10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2021; 17(2):460-474. doi:10.7150/ijbs.53050 This issue Cite
Research Paper
17β-Estradiol promotes LC3B-associated phagocytosis in trained immunity of female mice against sepsis
1. The State Key Laboratory of Pharmaceutical Biotechnology, Division of Immunology, Medical School, Nanjing University, Nanjing, China.
2. State Key Laboratory of Trauma, Burns and Combined Injury, Department of Wound Infection and Drug, Daping Hospital, Army Medical University, Chongqing, China.
3. Jiangsu Key Laboratory of Molecular Medicine, Division of Immunology, Medical School, Nanjing University, Nanjing, China.
Abstract
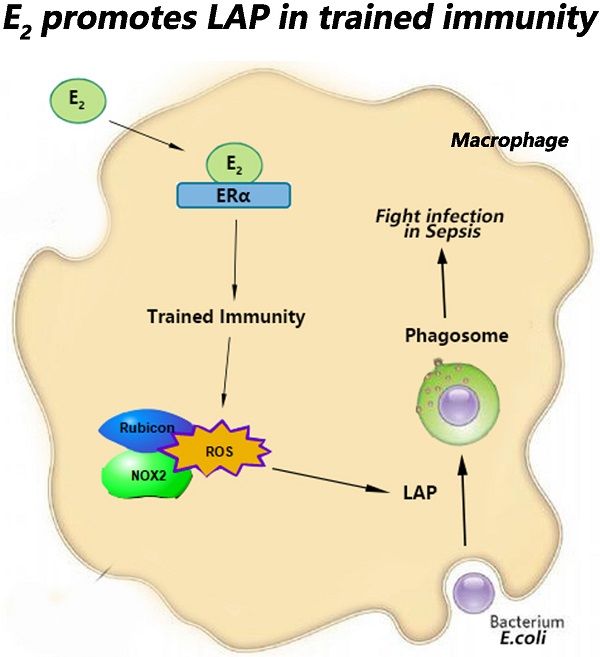
Sepsis is a common serious clinical infectious disease accompanied by more severe injuries and higher mortality rates in men than women. The much higher level of 17β-estradiol (E2) in female is one of the significant reasons for better sepsis resistance ability. Trained immunity is a novel way to fight against infection by improving innate immunity. However, whether β-glucan-induced trained immunity can promote macrophage phagocytosis to clear infections in early sepsis has not been clarified. And whether E2 involved in this process needs further investigation. Symptoms among male, female and ovariectomized (OVX) C57BL/6 mice in early sepsis were detected. The effect of trained immunity on macrophage LC3B-associated phagocytosis (LAP) and the mechanism of E2 functioned in this process have also been explored. We demonstrated compared with male mice, female has significantly more mild symptoms and more reactive oxygen species (ROS) production and stronger NADPH oxidase 2 (NOX2) expression in the macrophage of major organs. In contrary, these characteristics are disappeared in OVX mice. Furthermore, in macrophage cell lines and primary bone marrow- derived macrophages (BMDMs), β-glucan-induced trained immunity can increase ROS production by activating NOX2 to promote macrophage LAP. E2 can up-regulate RUBICON through estrogen receptor α (ERα) to further facilitate macrophage LAP. These results indicated that trained immunity can improve sepsis resistance ability by stimulating macrophage LAP. E2 can boost ROS production and RUBICON expression to further promote macrophage LAP, which can provide a new perspective to recognize the mechanism of trained immunity in gender differences when responding to sepsis.
Keywords: estradiol, trained immunity, sepsis, LAP, RUBICON, ROS

 Global reach, higher impact
Global reach, higher impact