ISSN: 1449-2288
Int J Biol Sci 2021; 17(8):1940-1952. doi:10.7150/ijbs.58062 This issue Cite
Research Paper
MicroRNA-135b/CAMK2D Axis Contribute to Malignant Progression of Gastric Cancer through EMT Process Remodeling
1. Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education), Division of Gastrointestinal Cancer Translational Research Laboratory, Peking University Cancer Hospital, Fu-Cheng Road, Beijing, 100142, China.
2. Department of Gastrointestinal Surgery, Peking University Cancer Hospital, Beijing, Fu-Cheng Road, Beijing, 100142, China.
3. Center of Minimally Invasive Gastrointestinal Surgery, Shanxi Cancer Hospital, Zhigong New Street, Taiyuan, Shanxi, China.
4. Department of Orthopedics, The First Affiliated Hospital of Shenzhen University, Shenzhen Second People's Hospital, Shenzhen, 518000, China.
Abstract
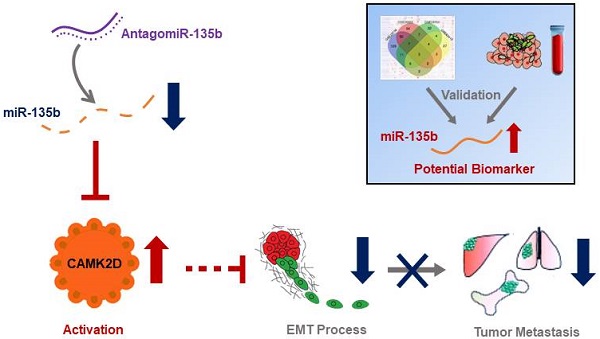
There is a continued need for investigating the roles of microRNAs (miRNAs) and their targets on the progression of gastric cancer (GC), especially metastasis. Here, we performed an integrated study to identify dysregulated miRNAs critical for GC development and progression. miR-135b was determined as a promising biomarker for GC. The expression level of miR-135b was increased among GC cell lines, patient tumor tissues, serum samples, and correlation with aggravation of the GC patients. The in vitro functional assays demonstrated overexpression of miR-135b promoted cell proliferation, migration and invasion in GC, while miR-135b inhibition led to the opposite results. CAMK2D was found to be the direct target of miR-135b, serving as a tumor suppressor in GC cells. Based on our and public datasets, we confirmed the attenuation of CAMK2D expression in GC tissues. And, the expression levels of miR-135b and CAMK2D were closely associated with prognosis of GC patients. Ectopic expression of miR-135b resulted in the down-regulation of CAMK2D. Additionally, CAMK2D was a prerequisite for miR-135b to promote GC cells proliferation and migration by regulating the EMT process, which was confirmed by the in vivo experiments. Importantly, in vivo injection of miR-135b antagomir significantly repressed the tumor growth and metastasis of xenograft models, which suggested that the miR-135b antagomir were promising for clinical applications. Taken together, these results indicate that miR-135b/CAMK2D axis drives GC progression by EMT process remodeling, suggesting that miR-135b may be utilized as a new therapeutic target and prognostic marker for GC patients.
Keywords: MicroRNA-135b, CAMK2D, EMT, Oligonucleotide therapy, Gastric cancer.

 Global reach, higher impact
Global reach, higher impact