: anti-microbial peptide; IgA: immunoglobulin A; IL-10: interleukin 10; IL-17: interleukin 17; IL-22: interleukin 22; M cell: microfold cell; TNFα: tumor necrosis factor-alpha.
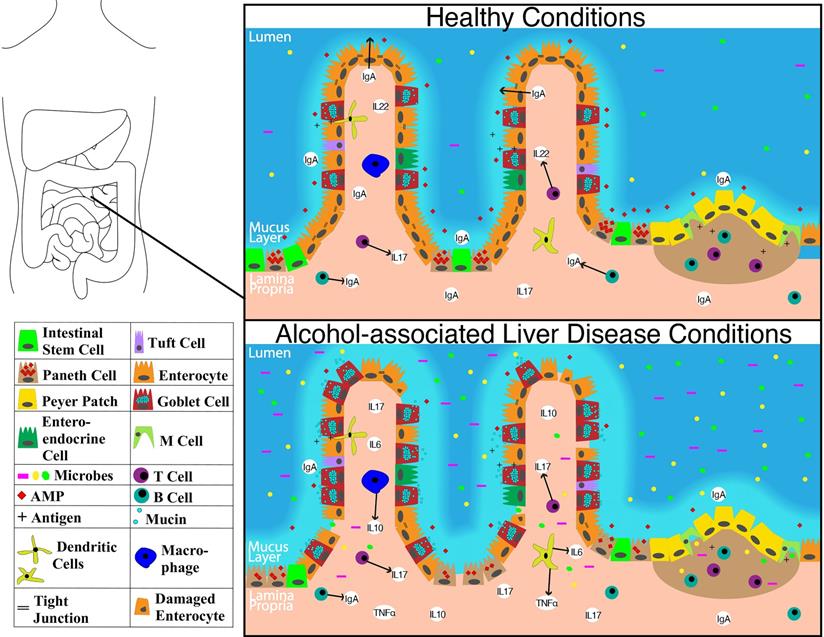
The overall structure of the small intestine with changes on a cellular and molecular level in the context of ALD is illustrated in Figure 1. The impact of alcohol abuse on the colon is illustrated in Figure 2. Major cells in the intestinal epithelium along with functions and associations with ALD are summarized in Table 1.
The lamina propria and maintenance of the intestinal homeostasis
The lamina propria is another major physical barrier underneath of the intestinal epithelial cells consisting of noncellular connective tissue elements (collagen and elastin), myofibroblasts, and diverse immune cells such as CD4+ T lymphocytes, NKT lymphocytes, IgA plasma cells, B lymphocytes, innate lymphoid cells (ILCs), macrophages, mast cells, and DCs [80-85]. The lamina propria contains also vascular elements such as large capillary loops, lymphatic vessels, nerves, and nerve endings [86].
LP-DCs within the lamina propria are classified based on their expression. DCs are defined as CD11chigh, major histocompatibility class (MHC)IIhigh [87]. The main subgroups of LP-DCs include those that are CD103+ CD11b+, CD103+ CD11b-, CD103- CD11b+ and CD103- CD11b- [87, 88]. CD103+ CD11b+ LP DCs are CX3CR1- and can extend their dendrites outward to capture bacteria in the lumen and later present antigens to the T cells in the mesenteric lymph nodes [89]. CD103+ CD11b+ LP DCs can also initiate the intestinal immune system adaptive response via recognition of flagellin through TLR5 [90]. TLR5 identification of flagellin prompts the production of IL-23 causing a cascading effect of ILC3 production of IL-22 and AMPs for defense against bacteria [90]. During inflammation, TLRs such as TLR4 activate CD103+ CD11b+ DCs causing secretion of IL-6 which, in turn, induce T helper cells (Th) 17 differentiation [91]. However, CD103+ CD11b+ DCs have characteristic tolerogenic properties, induce IgA, regulate gut-specific homing on lymphocytes [92], and stimulate the differentiation to regulatory T (Treg) cells [93]. Th17 and forkhead box P3 (FOXP3)+ Tregs share developmental properties. They can transdifferentiate in response to different cytokines [94]. The activation of the transcription factor FoxP3 in naïve T cells by TGF-β promotes Treg differentiation while IL6 inhibits FOXP3 [95]. Tolerance is crucial towards preventing autoimmunity as well as depleting commensal bacteria of the gut. Through the training of the DCs to selectively destroy harmful pathogens, a balanced immunity versus tolerance allows for proper gut function with the microbiome. CD103+ CD11b+ tolerogenic DCs help to regulate intestinal immune tolerance through promoting CD4+ T cell differentiation into Tregs [96]. Notably, CD103+ CD11b+ DCs play the most prominent role in the communication with GAPs in the small intestine and the majority of antigens are delivered almost specifically to this subset [97]. This subset of LP-DCs induce specific adaptive immune responses that control the intestinal homeostasis [98]. Of note, this subset is rare in the colon where GAPs are closed. CD103+ CX3CR1- DCs produce retinoic acid and TGF-ß to stop any unnecessary inflammation [99].
Representation of molecular level changes between the colon in healthy condition alcohol-associated liver disease condition. Abbreviations: AMP: anti-microbial peptide; IgA: immunoglobulin A; IL-10: interleukin 10; IL-17: interleukin 17; IL-22: interleukin 22; TNFα: tumor necrosis factor-alpha.
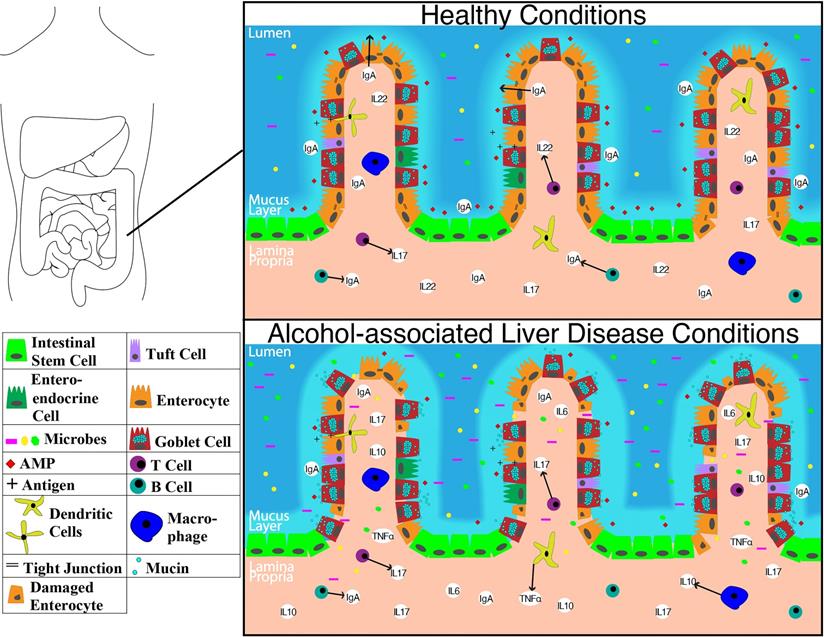
The CD103+ CD11b- subset of LP-DCs is similar to CD8+ DCs in lymphoid organs [100, 101]. They can migrate in a CCR7 dependent manner to the mesenteric lymph nodes (MLN) to present luminal antigens to T cells [101]. These cells are more predominant in lymphoid tissues, Peyer's patches, and also in intestinal draining lymph nodes such as MLNs [102]. Differentiation of CD8+ effector T cells under inflammatory condition seems to be dependent on these cells that migrate into the lymph [101].
The CD103- CD11b+ DCs subset is closely related to macrophages, is always found in the lamina propria, and activate nearby immune cells by releasing inflammatory cytokines [102]. CD103-CX3CR1+ DCs promote inflammation through IL-6 and tumor necrosis factor-alpha (TNFα) [97, 103]. Furthermore, this subset stimulates IFN-γ and IL-17 producing effector cells after lymphadenectomy [104]. It has also been reported that CD103+ CD11b- and CD103- CD11b+ cDCs can drive Th1 differentiation rather than the CD103+ CD11b+ DCs [104-106]. IL-10 production from monocyte-derived DC and macrophages has also been reported to diminish inflammatory responses in the intestinal lamina propria [107]. CX3CR1+ LP DCs can extend their dendrites to receive and monitor antigens from the lumen [89]. Along with capturing, CX3CR1 also allows for proper transportation of these antigens to other DCs, as mice without CX3CR1 have reduced capability of pathogen uptake and transportation from DCs to areas such as the mesenteric lymph nodes [108]. Information on the CD103- CD11b- subset which is believed to have originated from Peyer's patches or SILT is currently limited aside from their transport of luminal antigens via afferent lymphatics to the mesenteric lymph nodes [104].
Uncovering the structure and function of LP-DCs has offered insight into one of the crucial components of the intestinal immune systems adaptive response. Antigen-specific T cell responses and accessory cell function of myeloid DCs is greatly strained in human blood by alcohol drinking [109]. In Rhesus macaques and mice, alcohol-reduced the number of DC pools found in the bone marrow and a noted reduction in the ability to initiate T-cell development [110]. Despite this information, the effect of alcohol abuse on LP-DCs remains to be characterized.
ILCs are also present in the lamina propria and include ILC1s, ILC2s, and ILC3s [4]. ILC1s are distinctive in their production of IFN-γ and found at mucosal inflammation locations while ILC2s are noted for their response to parasitic infections [4]. ILC3s are the focal group when referencing the intestinal immune system due to the interleukins it produces and the impacts on the gut. RAR-related orphan receptor gamma (RORγt) is required by ILC3s for proper function [111]. ILC3s can generate interleukins IL-17 and IL-22, each causing a cascading innate immune reaction in the intestinal system. IL-22 production in ILC3s is stimulated by CD103+ dendritic cells after TLR5 activation and release of IL-23 [112]. This IL-22 production has also been linked to gut microbe influenced epithelial fucosylation along with REG3γ and REG3ß expression [113-115]. IL-17 accumulation causes a corresponding increase in neutrophil chemokines and a proinflammatory response, whereas IL-22 buildup offers a regenerative response [114]. In the lamina propria, IL-22 production is impaired in mice with ethanol-induced liver disease, thus compromising the epithelial barrier in the gut, decreasing the production of REG3, and compounding issues such as increased intestinal permeability and bacterial translocation [116, 117].
Major cell types found in the intestinal epithelium, their major function(s), and impacts seen in the cells during ALD
| Cell type | Layer | Major function(s) | Ald impacts | References |
|---|---|---|---|---|
| Enterocytes | Intestinal epithelium | Cytokine secretion (APRIL, BAFF, TSLP) and response; | Reduced expression of tight junction proteins; | [4-16, 18-27, 30, 31] |
| AMP secretion (REG3γ, REG3ß, cathelicins); | Increased permeability; | |||
| IgA transport (pIgR); | Reduction in expression of REG3γ and REG3ß; | |||
| Formation of tight junctions (occluding, claudins, Junction Adhesion Molecules); | IgA levels reduced; | |||
| Express Lypd8 protein to prevent invasion of flagellated microbiota; | Apoptosis of enterocytes; | |||
| Express fucosylated carbohydrate structures. | α1-2-fucosylation downregulated. | |||
| Goblet cells | Intestinal epithelium | Mucin secretion to form mucus layer; | Mucin production increases in the small intestine; | [46, 51-63] |
| Disperse AMPs (lysozyme C, α-defensins, phospholipases, cryptdins, lectins), IgA, and mucin for mucus protective layer; | Thickening of mucus layer. | |||
| Secrete AMPs (RELM-ß, TFF); | ||||
| Create goblet cell associated-passages (GAPs). | ||||
| M cells | Intestinal epithelium | Transporters of antigens to macrophages and dendritic cells through endocytosis and transcytosis; | Structural changes in M cells; | [64-66, 70] |
| Partner with B lymphocyte. | Further research needed. | |||
| Enteroendocrine cells | Intestinal epithelium | Help with gastrointestinal process through release of peptide hormones; | Increase in the numbers of glucagon and gastric inhibitory peptide (GIP) cells; | [72-77] |
| Release cytokines; | Decreased somatostatin. | |||
| Recognize SCFAs through GPR41, GPR43. | ||||
| Tuft cells | Intestinal epithelium | Produce cytokines (IL-25) to allow communication with intestinal immune cells | Increase in number | [78, 79] |
| Intestinal stem cells | Intestinal epithelium | Replenish themselves along all cell types in the intestinal epithelium; | Dysfunction of signaling; | [32] |
| Maintains barrier. | Reduces replacement of cells. | |||
| Paneth cells | Intestinal epithelium | Produce and release AMPs (lysozyme C, α-defensins, phospholipases, cryptdins, and lectins) | Increase in number in the proximal small intestine; | [10, 11, 35, 40-44, 46-50] |
| AMP α-defensins reduced; | ||||
| REG3 expression reduced. |
Impacts seen during ALD include evidence from human patients and/or evidence from mouse models fed various ethanol diets. Abbreviations: ALD: alcohol-associated liver disease; AMP: anti-microbial peptide; APRIL: A proliferation-inducing ligand; BAFF: B cell-activating factor of the tumor necrosis factor family; GAPs: goblet cell associated-antigen passages; GIP: gastric inhibitory peptide; GPR: G protein receptors; IgA: immunoglobulin A; Lypd8: Ly6/Plaur domain-containing 8; M cell: microfold cell; pIgR: polymeric immunoglobulin receptor; REG3: regenerating islet-derived 3; RELM- ß resistin-like molecule ß; SCFAs: Short-chain fatty acids; TFF: trefoil factor; TSLP: thymic stromal lymphopoietin.
Intestinal macrophages throughout the lamina propria have similarities in the membrane gene expression profile to macrophages in other tissues as well as monocytes, however, several key differences differentiate intestinal macrophages from macrophages of other tissues [118, 119]. Intestinal macrophages do not have membrane expression of CD14 or CD89 and produce markedly fewer cytokines in response to lipopolysaccharide (LPS) [119]. Intestinal macrophages can also secrete anti-inflammatory cytokines such as IL-10 [120, 121]. IL-10 acts together with TGF-ß to promote Treg differentiation [122]. Chronic alcohol exposure increased the frequencies of CD64+F4/80+CD11b+ macrophage in the lamina propria [123]. Further studies regarding the impact of alcohol abuse on intestinal macrophages are needed to understand the functionality and role during ALD.
CD4+ T cells are another important component of immune cells within the lamina propria. Th1 cells, a subtype of CD4+ T cells are noted for controlling immune responses responsible for killing intracellular parasites and perpetuating autoimmune responses through inflammatory cell activation via IFN-γ and for T and B cell growth through IL-2 interaction with T and B cells [124-126]. Th2 cells producing IL-4, IL-5, IL-13 and also IL-10 are another subtype of CD4+ T cells. Th2 cells have noted involvement in allergy and humoral immune responses [127]. Th1 and Th2 cells induce chronic intestinal inflammation [128, 129]. IL-4 can create a healthy balance among the Th1 and Th2 cells due to its anti-inflammation properties and ability to regulate T and B cell growth, contrary to IL-2 [129, 130]. IL-10, as previously discussed also can have anti-inflammatory properties and can help to abate the production of IL-2 and IFN-γ [131]. Other Th2 cytokines such as IL-5 assist with epithelial cell growth in the colon during inflammation through activation of macrophages [132]. IL-13 expression, on the other hand, has been linked to higher incidences of apoptosis in intestinal epithelial cells during inflammation [132]. Exacerbation of Th2 responses neutralizes the Th1 mediated microbial action. Thus, a balanced Th1 and Th2 response is required to maintain homeostasis. During ethanol-induced liver disease in mice, higher levels of a Th1 cytokine, IL-12, were increased in the liver, serum, and lungs stimulating IFN-γ production [133]. However other studies found that alcohol consumption decreases IFN-γ and IL-12 in lymph nodes and spleen [134, 135] while the expression of Th2 cytokines is stable or augmented [134, 135]. Therefore, ethanol drinking could be related to an immune shift toward Th2 cytokines that could contribute to damage to the intestinal immune system and the liver.
Another type of CD4+ T cell, Th17, plays a key role in inflammation. Th17 cells, along with Paneth cells in the intestine produce IL-17 which was previously discussed as a pro-inflammatory cytokine [47, 136, 137]. Th17 responses can be induced by CD103+ DCs through the expression of TLR5 [90]. Based on a mouse NIAAA model of AH [138], IL-17 is increased in the proximal small intestine during ethanol feeding stemming from the increase in Paneth cells [47]. In a study using RORγt-deficient mice, sphingosine kinase-deficient mice, and local gut anti-inflammatory, 5-aminosalicyclic acid (5-ASA)-treated mice were used to abrogate Th17 cells. These mice experienced less intestinal inflammation and less ethanol-induced liver disease [139]. However, it remains unknown what would be the specific trigger of Th17 regulation locally in the intestine. In the same line, it is well known that hepatic IL-17 is induced after chronic alcohol abuse in humans and mice [136, 140].
IgA was previously discussed due to its importance in controlling bacteria after being transported to the lumen with the assistance of pIgR. IgA production in the intestine is markedly higher than other areas in the body, with two subclasses IgA1 and IgA2 existing in humans [141]. IgA2 has been found to have advantages over IgA1 in the presence of bacteria due to its higher resistance to bacterial products [141]. IgA1 and IgA2 are present in similar numbers within the colon lamina propria, however, IgA2 increases further down the small intestine with IgA1 found consistently throughout the small intestine lamina propria [141]. Production of IgA is critical for proper maintenance of diverse gut microbiota, stopping unwarranted access of microbes, and destroying pathogenic microbes [142]. In different mouse models of ethanol-induced liver disease, intestinal LP IgA-secreting plasma cells and IgA levels are reduced [143-146]. In patients with severe AC, SIgA was reported reduced [147, 148]. However, other studies have shown no variation in levels of jejunal SIgA secretion in patients with ALD [149] or duodenal IgA-secreting cells [148]. These conflicting reports may result from subjects in different stages of the disease or different methods of IgA measurement. Future studies should be conducted to understand the causation of this variability.
Mucosa-associated invariant T (MAIT) cells also play an important role in immune response as once activated in response to bacterial infection, MAIT cells secrete cytokines such as IFN-γ, TNFα, and IL-17 to destroy bacteria-infected cells [150]. During AH and AC, fewer MAIT cells are present along with a diminishing of their functional efficiency in destroying infected cells due to dysfunctional cytokine responses [150]. The authors concluded that microbial products and microbiota compromise MAIT cells' antibacterial potency [150]. A summary of the major cells of the lamina propria, their functions, and associations with ALD are listed in Table 2.
While these physical barriers and their components offer critical defense from pathogen invasion of the gut, they must also work in harmony with the microbiome for optimal health. The influence of the gut microbiome in training the immune system is a hallmark of a healthy intestinal immune system.
Interactions of the intestinal immune system and the microbiome
The presence of a diverse set of microbes in the gut creates a mutualistic relationship with the host allowing proper gut and immune function. Intestinal microbiota gain a habitable environment and nutrients in exchange for their functions within the gut. These functions include nutrient metabolism, xenobiotic and drug metabolism, protection against pathogens, maintenance of the integrity of the mucosal barrier, and regulation at the molecular, genetic, and cellular levels of the intestinal environment which impacts the immune system [151].
The cruciality of the intestinal microbiome has long been accepted for health and proper immune response. This has been illustrated in many studies done on germ-free mice lacking microbiota. Germ-free mice have significant deficiencies in the immune system. Specifically, the lack of gut microbiome is associated with fewer intraepithelial lymphocytes, less IgA secreting plasma in the lamina propria, smaller Peyer's patches, fewer Tregs, and markedly reduced mRNA expression of AMPs from the Paneth cells such as Angiogenin-4 [152-156]. These deficiencies of the immune system in germ-free mice result in severe compromising of the intestine due to the inability to detect and respond to potential pathogens. Germ-free mice are more susceptible to liver fibrosis and damage in a toxic model using thioacetamide or carbon tetrachloride and in a model of acute ethanol exposure that mimics binge drinking [157, 158].
A common method of treatment in preclinical models of ethanol-induced liver disease is the use of antibiotics. The main purpose of antibiotics is to combat bacterial overgrowth and avoid translocating bacteria. The differences in antibiotics and the history of their success have led to the many different viewpoints about their current use and effectiveness in treating ALD. Antibiotics have direct impacts on the immune system within the gut due to changes in the GAPs in the colon. Colonic GAPs are typically closed to prevent bacterial translocation; however, following the administration of antibiotics, GAPs can be opened leading to a higher level of inflammation by induction of CX3CR1+ cells after recognizing higher levels of microbes and antigens [57]. A potential caveat to antibiotic treatment is the development of resistance within the gut microbes. The gut microbiome resistance to antibiotics, known as the gut resistome, could occur with the long-term administration of antibiotic treatment where resistant genes transfer over amongst gut microbes including pathogens [159]. This would further dampen any impact of antibiotics preventing the overgrowth of bacteria and the potential translocation of bacteria to the liver and other tissues. The result of these potential issues with antibiotics has increased the calls for a more individualized approach to prevent ALDs and alternative therapeutic options which are discussed in a later section.
A major function of gut microbes is converting dietary carbohydrates into organic acids. Lactate and SCFAs such as acetate, propionate, and butyrate can all be end-products created by the gut microbes [160]. Each of these can be used for communication amongst microbes but can also be used for other purposes. Butyrate serves as an energy substrate for intestinal epithelial cells, as it allows for enhancement of the physical barrier in the gut and can increase tight junction assembly, leading to less translocation of bacteria across this barrier [161]. Butyrate also can promote mucin synthesis strengthening the mucus barrier against pathogens along with reducing the expression of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), a transcription factor that can activate several genes to promote inflammation [162]. Other SCFAs, such as acetate and propionate can slow inflammation through binding to receptors such as GPR43 [163]. In addition to SCFAs, gut microbes also synthesize long-chain fatty acids (LCFAs) and medium-chain fatty acids (MCFAs). In a mouse model of intragastric feeding of alcohol for 3 weeks, the synthesis of saturated LCFAs was found markedly reduced and was linked to an imbalance in gut microbes [164]. The synthesis of saturated LCFAs by the microbiome is an important component of maintaining homeostasis within the gut microbiota along with maintaining a protective intestinal gut physical barrier [164].
The gut microbiota influences the expression of neutrophil chemokines through the promotion of naïve CD8+ to CD4+ T cells. In the mucosa of the small intestine, specific microbiota induces the differentiation of T-helper cells and secretion of IL-22 along with IL-17 which specifically induces the expression of neutrophil chemokines [163, 165, 166].
Major cell types found in the lamina propria, their major function(s), and impacts seen in the cells during ALD
| Cell type | Layer | Major function(s) | ALD impacts | References |
|---|---|---|---|---|
| Dendritic cells | Lamina propria | Microbe sampling, seize harmful pathogens in the lumen; | Fewer pools of dendritic cells | [89-93, 96, 97, 101, 102, 109, 110] |
| Instigate intestinal adaptive immune response through TLR5; | ||||
| Induce IgA, stimulate Treg cell and Th17 cell differentiation; | ||||
| Specific subsets of DC associate with GAPs; | ||||
| Present luminal antigens to T cells within mesenteric lymph nodes inducing tolerance or immunity. | ||||
| ILC3s | Lamina propria | Interleukin production (IL-17, IL-22) in the intestine spurring an innate immune response | Interleukin production is hindered Amplifies bacteria translocation. | [90, 112, 114, 116, 117] |
| Macrophages | Lamina propria | Produce fewer cytokines in response to LPS compared to other tissue macrophages; | Increase in number | [119-122] |
| Secrete anti-inflammatory cytokines (IL-10) that can assist with differentiation of Tregs. | ||||
| Th1 and Th2 cells | Lamina propria | In balance, assist with immune response through inflammation and regulate T and B cell proliferation. | Balance is compromised, favoring Th2 cells and promoting tissue damage. | [129, 130, 133-135] |
| Th17 cells | Lamina propria | Key player in inflammation; | Increase in number; | [47, 90, 136, 137, 139, 140] |
| Activated by lamina propria dendritic cells. | Pro-inflammatory. | |||
| IgA | Lamina propria | Prevents invading microbes from gaining access through barrier; | Conflicting findings, some have reported overall decrease and other findings have shown no change; | [142-149] |
| Destroys pathogenic microbes | Further research needed. | |||
| MAIT cells | Lamina propria | Release cytokines (IFN-γ, TNFα, IL-17) Destroy cells infected with bacteria | Fewer presense and less efficient in function. | [150] |
Impacts seen during ALD include evidence from human patients and/or evidence from mouse models fed various ethanol diets. Abbreviations: ALD: alcohol-associated liver disease; GAPs: goblet cell associated-antigen passages; IFN-γ: interferon-gamma; IgA: immunoglobulin A; IL: interleukin; ILCs: innate lymphoid cells; LPS: lipopolysaccharide; MAIT: mucosa-associated invariant T cells; Th: T helper cells; TLRs: toll-like receptors; TNF: tumor necrosis factor; Treg: regulatory T cells.
Gut microbiota can also influence DCs through Fut2 mediated α1,2-fucosylation. Certain bacteria can produce energy from fucose metabolism. This fucosylation has been found in the intestinal epithelial cells to increase during acute inflammation which causes the DCs to produce IL-23 [167]. IL-23 then triggers IL-22 production of RORγT+ ILCs [167]. This fucosylation also has been found to help with pathogenic microbes by reducing their virulent genetic expression and improving the tolerance of the host to pathogens [167]. Fucosylation is linked to bacteria located within the gut as studies on germ-free mice found no gut fucosylation after weaning pups from their mother [31]. Bacteria such as segmented filamentous bacteria have been linked to increases in fucosylation in the intestine, thus impacting the production of cytokines and immune response in the host [31].
Gut microbes can also provide influential benefits on gene-level expression in the intestine. An example of this is the regulation of the immune response via regulation of the retinoic acid receptor alpha (RARα) [168]. Retinaldehyde dehydrogenase (RALDH) enzymes form retinoic acid (RA) which is produced by DCs, macrophages, epithelial cells, and stromal cells. RA is consequential for Treg cell differentiation and reciprocal inhibition of Th17 cell development [169, 170]. RALDH enzymes are expressed by DCs and macrophages along the intestine and its expression decreases during a high dose of chronic T. muris infection invading pathogens to fight the infection [171]. In addition, bacteria from Clostridia class modulates the levels of RA in the gut by reducing the expression of retinal dehydrogenase 7 (Rdh7) in intestinal epithelial cells and consequently disrupting the RA signaling immune cells, reducing the levels of IL-22 and altering antimicrobial responses which in turn protects the commensal microbiota [172]. These functions show the importance of RA signaling in intestinal immune system regulation. RA deficiency is associated with colitis-associated colon cancer and inflammatory bowel disease (IBD) [173]. Alcohol abuse has been linked with the reduction of RA in the liver while it increases the levels in extrahepatic tissues including the colon [174, 175]. However, the implications of the intestinal reduction of RA during ALD remain to be studied.
Segmented filamentous bacteria of the microbiota lead to the induction of Th17 cells through the stimulation of reactive oxygen species (ROS) enhancing IL-1β secretion, IL-6, and TGF-β [176, 177]. Th17 cells are a subset of T helper cells that produce IL-17A. IL-17A stimulates epithelial cells to release pro-inflammatory factors such as C-X-C Motif Chemokine Ligand 1 (CXCL1) and C-C Motif Chemokine Ligand 20 (CCL20), allowing recruitment of immune cells to areas of inflammation and can promote tissue damage [178, 179]. Other proinflammatory cytokines such as IL-6 can drive the development of Th17 cells while stymying Tregs [180]. The response of Th17 cells against harmful pathogens is critical and requires a fine balance as too strong of response can lead to damaging inflammatory or autoimmune diseases. The impact on Th17 cells due to ethanol-induced dysbiosis associated with ALD remains to be further characterized at this point. Clostridia production of SCFAs has been related to the induction of TGF-β and induction of Treg cells [181]. Bacteroides fragilis is required for the differentiation of functional Tregs producing IL-10 expression and prevent colitis [181]. Bifidobacteria has also been involved in Th17 differentiation along with regulating the balance of Th1 and Th2 responses and stimulation of CD8+ cells [182, 183].
The microbiota also can perform quorum sensing in the gut which regulates gene expression due to microbe cell-population density fluctuations. Microbiota creates and releases chemical signals called autoinducers that operate on a positive feedback loop with cell density [184, 185]. Through sensing cell population density, bacteria can effectively communicate with one another through the autoinducers and build a coordinate response through altering gene expression to effectively help regulate immune responses in cells [186].
During ALD, disruptions occur within the microbiome that accelerates and exploit damage previously mentioned in the intestinal barriers. The most common impacts involve bacterial overgrowth within the intestine along with compositional changes [187, 188]. Increased harmful microbes can penetrate the gut barriers damaging the intestine and leading to translocation of bacteria to the liver where damage may also occur [189]. TLRs and other pattern recognition receptors within the gut activate an innate immune system response due to the abundance of bacteria in the gut during ALD, causing a pro-inflammatory response in human patients with severe AH [190, 191].
The collective intestinal fungi that act symbiotically with the host known as the intestinal mycobiome contributes to the immune system and also undergo major impacts in disease conditions [192]. C-type lectin receptors (CLRs) are instrumental in the immune response to fungi and include such receptors as dectin 1 and dectin 2 [193, 194]. In mice, dectin 1 amplifies cytokine production and phagocytosis in response to fungal overgrowth [193]. In mice and alcohol-dependent patients, a long-term ethanol-containing Lieber-DeCarli liquid diet has been associated with the overgrowth of Candida albicans (C. albicans) causing a decrease in intestinal fungal diversity [192, 195]. Levels of the toxin candidalysin, which is a peptide toxin produced by C. albicans were associated with progression of ethanol-induced liver disease in mice [195]. Studies into the role of dectin 2 have shown its importance for immune system response to dysbiosis of the mycobiome through inducing Th17 production [194]. The inflammatory cytokine produced by Th17 cells and neutrophils, IL-17 is a key factor in the immune system defense against fungal dysbiosis and overgrowth [196]. During AH and AC, the overgrowth of C. albicans and the resulting pro-inflammatory and immune response contributes to increased intestinal permeability further exacerbating liver damage and was associated with increased mortality in patients with AH, and increased fecal levels of candidalysin which were also associated with higher mortality [192, 197].
Interactions of the microbiome and the liver immune system
The liver immune system has limited interaction with the microbiome under normal healthy conditions. With the main connection points between the liver and microbiome being only through bile ducts and the portal vein, only select substances can get through the intestinal barrier and move into the liver. This is illustrated in Figure 3 with the structure of the liver under healthy conditions and the impacts of ALD changes on the molecular structure. Dendritic cells in the intestine can sample and transport some bacteria to the mesenteric lymph nodes through afferent lymphatics, allowing a more localized immune response [198, 199]. The vessels of the lymphatic system proliferate during chronic liver disease due to an increase in the expression of CCL21 [200]. Prolonged inflammation alters the functionality of the lymphatic system, compromising even further the liver immune system [200]. A major protection mechanism of the liver for the host is through secreting IgA in the bile which attaches to bacteria [201].
Disturbances within the host can push the liver immune system to act further in regulating the microbiome to protect itself from a compromised gut barrier. Dysbiosis in patients with AH has been related to overgrowth of E. faecalis, which is linked to a higher incidence of liver damage and mortality due to the release of the pore-forming toxin cytolysin [202]. Proton pump inhibitors (PPIs), a commonly prescribed medication, have also been linked to promoting dysbiosis through E. faecalis overgrowth in mice with ethanol-induced liver disease fed a long-term ethanol diet and it is a risk factor for developing ALD in alcohol-dependent patients [203]. Translocation of E. faecalis to the liver was associated with an induction of IL1B secretion by hepatic macrophages and Kupffer cells which further contribute to the disease [203]. Bacterial translocation is a common occurrence under these conditions where viable bacteria pass from the lumen of the intestine through the barriers [201, 203]. The liver immune system responds through the activation of Kupffer cells via receptors such as TLR4 [204, 205]. Kupffer cells activate an innate immune response releasing cytokines and ROS production. The released cytokines attract immune cells such as neutrophils and monocytes to the liver to combat the invading microbes, but also promote damage in the liver as well. Recruitment of T-lymphocytes and neutrophils can occur as well as hepatic stellate cell activation, ultimately leading to collagen production and consequently fibrosis [206]. Finally, invariant natural killer (iNKT) cells, commonly found in the intestine, can also migrate to the liver, during bacterial translocation induced by chronic alcohol abuse, and has been found to assist in liver cell apoptosis [207].
The liver can also have an impact on the Th17 and Treg balance in the lamina propria through derivates of the bile acid metabolite lithocholic acid (LCA). 3-oxoLCA can bind to RORγT to prevent Th17 cell proliferation, whereas isoalloLCA produces mitochondrial ROS leading to an uptick in FOXP3 expression which is a master regulator of the differentiation of Tregs [208]. In mice fed Lieber DeCarli ethanol diet, farnesoid X receptor (FXR), a regulator of bile acids, lipids, and glucose metabolism, is downregulated, leading to a higher amount of bile acids being produced and secreted by the liver [209]. Mice with whole-body depletion of FXR treated with a chronic plus binge ethanol feeding model were susceptible to hepatic steatosis and ethanol-induced liver disease [209-211]. Experimental treatment of isoalloLCA and 3-oxoLCA to naïve CD4+ T cells isolated from FXR deficient mice, did not change FOXP3 expression when compared to the controls [208]. In the 3-oxoLCA treated cells, FXR did not assist with the inhibition of Th17 cells [208].
Image of differences within the liver between healthy condition and alcohol-associated liver disease. Abbreviations: IL1-ß: interleukin 1 beta; TNFα: tumor necrosis factor-alpha.

Alternative therapeutic approaches that modulate the intestinal homeostasis to prevent alcohol-associated liver disease
Under healthy conditions, the intestinal immune system finds a balance between proinflammatory response to pathogens and preventing the pro-inflammatory responses from causing damage to vital organs. As previously mentioned, this is regulated by the gut microbiota, crucial for the maintenance of intestinal homeostasis. However, under certain conditions, this balance can become skewed, leading to a compromised intestinal immune system. Chronic alcohol abuse causes consequential impacts on the immune system, liver, intestine, and gut microbiota. Excessive alcohol use increases the permeability of the intestine, leads to inflammation, and causes tight junction dysfunction. Intestinal bacterial overgrowth and dysbiosis is also a common effect of alcohol abuse which can lead to high levels of bacterial translocation to the liver, causing irreparable damage [50, 188]. Over recent years, some therapeutic approaches have targeted cells that are consequential in the immune response and regulation of the microbiome.
Therapeutic approaches targeting ILC3s in mice have become an emerging area of research over recent years. Ethanol-induced liver disease is typically associated with impairment of ILC3s in the gut from properly producing IL-22, thus fueling bacterial translocation [117]. By targeting ILC3s and therefore reducing bacterial translocation, progressive liver damage could be avoided allowing for the immune system to focus the response in the gut. One such therapeutic approach on ILC3s done in mice involves Aryl-hydrocarbon receptor-ligand (AHR) indole-3-acetic acid (IAA). This approach has been found to promote the expression of REG3γ through supplementing bacteria engineered to produce IL-22, reducing the translocation of bacteria to the liver [117]. Another therapeutic approach is through the administration of IL-22 producing bacteria which was also found to reduce bacterial translocation [117]. Preliminary clinical data show a correlation to the overall survival rate in patients with AH to the density of IL-22 cells from peripheral blood [212, 213]. F-652 is a recombinant fusion protein of human IL-22 and immunoglobulin G2 fragment crystallizable. The first human phase I clinical trial using F-652 in healthy subjects was conducted [214] and phase II clinical trial (NCT02655510) to treat patients with AH and severe AD is now completed [215]. AH patients treated with F-652 presented a significant decrease in the MELD score, total bilirubin, ALT and AST, a high rate of Lille score, and reduction of marker of inflammation in plasma [216].
The cytokine osteopontin has shown controversial results in animal studies in ethanol-induced liver disease [217-219]. Osteopontin is normally increased in patients with AH, however, pushing levels of expression in hepatocytes experimentally beyond the natural increase blocked TNFα and its damaging impacts on the liver [217]. However, therapies targeting inflammatory cytokines such as TNFα have been demonstrated to be ineffective and increase mortality due to infections [220, 221].
Th17 offers an intriguing option as an alternative therapy approach as their presence within the immune system fights invading pathogens through promoting inflammatory responses [222-226]. These inflammatory responses, however, can be problematic during chronic events such as ALD. The pro-inflammatory positive feedback response can be harmful to the liver where inflammation causes a buildup of tissue damage [140, 227] and can also promote an autoimmune response [228] and compromise the immune system tolerance of commensal gut microbes [229]. Several recent studies have shown the positive impacts of stymying a Th17 cell buildup. Halofugine, an antifibrotic drug, was found to suppress Th17 differentiation and thus lower inflammation and collagen synthesis in a model of concanavalin A-induced liver fibrosis in the rat [230]. This was accomplished due to Halofugine downregulates TGF-ß signaling and other reactive cytokines, leading to less inflammation and collagen buildup [230]. Similarly, as mention above mouse models with a reduced number of Th17 cells presented less intestinal inflammation and reduced ethanol-induced liver injury [139]. Caution should be considered when using IL-17 targeted therapies as some studies with inflammatory bowel disease and Crohn's disease have resulted in isolated cases of exacerbation of the disease or new-onset [231-233].
Lactobacillus rhamnosus GG supernatant (LGG-s) therapy was also found to increase mRNA expression of tight junction proteins and reducing intestinal permeability in a mouse model of chronic-binge alcohol feeding. This therapy led to a decrease of Escherichia coli in the liver and an overall balance restored of Tregs, Th17s, and IL-17 [234, 235]. Most importantly, there is a completed, yet to be published clinical trial in phase II for acute AH patients (NCT01922895) that has studied whether Lactobacillus rhamnosus GG supplementation will improve their condition and intestinal complications. While some alternative therapies seek to address ALD treatment by significantly reducing bacteria levels overall within the host, prebiotic and probiotic therapies aim to alleviate gut microbe dysbiosis. Prebiotic therapies aim to restore commensal gut microbes through the introduction of dietary supplements. Studies using fructooligosaccharides (FOS) prebiotics in mice after the intragastric feeding model of continuous ethanol infusion showed a lessening of bacterial overgrowth and restoration of AMP levels such as REG3γ [50]. FOS administration has been shown to increase ileal IgA secretion, B220+IgA+ cells, and pIgR expression in the small intestine and colon [236, 237] and induced immunoregulatory DC responses [238]. The use of pectin in mice fed alcohol with a Lieber DeCarli diet avoided ethanol-induced downregulation of Bacteroides and protected from ethanol-induced liver disease [239]. Administration of Lactobacillus species GG in rats, administration of Akkermansia muciniphila in mice, short-term oral supplementation with Bifidobacterium bifidum in preclinical studies have shown improvement of gut microbe homeostasis and reducing liver damage [240, 241]. A clinical study where administration of Lactobacillus plantarum 8PA3 to AH patients, and Lactobacillus subtilis/Streptococcus faecium supplementation in AH patients also have shown improvement of gut microbe homeostasis, less liver damage, and balancing immune response and cytokine production [242-244]. Furthermore, probiotic administration of Lactobacillus rhamnosus R0011 and acidophilus R0052 in mice with ethanol-induced liver disease has also been linked to a reduction in TNFα, IL-1β TLR-4 in the liver [245].
Fecal microbiota transplantation (FMT) is a growing interest as an alternative therapy to treat conditions such as AH to improve the long-term mortality rate of affected patients. Initial findings from a limited number of studies performing FMT on patients with severe AH found higher survival rates and less toxin buildup in the bloodstream in patients receiving the transplantation versus other patients that received other treatments such as corticosteroids and pentoxifylline [246, 247]. FMT data to date offers a promising option for alternative therapy for AH, however, further studies are needed to confirm these results. There is a recently completed phase 1, randomized, single-blind, interventional clinical trial (NCT03416751) that included patients with cirrhosis and alcohol use disorder to assess whether FMT improves the prognosis of these patients. The study showed that FMT is associated with lower alcohol consumption and craving which was accompanied by reduced levels of serum IL-6 and lipopolysaccharide-binding protein (LBP), an increased amount of butyrate/isobutyrate, and increased microbial diversity [248]. FMT has been related to the restoration of CD4+, CD8+, and B220+ in the small intestine and CD4+ in the colon after broad-spectrum antibiotics for 8 weeks [249]. The study found with those receiving an FMT a reduction in short-term alcohol use along with improvements in microbial diversity which has been inversely linked to the severity of alcohol-use related diseases [248]. It would be relevant to study the impact of FMT in the LP immune system of patients with ALD and establish a deeper relationship between certain bacterial composition and the mucosal immune system.
The use of antibiotics to control ethanol-induced bacterial overgrowth and prevent ethanol-induced liver disease in rodent models has been previously demonstrated [249, 250], however, the short term use of paromomycin in patients with ALD did not result in improvement of liver function tests [251]. The use of antibiotics has been related to changes in DC, memory/effector T cells, Th17, Tregs ILC3, IL-22, Th1 and Th2 in the small intestine, large intestine, spleen, and mesenteric lymph nodes in mice [249, 252], changes in immunity to vaccines in humans and it has been related with food allergies in humans [253, 254]. Depletion of gram-negative bacteria compromises TLR4 and MyD88 signaling while depletion of gram-positive bacteria reduces TLR2 signaling and both effects result in a reduction of Reg3g levels [252, 255]. These results indicate that despite antibiotics are a good alternative to reduce bacterial overgrowth, its use could promote drastic changes in the immune-microbiome interactions vital for health. However, some antibiotics such as rifaximin have been shown beneficial for hepatic encephalopathy in cirrhosis and had an impact on gut bacterial metabolites such as an increase in saturated and unsaturated fatty acids without significant changes in microbial abundance [256]. There is a completed but yet to be published phase 3, interventional, randomized clinical trial (NCT02281929) that has proposed the use of augmentin (amoxicillin, clavulanic acid) in conjunction with oral corticotheraoy (prednisolone) to test whether it has positive effects in patients with AH. This is a very interesting approach however levels of intestinal C. albicans have been related to the use of certain antibiotics, among them amoxicillin [257, 258].
As previously mentioned, during ALD overgrowth of certain bacteria such a cytolytic E. faecalis contributes to liver damage after translocation. The presence of cytolysin in stools correlates with mortality in patients with AH. Mice gavaged with cytolytic E. faecalis exhibited increased levels of cytolysin in the liver and developed a more severe ethanol-induced liver injury. Cytolysin forms pores [259] and induces hepatocyte death [202]. To counteract this dysbiosis, bacteriophages targeting cytolytic E. faecalis were found to significantly diminish cytolysin and the impacts of AH such as liver damage and hepatic cell death in mice [202]. Future studies can determine if this alternative therapy can translate to humans.
The connection of the bile ducts between the liver and intestine offers a potential option to alternative therapy approaches. Restoring the homeostasis of bile acids is a necessary step to preventing further liver injury in patients with ALDs. The potential key target to restoring homeostasis within bile acids is FXR, with a study reporting positive results of reduced liver injury in rats [260]. By using Dihydroartemisinin (DHA), an anti-malaria drug, FXR expression was restored suggesting FXR as a molecular target of DHA [260]. In another study, ethanol-induced liver disease was reduced in mice using an intestine-restricted FXR agonist fexaramine through the reduction of IL-1ß and TNF in the liver [261]. Further studies can help to uncover the mechanisms for upregulating FXR expression and the reestablishment of bile acid homeostasis. There is an interventional phase 2 completed a clinical trial that is using obeticholic acid (OCA), an FXR agonist, in moderately severe AH patients (NCT02039219). Early termination of the study did not allow researchers to collect enough samples to study changes in innate immunity. However, studies in rodent models have shown that OCA reduces the production of TNF-α by intestinal DC and their differentiation into mature DCs in models of colitis avoiding intestinal inflammation [262, 263].
Nutrition-based therapies are another targeted alternative therapeutic approach to ALD. Given the influence on diet and microbiota derived-metabolites such a SCFAs, MCFAs, and LCFAs on the host, these potential therapies could offer promising results for attenuating ALD. Supplementation of SCFAs, MCFAs, or LCFAs in both mice and rats, have all been linked to reduce liver damage and improve the intestinal gut barrier [264, 265]. MCFAs and LCFAs in particular have been found to enhance the expression of intestinal tight junctions [164, 266, 267]. Future research on the impacts of SCFAs, MCFAs, and LCFAs on humans with ALD can further determine both the short-term and long-term implications of nutritional therapy. In addition, the administration of SCFA facilitates C. albicans clearance and Tregs inductions in mice treated with an antibiotic [268]. SCFAs derived from Clostridia are also related to the induction of TGF-β and Treg cells [181].
Other novel therapies have emerged with promising results towards potential ways of alleviating ALD. Purified hyperimmune bovine colostrum (IMM-124E) therapy has been used in phase 3 human clinical trial (NCT02473341) in severe AH patients to reduce bacterial translocation to the liver as it contains IgG anti-LPS antibodies.
Therapies using agonists and antagonists have also shown promise and are currently moving through human trial phases. Anakinra, an IL-1 receptor antagonist is currently being tested in phase 2 clinical trial (NCT04072822) in human subjects with AH. If successful, anakinra will be able to reduce TLR4-induced inflammation which depends on signaling from IL-1. Inhibition of IL-1R signally is related with reducing expression of IL-17A by Th17 cells in mice and humans [269]. Another antagonist, Selonsertib (GS-4997) in combination with prednisolone, completed phase 3 clinical trial (NCT03053063) but was not found to reduce fibrosis in affected patients [270].
Antioxidant therapy clinical trial results in AH patients using such antioxidants as N-acetylcysteine (NAC) and metadoxine have shown higher survival rates short term, however, the long-term effects and overall efficacy require further study [212]. A current phase 3 clinical trial (NCT03069300) to study the effect of NAC in conjunction with prednisolone in AH patients is currently ongoing. NAC treatment under LPS challenge has been associated with changes in the immune and inflammatory response [271]. In addition, mucus hypersecretion is controlled by NAC as NAC functions as a mucolytic through depolymerization of the mucin, and decreasing viscosity which decreases the adhesion of bacteria [272]. The benefits of mucin depletion accompanied by a possible induction of Reg3g during ethanol-induced liver disease has been discussed above [49].
Human clinical trials using regenerative methods such as the previously mentioned IL-22 therapies are also offering positive initial outcomes [212]. A current clinical trial in patients with severe AH found treatment using granulocyte colony-stimulating factor (G-CSF) in phase 4 (NCT03703674). Previous clinical trial phases showed that G-CSF leads to a higher rate of survival due to improved liver regeneration through stimulation of hematopoietic stem cells present in the liver and more neutrophils in the blood [212].
Conclusion
While many recent studies show promise in advancing treatment towards ALD, further research is needed to combat the disease. Along with targeting ILC3s, and Th17 cells functionality for alternative therapeutic approaches, the LP-associated immune system also offers other potential targets for effective therapy. Continuing research into alternative therapies to these targets offers a promising outlook to both better understanding and treating diseases impacting gut and liver health.
Abbreviations
5-ASA: 5-aminosalicyclic acid; AC: alcohol-associated cirrhosis; AH: alcoholic hepatitis; ALD: alcohol-associated liver disease; AMPs: antimicrobial peptides; AHR: Aryl-hydrocarbon receptor-ligand; APRIL: A proliferation-inducing ligand; AUD: alcohol use disorder; BAFF: B cell-activating factor of the tumor necrosis factor family; C. albicans: Candida albicans; CLRs: C-type lectin receptors; CCL20: C-C Motif Chemokine Ligand 20; CXCL1: C-X-C Motif Chemokine Ligand 1; DCs: dendritic cells; DHA: Dihydroartemisinin; E. faecalis: Enterococcus faecalis; EECs: enteroendocrine cells; FMT: fecal microbiota transplantation; FOS: fructooligosaccharides; FOXP3: forkhead box P3; FXR: farnesoid X receptor; G-CSF: granulocyte colony stimulating factor; GAPs: goblet cell associated-antigen passages; GIP: gastric inhibitory peptide; gp130: glycoprotein 130; GPR: G protein receptors; IAA: indole-3-acetic acid; IBD: inflammatory bowel disease; IFN-γ: interferon-gamma; IgA: immunoglobulin A; IL: interleukin; ILCs: innate lymphoid cells; ISCs: intestinal stem cells; LCA: lithocholic acid; LCFAs: long-chain fatty acids; LGG-s: Lactobacillus rhamnosus GG; Lgr5: leucine-rich repeat-containing G-protein couple receptor 5; LPS: lipopolysaccharide; LP-DCs: lamina propria dendritic cells; Lypd8: Ly6/Plaur domain-containing 8; M cells: microfold cells; MAIT: mucosa-associated invariant T cells; MCFAs: medium-chain fatty acids; MHC: major histocompatibility class; MLN: mesenteric lymph nodes; NAC: N-acetylcysteine; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; NODs: nucleotide-binding oligomerization domain-containing proteins; OCA: obeticholic acid; pIgR: polymeric immunoglobulin receptor; PPI: Proton pump inhibitors; RA: retinoic acid; RALDH: retinaldehyde dehydrogenase; RANKL: receptor activator of nuclear factor-kappa-Β ligand; RARα: retinoic acid receptor alpha; Rdh7: retinal dehydrogenase 7; REG3: regenerating islet-derived 3; RELM- ß: resistin-like molecule ß; RORγt: RAR-related orphan receptor gamma; RAR: related orphan receptor gamma; ROS: reactive oxygen species; SCFAs: Short-chain fatty acids; TFF: trefoil factor; Th: T helper cells; TLRs: toll-like receptors; TNF: tumor necrosis factor; TNFR2: Tumor necrosis factor receptor 2; Treg: regulatory T cells; TSLP: (thymic stromal lymphopoietin); VLDL: very low-density lipoprotein.
Acknowledgements
C.L. was supported by an AASLD Pinnacle Research Award in Liver Disease and a pilot project award from Southern California Research Center for Alcoholic Liver and Pancreatic Disease (ALPD) and Cirrhosis (P50 AA011999). This study was also supported by services provided by P30 DK120515.
Author contributions
R.B. was responsible for writing of the manuscript; C.L. was responsible for guidance and editing of manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Gao B, Ahmad MF, Nagy LE, Tsukamoto H. Inflammatory pathways in alcoholic steatohepatitis. J Hepatol. 2019;70(2):249-59
2. Avila MA, Dufour JF, Gerbes AL, Zoulim F, Bataller R, Burra P. et al. Recent advances in alcohol-related liver disease (ALD): summary of a Gut round table meeting. Gut. 2020;69(4):764-80
3. Mendenhall CL, Seeff L, Diehl AM, Ghosn SJ, French SW, Gartside PS. et al. Antibodies to hepatitis B virus and hepatitis C virus in alcoholic hepatitis and cirrhosis: their prevalence and clinical relevance. The VA Cooperative Study Group (No. 119). Hepatology. 1991;14(4 Pt 1):581-9
4. McDermott AJ, Huffnagle GB. The microbiome and regulation of mucosal immunity. Immunology. 2014;142(1):24-31
5. Yang SK, Eckmann L, Panja A, Kagnoff MF. Differential and regulated expression of C-X-C, C-C, and C-chemokines by human colon epithelial cells. Gastroenterology. 1997;113(4):1214-23
6. Onyiah JC, Colgan SP. Cytokine responses and epithelial function in the intestinal mucosa. Cell Mol Life Sci. 2016;73(22):4203-12
7. He B, Xu W, Santini PA, Polydorides AD, Chiu A, Estrella J. et al. Intestinal bacteria trigger T cell-independent immunoglobulin A(2) class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity. 2007;26(6):812-26
8. Mukherjee S, Zheng H, Derebe MG, Callenberg KM, Partch CL, Rollins D. et al. Antibacterial membrane attack by a pore-forming intestinal C-type lectin. Nature. 2014;505(7481):103-7
9. Hendrikx T, Schnabl B. Antimicrobial proteins: intestinal guards to protect against liver disease. J Gastroenterol. 2019;54(3):209-17
10. Yan AW, Fouts DE, Brandl J, Starkel P, Torralba M, Schott E. et al. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology. 2011;53(1):96-105
11. Wang L, Fouts DE, Starkel P, Hartmann P, Chen P, Llorente C. et al. Intestinal REG3 Lectins Protect against Alcoholic Steatohepatitis by Reducing Mucosa-Associated Microbiota and Preventing Bacterial Translocation. Cell Host Microbe. 2016;19(2):227-39
12. Dürr UH, Sudheendra US, Ramamoorthy A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim Biophys Acta. 2006;1758(9):1408-25
13. Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nuñez G. et al. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307(5710):731-4
14. Bruno ME, Frantz AL, Rogier EW, Johansen FE, Kaetzel CS. Regulation of the polymeric immunoglobulin receptor by the classical and alternative NF-κB pathways in intestinal epithelial cells. Mucosal Immunol. 2011;4(4):468-78
15. Kaetzel CS, Robinson JK, Chintalacharuvu KR, Vaerman JP, Lamm ME. The polymeric immunoglobulin receptor (secretory component) mediates transport of immune complexes across epithelial cells: a local defense function for IgA. Proc Natl Acad Sci U S A. 1991;88(19):8796-800
16. Schneeman TA, Bruno ME, Schjerven H, Johansen FE, Chady L, Kaetzel CS. Regulation of the polymeric Ig receptor by signaling through TLRs 3 and 4: linking innate and adaptive immune responses. J Immunol. 2005;175(1):376-84
17. Macpherson AJ, Gatto D, Sainsbury E, Harriman GR, Hengartner H, Zinkernagel RM. A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science. 2000;288(5474):2222-6
18. Okumura R, Kurakawa T, Nakano T, Kayama H, Kinoshita M, Motooka D. et al. Lypd8 promotes the segregation of flagellated microbiota and colonic epithelia. Nature. 2016;532(7597):117-21
19. Zhong W, Zhao Y, Sun X, Song Z, McClain CJ, Zhou Z. Dietary zinc deficiency exaggerates ethanol-induced liver injury in mice: involvement of intrahepatic and extrahepatic factors. PLoS One. 2013;8(10):e76522
20. Zhong W, McClain CJ, Cave M, Kang YJ, Zhou Z. The role of zinc deficiency in alcohol-induced intestinal barrier dysfunction. Am J Physiol Gastrointest Liver Physiol. 2010;298(5):G625-33
21. Keshavarzian A, Holmes EW, Patel M, Iber F, Fields JZ, Pethkar S. Leaky gut in alcoholic cirrhosis: a possible mechanism for alcohol-induced liver damage. Am J Gastroenterol. 1999;94(1):200-7
22. Fukui H, Brauner B, Bode JC, Bode C. Plasma endotoxin concentrations in patients with alcoholic and non-alcoholic liver disease: reevaluation with an improved chromogenic assay. J Hepatol. 1991;12(2):162-9
23. Szabo G. Consequences of alcohol consumption on host defence. Alcohol Alcohol. 1999;34(6):830-41
24. Cho YE, Yu LR, Abdelmegeed MA, Yoo SH, Song BJ. Apoptosis of enterocytes and nitration of junctional complex proteins promote alcohol-induced gut leakiness and liver injury. J Hepatol. 2018;69(1):142-53
25. Zhou R, Llorente C, Cao J, Gao B, Duan Y, Jiang L. et al. Deficiency of Intestinal α1-2-fucosylation Exacerbates Ethanol-Induced Liver Disease in Mice. Alcohol Clin Exp Res. 2020
26. Tong M, McHardy I, Ruegger P, Goudarzi M, Kashyap PC, Haritunians T. et al. Reprograming of gut microbiome energy metabolism by the FUT2 Crohn's disease risk polymorphism. ISME J. 2014;8(11):2193-206
27. Rausch P, Kunzel S, Suwandi A, Grassl GA, Rosenstiel P, Baines JF. Multigenerational Influences of the Fut2 Gene on the Dynamics of the Gut Microbiota in Mice. Front Microbiol. 2017;8:991
28. Pham TA, Clare S, Goulding D, Arasteh JM, Stares MD, Browne HP. et al. Epithelial IL-22RA1-mediated fucosylation promotes intestinal colonization resistance to an opportunistic pathogen. Cell Host Microbe. 2014;16(4):504-16
29. Kelly RJ, Rouquier S, Giorgi D, Lennon GG, Lowe JB. Sequence and expression of a candidate for the human Secretor blood group alpha(1,2)fucosyltransferase gene (FUT2). Homozygosity for an enzyme-inactivating nonsense mutation commonly correlates with the non-secretor phenotype. J Biol Chem. 1995;270(9):4640-9
30. Bry L, Falk PG, Midtvedt T, Gordon JI. A model of host-microbial interactions in an open mammalian ecosystem. Science. 1996;273(5280):1380-3
31. Pickard JM, Chervonsky AV. Intestinal fucose as a mediator of host-microbe symbiosis. J Immunol. 2015;194(12):5588-93
32. Lu R, Voigt RM, Zhang Y, Kato I, Xia Y, Forsyth CB. et al. Alcohol Injury Damages Intestinal Stem Cells. Alcohol Clin Exp Res. 2017;41(4):727-34
33. Taniguchi K, Wu LW, Grivennikov SI, de Jong PR, Lian I, Yu FX. et al. A gp130-Src-YAP module links inflammation to epithelial regeneration. Nature. 2015;519(7541):57-62
34. Deng F, Peng L, Li Z, Tan G, Liang E, Chen S. et al. YAP triggers the Wnt/β-catenin signalling pathway and promotes enterocyte self-renewal, regeneration and tumorigenesis after DSS-induced injury. Cell Death Dis. 2018;9(2):153
35. Tanaka M, Saito H, Kusumi T, Fukuda S, Shimoyama T, Sasaki Y. et al. Spatial distribution and histogenesis of colorectal Paneth cell metaplasia in idiopathic inflammatory bowel disease. J Gastroenterol Hepatol. 2001;16(12):1353-9
36. Gassler N. Paneth cells in intestinal physiology and pathophysiology. World J Gastrointest Pathophysiol. 2017;8(4):150-60
37. Bluemel S, Wang L, Martino C, Lee S, Wang Y, Williams B. et al. The Role of Intestinal C-type Regenerating Islet Derived-3 Lectins for Nonalcoholic Steatohepatitis. Hepatol Commun. 2018;2(4):393-406
38. Ayabe T, Satchell DP, Wilson CL, Parks WC, Selsted ME, Ouellette AJ. Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat Immunol. 2000;1(2):113-8
39. Jones DE, Bevins CL. Paneth cells of the human small intestine express an antimicrobial peptide gene. J Biol Chem. 1992;267(32):23216-25
40. Peeters T, Vantrappen G. The Paneth cell: a source of intestinal lysozyme. Gut. 1975;16(7):553-8
41. Fahlgren A, Hammarström S, Danielsson A, Hammarström ML. Increased expression of antimicrobial peptides and lysozyme in colonic epithelial cells of patients with ulcerative colitis. Clin Exp Immunol. 2003;131(1):90-101
42. Com E, Bourgeon F, Evrard B, Ganz T, Colleu D, Jégou B. et al. Expression of antimicrobial defensins in the male reproductive tract of rats, mice, and humans. Biol Reprod. 2003;68(1):95-104
43. Harwig SS, Tan L, Qu XD, Cho Y, Eisenhauer PB, Lehrer RI. Bactericidal properties of murine intestinal phospholipase A2. J Clin Invest. 1995;95(2):603-10
44. Cash HL, Whitham CV, Behrendt CL, Hooper LV. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313(5790):1126-30
45. Natividad JM, Hayes CL, Motta JP, Jury J, Galipeau HJ, Philip V. et al. Differential induction of antimicrobial REGIII by the intestinal microbiota and Bifidobacterium breve NCC2950. Appl Environ Microbiol. 2013;79(24):7745-54
46. Vaishnava S, Yamamoto M, Severson KM, Ruhn KA, Yu X, Koren O. et al. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science. 2011;334(6053):255-8
47. Gyongyosi B, Cho Y, Lowe P, Calenda CD, Iracheta-Vellve A, Satishchandran A. et al. Alcohol-induced IL-17A production in Paneth cells amplifies endoplasmic reticulum stress, apoptosis, and inflammasome-IL-18 activation in the proximal small intestine in mice. Mucosal Immunol. 2019;12(4):930-44
48. Zhong W, Wei X, Hao L, Lin TD, Yue R, Sun X. et al. Paneth Cell Dysfunction Mediates Alcohol-related Steatohepatitis Through Promoting Bacterial Translocation in Mice: Role of Zinc Deficiency. Hepatology. 2020;71(5):1575-91
49. Hartmann P, Chen P, Wang HJ, Wang L, McCole DF, Brandl K. et al. Deficiency of intestinal mucin-2 ameliorates experimental alcoholic liver disease in mice. Hepatology. 2013;58(1):108-19
50. Yan AW, Fouts DE, Brandl J, Stärkel P, Torralba M, Schott E. et al. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology. 2011;53(1):96-105
51. Nair MG, Guild KJ, Du Y, Zaph C, Yancopoulos GD, Valenzuela DM. et al. Goblet cell-derived resistin-like molecule beta augments CD4+ T cell production of IFN-gamma and infection-induced intestinal inflammation. J Immunol. 2008;181(7):4709-15
52. Farrell JJ, Taupin D, Koh TJ, Chen D, Zhao CM, Podolsky DK. et al. TFF2/SP-deficient mice show decreased gastric proliferation, increased acid secretion, and increased susceptibility to NSAID injury. J Clin Invest. 2002;109(2):193-204
53. Halm DR, Halm ST. Secretagogue response of goblet cells and columnar cells in human colonic crypts. Am J Physiol. 1999;277(3):C501-22
54. Ermund A, Schütte A, Johansson ME, Gustafsson JK, Hansson GC. Studies of mucus in mouse stomach, small intestine, and colon. I. Gastrointestinal mucus layers have different properties depending on location as well as over the Peyer's patches. Am J Physiol Gastrointest Liver Physiol. 2013;305(5):G341-7
55. Propheter DC, Chara AL, Harris TA, Ruhn KA, Hooper LV. Resistin-like molecule β is a bactericidal protein that promotes spatial segregation of the microbiota and the colonic epithelium. Proc Natl Acad Sci U S A. 2017;114(42):11027-33
56. Knoop KA, McDonald KG, McCrate S, McDole JR, Newberry RD. Microbial sensing by goblet cells controls immune surveillance of luminal antigens in the colon. Mucosal Immunol. 2015;8(1):198-210
57. Knoop KA, Gustafsson JK, McDonald KG, Kulkarni DH, Kassel R, Newberry RD. Antibiotics promote the sampling of luminal antigens and bacteria via colonic goblet cell associated antigen passages. Gut Microbes. 2017;8(4):400-11
58. Elamin E, Masclee A, Troost F, Dekker J, Jonkers D. Cytotoxicity and metabolic stress induced by acetaldehyde in human intestinal LS174T goblet-like cells. Am J Physiol Gastrointest Liver Physiol. 2014;307(3):G286-94
59. Brooks PJ, Theruvathu JA. DNA adducts from acetaldehyde: implications for alcohol-related carcinogenesis. Alcohol. 2005;35(3):187-93
60. Rao RK. Acetaldehyde-induced barrier disruption and paracellular permeability in Caco-2 cell monolayer. Methods Mol Biol. 2008;447:171-83
61. Dunagan M, Chaudhry K, Samak G, Rao RK. Acetaldehyde disrupts tight junctions in Caco-2 cell monolayers by a protein phosphatase 2A-dependent mechanism. Am J Physiol Gastrointest Liver Physiol. 2012;303(12):G1356-64
62. Vakevainen S, Tillonen J, Agarwal DP, Srivastava N, Salaspuro M. High salivary acetaldehyde after a moderate dose of alcohol in ALDH2-deficient subjects: strong evidence for the local carcinogenic action of acetaldehyde. Alcohol Clin Exp Res. 2000;24(6):873-7
63. Hartmann P, Chen P, Wang HJ, Wang L, McCole DF, Brandl K. et al. Deficiency of intestinal mucin-2 ameliorates experimental alcoholic liver disease in mice. Hepatology. 2013;58(1):108-19
64. Gebert A, Steinmetz I, Fassbender S, Wendlandt KH. Antigen transport into Peyer's patches: increased uptake by constant numbers of M cells. Am J Pathol. 2004;164(1):65-72
65. de Lau W, Kujala P, Schneeberger K, Middendorp S, Li VS, Barker N. et al. Peyer's patch M cells derived from Lgr5(+) stem cells require SpiB and are induced by RankL in cultured “miniguts”. Mol Cell Biol. 2012;32(18):3639-47
66. Rol N, Favre L, Benyacoub J, Corthésy B. The role of secretory immunoglobulin A in the natural sensing of commensal bacteria by mouse Peyer's patch dendritic cells. J Biol Chem. 2012;287(47):40074-82
67. Obata T, Shibata N, Goto Y, Ishikawa I, Sato S, Kunisawa J. et al. Critical role of dendritic cells in T cell retention in the interfollicular region of Peyer's patches. J Immunol. 2013;191(2):942-8
68. Rey J, Garin N, Spertini F, Corthésy B. Targeting of secretory IgA to Peyer's patch dendritic and T cells after transport by intestinal M cells. J Immunol. 2004;172(5):3026-33
69. Parnell EA, Walch EM, Lo DD. Inducible Colonic M Cells Are Dependent on TNFR2 but Not Ltβr, Identifying Distinct Signalling Requirements for Constitutive Versus Inducible M Cells. J Crohns Colitis. 2017;11(6):751-60
70. Eversole R, Beuving L, Ulrich R. Ultrastructural alterations in ileal M cells of rats after chronic ethanol ingestion: reversal after cessation of ethanol. J Stud Alcohol. 1992;53(5):519-23
71. Lopez MC, Watzl B, Colombo LL, Watson RR. Alterations in mouse Peyer's patch lymphocyte phenotype after ethanol consumption. Alcohol. 1997;14(2):107-10
72. Worthington JJ, Reimann F, Gribble FM. Enteroendocrine cells-sensory sentinels of the intestinal environment and orchestrators of mucosal immunity. Mucosal Immunol. 2018;11(1):3-20
73. Samuel BS, Shaito A, Motoike T, Rey FE, Backhed F, Manchester JK. et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci U S A. 2008;105(43):16767-72
74. Karaki S, Mitsui R, Hayashi H, Kato I, Sugiya H, Iwanaga T. et al. Short-chain fatty acid receptor, GPR43, is expressed by enteroendocrine cells and mucosal mast cells in rat intestine. Cell Tissue Res. 2006;324(3):353-60
75. Kim MH, Kang SG, Park JH, Yanagisawa M, Kim CH. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology. 2013;145(2):396-406.e1 -10
76. Hauge T, Persson J, Sjolund K. Neuropeptides in the duodenal mucosa of chronic alcoholic heavy drinkers. Alcohol Alcohol. 2001;36(3):213-8
77. Zhou C, Li J, Wang H, Tang C. Decreased somatostatin is related to the hypersensitivity of intestinal epithelia to LPS via upregulated TLR4-TBK1 pathway in rats chronically exposed to ethanol. Alcohol. 2009;43(4):293-303
78. Haber AL, Biton M, Rogel N, Herbst RH, Shekhar K, Smillie C. et al. A single-cell survey of the small intestinal epithelium. Nature. 2017;551(7680):333-9
79. von Moltke J, Ji M, Liang HE, Locksley RM. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature. 2016;529(7585):221-5
80. Davies MD, Parrott DM. Preparation and purification of lymphocytes from the epithelium and lamina propria of murine small intestine. Gut. 1981;22(6):481-8
81. Kim MS, Kim TS. IgA+ plasma cells in murine intestinal lamina propria as a positive regulator of Treg differentiation. J Leukoc Biol. 2014;95(3):461-9
82. Schulz O, Jaensson E, Persson EK, Liu X, Worbs T, Agace WW. et al. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J Exp Med. 2009;206(13):3101-14
83. Bianco C, Felice V, Panarese S, Marrocco R, Ostanello F, Brunetti B. et al. Quantitative immunohistochemical assessment of IgA, IgM, IgG and antigen-specific immunoglobulin secreting plasma cells in pig small intestinal lamina propria. Vet Immunol Immunopathol. 2014;160(3-4):281-7
84. Zeissig S, Blumberg RS. Commensal microbial regulation of natural killer T cells at the frontiers of the mucosal immune system. FEBS Lett. 2014;588(22):4188-94
85. Powell DW, Pinchuk IV, Saada JI, Chen X, Mifflin RC. Mesenchymal cells of the intestinal lamina propria. Annu Rev Physiol. 2011;73:213-37
86. Boudry G, Yang P-C, Perdue M. Small Intestine, Anatomy. In: Johnson L, editor. Encyclopedia of Gastroenterology: Academic Press. 2004 P: 404-9
87. Denning TL, Norris BA, Medina-Contreras O, Manicassamy S, Geem D, Madan R. et al. Functional specializations of intestinal dendritic cell and macrophage subsets that control Th17 and regulatory T cell responses are dependent on the T cell/APC ratio, source of mouse strain, and regional localization. J Immunol. 2011;187(2):733-47
88. Becker M, Güttler S, Bachem A, Hartung E, Mora A, Jäkel A. et al. Ontogenic, Phenotypic, and Functional Characterization of XCR1(+) Dendritic Cells Leads to a Consistent Classification of Intestinal Dendritic Cells Based on the Expression of XCR1 and SIRPα. Front Immunol. 2014;5:326
89. Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA. et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307(5707):254-8
90. Kinnebrew MA, Buffie CG, Diehl GE, Zenewicz LA, Leiner I, Hohl TM. et al. Interleukin 23 production by intestinal CD103(+)CD11b(+) dendritic cells in response to bacterial flagellin enhances mucosal innate immune defense. Immunity. 2012;36(2):276-87
91. Leal Rojas IM, Mok WH, Pearson FE, Minoda Y, Kenna TJ, Barnard RT. et al. Human Blood CD1c(+) Dendritic Cells Promote Th1 and Th17 Effector Function in Memory CD4(+) T Cells. Front Immunol. 2017;8:971
92. Hammerschmidt SI, Ahrendt M, Bode U, Wahl B, Kremmer E, Förster R. et al. Stromal mesenteric lymph node cells are essential for the generation of gut-homing T cells in vivo. J Exp Med. 2008;205(11):2483-90
93. Matteoli G, Mazzini E, Iliev ID, Mileti E, Fallarino F, Puccetti P. et al. Gut CD103+ dendritic cells express indoleamine 2,3-dioxygenase which influences T regulatory/T effector cell balance and oral tolerance induction. Gut. 2010;59(5):595-604
94. Sun L, Fu J, Zhou Y. Metabolism Controls the Balance of Th17/T-Regulatory Cells. Front Immunol. 2017;8:1632
95. Korn T, Anderson AC, Bettelli E, Oukka M. The dynamics of effector T cells and Foxp3+ regulatory T cells in the promotion and regulation of autoimmune encephalomyelitis. J Neuroimmunol. 2007;191(1-2):51-60
96. Maldonado R, Perdomo C, Vascotto F, Bauquet A, Sharpe A, Kuchroo V. et al. Induced-tolerogenic Dendritic Cells promote tolerance and de novo differentiation of regulatory T cells. The Journal of Immunology. 2012
97. McDole JR, Wheeler LW, McDonald KG, Wang B, Konjufca V, Knoop KA. et al. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature. 2012;483(7389):345-9
98. Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR. et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204(8):1775-85
99. Kulkarni DH, Gustafsson JK, Knoop KA, McDonald KG, Bidani SS, Davis JE. et al. Goblet cell associated antigen passages support the induction and maintenance of oral tolerance. Mucosal Immunol. 2020;13(2):271-82
100. Kushwah R, Hu J. Complexity of dendritic cell subsets and their function in the host immune system. Immunology. 2011;133(4):409-19
101. Johansson-Lindbom B, Svensson M, Pabst O, Palmqvist C, Marquez G, Forster R. et al. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J Exp Med. 2005;202(8):1063-73
102. Bogunovic M, Ginhoux F, Helft J, Shang L, Hashimoto D, Greter M. et al. Origin of the lamina propria dendritic cell network. Immunity. 2009;31(3):513-25
103. Coombes JL, Siddiqui KR, Arancibia-Cárcamo CV, Hall J, Sun CM, Belkaid Y. et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204(8):1757-64
104. Cerovic V, Houston SA, Scott CL, Aumeunier A, Yrlid U, Mowat AM. et al. Intestinal CD103(-) dendritic cells migrate in lymph and prime effector T cells. Mucosal Immunol. 2013;6(1):104-13
105. Persson EK, Scott CL, Mowat AM, Agace WW. Dendritic cell subsets in the intestinal lamina propria: ontogeny and function. Eur J Immunol. 2013;43(12):3098-107
106. Fujimoto K, Karuppuchamy T, Takemura N, Shimohigoshi M, Machida T, Haseda Y. et al. A new subset of CD103+CD8alpha+ dendritic cells in the small intestine expresses TLR3, TLR7, and TLR9 and induces Th1 response and CTL activity. J Immunol. 2011;186(11):6287-95
107. Morhardt TL, Hayashi A, Ochi T, Quirós M, Kitamoto S, Nagao-Kitamoto H. et al. IL-10 produced by macrophages regulates epithelial integrity in the small intestine. Sci Rep. 2019;9(1):1223
108. Lee M, Lee Y, Song J, Lee J, Chang SY. Tissue-specific Role of CX. Immune Netw. 2018;18(1):e5
109. Mandrekar P, Catalano D, Dolganiuc A, Kodys K, Szabo G. Inhibition of myeloid dendritic cell accessory cell function and induction of T cell anergy by alcohol correlates with decreased IL-12 production. J Immunol. 2004;173(5):3398-407
110. Siggins RW, Bagby GJ, Molina P, Dufour J, Nelson S, Zhang P. Alcohol exposure impairs myeloid dendritic cell function in rhesus macaques. Alcohol Clin Exp Res. 2009;33(9):1524-31
111. Takatori H, Kanno Y, Watford WT, Tato CM, Weiss G, Ivanov II. et al. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J Exp Med. 2009;206(1):35-41
112. Longman RS, Diehl GE, Victorio DA, Huh JR, Galan C, Miraldi ER. et al. CX₃CR1⁺ mononuclear phagocytes support colitis-associated innate lymphoid cell production of IL-22. J Exp Med. 2014;211(8):1571-83
113. Goto Y, Obata T, Kunisawa J, Sato S, Ivanov II, Lamichhane A. et al. Innate lymphoid cells regulate intestinal epithelial cell glycosylation. Science. 2014;345(6202):1254009
114. McDermott AJ, Falkowski NR, McDonald RA, Pandit CR, Young VB, Huffnagle GB. Interleukin-23 (IL-23), independent of IL-17 and IL-22, drives neutrophil recruitment and innate inflammation during Clostridium difficile colitis in mice. Immunology. 2016;147(1):114-24
115. Moyat M, Bouzourene H, Ouyang W, Iovanna J, Renauld JC, Velin D. IL-22-induced antimicrobial peptides are key determinants of mucosal vaccine-induced protection against H. pylori in mice. Mucosal Immunol. 2017;10(1):271-81
116. Rendon JL, Li X, Akhtar S, Choudhry MA. Interleukin-22 modulates gut epithelial and immune barrier functions following acute alcohol exposure and burn injury. Shock. 2013;39(1):11-8
117. Hendrikx T, Duan Y, Wang Y, Oh JH, Alexander LM, Huang W. et al. Bacteria engineered to produce IL-22 in intestine induce expression of REG3G to reduce ethanol-induced liver disease in mice. Gut. 2019;68(8):1504-15
118. Grunwald U, Fan X, Jack RS, Workalemahu G, Kallies A, Stelter F. et al. Monocytes can phagocytose Gram-negative bacteria by a CD14-dependent mechanism. J Immunol. 1996;157(9):4119-25
119. Smith PD, Smythies LE, Mosteller-Barnum M, Sibley DA, Russell MW, Merger M. et al. Intestinal macrophages lack CD14 and CD89 and consequently are down-regulated for LPS- and IgA-mediated activities. J Immunol. 2001;167(5):2651-6
120. Kamada N, Hisamatsu T, Okamoto S, Sato T, Matsuoka K, Arai K. et al. Abnormally differentiated subsets of intestinal macrophage play a key role in Th1-dominant chronic colitis through excess production of IL-12 and IL-23 in response to bacteria. J Immunol. 2005;175(10):6900-8
121. Erkelens MN, Goverse G, Konijn T, Molenaar R, Beijer MR, Van den Bossche J. et al. Intestinal Macrophages Balance Inflammatory Expression Profiles via Vitamin A and Dectin-1-Mediated Signaling. Front Immunol. 2020;11:551
122. Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8(10):1086-94
123. Hao L, Zhong W, Sun X, Zhou Z. Small intestinal lamina propria immune cells dyshomeostasis is associated with alcoholic steatohepatitis in the mouse. The Journal of Immunology. 2019;202(1 Supplement):182.33-33
124. Brynskov J, Nielsen OH, Ahnfelt-Ronne I, Bendtzen K. Cytokines in inflammatory bowel disease. Scand J Gastroenterol. 1992;27(11):897-906
125. Kjerrulf M, Grdic D, Ekman L, Schon K, Vajdy M, Lycke NY. Interferon-gamma receptor-deficient mice exhibit impaired gut mucosal immune responses but intact oral tolerance. Immunology. 1997;92(1):60-8
126. Cantrell DA, Smith KA. The interleukin-2 T-cell system: a new cell growth model. Science. 1984;224(4655):1312-6
127. Romagnani S. Th1/Th2 Cells. Inflammatory Bowel Diseases. 1999;5(4):285-94
128. Mizoguchi A, Mizoguchi E, Bhan AK. The critical role of interleukin 4 but not interferon gamma in the pathogenesis of colitis in T-cell receptor alpha mutant mice. Gastroenterology. 1999;116(2):320-6
129. Iqbal N, Oliver JR, Wagner FH, Lazenby AS, Elson CO, Weaver CT. T helper 1 and T helper 2 cells are pathogenic in an antigen-specific model of colitis. J Exp Med. 2002;195(1):71-84
130. Paul WE, Seder RA. Lymphocyte responses and cytokines. Cell. 1994;76(2):241-51
131. Fiorentino DF, Zlotnik A, Vieira P, Mosmann TR, Howard M, Moore KW. et al. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991;146(10):3444-51
132. Okumura R, Takeda K. Roles of intestinal epithelial cells in the maintenance of gut homeostasis. Exp Mol Med. 2017;49(5):e338
133. Olleros ML, Martin ML, Vesin D, Fotio AL, Santiago-Raber ML, Rubbia-Brandt L. et al. Fat diet and alcohol-induced steatohepatitis after LPS challenge in mice: role of bioactive TNF and Th1 type cytokines. Cytokine. 2008;44(1):118-25
134. Waltenbaugh C, Vasquez K, Peterson JD. Alcohol consumption alters antigen-specific Th1 responses: mechanisms of deficit and repair. Alcohol Clin Exp Res. 1998;22(5 Suppl):220S-3S
135. Geissler M, Gesien A, Wands JR. Inhibitory effects of chronic ethanol consumption on cellular immune responses to hepatitis C virus core protein are reversed by genetic immunizations augmented with cytokine-expressing plasmids. J Immunol. 1997;159(10):5107-13
136. Lemmers A, Moreno C, Gustot T, Marechal R, Degre D, Demetter P. et al. The interleukin-17 pathway is involved in human alcoholic liver disease. Hepatology. 2009;49(2):646-57
137. Mandrekar P, Ambade A. Immunity and inflammatory signaling in alcoholic liver disease. Hepatol Int. 2014;8(Suppl 2):439-46
138. Bertola A, Mathews S, Ki SH, Wang H, Gao B. Mouse model of chronic and binge ethanol feeding (the NIAAA model). Nat Protoc. 2013;8(3):627-37
139. Chu S, Sun R, Gu X, Chen L, Liu M, Guo H. et al. Inhibition of sphingosine-1-phosphate-induced Th17 cells ameliorates alcoholic steatohepatitis in mice. Hepatology. 2020
140. Xu J, Ma HY, Liu X, Rosenthal S, Baglieri J, McCubbin R. et al. Blockade of IL-17 signaling reverses alcohol-induced liver injury and excessive alcohol drinking in mice. JCI Insight. 2020 5(3)
141. Lin M, Du L, Brandtzaeg P, Pan-Hammarström Q. IgA subclass switch recombination in human mucosal and systemic immune compartments. Mucosal Immunol. 2014;7(3):511-20
142. Gutzeit C, Magri G, Cerutti A. Intestinal IgA production and its role in host-microbe interaction. Immunol Rev. 2014;260(1):76-85
143. Moro-Sibilot L, Blanc P, Taillardet M, Bardel E, Couillault C, Boschetti G. et al. Mouse and Human Liver Contain Immunoglobulin A-Secreting Cells Originating From Peyer's Patches and Directed Against Intestinal Antigens. Gastroenterology. 2016;151(2):311-23
144. Inamine T, Yang AM, Wang L, Lee KC, Llorente C, Schnabl B. Genetic Loss of Immunoglobulin A Does Not Influence Development of Alcoholic Steatohepatitis in Mice. Alcohol Clin Exp Res. 2016;40(12):2604-13
145. Souza HS, Elia CC, Braulio VB, Cortes MQ, Furtado VC, Garrofe HC. et al. Effects of ethanol on gut-associated lymphoid tissues in a model of bacterial translocation: a possible role of apoptosis. Alcohol. 2003;30(3):183-91
146. Lopez MC. Chronic alcohol consumption regulates the expression of poly immunoglobulin receptor (pIgR) and secretory IgA in the gut. Toxicol Appl Pharmacol. 2017;333:84-91
147. Pelletier G, Briantais MJ, Buffet C, Pillot J, Etienne JP. Serum and intestinal secretory IgA in alcoholic cirrhosis of the liver. Gut. 1982;23(6):475-80
148. Maier A, Bode C, Fritz P, Bode JC. Effects of chronic alcohol abuse on duodenal mononuclear cells in man. Dig Dis Sci. 1999;44(4):691-6
149. Colombel JF, Vaerman JP, Mesnard B, Dehennin JP, Dive C, Rambaud JC. Jejunal immunoglobulin secretion in alcoholic patients with and without cirrhosis. J Hepatol. 1991;12(2):145-9
150. Riva A, Patel V, Kurioka A, Jeffery HC, Wright G, Tarff S. et al. Mucosa-associated invariant T cells link intestinal immunity with antibacterial immune defects in alcoholic liver disease. Gut. 2018;67(5):918-30
151. Valdes AM, Walter J, Segal E, Spector TD. Role of the gut microbiota in nutrition and health. BMJ. 2018;361:k2179
152. Bandeira A, Mota-Santos T, Itohara S, Degermann S, Heusser C, Tonegawa S. et al. Localization of gamma/delta T cells to the intestinal epithelium is independent of normal microbial colonization. J Exp Med. 1990;172(1):239-44
153. Crabbé PA, Bazin H, Eyssen H, Heremans JF. The normal microbial flora as a major stimulus for proliferation of plasma cells synthesizing IgA in the gut. The germ-free intestinal tract. Int Arch Allergy Appl Immunol. 1968;34(4):362-75
154. Hooper LV, Stappenbeck TS, Hong CV, Gordon JI. Angiogenins: a new class of microbicidal proteins involved in innate immunity. Nat Immunol. 2003;4(3):269-73
155. Ostman S, Rask C, Wold AE, Hultkrantz S, Telemo E. Impaired regulatory T cell function in germ-free mice. Eur J Immunol. 2006;36(9):2336-46
156. Pollard M, Sharon N. Responses of the Peyer's Patches in Germ-Free Mice to Antigenic Stimulation. Infect Immun. 1970;2(1):96-100
157. Chen P, Miyamoto Y, Mazagova M, Lee KC, Eckmann L, Schnabl B. Microbiota Protects Mice Against Acute Alcohol-Induced Liver Injury. Alcohol Clin Exp Res. 2015;39(12):2313-23
158. Mazagova M, Wang L, Anfora AT, Wissmueller M, Lesley SA, Miyamoto Y. et al. Commensal microbiota is hepatoprotective and prevents liver fibrosis in mice. FASEB J. 2015;29(3):1043-55
159. D'Costa VM, McGrann KM, Hughes DW, Wright GD. Sampling the antibiotic resistome. Science. 2006;311(5759):374-7
160. Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27(2):104-19
161. Lewis K, Lutgendorff F, Phan V, Söderholm JD, Sherman PM, McKay DM. Enhanced translocation of bacteria across metabolically stressed epithelia is reduced by butyrate. Inflamm Bowel Dis. 2010;16(7):1138-48
162. Säemann MD, Parolini O, Böhmig GA, Kelemen P, Krieger PM, Neumüller J. et al. Bacterial metabolite interference with maturation of human monocyte-derived dendritic cells. J Leukoc Biol. 2002;71(2):238-46
163. Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D. et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461(7268):1282-6
164. Chen P, Torralba M, Tan J, Embree M, Zengler K, Starkel P. et al. Supplementation of saturated long-chain fatty acids maintains intestinal eubiosis and reduces ethanol-induced liver injury in mice. Gastroenterology. 2015;148(1):203-14 e16
165. Chewning JH, Weaver CT. Development and survival of Th17 cells within the intestines: the influence of microbiome- and diet-derived signals. J Immunol. 2014;193(10):4769-77
166. Ivanov II, Frutos ReL, Manel N, Yoshinaga K, Rifkin DB, Sartor RB. et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4(4):337-49
167. Pickard JM, Maurice CF, Kinnebrew MA, Abt MC, Schenten D, Golovkina TV. et al. Rapid fucosylation of intestinal epithelium sustains host-commensal symbiosis in sickness. Nature. 2014;514(7524):638-41
168. Bene K, Varga Z, Petrov VO, Boyko N, Rajnavolgyi E. Gut Microbiota Species Can Provoke both Inflammatory and Tolerogenic Immune Responses in Human Dendritic Cells Mediated by Retinoic Acid Receptor Alpha Ligation. Front Immunol. 2017;8:427
169. Collins CB, Aherne CM, Kominsky D, McNamee EN, Lebsack MD, Eltzschig H. et al. Retinoic acid attenuates ileitis by restoring the balance between T-helper 17 and T regulatory cells. Gastroenterology. 2011;141(5):1821-31
170. Bakdash G, Vogelpoel LT, van Capel TM, Kapsenberg ML, de Jong EC. Retinoic acid primes human dendritic cells to induce gut-homing, IL-10-producing regulatory T cells. Mucosal Immunol. 2015;8(2):265-78
171. Hurst RJ, Else KJ. The retinoic acid-producing capacity of gut dendritic cells and macrophages is reduced during persistent T. muris infection. Parasite Immunol. 2013;35(7-8):229-33
172. Grizotte-Lake M, Zhong G, Duncan K, Kirkwood J, Iyer N, Smolenski I. et al. Commensals Suppress Intestinal Epithelial Cell Retinoic Acid Synthesis to Regulate Interleukin-22 Activity and Prevent Microbial Dysbiosis. Immunity. 2018;49(6):1103-15 e6
173. Xu YL, Ding CL, Qian CL, Qi ZT, Wang W. Retinoid acid induced 16 deficiency aggravates colitis and colitis-associated tumorigenesis in mice. Cell Death Dis. 2019;10(12):958
174. Mobarhan S, Seitz HK, Russell RM, Mehta R, Hupert J, Friedman H. et al. Age-related effects of chronic ethanol intake on vitamin A status in Fisher 344 rats. J Nutr. 1991;121(4):510-7
175. Clugston RD, Blaner WS. The adverse effects of alcohol on vitamin A metabolism. Nutrients. 2012;4(5):356-71
176. Ravindran R, Loebbermann J, Nakaya HI, Khan N, Ma H, Gama L. et al. The amino acid sensor GCN2 controls gut inflammation by inhibiting inflammasome activation. Nature. 2016;531(7595):523-7
177. Goto Y, Panea C, Nakato G, Cebula A, Lee C, Diez MG. et al. Segmented filamentous bacteria antigens presented by intestinal dendritic cells drive mucosal Th17 cell differentiation. Immunity. 2014;40(4):594-607
178. Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C. et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183(6):2593-603
179. Li X, Qi Y, Ma X, Huang F, Guo H, Jiang X. et al. Chemokine (C-C motif) ligand 20, a potential biomarker for Graves' disease, is regulated by osteopontin. PLoS One. 2013;8(5):e64277
180. Cheng H, Guan X, Chen D, Ma W. The Th17/Treg Cell Balance: A Gut Microbiota-Modulated Story. Microorganisms. 2019 7(12)
181. Narushima S, Sugiura Y, Oshima K, Atarashi K, Hattori M, Suematsu M. et al. Characterization of the 17 strains of regulatory T cell-inducing human-derived Clostridia. Gut Microbes. 2014;5(3):333-9
182. Tanabe S. The effect of probiotics and gut microbiota on Th17 cells. Int Rev Immunol. 2013;32(5-6):511-25
183. Tan TG, Sefik E, Geva-Zatorsky N, Kua L, Naskar D, Teng F. et al. Identifying species of symbiont bacteria from the human gut that, alone, can induce intestinal Th17 cells in mice. Proc Natl Acad Sci U S A. 2016;113(50):E8141-E50
184. Lin L, Zhang J. Role of intestinal microbiota and metabolites on gut homeostasis and human diseases. BMC Immunol. 2017;18(1):2
185. Bandyopadhaya A, Kesarwani M, Que YA, He J, Padfield K, Tompkins R. et al. The quorum sensing volatile molecule 2-amino acetophenon modulates host immune responses in a manner that promotes life with unwanted guests. PLoS Pathog. 2012;8(11):e1003024
186. Rumbaugh KP, Kaufmann GF. Exploitation of host signaling pathways by microbial quorum sensing signals. Curr Opin Microbiol. 2012;15(2):162-8
187. Dubinkina VB, Tyakht AV, Odintsova VY, Yarygin KS, Kovarsky BA, Pavlenko AV. et al. Links of gut microbiota composition with alcohol dependence syndrome and alcoholic liver disease. Microbiome. 2017;5(1):141
188. Yan AW, Schnabl B. Bacterial translocation and changes in the intestinal microbiome associated with alcoholic liver disease. World J Hepatol. 2012;4(4):110-8
189. Tuomisto S, Pessi T, Collin P, Vuento R, Aittoniemi J, Karhunen PJ. Changes in gut bacterial populations and their translocation into liver and ascites in alcoholic liver cirrhotics. BMC Gastroenterol. 2014;14:40
190. Gustot T, Lemmers A, Moreno C, Nagy N, Quertinmont E, Nicaise C. et al. Differential liver sensitization to toll-like receptor pathways in mice with alcoholic fatty liver. Hepatology. 2006;43(5):989-1000
191. Stadlbauer V, Mookerjee RP, Wright GA, Davies NA, Jürgens G, Hallström S. et al. Role of Toll-like receptors 2, 4, and 9 in mediating neutrophil dysfunction in alcoholic hepatitis. Am J Physiol Gastrointest Liver Physiol. 2009;296(1):G15-22
192. Yang AM, Inamine T, Hochrath K, Chen P, Wang L, Llorente C. et al. Intestinal fungi contribute to development of alcoholic liver disease. J Clin Invest. 2017;127(7):2829-41
193. Taylor PR, Tsoni SV, Willment JA, Dennehy KM, Rosas M, Findon H. et al. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat Immunol. 2007;8(1):31-8
194. Saijo S, Ikeda S, Yamabe K, Kakuta S, Ishigame H, Akitsu A. et al. Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity. 2010;32(5):681-91
195. Chu H, Duan Y, Lang S, Jiang L, Wang Y, Llorente C. et al. The Candida albicans exotoxin candidalysin promotes alcohol-associated liver disease. J Hepatol. 2020;72(3):391-400
196. Eyerich K, Foerster S, Rombold S, Seidl HP, Behrendt H, Hofmann H. et al. Patients with chronic mucocutaneous candidiasis exhibit reduced production of Th17-associated cytokines IL-17 and IL-22. J Invest Dermatol. 2008;128(11):2640-5
197. Lang S, Duan Y, Liu J, Torralba MG, Kuelbs C, Ventura-Cots M. et al. Intestinal Fungal Dysbiosis and Systemic Immune Response to Fungi in Patients With Alcoholic Hepatitis. Hepatology. 2020;71(2):522-38
198. Diehl GE, Longman RS, Zhang JX, Breart B, Galan C, Cuesta A. et al. Microbiota restricts trafficking of bacteria to mesenteric lymph nodes by CX(3)CR1(hi) cells. Nature. 2013;494(7435):116-20
199. Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004;303(5664):1662-5
200. Tamburini BAJ, Finlon JM, Gillen AE, Kriss MS, Riemondy KA, Fu R. et al. Chronic Liver Disease in Humans Causes Expansion and Differentiation of Liver Lymphatic Endothelial Cells. Front Immunol. 2019;10:1036
201. Wahlström A. Outside the liver box: The gut microbiota as pivotal modulator of liver diseases. Biochim Biophys Acta Mol Basis Dis. 2019;1865(5):912-9
202. Duan Y, Llorente C, Lang S, Brandl K, Chu H, Jiang L. et al. Bacteriophage targeting of gut bacterium attenuates alcoholic liver disease. Nature. 2019;575(7783):505-11
203. Llorente C, Jepsen P, Inamine T, Wang L, Bluemel S, Wang HJ. et al. Gastric acid suppression promotes alcoholic liver disease by inducing overgrowth of intestinal Enterococcus. Nat Commun. 2017;8(1):837
204. Fukui H. Role of Gut Dysbiosis in Liver Diseases: What Have We Learned So Far? Diseases. 2019 7(4)
205. Uesugi T, Froh M, Arteel GE, Bradford BU, Thurman RG. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology. 2001;34(1):101-8
206. Holt AP, Salmon M, Buckley CD, Adams DH. Immune interactions in hepatic fibrosis. Clin Liver Dis. 2008;12(4):861-82 x
207. Lee KC, Chen P, Maricic I, Inamine T, Hu J, Gong S. et al. Intestinal iNKT cells migrate to liver and contribute to hepatocyte apoptosis during alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2019;316(5):G585-G97
208. Hang S, Paik D, Yao L, Kim E, Trinath J, Lu J. et al. Bile acid metabolites control TH17 and Treg cell differentiation. Nature. 2019;576(7785):143-8
209. Wu W, Zhu B, Peng X, Zhou M, Jia D, Gu J. Activation of farnesoid X receptor attenuates hepatic injury in a murine model of alcoholic liver disease. Biochem Biophys Res Commun. 2014;443(1):68-73
210. Manley S, Ding W. Role of farnesoid X receptor and bile acids in alcoholic liver disease. Acta Pharm Sin B. 2015;5(2):158-67
211. Kong B, Zhang M, Huang M, Rizzolo D, Armstrong LE, Schumacher JD. et al. FXR deficiency alters bile acid pool composition and exacerbates chronic alcohol induced liver injury. Dig Liver Dis. 2019;51(4):570-6
212. Singal AK, Shah VH. Current trials and novel therapeutic targets for alcoholic hepatitis. J Hepatol. 2019;70(2):305-13
213. Stoy S, Sandahl TD, Dige AK, Agnholt J, Rasmussen TK, Gronbaek H. et al. Highest frequencies of interleukin-22-producing T helper cells in alcoholic hepatitis patients with a favourable short-term course. PLoS One. 2013;8(1):e55101
214. Tang KY, Lickliter J, Huang ZH, Xian ZS, Chen HY, Huang C. et al. Safety, pharmacokinetics, and biomarkers of F-652, a recombinant human interleukin-22 dimer, in healthy subjects. Cell Mol Immunol. 2019;16(5):473-82
215. Gao B, Xiang X. Interleukin-22 from bench to bedside: a promising drug for epithelial repair. Cell Mol Immunol. 2019;16(7):666-7
216. Arab JP, Sehrawat TS, Simonetto DA, Verma VK, Feng D, Tang T. et al. An Open-Label, Dose-Escalation Study to Assess the Safety and Efficacy of IL-22 Agonist F-652 in Patients With Alcohol-associated Hepatitis. Hepatology. 2020;72(2):441-53
217. Ge X, Leung TM, Arriazu E, Lu Y, Urtasun R, Christensen B. et al. Osteopontin binding to lipopolysaccharide lowers tumor necrosis factor-α and prevents early alcohol-induced liver injury in mice. Hepatology. 2014;59(4):1600-16
218. Ge X, Leung TM, Arriazu E, Lu Y, Urtasun R, Christensen B. et al. Osteopontin binding to lipopolysaccharide lowers tumor necrosis factor-alpha and prevents early alcohol-induced liver injury in mice. Hepatology. 2014;59(4):1600-16
219. Banerjee A, Apte UM, Smith R, Ramaiah SK. Higher neutrophil infiltration mediated by osteopontin is a likely contributing factor to the increased susceptibility of females to alcoholic liver disease. J Pathol. 2006;208(4):473-85
220. Boetticher NC, Peine CJ, Kwo P, Abrams GA, Patel T, Aqel B. et al. A randomized, double-blinded, placebo-controlled multicenter trial of etanercept in the treatment of alcoholic hepatitis. Gastroenterology. 2008;135(6):1953-60
221. Naveau S, Chollet-Martin S, Dharancy S, Mathurin P, Jouet P, Piquet MA. et al. A double-blind randomized controlled trial of infliximab associated with prednisolone in acute alcoholic hepatitis. Hepatology. 2004;39(5):1390-7
222. Orgun NN, Mathis MA, Wilson CB, Way SS. Deviation from a strong Th1-dominated to a modest Th17-dominated CD4 T cell response in the absence of IL-12p40 and type I IFNs sustains protective CD8 T cells. J Immunol. 2008;180(6):4109-15
223. Wozniak TM, Ryan AA, Britton WJ. Interleukin-23 restores immunity to Mycobacterium tuberculosis infection in IL-12p40-deficient mice and is not required for the development of IL-17-secreting T cell responses. J Immunol. 2006;177(12):8684-92
224. Bai H, Cheng J, Gao X, Joyee AG, Fan Y, Wang S. et al. IL-17/Th17 promotes type 1 T cell immunity against pulmonary intracellular bacterial infection through modulating dendritic cell function. J Immunol. 2009;183(9):5886-95
225. Schulz SM, Kohler G, Holscher C, Iwakura Y, Alber G. IL-17A is produced by Th17, gammadelta T cells and other CD4- lymphocytes during infection with Salmonella enterica serovar Enteritidis and has a mild effect in bacterial clearance. Int Immunol. 2008;20(9):1129-38
226. Wu Q, Martin RJ, Rino JG, Breed R, Torres RM, Chu HW. IL-23-dependent IL-17 production is essential in neutrophil recruitment and activity in mouse lung defense against respiratory Mycoplasma pneumoniae infection. Microbes Infect. 2007;9(1):78-86
227. Bacher P, Hohnstein T, Beerbaum E, Rocker M, Blango MG, Kaufmann S. et al. Human Anti-fungal Th17 Immunity and Pathology Rely on Cross-Reactivity against Candida albicans. Cell. 2019;176(6):1340-55 e15
228. Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008;9(6):641-9
229. Omenetti S, Pizarro TT. The Treg/Th17 Axis: A Dynamic Balance Regulated by the Gut Microbiome. Front Immunol. 2015;6:639
230. Liang J, Zhang B, Shen RW, Liu JB, Gao MH, Geng X. et al. The effect of antifibrotic drug halofugine on Th17 cells in concanavalin A-induced liver fibrosis. Scand J Immunol. 2014;79(3):163-72
231. Fieldhouse KA, Ukaibe S, Crowley EL, Khanna R, O'Toole A, Gooderham MJ. Inflammatory bowel disease in patients with psoriasis treated with interleukin-17 inhibitors. Drugs Context. 2020 9
232. Smith MK, Pai J, Panaccione R, Beck P, Ferraz JG, Jijon H. Crohn's-like disease in a patient exposed to anti-Interleukin-17 blockade (Ixekizumab) for the treatment of chronic plaque psoriasis: a case report. BMC Gastroenterol. 2019;19(1):162
233. Wang J, Bhatia A, Krugliak Cleveland N, Gupta N, Dalal S, Rubin DT. et al. Rapid Onset of Inflammatory Bowel Disease after Receiving Secukinumab Infusion. ACG Case Rep J. 2018;5:e56
234. Chen RC, Xu LM, Du SJ, Huang SS, Wu H, Dong JJ. et al. Lactobacillus rhamnosus GG supernatant promotes intestinal barrier function, balances Treg and TH17 cells and ameliorates hepatic injury in a mouse model of chronic-binge alcohol feeding. Toxicol Lett. 2016;241:103-10
235. Bull-Otterson L, Feng W, Kirpich I, Wang Y, Qin X, Liu Y. et al. Metagenomic analyses of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of Lactobacillus rhamnosus GG treatment. PLoS One. 2013;8(1):e53028
236. Nakamura Y, Nosaka S, Suzuki M, Nagafuchi S, Takahashi T, Yajima T. et al. Dietary fructooligosaccharides up-regulate immunoglobulin A response and polymeric immunoglobulin receptor expression in intestines of infant mice. Clin Exp Immunol. 2004;137(1):52-8
237. Hosono A, Ozawa A, Kato R, Ohnishi Y, Nakanishi Y, Kimura T. et al. Dietary fructooligosaccharides induce immunoregulation of intestinal IgA secretion by murine Peyer's patch cells. Biosci Biotechnol Biochem. 2003;67(4):758-64
238. Lindsay JO, Whelan K, Stagg AJ, Gobin P, Al-Hassi HO, Rayment N. et al. Clinical, microbiological, and immunological effects of fructo-oligosaccharide in patients with Crohn's disease. Gut. 2006;55(3):348-55
239. Ferrere G, Wrzosek L, Cailleux F, Turpin W, Puchois V, Spatz M. et al. Fecal microbiota manipulation prevents dysbiosis and alcohol-induced liver injury in mice. J Hepatol. 2017;66(4):806-15
240. Nanji AA, Khettry U, Sadrzadeh SM. Lactobacillus feeding reduces endotoxemia and severity of experimental alcoholic liver (disease). Proc Soc Exp Biol Med. 1994;205(3):243-7
241. Grander C, Adolph TE, Wieser V, Lowe P, Wrzosek L, Gyongyosi B. et al. Recovery of ethanol-induced Akkermansia muciniphila depletion ameliorates alcoholic liver disease. Gut. 2018;67(5):891-901
242. Han SH, Suk KT, Kim DJ, Kim MY, Baik SK, Kim YD. et al. Effects of probiotics (cultured Lactobacillus subtilis/Streptococcus faecium) in the treatment of alcoholic hepatitis: randomized-controlled multicenter study. Eur J Gastroenterol Hepatol. 2015;27(11):1300-6
243. Kirpich IA, Solovieva NV, Leikhter SN, Shidakova NA, Lebedeva OV, Sidorov PI. et al. Probiotics restore bowel flora and improve liver enzymes in human alcohol-induced liver injury: a pilot study. Alcohol. 2008;42(8):675-82
244. Stadlbauer V, Mookerjee RP, Hodges S, Wright GA, Davies NA, Jalan R. Effect of probiotic treatment on deranged neutrophil function and cytokine responses in patients with compensated alcoholic cirrhosis. J Hepatol. 2008;48(6):945-51
245. Hong M, Kim SW, Han SH, Kim DJ, Suk KT, Kim YS. et al. Probiotics (Lactobacillus rhamnosus R0011 and acidophilus R0052) reduce the expression of toll-like receptor 4 in mice with alcoholic liver disease. PLoS One. 2015;10(2):e0117451
246. Philips CA, Pande A, Shasthry SM, Jamwal KD, Khillan V, Chandel SS. et al. Healthy Donor Fecal Microbiota Transplantation in Steroid-Ineligible Severe Alcoholic Hepatitis: A Pilot Study. Clin Gastroenterol Hepatol. 2017;15(4):600-2
247. Philips CA, Phadke N, Ganesan K, Ranade S, Augustine P. Corticosteroids, nutrition, pentoxifylline, or fecal microbiota transplantation for severe alcoholic hepatitis. Indian J Gastroenterol. 2018;37(3):215-25
248. Bajaj JS, Gavis EA, Fagan A, Wade JB, Thacker LR, Fuchs M. et al. A Randomized Clinical Trial of Fecal Microbiota Transplant for Alcohol Use Disorder. Hepatology. 2020
249. Ekmekciu I, von Klitzing E, Fiebiger U, Escher U, Neumann C, Bacher P. et al. Immune Responses to Broad-Spectrum Antibiotic Treatment and Fecal Microbiota Transplantation in Mice. Front Immunol. 2017;8:397
250. Adachi Y, Moore LE, Bradford BU, Gao W, Thurman RG. Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology. 1995;108(1):218-24
251. Bode C, Schafer C, Fukui H, Bode JC. Effect of treatment with paromomycin on endotoxemia in patients with alcoholic liver disease - a double-blind, placebo-controlled trial. Alcohol Clin Exp Res. 1997;21(8):1367-73
252. Becattini S, Taur Y, Pamer EG. Antibiotic-Induced Changes in the Intestinal Microbiota and Disease. Trends Mol Med. 2016;22(6):458-78
253. Metsala J, Lundqvist A, Virta LJ, Kaila M, Gissler M, Virtanen SM. Mother's and offspring's use of antibiotics and infant allergy to cow's milk. Epidemiology. 2013;24(2):303-9
254. Savage JH, Matsui EC, Wood RA, Keet CA. Urinary levels of triclosan and parabens are associated with aeroallergen and food sensitization. J Allergy Clin Immunol. 2012;130(2):453-60 e7
255. Willing BP, Russell SL, Finlay BB. Shifting the balance: antibiotic effects on host-microbiota mutualism. Nat Rev Microbiol. 2011;9(4):233-43
256. Bajaj JS, Heuman DM, Sanyal AJ, Hylemon PB, Sterling RK, Stravitz RT. et al. Modulation of the metabiome by rifaximin in patients with cirrhosis and minimal hepatic encephalopathy. PLoS One. 2013;8(4):e60042
257. Samonis G, Anastassiadou H, Dassiou M, Tselentis Y, Bodey GP. Effects of broad-spectrum antibiotics on colonization of gastrointestinal tracts of mice by Candida albicans. Antimicrob Agents Chemother. 1994;38(3):602-3
258. Vlaspolder F, de Zeeuw G, Rozenberg-Arska M, Egyedi P, Verhoef J. The influence of flucloxacillin and amoxicillin with clavulanic acid on the aerobic flora of the alimentary tract. Infection. 1987;15(4):241-4
259. Van Tyne D, Martin MJ, Gilmore MS. Structure, function, and biology of the Enterococcus faecalis cytolysin. Toxins (Basel). 2013;5(5):895-911
260. Xu W, Lu C, Yao L, Zhang F, Shao J, Zheng S. Dihydroartemisinin protects against alcoholic liver injury through alleviating hepatocyte steatosis in a farnesoid X receptor-dependent manner. Toxicol Appl Pharmacol. 2017;315:23-34
261. Hartmann P, Hochrath K, Horvath A, Chen P, Seebauer CT, Llorente C. et al. Modulation of the intestinal bile acid/farnesoid X receptor/fibroblast growth factor 15 axis improves alcoholic liver disease in mice. Hepatology. 2018;67(6):2150-66
262. Vavassori P, Mencarelli A, Renga B, Distrutti E, Fiorucci S. The bile acid receptor FXR is a modulator of intestinal innate immunity. J Immunol. 2009;183(10):6251-61
263. Massafra V, Ijssennagger N, Plantinga M, Milona A, Ramos Pittol JM, Boes M. et al. Splenic dendritic cell involvement in FXR-mediated amelioration of DSS colitis. Biochim Biophys Acta. 2016;1862(2):166-73
264. Cresci GA, Bush K, Nagy LE. Tributyrin supplementation protects mice from acute ethanol-induced gut injury. Alcohol Clin Exp Res. 2014;38(6):1489-501
265. Cresci GA, Glueck B, McMullen MR, Xin W, Allende D, Nagy LE. Prophylactic tributyrin treatment mitigates chronic-binge ethanol-induced intestinal barrier and liver injury. J Gastroenterol Hepatol. 2017;32(9):1587-97
266. Zhong W, Li Q, Xie G, Sun X, Tan X, Jia W. et al. Dietary fat sources differentially modulate intestinal barrier and hepatic inflammation in alcohol-induced liver injury in rats. Am J Physiol Gastrointest Liver Physiol. 2013;305(12):G919-32
267. Kirpich IA, Feng W, Wang Y, Liu Y, Barker DF, Barve SS. et al. The type of dietary fat modulates intestinal tight junction integrity, gut permeability, and hepatic toll-like receptor expression in a mouse model of alcoholic liver disease. Alcohol Clin Exp Res. 2012;36(5):835-46
268. Bhaskaran N, Quigley C, Paw C, Butala S, Schneider E, Pandiyan P. Role of Short Chain Fatty Acids in Controlling Tregs and Immunopathology During Mucosal Infection. Front Microbiol. 2018;9:1995
269. Jain A, Song R, Wakeland EK, Pasare C. T cell-intrinsic IL-1R signaling licenses effector cytokine production by memory CD4 T cells. Nat Commun. 2018;9(1):3185
270. Harrison SA, Wong VW, Okanoue T, Bzowej N, Vuppalanchi R, Younes Z. et al. Selonsertib for patients with bridging fibrosis or compensated cirrhosis due to NASH: Results from randomized phase III STELLAR trials. J Hepatol. 2020;73(1):26-39
271. Lee SI, Kang KS. N-acetylcysteine modulates lipopolysaccharide-induced intestinal dysfunction. Sci Rep. 2019;9(1):1004
272. Sadowska AM. N-Acetylcysteine mucolysis in the management of chronic obstructive pulmonary disease. Ther Adv Respir Dis. 2012;6(3):127-35
Author contact
![]() Corresponding author: Cristina Llorente, Ph.D., Department of Medicine, University of California San Diego, MC0063, 9500 Gilman Drive, La Jolla, CA 92093, Phone 858-366-3173, Fax 858-822-5370, Email allorenteizquierdoucsd.edu.
Corresponding author: Cristina Llorente, Ph.D., Department of Medicine, University of California San Diego, MC0063, 9500 Gilman Drive, La Jolla, CA 92093, Phone 858-366-3173, Fax 858-822-5370, Email allorenteizquierdoucsd.edu.

 Global reach, higher impact
Global reach, higher impact