10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2021; 17(4):926-941. doi:10.7150/ijbs.57445 This issue Cite
Research Paper
Macrophage contributes to radiation-induced anti-tumor abscopal effect on transplanted breast cancer by HMGB1/TNF-α signaling factors
1. Institute of Radiation Medicine, Shanghai Medical College, Fudan University, Shanghai 200032, China.
2. State Key Laboratory of Radiation Medicine and Protection, School of Radiation Medicine and Protection, Medical College of Soochow University, Suzhou 215123, China.
3. Department of Radiation Oncology, Shanghai Pulmonary Hospital, School of Medicine, Tongji University, Shanghai 200433, China.
* These authors contributed equally to this work.
Abstract
Objectives: The roles of innate immunity including macrophages in radiation-induced abscopal effect (RIAE) are ambiguous. In this study, we evaluated the role of macrophage in RIAE and the interaction of cytokines in tumor microenvironment after irradiation.
Materials and Methods: Transplanted tumor of breast cancer cells in BalB/C mice, severe combined immunodeficiency (SCID) mice and non-obese diabetic (NOD)-SCID mice were irradiated with fractionation doses to observe anti-tumor abscopal effect. The underlying mechanism of RIAE was investigated by treating the mice with TNF-α inhibitor or macrophage depletion drug and analyzing the alteration of macrophage distribution in tumors. A co-culture system of breast cancer cells and macrophages was applied to disclose the signaling factors and related pathways involved in the RIAE.
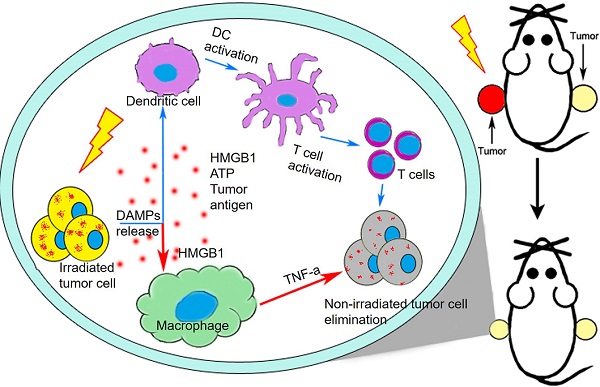
Results: The growth of nonirradiated tumor was effectively suppressed in mice with normal or infused macrophages but not in mice with insufficiency/depletion of macrophage or TNF-α inhibition, where M1-macrophage was mainly involved. Investigation of the bystander signaling factors in vitro demonstrated that HMGB1 released from irradiated breast cancer cells promoted bystander macrophages to secret TNF-α through TLR-4 pathway and further inhibited the proliferation and migration of non-irradiated cancer cells by PI3K-p110γ suppression.
Conclusions: HMGB1 and TNF-α contributes to M1-macrophages facilitated systemic anti-tumor abscopal response triggered by radiotherapy in breast cancer, indicating that the combination of immunotherapy and radiotherapy may has important implication in enhancing the efficiency of tumor treatment.
Keywords: Radiation-induced abscopal effect, Breast cancer cells, Macrophages, HMGB1, TNF-α.

 Global reach, higher impact
Global reach, higher impact