Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2021; 17(13):3428-3440. doi:10.7150/ijbs.62728 This issue Cite
Review
NEAT1 as a competing endogenous RNA in tumorigenesis of various cancers: Role, mechanism and therapeutic potential
1. Department of Nuclear Medicine, The First Affiliated Hospital of Shandong First Medical University & Shandong Provincial Qianfoshan Hospital, Jinan 250014, China.
2. Biomedical Sciences College & Shandong Medicinal Biotechnology Centre, Shandong First Medical University & Shandong Academy of Medical Sciences, Jinan 250062, China.
#These authors contributed equally to this work.
Received 2021-5-13; Accepted 2021-7-24; Published 2021-8-3
Abstract
The nuclear paraspeckle assembly transcript 1 (NEAT1) is a long non-coding RNA (lncRNA) that is upregulated in a variety of human cancer types. Increasing evidence has shown that the elevation of NEAT1 in cancer cells promotes cell growth, migration, and invasion and inhibits cell apoptosis. It is also known that lncRNAs act as a competing endogenous RNA (ceRNA) by sponging microRNAs (miRNAs) to alter the expression levels of their target genes in the development of cancers. Therefore, it is important to understand the molecular mechanisms underlying this observation. In this review, specific emphasis was placed on NEAT1's role in tumor development. We also summarize and discuss the feedback roles of NEAT1/miRNA/target network in the progression of various cancers. As our understanding of the role of NEAT1 during tumorigenesis improves, its therapeutic potential as a biomarker and/or target for cancer also becomes clearer.
Keywords: NEAT1, competing endogenous RNA, long non-coding RNA, microRNA, cancer, therapeutic target
Introduction
Nuclear paraspeckle assembly transcript 1 (NEAT1) is a long non-coding RNA (lncRNA) located in nuclear paraspeckles. It functions as a frame for paraspeckle formation by associating with the paraspeckle proteins, paraspeckle component 1 (PSPC1), splicing factor proline/glutamine rich (SFPQ), and 54 kDa nuclear RNA- and DNA-binding protein (p54nrb) [1,2]. Since the discovery of NEAT1 in 2007, many of its biological functions have been reported, including regulation of cell differentiation [3,4], immune response [5], and organ development [6,7]; NEAT1 also participates in the progression of a variety of disorders, such as cancer [8,9], metabolic diseases [10,11], and immunological diseases [12]. In addition, our previous studies revealed that NEAT1 is also involved in herpes simplex virus-1 (HSV-1) replication and the development of Alzheimer's disease (AD) by epigenetically regulating the expression of HSV-1 viral genes and endocytosis-related genes, respectively [13,14]. The key role of NEAT1 is to mediate gene expression through complex mechanisms. NEAT1 regulates target genes by recruiting and/or sequestering transcriptional factors and regulators to and from promoters and transcripts of target genes, thereby influencing their transcription, splicing, RNA stability, and translation [15].
There is growing evidence that lncRNAs can act as competing endogenous RNAs (ceRNAs) by sponging microRNAs (miRNAs) to alter the expression levels of their target genes in the development of human diseases [16,17]. During tumorigenesis and cancer progression, many oncogenic lncRNAs exhibit dysregulated expression, which promotes the development of cancer and is associated with poor overall survival. This occurs through lncRNA's enhancing of cancer cell proliferation, migration, invasion, and apoptosis inhibition. Researchers discovered that lncRNAs regulate the expression of tumor-related genes by interacting with lncRNA-specific miRNAs, thereby preventing the degradation of tumor-related gene transcripts and promoting their translation [18]. These findings suggest that lncRNA-mediated ceRNA networks have great potential as biomarkers and therapeutic targets for cancer.
In this review, we discuss the roles of NEAT1 in the progression of different types of tumors by describing a universal regulatory pattern of NEAT1 in tumor-related gene expression. We have focused on the function of NEAT1 as a ceRNA to upregulate these gene expression levels through sponging miRNAs. This gene upregulation results in the promotion of tumor cell proliferation, migration, invasion, epithelial-mesenchymal transition (EMT), and cell apoptosis inhibition. We also discuss the potential clinical applications of NEAT1-mediated ceRNA networks in overcoming chemo- and radioresistance in cancer treatment.
NEAT1 role in the tumorigenesis in respiratory system tumors
In this section, we summarize the roles of the NEAT1/miRNA/target axis in respiratory system tumors, including nasopharyngeal carcinoma, sinonasal squamous cell carcinoma, laryngeal squamous cell cancer, and non-small cell lung cancer (Table 1).
Nasopharyngeal carcinoma
Nasopharyngeal carcinoma (NPC), a common head and neck cancer originating from the nasopharynx epithelium, is a leading cause of cancer-related deaths worldwide [19]. In a study of the underlying mechanism of NEAT1 in NPC progression, NEAT1 expression was found to be upregulated in NPC tissues and cells, and NEAT1 knockdown resulted in an inhibition of tumor cell proliferation, migration, invasion, and EMT by blocking Wnt/β-catenin signaling, a trigger for tumorigenesis [20], via targeting miR-34a-5p [21].
NF-κB signaling is another pathway that is influenced by NEAT1 in NPC progression. Cheng et al. reported that miR-124 inhibits NPC cell proliferation and promotes cell apoptosis by binding to and repressing the expression of NEAT1 and NF-κB, suggesting that NEAT1 functions as a potential ceRNA for NF-κB expression [22].
Sinonasal squamous cell carcinoma
Sinonasal squamous cell carcinoma (SNSCC) is the most common type of sinonasal malignancy, an aggressive tumor type characterized by late discovery and rapid progression [23,24]. A study on the molecular mechanisms of SNSCC development revealed that the expression of lncRNA NEAT1 and vascular endothelial growth factor A (VEGFA) were both upregulated in SNSCC tissues and cells, which resulted in a promotion of SNSCC cell viability and a reduction in SNSCC cell apoptosis. Moreover, upregulation of miR-195-5p in SNSCC cells decreased cell viability by directly binding with NEAT1 and VEGFA and decreasing their expression levels, suggesting an important role of the NEAT1/miR-195-5p/VEGFA axis in SNSCC progression [25].
Laryngeal squamous cell cancer
Laryngeal squamous cell carcinoma (LSCC) is the most common malignant tumor occurring in the head and neck, and it also is the leading cause of cancer-related deaths in this category [26]. Wang et al. [27] studied the role of NEAT1 in human LSCC progression, and the authors found that NEAT1 expression was significantly induced in LSCC with a positive relationship with grade, lymph node metastasis, and clinical stages. Moreover, NEAT1 was shown to promote LSCC cell proliferation and invasion and inhibit LSCC cell apoptosis and cell cycle arrest at the G1 phase. Further investigation of the molecular mechanism demonstrated that NEAT1 sponges miR-107 to upregulate the expression of cyclin-dependent kinase 6 (CDK6), a member of the CDK family that significantly correlates with head and neck squamous cell carcinoma progression [28].
Roles of NEAT1/miRNA/target axis in respiratory system tumors
| Cancer type | MiRNA | Target | Role | Reference |
|---|---|---|---|---|
| NPC | 34a-5p | Wnt/β-catenin | Promoting NPC cell proliferation, migration, invasion, and EMT | [21] |
| 124 | NF-κB | Promoting NPC cell proliferation and inhibiting cell apoptosis | [22] | |
| SNSCC | 195-5p | VEGFA | Enhancing SNSCC cell viability and inhibiting SNSCC cell apoptosis | [25] |
| LSCC | 107 | CDK6 | Promoting LSCC cells proliferation and invasion, and inhibiting LSCC cells apoptosis and cell cycle arrest at G1 phase | [28] |
| NSCLC | 377-3p | E2F3 | Promoting NSCLC cells growth and metastasis | [30] |
| 98-5p | MAPK6 | Promoting NSCLC cell growth, migration, and invasion | [31] | |
| 101-3p | SOX9 | Promoting NSCLC cell proliferation, migration and invasion | [32] | |
| 376b-3p | SURF1 | Promoting NSCLC cell proliferation, migration, and invasion and inhibiting cell apoptosis | [33] | |
| LUAD | 193a-3p | USF1 | Promoting LUAD cell proliferation, invasion, and migration and inhibiting cell apoptosis | [40] |
Roles of NEAT1/miRNA/target axis in digestive system tumors
| Cancer type | MiRNA | Target | Role | Reference |
|---|---|---|---|---|
| OSCC | 365 | RGS20 | Promoting OSCC cells proliferation and invasion, and inhibiting cell cycle arrest at the G0/G1 phase and apoptosis | [45] |
| ESCC | 129 | CTBP2 | Promoting ESCC cell viability and invasion | [47] |
| GC | 497-5p | PIK3R | Promoting GC cells proliferation, and inhibited GC cells apoptosis | [54] |
| 506 | STAT3 | Promoting GC cells proliferation, migration and invasion | [53] | |
| 335-5p | ROCK1 | [55] | ||
| 103a | STAMBPL1 | [56] | ||
| 365a-3p | ABCC4 | Promoting GC cells proliferation, colony formation, invasion, and cell cycle | [57] | |
| HCC | 296-5p | CNN2 | Promoting HCC cells growth, migration, and invasion | [60] |
| 139-5p | TGF-β1 | [61] | ||
| 485 | STAT3 | [62] | ||
| CRC | 495-3p | CDK6 | Promoting colon cancer cell proliferation, cell cycle, cell migration, and invasion and inhibiting cell apoptosis | [65] |
| 185-5p | IGF2 | Promoting colon cancer cell migration and invasion | [66] | |
| 34a | SIRT1 | Promoting CRC cells proliferation, migration, and invasion | [67] | |
| 196a-5p | GDNF | [68] | ||
| 205-5p | VEGFA | [69] | ||
| 193a | IL17RD | Promoting CRC cells proliferation, migration, and invasion, and inhibiting CRC cells apoptosis | [70] | |
| 193a-3p | KRAS | [72] | ||
| 138 | SLC38A | [71] | ||
| 195-5p | CEP55 | [73] |
Non-small cell lung cancer
Lung cancer remains one of the most prevalent malignant tumors and the leading cause of cancer-related deaths all over the world [29]. The most prevalent form of lung cancer (~80%) is the non-small cell lung cancer (NSCLC). Sun et al. [30] reported that high NEAT1 expression in NSCLC is related to a short overall survival of patients with NSCLC by promoting cancer cell growth and metastasis. Further investigation revealed that NEAT1 functions as a ceRNA for E2F3, a core oncogene in promoting NSCLC progression, by sponging hsa-miR-377-3p and antagonizing its functions of binding with E2F3 to repress E2F3 expression. Furthermore, NEAT1 was found to promote growth, migration, and invasion of NSCLC by sponging miR-98-5p, miR-101-3p, and miR-376b-3p to upregulate mitogen-activated protein kinase 6 (MAPK6) [31], SRY-box transcription factor 9 (SOX9) [32], and sulfatase 1 (SULF1) [33], respectively. These targets play a vital role in cancer progression [34-36].
Lung adenocarcinoma (LUAD) is a histopathological subtype of NSCLC that accounts for nearly 40% of lung cancer cases [37,38]. Xiong et al. [39] showed that NEAT1 accelerated LUAD cell proliferation, invasion, and migration and inhibited cell apoptosis by upregulating the expression of upstream stimulating factor 1 (USF1), a basic helix-loop-helix-zipper transcription factor that promotes lung adenocarcinoma progression [40], by sponging miR-193a-3p.
NEAT1 role in the tumorigenesis in digestive system tumors
In this section, we summarize the roles of the NEAT1/miRNA/target axis in digestive system tumors, including oral squamous cell carcinoma, esophageal squamous cell carcinoma, gastric cancer, hepatocellular carcinoma, and colorectal cancer (Table 2).
Oral squamous cell carcinoma
Oral squamous cell carcinoma (OSCC) is the most prevalent type of head and neck squamous cell carcinoma (HNSCC), the sixth most common cancer worldwide in 2018 [41,42]. In a study to determine the function and mechanism of lncRNA NEAT1 in OSCC, NEAT1 expression was found to be significantly upregulated in OSCC cells and tissues. This high expression of NEAT1 was positively correlated with advanced TNM stage (a system used to classify Tumor size, Node location, and Metastasis status) and poor survival of patients by promoting tumor cells proliferation and invasion, and inhibiting cell cycle arrest at the G0/G1 phase and apoptosis. It is proposed that NEAT1 could positively regulate the expression of the regulator of G protein signaling 20 (RGS20), an accelerator for the proliferation and migration of cancer cells [43,44], by interacting with miR-365 to suppress the repressive effects of miR-365 on the expression of RGS20 [45].
Esophageal squamous cell carcinoma
Esophageal squamous cell carcinoma (ESCC) is the predominant histological type of esophageal cancer and is one of the most common and leading aggressive malignancies, with a five-year survival rate of less than 10% [46]. A study investigating the molecular mechanism of the NEAT1 regulatory network in ESCC progression revealed that the expression of NEAT1 and C-terminal-binding protein 2 (CTBP2) was upregulated, while expression of miR-129 was downregulated in ESCC cells. Further studies validated that miR-129 could target NEAT1 and CTBP2 to decrease their expression levels. In addition, cellular function investigation confirmed that either NEAT1 knockdown, CTBP2 knockdown, or miR-129 upregulation resulted in an inhibition of ESCC cell viability and invasion, suggesting a NEAT1/miR-129/CTBP2 regulatory network in ESCC progression [47].
Gastric cancer
Gastric cancer (GC) remains the third leading cause of cancer-related deaths all over the world and is the most common type of digestive malignancies [48-50]. In addition, most patients with GC exhibit malignant metastasis with poor overall survival [51,52].
Tan et al. [53] explored the detailed roles and molecular mechanisms of NEAT1 in GC progression. The authors found that the expression of NEAT1 and signal transducer and activator of transcription 3 (STAT3) were significantly upregulated in human GC cells, while expression of miR-506 was downregulated. NEAT1 and STAT3 are two targets of miR-506. Moreover, NEAT1 knockdown repressed GC cell growth, migration, and invasion by decreasing the expression level of STAT3 via miR-506 upregulation.
In addition, other NEAT1 sponging-miRNAs and targets of these miRNAs that play roles in GC progression have been discovered. For example, the NEAT1/miR-497-5p/phosphoinositide-3-kinase regulatory subunit 1 (PIK3R1) axis promotes GC cell proliferation and inhibits GC cell apoptosis [54]; the NEAT1/miR-335-5p/rho associated coiled-coil containing protein kinase 1 (ROCK1) axis promotes GC cell proliferation, migration, and invasion [55]; the NEAT1/miR-103a/STAM binding protein like 1 (STAMBPL1) axis promotes GC cell proliferation and cell invasion [56]; NEAT1/miR-365a-3p/ATP binding cassette subfamily C member 4 (ABCC4) axis promotes GC cell proliferation, colony formation, invasion, and cell cycle progression [57].
Hepatocellular carcinoma
Hepatocellular carcinoma (HCC) is the fifth most common cancer in men and seventh in women and is the second most common cause of cancer-related deaths worldwide [58,59]. Increasing evidence has demonstrated that NEAT1 expression is induced and NEAT1 upregulation promotes HCC progression. Molecular mechanism investigations have shown that NEAT1 promotes HCC cell proliferation, migration, and invasion by upregulating the expression of calponin 2 (CNN2), transforming growth factor-β1 (TGF-β1), and STAT3 by targeting miR-296-5p, miR-139-5p, and miR-485, respectively [60-62].
Colorectal cancer
Colorectal cancer (CRC) remains the second most common cause of cancer-related deaths in the United States [63]. He et al. reported that NEAT1 knockdown inhibited colon cancer cell proliferation, cell cycle, cell migration/invasion, and promoted colon cancer cell apoptosis by repressing the expression of CDK6 via interaction with miR-495-3p [64]. CDK6 is a member of the CDK family whose dysregulation in cancers results in continued proliferation and unscheduled cell cycle [65]. Moreover, the NEAT1/miR-185-5p/insulin-like growth factor 2 (IGF2) axis is another pathway that induces the invasion and migration of colon cancer [66].
In addition, NEAT1 upregulation in CRC was significantly correlated with poor TNM staging, survival, and tumor recurrence in patients with CRC. By upregulating the expression of sirtuin-1 (SIRT1) via miR-34a [67], glial cell-derived neurotrophic factor (GDNF) via miR-196a-5p [68], and VEGFA via miR-205-5p [69], NEAT1 enhanced CRC cell proliferation, colony formation, and invasive potential. By upregulating the expression of interleukin 17 receptor D (IL17RD) via miR-193a [70], solute carrier family 38 member 1 (SLC38A1) via miR-138 [71], KRAS via miR-193a-3p [72], and centrosomal protein 55 (CEP55) via miR-195-5p [73], NEAT1 promotes CRC cell proliferation, migration, and invasion and inhibits apoptosis.
NEAT1 role in the tumorigenesis in reproductive system tumors
In this section, we summarize the roles of the NEAT1/miRNA/target axis in reproductive system tumors, including breast cancer, ovarian cancer, cervical cancer, endometrial carcinoma, and prostate cancer (Table 3).
Breast cancer
Breast cancer (BC) remains the leading cause of cancer death in women and occurs in the epithelial tissue of the mammary gland [74]. Researchers found that NEAT1 overexpression in BC was correlated with poor prognosis of patients and the feedback loop of NEAT1/miR-107/carnitine palmitoyltransferase 1A (CPT1A) [75], NEAT1/miR-124/STAT3 [76], NEAT1/miR-448/zinc finger E-box binding homeobox 1 (ZEB1) [77], and NEAT1/miR-101/enhancer of zeste homolog 2 (EZH2) [78], which promotes BC cell proliferation, migration, invasion, and cell cycle progression. In addition, NEAT1 upregulation in BC enhances EMT and inhibits cell apoptosis by sponging miR-410-3p to upregulate the expression of cyclin D1 (CCND1) [79] and sponging miR-138-5p to upregulate the expression of zinc finger protein X-linked (ZFX) [80].
Roles of NEAT1/miRNA/target axis in reproductive system tumors
| Cancer type | MiRNA | Target | Role | Reference |
|---|---|---|---|---|
| BC | 107 | CPT1A | Promoting BC cells proliferation, migration, invasion and cell cycle | [75] |
| 124 | STAT3 | [76] | ||
| 448 | ZEB1 | [77] | ||
| 101 | EZH2 | [78] | ||
| 410-3p | CCND1 | Promoting BC cells proliferation, migration, invasion, and EMT | [79] | |
| 138-5p | ZFX | Promoting BC cells proliferation, migration, invasion, and inhibiting apoptosis | [80] | |
| OC | 34a-5p | BCL2 | Promoting OC cells proliferation and inhibited apoptosis | [82] |
| 382-3p | ROCK1 | Promoting OC cells metastasis | [83] | |
| 4500 | BZW1 | Promoting OC cells proliferation, migration, invasion, and inhibiting apoptosis | [84] | |
| 1321 | TJP3 | Promoting OC cells proliferation, migration, invasion, and EMT | [85] | |
| CC | 133a | SOX4 | Promoting CC cells proliferation, migration, invasion, and inhibiting apoptosis | [88] |
| 9-5p | POU2F1 | Promoting CC cells proliferation and migration | [87] | |
| 361 | HSP90 | Promoting CC cells proliferation, migration, and EMT | [90] | |
| EC | 214-3p | HMGA1 | Promoting EC cells proliferation, migration and invasion | [93] |
| 144-3p | EZH2 | Promoting EC cells proliferation, migration and invasion | [94] | |
| PCa | 98-5p | HMGA2 | Promoting PCa cells proliferation and invasion | [96] |
Ovarian cancer
Ovarian cancer (OC) is another leading cause of cancer-related deaths in the female population worldwide [81]. Ding et al. [82] reported that NEAT1 overexpression in OC promoted proliferation and inhibited apoptosis of OC cells by negatively regulating miR-34a-5p expression and positively regulating B-cell lymphoma-2 (BCL2), a target of miR-34a-5p. Moreover, NEAT1 enhanced the metastasis of OC cells by upregulating the expression of ROCK1 by sponging miR-382-3p [83].
In addition to the promotion of OC cell proliferation, migration, and invasion, NEAT1 was shown to inhibit OC cell apoptosis by upregulating the expression of basic leucine zipper and W2 domain‑containing protein 1 (BZW1) via interaction with miR-4500 [84]. NEAT1 also enhanced EMT of OC cells by upregulating the expression of tight junction protein 3 (TJP3) via interaction with miR-1321 [85].
Cervical cancer
Cervical cancer (CC) remains the second most common and serious malignant tumor among women all over the world [86]. Xie et al. studied the role of NEAT1 in CC progression, and the authors reported that NEAT1 upregulation in CC tissue enhanced CC cell proliferation and migration [87]. Mechanistically, NEAT1 functions as a ceRNA to bind miR-9-5p and increase the expression level of POU class 2 homeobox 1 (POU2F1), a target of miR-9-5p. Moreover, overexpression of NEAT1 could inhibit CC cell apoptosis and EMT by targeting miR-133a, thereby increasing the expression of SRY-box transcription factor 4 (SOX4), an important epigenetic regulator in tumorigenesis [88, 89], and targeting miR-361 to increase expression of the 90-kDa heat shock proteins (HSP90s), an essential factor contributing to the tumor metastatic phenotype [90,91].
Endometrial carcinoma
Endometrial carcinoma (EC) is a commonly diagnosed gynecological cancer worldwide, and its incidence is increasing [92]. Researchers investigated the function and mechanism of lncRNA NEAT1 in EC progression, and they found that NEAT1 promotes EC cell proliferation, migration, and invasion by sponging miR-214-3p and miR-144-3p to upregulate the expression of high mobility group AT-hook 1 (HMGA1) [93] and EZH2 [94], respectively.
Prostate cancer
Prostate cancer (PCa) is the second most common tumor and the fifth leading cause of cancer-related deaths among men [42]. Guo et al. reported that NEAT1 expression was significantly upregulated in PCa tissues and PCa cell lines, and NEAT1 knockdown inhibited the growth and invasion of PCa cells [95]. Mechanistically, NEAT1 upregulates the expression of high mobility group AT-hook 2 (HMGA2), an important transcription factor for genes that modulate cell cycle process, DNA damage, apoptosis, and EMT [96], by binding miR-98-5p and decreasing the expression level of miR-98-5p.
NEAT1 in the tumorigenesis in circulatory system tumors
In this section, we summarize and discuss the role of the NEAT1/miRNA/target axis in circulatory system tumors, including hemangioma, acute myeloid leukemia, T-cell acute lymphoblastic leukemia, diffuse large B-cell lymphoma, Hodgkin's lymphoma, and multiple myeloma (Table 4).
Roles of NEAT1/miRNA/target axis in circulatory system tumors
| Cancer type | MiRNA | Target | Role | Reference |
|---|---|---|---|---|
| HA | 361-5p | VEGFA | Promoting HemECs proliferation and migration, and inhibiting HemECs apoptosis | [100] |
| 33-5p | HIF1α | Promoting HemECs proliferation, migration, and invasion | [98] | |
| AML | 23a-3p | SMC1A | Inhibiting AML cells proliferation, decreasing the number of cells in the G2/M phase, and inducing cell apoptosis | [103] |
| 338-3p | CREBRF | Inhibiting AML cells proliferation, migration and invasion, and enhancing AML cells apoptosis | [104] | |
| T-ALL | 146b-5p | NOTCH1 | Promoting T-ALL cells proliferation | [107] |
| DLBCL | 34b-5p | GLI1 | Promoting DLBCL cells proliferation, and inhibiting DLBCL cells apoptosis | [109] |
| HL | 448 | DCLK1 | Promoting HL cells proliferation and invasion | [113] |
| MM | 214 | B7-H3 | Promoting M2 macrophage polarization | [116] |
Hemangioma
Hemangioma (HA) is one of the most common benign vascular neoplasms of infancy due to the abnormal proliferation of hemangioma endothelial cells (HemECs) [97]. Yu et al. studied the roles and molecular mechanisms of NEAT1 in HA progression; the authors found that NEAT1 expression is increased in hemangiomas and depletion of NEAT1 results in the inhibition of HemEC proliferation, migration, and invasion [98]. Investigation of the mechanism revealed that NEAT1 upregulated the expression of HIF1α by sponging miR-33a-5p, thus activating NF-κB signaling, a critical pathway for tumorigenesis [99]. In addition, NEAT1 was found to inhibit the apoptosis of HemECs, thereby contributing to HA progression, by interacting with miR-361-5p to upregulate the expression of VEGFA, an essential factor in promoting cancer progression by increasing the proliferation and migration of cancer cells [100,101].
Acute myeloid leukemia
Acute myeloid leukemia (AML) is a representative hematologic malignancy characterized by an abnormal abundance of aberrantly differentiated myeloid cells in the bone marrow [102]. Researchers investigated the regulatory influence of the NEAT1/miRNA/target axis in AML progression; they found that NEAT1 expression was downregulated in AML cells and that overexpression of NEAT1 inhibited cell proliferation, migration, and invasion, decreased the number of cells in the G2/M phase, and significantly induced cell apoptosis through the NEAT1/miR-23a-3p/structural maintenance of chromosomes 1A (SMC1A) axis [103] and NEAT1/miR-338-3p/CREB3 regulatory factor (CREBRF) axis [104].
T-cell acute lymphoblastic leukemia
T-cell acute lymphoblastic leukemia (T-ALL) is an aggressive leukemia originating from T-lymphocytes in the bone marrow. Patients show symptoms of weakness, enlarged lymph nodes, fatigue, and weight loss [105]. Luo et al. studied the regulatory mechanism of NEAT1 in the process of T-ALL [106]. The authors found that NEAT1 expression levels were markedly increased in T-ALL cells. NEAT1 promotes the proliferation of T-ALL cells by upregulating the expression of NOTCH1, a driving oncogene that induces the development of pre-T cells to leukemia [107], by sponging miR-146b-5p and decreasing its expression level.
Diffuse large B-cell lymphoma
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphoma, and it is typically considered an aggressive lymphoma [108]. A study to investigate the underlying mechanism of NEAT1 in DLBCL progression found that NEAT1 transcriptionally regulated by MYC was upregulated in DLBCL tissues and cell lines, and NEAT1 knockdown resulted in the inhibition of DLBCL cell proliferation and a promotion of DLBCL cell apoptosis. Mechanistically, NEAT1 functions as a ceRNA to target miR-34b-5p and, thus, increases the expression level of the GLI family zinc finger 1 (GLI1), an oncogene that contributes to cell survival of DLBCL [109, 110].
Hodgkin's lymphoma
Hodgkin's lymphoma (HL) is the most common malignant lymphoma originating in the lymphoid hematopoietic system, especially in young adults [111]. Fan et al. [112] showed that NEAT1 expression was significantly enhanced in HL tissues and cell lines, and NEAT1 downregulation resulted in inhibition of HL cell proliferation and invasion through the downregulation of doublecortin-like kinase 1 (DCLK1), an accelerator in tumor cell invasion, metastasis, and EMT [113], via interaction with miR-448.
Multiple myeloma
Multiple myeloma (MM) is one of the most common hematological malignancies characterized by aberrant proliferation of plasma cells and secretion of monoclonal immunoglobulin proteins [114]. Gao et al. [115] reported that NEAT1 upregulation in MM patients promoted M2 macrophage polarization, a contributor to tumor progression that promotes angiogenesis to support tumor growth [116], by upregulating the expression and release of B7-H3 and then activating JAK2/STAT3 signaling via direct targeting of miR-214.
NEAT1 in the tumorigenesis in nervous system tumors
In this section, we summarize and discuss the role of the NEAT1/miRNA/target axis in nervous system tumors, including glioma, retinoblastoma, and neuroblastoma (Table 5).
Roles of NEAT1/miRNA/target axis in nervous system tumors
| Cancer type | MiRNA | Target | Role | Reference |
|---|---|---|---|---|
| Glioma | 107 | CDK14 | Promoting glioma cells proliferation, migration, and invasion | [118] |
| 132 | SOX2 | [119] | ||
| 449b-5 | c-Met | [120] | ||
| 152-3p | CCT6A | Promoting glioma cells proliferation, migration, and invasion, inhibiting cells apoptosis | [121] | |
| 139-5p | CDK6 | [122] | ||
| 185-5p | DNMT1 | Promoting glioma cells proliferation, migration, invasion, and EMT, inhibiting cells apoptosis | [123] | |
| RB | 204 | CXCR4 | Promoting RB cells proliferation and migration, and inhibiting cells apoptosis | [125] |
| NB | 326 | JAK1, STAT3 | Promoting NB cells proliferation, and inhibiting cells apoptosis | [127] |
Glioma
Gliomas are the most common and aggressive tumors of the central nervous system and characterized by extremely poor prognosis outcomes [117]. Researchers studied the molecular mechanisms underlying gliomas. They observed that NEAT1 was upregulated in glioma tissues and cell lines, and this upregulation contributed to glioma progression by inducing glioma cell survival, promoting cell proliferation, migration, and invasion by sponging miR-107 [118], miR-132 [119], and miR-449b-5 [120] to elevate the expression levels of cyclin-dependent kinase 14 (CDK14), SRY-box transcription factor 2 (SOX2), and c-Met, respectively. In addition, NEAT1/miR-152-3p/chaperonin containing the TCP1 subunit 6A (CCT6A) axis [121] and NEAT1/miR-139-5p/CDK6 axis [122] were shown to inhibit cell apoptosis, while the NEAT1/miR-185-5p/DNA methyltransferase 1 (DNMT1) axis [123] promoted EMT in glioma cells and inhibited cell apoptosis.
Retinoblastoma
Retinoblastoma (RB) is an aggressive retinal cancer that is initiated in response to biallelic loss of the tumor suppressor gene RB1 in almost all cases and develops after additional genetic/epigenetic alterations [124]. A study on the role of NEAT1 in RB progression revealed that NEAT1 expression levels were elevated in RB tissues and cells; NEAT1 knockdown significantly inhibited RB cell proliferation and migration and promoted cell apoptosis by competitively binding with miR-204 to regulate the expression of C-X-C chemokine receptor type 4 (CXCR4) [125].
Neuroblastoma
Neuroblastoma (NB) is the most common pediatric solid tumor that arises in the sympathetic nervous system. NB accounts for 7%-8% of childhood malignancies and ~15% of childhood cancer-related deaths [126]. Yang et al. explored the mechanism of NEAT1 in NB progression. The authors observed that NEAT1 expression was induced in neuroblastoma cell lines, and overexpression of NEAT1 resulted in an increase in NB cell proliferation and a decrease in cell apoptosis through upregulating the expression of Janus kinase 1 (JAK1) and STAT3 by sponging miR-326 [127].
NEAT1 role in the tumorigenesis in endocrine system tumors
In this section, we summarize and discuss the role of the NEAT1/miRNA/target axis in thyroid cancer, a type of endocrine system tumor (Table 6).
Roles of NEAT1/miRNA/target axis in endocrine system, mobility system, and urinary system tumors
| System | Cancer type | miRNA | Target | Role | Reference |
|---|---|---|---|---|---|
| Endocrine system | Thyroid carcinoma | 592 | NOVA1 | Promoting thyroid cancer cells proliferation, migration, and invasion | [129] |
| PTC | 129-5p | KLK7 | Promoting PTC cells proliferation, migration, and invasion, and inhibiting cells apoptosis | [130] | |
| 106b-5p | ATAD2 | [131] | |||
| Mobility system | OS | 339-5p | TGF-β1 | Promoting OS cells proliferation, migration, and invasion | [133] |
| 34a-5p | HOXA13 | Promoting OS cells proliferation and inhibiting cells apoptosis | [135] | ||
| 186-5p | HIF-1α | Promoting OS cells proliferation, invasion, and EMT | [134] | ||
| Urinary system | Bladder cancer | 410 | HMGB1 | Promoting bladder cancer cells proliferation and inhibiting cell apoptosis and cell arrest | [137] |
| RCC | 34a | c-Met | Promoting RCC cells proliferation, migration, invasion,and EMT, and inhibiting cell cycle progression | [140] |
Thyroid cancer is the most commonly diagnosed endocrine tumor worldwide, with an increasing incidence in the past 20 years [128]. To date, a number of miRNAs have been reported to be aberrantly expressed in thyroid cancer and play a vital role in its progression. A study to understand the roles of miR-592 in thyroid cancer found that downregulated miR-592 in thyroid cancer exhibited a short overall survival of patients by promoting cell proliferation, migration, and invasion of thyroid cancer cells. The investigation showed that NEAT1 and neuro-oncological ventral antigen 1 (NOVA1) are targets of miR-592, and the knockdown of NEAT1 and NOVA1 effectively abolish the promotion effects of miR-592 downregulation in thyroid cancer cells, suggesting a vital role of NEAT1/miR-592/NOVA1 axis in thyroid cancer progression [129].
Papillary thyroid cancer (PTC) is the most common form of thyroid cancer, accounting for >80% of thyroid cancer cases. Investigation of the roles of NEAT1 in PTC progression showed that NEAT1 expression was significantly upregulated in PTC tissues and cell lines, and NEAT1 overexpression promoted PTC cell proliferation, invasion, and migration, and inhibited cell apoptosis by increasing the expression level of kallikrein-related peptidase 7 (KLK7) [130] and ATPase family AAA domain-containing protein 2 (ATAD2) [131] via sponging miR-129-5p and miR-106b-5p, respectively.
NEAT1 role in the tumorigenesis in mobility system tumors
In this section, we summarize and discuss the role of the NEAT1/miRNA/target axis in osteosarcoma, a type of mobility system tumor (Table 6).
Osteosarcoma (OS) is the most common primary malignant bone tumor in children and teenagers. Somatic mutations and epigenetic mechanisms contribute to the progression of OS, such as aberrant activation of oncogenes and dysregulation of ncRNAs [132]. Several studies on the role of NEAT1 in OS progression showed that upregulation of NEAT1 in osteosarcoma tissues promoted OS cell proliferation, migration, and invasion, EMT, and inhibited cell apoptosis. Investigation of the mechanism revealed that NEAT1 acts as a ceRNA to regulate the expression of TGF-β1 [133], human hypoxia-inducible factor 1α (HIF-1α) [134], and homeobox A13 (HOXA13) [135] by sponging miR-339-5p, miR-186-5p, and miR-34a-5p.
NEAT1 role in the tumorigenesis in urinary system tumors
In this section, we summarize and discuss the role of the NEAT1/miRNA/target axis in urinary system tumors, including bladder cancer and renal cell carcinoma (Table 6).
Bladder cancer
Bladder cancer is a common urological malignant tumor in men worldwide and is characterized by a high rate of early systemic dissemination and nearly 170,000 deaths annually [136]. Shan et al. [137] revealed that the upregulation of NEAT1 in bladder cancer promotes bladder cancer cell proliferation and inhibits cell apoptosis and cell arrest by sponging miR-410, thereby upregulating the expression of high mobility group box 1 (HMGB1), an accelerator for tumor progression by its immune protective and suppressive functions [138].
Renal cell carcinoma
Renal cell carcinoma (RCC) is the most common type of kidney cancer and accounts for nearly 95% of all kidney cancer diagnoses [42]. In a study to determine the role of NEAT1 in RCC progression, Liu et al. [139] found that NEAT1 expression is upregulated in RCC tissue and cell lines, and high NEAT1 expression is correlated with poor prognosis. Further investigation revealed that NEAT1 enhanced RCC cell proliferation, migration, invasion, and EMT, and inhibited cell cycle progression by sponging miR-34a, thus increasing the expression level of c-Met, a potential therapeutic target in cancers [140].
NEAT1 in cancer therapy
Conventional treatments for cancer include surgery, chemotherapy, and radiotherapy. However, there is a subset of cancer patients that exhibit metastases and are unresponsive to chemotherapy or radiotherapy owing to tumor heterogeneity, tumor microenvironment, and dysfunction of therapeutic resistance-related genes [141-143]. To date, dozens of studies have reported an association between NEAT1 and resistance to chemotherapy or radiotherapy in various cancers (Table 7). They found that knockdown of NEAT1 could sensitize cancer cells to radiation or chemical drugs through NEAT1-mediated ceRNA networks. Therefore, targeting the feedback loop of NEAT1/miRNA/target may be a potential pathway to overcome therapeutic resistance in cancer.
Conclusions
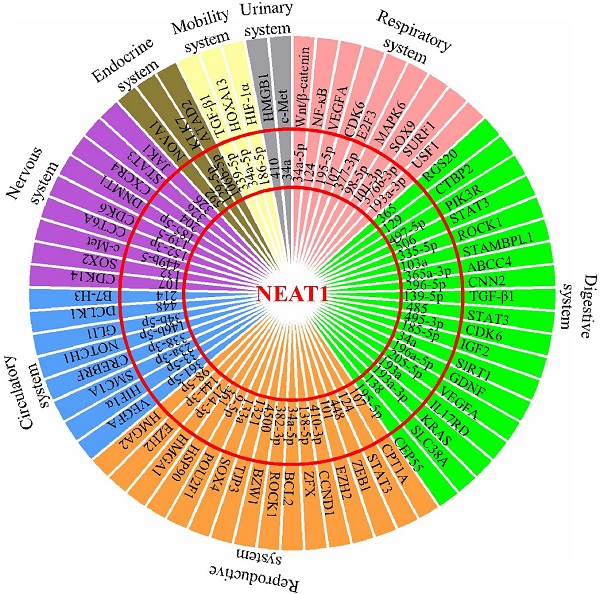
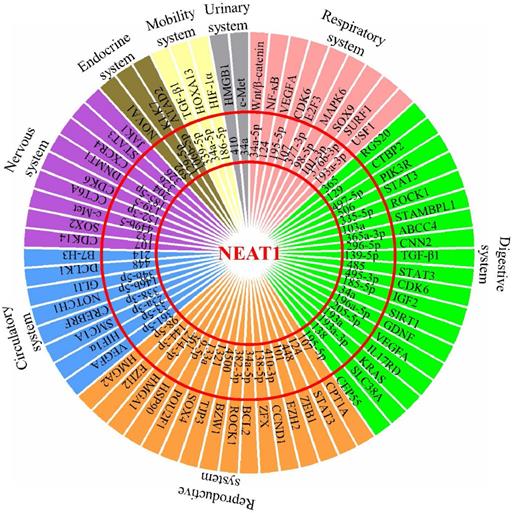
The effect of malignant cancers is devastating across all physiological systems. The interplay between uncontrolled cancer cell growth, migration, invasion, and inhibition of apoptosis results in inevitable metastasis affecting all organ systems. To date, lncRNA NEAT1 has been reported to be aberrantly expressed in different types of cancers. This review extensively summarized all existing information available on NEAT1's contribution in their development, as NEAT1's role as a ceRNA influences the miRNA environment during tumorigenesis (Figure 1). NEAT1 knockdown studies have revealed a therapeutic potential by redirecting the feedback loop between NEAT1/miRNA/target, thereby increasing efficacy of radio- and chemotherapy. It therefore highlights that NEAT1 is of relevant research interest and its role in therapeutic knockdown to enhance cancer therapies should be considered. However, as a nuclear enriched lncRNA, it should be clarified how NEAT1 sponges so many miRNAs to regulate expression of tumorigenesis-related genes, and whether NEAT1 in the peripheral blood could act as a biomarker for the diagnosis of cancers. In addition, more studies are needed in the future to characterize the role of NEAT1 in tumor microenvironments, such as whether NEAT1 affects the function of tumor infiltrating lymphocytes (TILs), and whether NEAT1 could function as a “messenger lncRNA” for the communication between tumor cells and these immune cells. Overall, this review summarizes and discusses the roles of the NEAT1-miRNA-target axis in the progression of various cancers and provides insight into its potential clinical utility in cancer treatment.
Roles of NEAT1/miRNA/target axis in therapeutic resistance of cancers
| Cancer type | MiRNA | Target | Chemical-/radio- resistance | Reference |
|---|---|---|---|---|
| BC | 211 | HMGA2 | 5-fluorouracil (5-FU) | [144] |
| CRC | 150-5p | CPSF4 | [145] | |
| OC | 770-5p | PARP1 | Cisplatin (CDDP) | [146] |
| ATC | 9-5p | SPAG9 | [147] | |
| OS | 34c | BCL-2 | [148] | |
| CCND1 | ||||
| PCA | 204-5p | ACSL4 | Docetaxel | [149] |
| 34a-5p | ||||
| Bladder cancer | 214-3p | Wnt/β-catenin | Doxorubicin (DOX) | [150] |
| OC | 194 | ZEB1 | Paclitaxel (PTX) | [151] |
| EC | 361 | STAT3 | [152] | |
| HCC | 204 | ATG3 | Sorafenib | [153] |
| 335 | c-Met | [154] | ||
| RCC | 34a | c-Met | [140] | |
| NPC | 129 | Bcl-2 | Suberoylanilide hydroxamic acid (SAHA) | [155] |
| NPC | 204 | ZEB1 | Radiation | [156] |
| HCC | 101-3p | WEE1 | [157] | |
| CC | 193b-3p | CCND1 | [158] |
Schematic model shows NEAT1's role as a ceRNA to influence the miRNA environment during development of multiple physiological system tumors.
Abbreviations
NEAT1: nuclear paraspeckle assembly transcript 1; LncRNA: Long non-coding RNA; CeRNA: competing endogenous RNA; MiRNAs: MicroRNAs; PSPC1: Paraspeckle component 1; SFPQ: Splicing factor proline/glutamine rich; P54nrb: 54 kDa nuclear RNA- and DNA-binding protein; AD: Alzheimer's disease; EMT: Epithelial-mesenchymal transition; NPC: Nasopharyngeal carcinoma; SNSCC: Sinonasal squamous cell carcinoma; VEGFA: Vascular endothelial growth factor A; LSCC: Laryngeal squamous cell carcinoma; CDK6: Cyclin-dependent kinase 6; NSCLC: Non-small cell lung cancer; MAPK6: Mitogen-activated protein kinase 6; LUAD: Lung adenocarcinoma; USF1: Upstream stimulating factor 1; OSCC: Oral squamous cell carcinoma; HNSCC: Head and neck squamous cell carcinoma; TNM: Tumor size, Node location, and Metastasis; RGS20: G protein signaling 20; ESCC: Esophageal squamous cell carcinoma; CTBP2: C-terminal-binding protein 2; GC: Gastric cancer; STAT3: Signal transducer and activator of transcription 3; PIK3R1: Phosphoinositide-3-kinase regulatory subunit 1; ROCK1: Rho associated coiled-coil containing protein kinase 1; STAMBPL1: STAM binding protein like 1; ABCC4: ATP binding cassette subfamily C member 4; HCC: Hepatocellular carcinoma; CNN2: Calponin 2; TGF-β1: Transforming growth factor-β1; CRC: Colorectal cancer; IGF2: Insulin-like growth factor 2; SIRT1: Sirtuin-1; GDNF: Glial cell-derived neurotrophic factor; IL17RD: Interleukin 17 receptor D; CEP55: Centrosomal protein 55; BC: Breast cancer; ZEB1: Zinc finger E-box binding homeobox 1; EZH2: Enhancer of zeste homolog 2; CCND1: Cyclin D1; ZFX: zinc finger protein X-linked; OC: Ovarian cancer; BCL2: B-cell lymphoma-2; BZW1: Basic leucine zipper and W2 domain‑containing protein 1; TJP3: Tight junction protein 3; CC: Cervical cancer; POU2F1: POU class 2 homeobox 1; SOX4: SRY-box transcription factor 4; HSP90s: 90-kDa heat shock proteins; EC: Endometrial carcinoma; HMGA1: High mobility group AT-hook 1; PCa: Prostate cancer; HMGA2: High mobility group AT-hook 2; HA: Hemangioma; HemECs: Hemangioma endothelial cells; AML: Acute myeloid leukemia; SMC1A: Structural maintenance of chromosomes 1A; CREBRF: CREB3 regulatory factor; T-ALL: T-cell acute lymphoblastic leukemia; DLBCL: Diffuse large B-cell lymphoma; GLI1: GLI family zinc finger 1; HL: Hodgkin's lymphoma; DCLK1: Doublecortin-like kinase 1; MM: Multiple myeloma; CDK14: Cyclin-dependent kinase 14; SOX2: SRY-box transcription factor 2; CCT6A: Chaperonin containing the TCP1 subunit 6A; DNMT1: DNA methyltransferase 1; RB: Retinoblastoma; CXCR4: C-X-C chemokine receptor type 4; NB: Neuroblastoma; JAK1: Janus kinase 1; NOVA1: Neuro‑oncological ventral antigen 1; PTC: Papillary thyroid cancer; KLK7: Kallikrein-related peptidase 7; ATAD2: ATPase family AAA domain-containing protein 2; OS: Osteosarcoma; HIF-1α: Hypoxia-inducible factor 1α; HOXA13: Homeobox A13; HMGB1: High mobility group box 1; RCC: Renal cell carcinoma; TILs: tumor infiltrating lymphocytes.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (32000878), and the Natural Science Foundation of Shandong Province (ZR2020LZL008).
Authors' contributions
KL, TY, YZ and WL drafted the manuscript. ZW contributed to conception, designed the figure, and revised the manuscript. All authors read and approved the final manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Mao YS, Sunwoo H, Zhang B. et al. Direct visualization of the co-transcriptional assembly of a nuclear body by noncoding RNAs. Nat Cell Biol. 2011;13(1):95-101
2. Clemson CM, Hutchinson JN, Sara SA. et al. An architectural role for a nuclear noncoding RNA: NEAT1 RNA is essential for the structure of paraspeckles. Mol Cell. 2009;33(6):717-26
3. Mercer TR, Qureshi IA, Gokhan S. et al. Long noncoding RNAs in neuronal-glial fate specification and oligodendrocyte lineage maturation. BMC Neurosci. 2010;11:14
4. Sunwoo H, Dinger ME, Wilusz JE. et al. MEN epsilon/beta nuclear-retained non-coding RNAs are up-regulated upon muscle differentiation and are essential components of paraspeckles. Genome Res. 2009;19(3):347-59
5. Morchikh M, Cribier A, Raffel R. et al. HEXIM1 and NEAT1 Long Non-coding RNA Form a Multi-subunit Complex that Regulates DNA-Mediated Innate Immune Response. Mol Cell. 2017;67(3):387-399.e5
6. Nakagawa S, Shimada M, Yanaka K. et al. The lncRNA Neat1 is required for corpus luteum formation and the establishment of pregnancy in a subpopulation of mice. Development. 2014;141(23):4618-27
7. Standaert L, Adriaens C, Radaelli E. et al. The long noncoding RNA Neat1 is required for mammary gland development and lactation. RNA. 2014;20(12):1844-9
8. Li Y, Cheng C. Long noncoding RNA NEAT1 promotes the metastasis of osteosarcoma via interaction with the G9a-DNMT1-Snail complex. Am J Cancer Res. 2018;8(1):81-90
9. Li W, Zhang Z, Liu X. et al. The FOXN3-NEAT1-SIN3A repressor complex promotes progression of hormonally responsive breast cancer. J Clin Invest. 2017;127(9):3421-3440
10. Wang X. Down-regulation of lncRNA-NEAT1 alleviated the non-alcoholic fatty liver disease via mTOR/S6K1 signaling pathway. J Cell Biochem. 2018;119(2):1567-1574
11. Lin Z, Li X, Zhan X. et al. Construction of competitive endogenous RNA network reveals regulatory role of long non-coding RNAs in type 2 diabetes mellitus. J Cell Mol Med. 2017;21(12):3204-3213
12. Zhang F, Wu L, Qian J. et al. Identification of the long noncoding RNA NEAT1 as a novel inflammatory regulator acting through MAPK pathway in human lupus. J Autoimmun. 2016;75:96-104
13. Wang Z, Fan P, Zhao Y. et al. NEAT1 modulates herpes simplex virus-1 replication by regulating viral gene transcription. Cell Mol Life Sci. 2017;74(6):1117-1131
14. Wang Z, Zhao Y, Xu N. et al. NEAT1 regulates neuroglial cell mediating Aβ clearance via the epigenetic regulation of endocytosis-related genes expression. Cell Mol Life Sci. 2019;76(15):3005-3018
15. Wang Z, Li K, Huang W. Long non-coding RNA NEAT1-centric gene regulation. Cell Mol Life Sci. 2020;77(19):3769-3779
16. Moreno-García L, López-Royo T, Calvo AC. et al. Competing Endogenous RNA Networks as Biomarkers in Neurodegenerative Diseases. Int J Mol Sci. 2020;21(24):9582
17. Niu ZS, Wang WH, Dong XN. et al. Role of long noncoding RNA-mediated competing endogenous RNA regulatory network in hepatocellular carcinoma. World J Gastroenterol. 2020;26(29):4240-4260
18. Braga EA, Fridman MV, Moscovtsev AA. et al. LncRNAs in Ovarian Cancer Progression, Metastasis, and Main Pathways: ceRNA and Alternative Mechanisms. Int J Mol Sci. 2020;21(22):8855
19. Lee VH, Lam KO, Chang AT. et al. Management of Nasopharyngeal Carcinoma: Is Adjuvant Therapy Needed? J Oncol Pract. 2018;14(10):594-602
20. Fodde R, Brabletz T. Wnt/beta-catenin signaling in cancer stemness and malignant behavior. Curr Opin Cell Biol. 2007;19(2):150-8
21. Ji Y, Wang M, Li X. et al. The Long Noncoding RNA NEAT1 Targets miR-34a-5p and Drives Nasopharyngeal Carcinoma Progression via Wnt/β-Catenin Signaling. Yonsei Med J. 2019;60(4):336-345
22. Cheng N, Guo Y. Long noncoding RNA NEAT1 promotes nasopharyngeal carcinoma progression through regulation of miR-124/NF-κB pathway. Onco Targets Ther. 2017;10:5843-5853
23. Oliver JR, Lieberman SM, Tam MM. et al. Human papillomavirus and survival of patients with sinonasal squamous cell carcinoma. Cancer. 2020;126(7):1413-1423
24. Robin TP, Jones BL, Gordon OM. et al. A comprehensive comparative analysis of treatment modalities for sinonasal malignancies. Cancer. 2017;123(16):3040-3049
25. Lu H, Kang F. Down-regulating NEAT1 inhibited the viability and vasculogenic mimicry formation of sinonasal squamous cell carcinoma cells via miR-195-5p/VEGFA axis. Biosci Rep. 2020;40(11):BSR20201373
26. Chen L, Xu Z, Zhao J. et al. H19/miR-107/HMGB1 axis sensitizes laryngeal squamous cell carcinoma to cisplatin by suppressing autophagy in vitro and in vivo. Cell Biol Int. 2021;45(3):674-685
27. Wang P, Wu T, Zhou H. et al. Long noncoding RNA NEAT1 promotes laryngeal squamous cell cancer through regulating miR-107/CDK6 pathway. J Exp Clin Cancer Res. 2016;35:22
28. Poomsawat S, Sanguansin S, Punyasingh J. et al. Expression of cdk6 in head and neck squamous cell carcinoma. Clin Oral Investig. 2016;20(1):57-63
29. Chen W, Zheng R, Baade PD. et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115-32
30. Sun C, Li S, Zhang F. et al. Long non-coding RNA NEAT1 promotes non-small cell lung cancer progression through regulation of miR-377-3p-E2F3 pathway. Oncotarget. 2016;7(32):51784-51814
31. Wu F, Mo Q, Wan X. et al. NEAT1/hsa-mir-98-5p/MAPK6 axis is involved in non-small-cell lung cancer development. J Cell Biochem. 2019;120(3):2836-2846
32. Kong X, Zhao Y, Li X. et al. Overexpression of HIF-2α-Dependent NEAT1 Promotes the Progression of Non-Small Cell Lung Cancer through miR-101-3p/SOX9/Wnt/β-Catenin Signal Pathway. Cell Physiol Biochem. 2019;52(3):368-381
33. Chen LM, Niu YD, Xiao M. et al. LncRNA NEAT1 regulated cell proliferation, invasion, migration and apoptosis by targeting has-miR-376b-3p/SULF1 axis in non-small cell lung cancer. Eur Rev Med Pharmacol Sci. 2020;24(9):4810-4821
34. Long W, Foulds CE, Qin J. et al. ERK3 signals through SRC-3 coactivator to promote human lung cancer cell invasion. J Clin Invest. 2012;122(5):1869-80
35. Huang JQ, Wei FK, Xu XL. et al. SOX9 drives the epithelial-mesenchymal transition in non-small-cell lung cancer through the Wnt/β-catenin pathway. J Transl Med. 2019;17(1):143
36. Lee KW, Lee SS, Hwang JE. et al. Development and Validation of a Six-Gene Recurrence Risk Score Assay for Gastric Cancer. Clin Cancer Res. 2016;22(24):6228-6235
37. Kleczko EK, Kwak JW, Schenk EL. et al. Targeting the Complement Pathway as a Therapeutic Strategy in Lung Cancer. Front Immunol. 2019;10:954
38. Ali SA, Justilien V, Jamieson L. et al. Protein Kinase Cι Drives a NOTCH3-dependent Stem-like Phenotype in Mutant KRAS Lung Adenocarcinoma. Cancer Cell. 2016;29(3):367-378
39. Xiong DD, Li ZY, Liang L. et al. The LncRNA NEAT1 Accelerates Lung Adenocarcinoma Deterioration and Binds to Mir-193a-3p as a Competitive Endogenous RNA. Cell Physiol Biochem. 2018;48(3):905-918
40. Ren P, Hong X, Chang L. et al. USF1-induced overexpression of long noncoding RNA WDFY3-AS2 promotes lung adenocarcinoma progression via targeting miR-491-5p/ZNF703 axis. Mol Carcinog. 2020;59(8):875-885
41. Ferlay J, Colombet M, Soerjomataram I. et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941-1953
42. Bray F, Ferlay J, Soerjomataram I. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424
43. Yang L, Lee MM, Leung MM. et al. Regulator of G protein signaling 20 enhances cancer cell aggregation, migration, invasion and adhesion. Cell Signal. 2016;28(11):1663-72
44. Li G, Wang M, Ren L. et al. Regulator of G protein signaling 20 promotes proliferation and migration in bladder cancer via NF-κB signaling. Biomed Pharmacother. 2019;117:109112
45. Huang G, He X, Wei XL. lncRNA NEAT1 promotes cell proliferation and invasion by regulating miR-365/RGS20 in oral squamous cell carcinoma. Oncol Rep. 2018;39(4):1948-1956
46. Rustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med. 2014;371(26):2499-509
47. Li Y, Chen D, Gao X. et al. LncRNA NEAT1 Regulates Cell Viability and Invasion in Esophageal Squamous Cell Carcinoma through the miR-129/CTBP2 Axis. Dis Markers. 2017;2017:5314649
48. Torre LA, Bray F, Siegel RL. et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87-108
49. Chivu-Economescu M, Matei L, Necula LG. et al. New therapeutic options opened by the molecular classification of gastric cancer. World J Gastroenterol. 2018;24(18):1942-1961
50. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7-30
51. Pasechnikov V, Chukov S, Fedorov E. et al. Gastric cancer: prevention, screening and early diagnosis. World J Gastroenterol. 2014;20(38):13842-62
52. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30
53. Tan HY, Wang C, Liu G. et al. Long noncoding RNA NEAT1-modulated miR-506 regulates gastric cancer development through targeting STAT3. J Cell Biochem. 2019;120(4):4827-4836
54. Xia TF, Chen J, Wu K. et al. Long noncoding RNA NEAT1 promotes the growth of gastric cancer cells by regulating miR-497-5p/PIK3R1 axis. Eur Rev Med Pharmacol Sci. 2019;23(16):6914-6926
55. Wang H, Zhang M, Sun G. Long non-coding RNA NEAT1 regulates the proliferation, migration and invasion of gastric cancer cells via targeting miR-335-5p/ROCK1 axis. Pharmazie. 2018;73(3):150-155
56. Yu DJ, Guo CX, Qian J. et al. The Long Non-Coding RNA NEAT1 Promotes Gastric Cancer Cell Proliferation and Invasion by Regulating miR-103a/STAMBPL1 Axis. Technol Cancer Res Treat. 2020;19:1533033820964081
57. Gao M, Liu L, Zhang D. et al. Long Non-Coding RNA NEAT1 Serves as Sponge for miR-365a-3p to Promote Gastric Cancer Progression via Regulating ABCC4. Onco Targets Ther. 2020;13:3977-3985
58. Berretta M, Cavaliere C, Alessandrini L. et al. Serum and tissue markers in hepatocellular carcinoma and cholangiocarcinoma: clinical and prognostic implications. Oncotarget. 2017;8(8):14192-14220
59. El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365(12):1118-27
60. Li Y, Ding X, Xiu S. et al. LncRNA NEAT1 Promotes Proliferation, Migration And Invasion Via Regulating miR-296-5p/CNN2 Axis In Hepatocellular Carcinoma Cells. Onco Targets Ther. 2019;12:9887-9897
61. Tu J, Zhao Z, Xu M. et al. NEAT1 upregulates TGF-β1 to induce hepatocellular carcinoma progression by sponging hsa-mir-139-5p. J Cell Physiol. 2018;233(11):8578-8587
62. Zhang XN, Zhou J, Lu XJ. The long noncoding RNA NEAT1 contributes to hepatocellular carcinoma development by sponging miR-485 and enhancing the expression of the STAT3. J Cell Physiol. 2018;233(9):6733-6741
63. Siegel RL, Miller KD, Goding Sauer A. et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70(3):145-164
64. He Z, Dang J, Song A. et al. NEAT1 promotes colon cancer progression through sponging miR-495-3p and activating CDK6 in vitro and in vivo. J Cell Physiol. 2019;234(11):19582-19591
65. Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer. 2009;9(3):153-66
66. Zhuang ST, Cai YJ, Liu HP. et al. LncRNA NEAT1/miR-185-5p/IGF2 axis regulates the invasion and migration of colon cancer. Mol Genet Genomic Med. 2020;8(4):e1125
67. Luo Y, Chen JJ, Lv Q. et al. Long non-coding RNA NEAT1 promotes colorectal cancer progression by competitively binding miR-34a with SIRT1 and enhancing the Wnt/β-catenin signaling pathway. Cancer Lett. 2019;440-441:11-22
68. Zhong F, Zhang W, Cao Y. et al. LncRNA NEAT1 promotes colorectal cancer cell proliferation and migration via regulating glial cell-derived neurotrophic factor by sponging miR-196a-5p. Acta Biochim Biophys Sin (Shanghai). 2018;50(12):1190-1199
69. Liu H, Li A, Sun Z. et al. Long non-coding RNA NEAT1 promotes colorectal cancer progression by regulating miR-205-5p/VEGFA axis. Hum Cell. 2020;33(2):386-396
70. Yu HM, Wang C, Yuan Z. et al. LncRNA NEAT1 promotes the tumorigenesis of colorectal cancer by sponging miR-193a-3p. Cell Prolif. 2019;52(1):e12526
71. Wang S, Du H, Sun P. Long Noncoding RNA NEAT1 Contributes to the Tumorigenesis of Colorectal Cancer Through Regulating SLC38A1 Expression by Sponging miR-138. Cancer Biother Radiopharm. 2020 doi: 10.1089/cbr.2020.3608
72. Zhu Z, Du S, Yin K. et al. Knockdown long noncoding RNA nuclear paraspeckle assembly transcript 1 suppresses colorectal cancer through modulating miR-193a-3p/KRAS. Cancer Med. 2019;8(1):261-275
73. Cheng H, Malhotra A. Evaluation of Potential of Long Noncoding RNA NEAT1 in Colorectal Cancer. J Environ Pathol Toxicol Oncol. 2020;39(2):101-111
74. DeSantis CE, Ma J, Gaudet MM. et al. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69(6):438-451
75. Xiong Y, Liu Z, Li Z. et al. Long non-coding RNA nuclear paraspeckle assembly transcript 1 interacts with microRNA-107 to modulate breast cancer growth and metastasis by targeting carnitine palmitoyltransferase-1. Int J Oncol. 2019;55(5):1125-1136
76. Pang Y, Wu J, Li X. et al. NEAT1/miR-124/STAT3 feedback loop promotes breast cancer progression. Int J Oncol. 2019;55(3):745-754
77. Jiang X, Zhou Y, Sun AJ. et al. NEAT1 contributes to breast cancer progression through modulating miR-448 and ZEB1. J Cell Physiol. 2018;233(11):8558-8566
78. Qian K, Liu G, Tang Z. et al. The long non-coding RNA NEAT1 interacted with miR-101 modulates breast cancer growth by targeting EZH2. Arch Biochem Biophys. 2017;615:1-9
79. Liu X, Yao W, Xiong H. et al. LncRNA NEAT1 accelerates breast cancer progression through regulating miR-410-3p/ CCND1 axis. Cancer Biomark. 2020;29(2):277-290
80. Yao L, Chen L, Zhou H. et al. Long Noncoding RNA NEAT1Promotes the Progression of Breast Cancer by Regulating miR-138-5p/ZFX Axis. Cancer Biother Radiopharm. 2020 doi: 10.1089/cbr.2019.3515
81. Torre LA, Trabert B, DeSantis CE. et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68(4):284-296
82. Ding N, Wu H, Tao T. et al. NEAT1 regulates cell proliferation and apoptosis of ovarian cancer by miR-34a-5p/BCL2. Onco Targets Ther. 2017;10:4905-4915
83. Liu Y, Wang Y, Fu X. et al. Long non-coding RNA NEAT1 promoted ovarian cancer cells' metastasis through regulation of miR-382-3p/ROCK1 axial. Cancer Sci. 2018;109(7):2188-2198
84. Xu H, Sun X, Huang Y. et al. Long non-coding RNA NEAT1 modifies cell proliferation, colony formation, apoptosis, migration and invasion via the miR-4500/BZW1 axis in ovarian cancer. Mol Med Rep. 2020;22(4):3347-3357
85. Luo M, Zhang L, Yang H. et al. Long non-coding RNA NEAT1 promotes ovarian cancer cell invasion and migration by interacting with miR-1321 and regulating tight junction protein 3 expression. Mol Med Rep. 2020;22(4):3429-3439
86. Fontham ETH, Wolf AMD, Church TR. et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70(5):321-346
87. Xie Q, Lin S, Zheng M. et al. Long noncoding RNA NEAT1 promotes the growth of cervical cancer cells via sponging miR-9-5p. Biochem Cell Biol. 2019;97(2):100-108
88. Yuan LY, Zhou M, Lv H. et al. Involvement of NEAT1/miR-133a axis in promoting cervical cancer progression via targeting SOX4. J Cell Physiol. 2019;234(10):18985-18993
89. Hanieh H, Ahmed EA, Vishnubalaji R. et al. SOX4: Epigenetic regulation and role in tumorigenesis. Semin Cancer Biol. 2020;67(Pt 1):91-104
90. Xu D, Dong P, Xiong Y. et al. MicroRNA-361-Mediated Inhibition of HSP90 Expression and EMT in Cervical Cancer Is Counteracted by Oncogenic lncRNA NEAT1. Cells. 2020;9(3):632
91. Das JK, Xiong X, Ren X. et al. Heat Shock Proteins in Cancer Immunotherapy. J Oncol. 2019;2019:3267207
92. Brooks RA, Fleming GF, Lastra RR. et al. Current recommendations and recent progress in endometrial cancer. CA Cancer J Clin. 2019;69(4):258-279
93. Wang J, Zhao X, Guo Z. et al. Regulation of NEAT1/miR-214-3p on the growth, migration and invasion of endometrial carcinoma cells. Arch Gynecol Obstet. 2017;295(6):1469-1475
94. Wang W, Ge L, Xu XJ. et al. LncRNA NEAT1 promotes endometrial cancer cell proliferation, migration and invasion by regulating the miR-144-3p/EZH2 axis. Radiol Oncol. 2019;53(4):434-442
95. Guo Z, He C, Yang F. et al. Long non-coding RNA-NEAT1, a sponge for miR-98-5p, promotes expression of oncogene HMGA2 in prostate cancer. Biosci Rep. 2019;39(9):BSR20190635
96. Zhang S, Mo Q, Wang X. Oncological role of HMGA2 (Review). Int J Oncol. 2019;55(4):775-788
97. Léauté-Labrèze C, Harper JI, Hoeger PH. Infantile haemangioma. Lancet. 2017;390(10089):85-94
98. Yu L, Shu H, Xing L. et al. Silencing long non-coding RNA NEAT1 suppresses the tumorigenesis of infantile hemangioma by competitively binding miR-33a-5p to stimulate HIF1α/NF-κB pathway. Mol Med Rep. 2020;22(4):3358-3366
99. Eluard B, Thieblemont C, Baud V. NF-κB in the New Era of Cancer Therapy. Trends Cancer. 2020;6(8):677-687
100. Yu X, Liu X, Wang R. et al. Long non-coding RNA NEAT1 promotes the progression of hemangioma via the miR-361-5p/VEGFA pathway. Biochem Biophys Res Commun. 2019;512(4):825-831
101. Kanthou C, Tozer G. Targeting the vasculature of tumours: combining VEGF pathway inhibitors with radiotherapy. Br J Radiol. 2019;92(1093):20180405
102. Thomas D, Majeti R. Biology and relevance of human acute myeloid leukemia stem cells. Blood. 2017;129(12):1577-1585
103. Zhao C, Wang S, Zhao Y. et al. Long noncoding RNA NEAT1 modulates cell proliferation and apoptosis by regulating miR-23a-3p/SMC1A in acute myeloid leukemia. J Cell Physiol. 2019;234(5):6161-6172
104. Feng S, Liu N, Chen X. et al. Long non-coding RNA NEAT1/miR-338-3p axis impedes the progression of acute myeloid leukemia via regulating CREBRF. Cancer Cell Int. 2020;20:112
105. Girardi T, Vicente C, Cools J. et al. The genetics and molecular biology of T-ALL. Blood. 2017;129(9):1113-1123
106. Luo YY, Wang ZH, Yu Q. et al. LncRNA-NEAT1 promotes proliferation of T-ALL cells via miR-146b-5p/NOTCH1 signaling pathway. Pathol Res Pract. 2020;216(11):153212
107. Zheng R, Li M, Wang S. et al. Advances of target therapy on NOTCH1 signaling pathway in T-cell acute lymphoblastic leukemia. Exp Hematol Oncol. 2020;9(1):31
108. Sehn LH, Gascoyne RD. Diffuse large B-cell lymphoma: optimizing outcome in the context of clinical and biologic heterogeneity. Blood. 2015;125(1):22-32
109. Qian CS, Li LJ, Huang HW. et al. MYC-regulated lncRNA NEAT1 promotes B cell proliferation and lymphomagenesis via the miR-34b-5p-GLI1 pathway in diffuse large B-cell lymphoma. Cancer Cell Int. 2020;20:87
110. Agarwal NK, Kim CH, Kunkalla K. et al. Active IKKβ promotes the stability of GLI1 oncogene in diffuse large B-cell lymphoma. Blood. 2016;127(5):605-615
111. Townsend W, Linch D. Hodgkin's lymphoma in adults. Lancet. 2012;380(9844):836-847
112. Fan CB, Yan XH, Tian M. et al. Long non-coding RNA NEAT1 regulates Hodgkin's lymphoma cell proliferation and invasion via miR-448 mediated regulation of DCLK1. Eur Rev Med Pharmacol Sci. 2020;24(11):6219-6227
113. Cao Z, Weygant N, Chandrakesan P. et al. Tuft and Cancer Stem Cell Marker DCLK1: A New Target to Enhance Anti-Tumor Immunity in the Tumor Microenvironment. Cancers (Basel). 2020;12(12):E3801
114. Röllig C, Knop S, Bornhäuser M. Multiple myeloma. Lancet. 2015;385(9983):2197-208
115. Gao Y, Fang P, Li WJ. et al. LncRNA NEAT1 sponges miR-214 to regulate M2 macrophage polarization by regulation of B7-H3 in multiple myeloma. Mol Immunol. 2020;117:20-28
116. Shu Y, Qin M, Song Y. et al. M2 polarization of tumor-associated macrophages is dependent on integrin β3 via peroxisome proliferator-activated receptor-γ up-regulation in breast cancer. Immunology. 2020;160(4):345-356
117. Tan AC, Ashley DM, López GY. et al. Management of glioblastoma: State of the art and future directions. CA Cancer J Clin. 2020;70(4):299-312
118. Zhen Y, Nan Y, Guo S. et al. Knockdown of NEAT1 repressed the malignant progression of glioma through sponging miR-107 and inhibiting CDK14. J Cell Physiol. 2019;234(7):10671-10679
119. Zhou K, Zhang C, Yao H. et al. Knockdown of long non-coding RNA NEAT1 inhibits glioma cell migration and invasion via modulation of SOX2 targeted by miR-132. Mol Cancer. 2018;17(1):105
120. Zhen L, Yun-Hui L, Hong-Yu D. et al. Long noncoding RNA NEAT1 promotes glioma pathogenesis by regulating miR-449b-5p/c-Met axis. Tumour Biol. 2016;37(1):673-83
121. Li B, Lu X, Ma C. et al. Long non-coding RNA NEAT1 promotes human glioma tumor progression via miR-152-3p/CCT6A pathway. Neurosci Lett. 2020;732:135086
122. Wu DM, Wang S, Wen X. et al. Long noncoding RNA nuclear enriched abundant transcript 1 impacts cell proliferation, invasion, and migration of glioma through regulating miR-139-5p/ CDK6. J Cell Physiol. 2019;234(5):5972-5987
123. Yu H, Xu A, Wu B. et al. Long noncoding RNA NEAT1 promotes progression of glioma as a ceRNA by sponging miR-185-5p to stimulate DNMT1/mTOR signaling. J Cell Physiol. 2021;236(1):121-130
124. Kaewkhaw R, Rojanaporn D. Retinoblastoma: Etiology, Modeling, and Treatment. Cancers (Basel). 2020;12(8):2304
125. Zhong W, Yang J, Li M. et al. Long noncoding RNA NEAT1 promotes the growth of human retinoblastoma cells via regulation of miR-204/CXCR4 axis. J Cell Physiol. 2019;234(7):11567-11576
126. Zafar A, Wang W, Liu G. et al. Molecular targeting therapies for neuroblastoma: Progress and challenges. Med Res Rev. 2021;41(2):961-1021
127. Yang B, Ye X, Wang J. et al. Long noncoding RNA nuclear-enriched abundant transcript 1 regulates proliferation and apoptosis of neuroblastoma cells treated by cisplatin by targeting miR-326 through Janus kinase/signal transducer and activator of transcription 3 pathway. Neuroreport. 2020;31(17):1189-1198
128. Fallahi P, Ferrari SM, Galdiero MR. et al. Molecular targets of tyrosine kinase inhibitors in thyroid cancer. Semin Cancer Biol. 2020: S1044-579X(20)30249-2.
129. Luo Y, Hao T, Zhang J. et al. MicroRNA-592 suppresses the malignant phenotypes of thyroid cancer by regulating lncRNA NEAT1 and downregulating NOVA1. Int J Mol Med. 2019;44(3):1172-1182
130. Zhang H, Cai Y, Zheng L. et al. Long noncoding RNA NEAT1 regulate papillary thyroid cancer progression by modulating miR-129-5p/KLK7 expression. J Cell Physiol. 2018;233(10):6638-6648
131. Sun W, Lan X, Zhang H. et al. NEAT1_2 functions as a competing endogenous RNA to regulate ATAD2 expression by sponging microRNA-106b-5p in papillary thyroid cancer. Cell Death Dis. 2018;9(3):380
132. Li Z, Li X, Xu D. et al. An update on the roles of circular RNAs in osteosarcoma. Cell Prolif. 2020: e12936.
133. Zhang L, Lu XQ, Zhou XQ. et al. NEAT1 induces osteosarcoma development by modulating the miR-339-5p/TGF-β1 pathway. J Cell Physiol. 2019;234(4):5097-5105
134. Tan H, Zhao L. lncRNA nuclear-enriched abundant transcript 1 promotes cell proliferation and invasion by targeting miR-186-5p/HIF-1α in osteosarcoma. J Cell Biochem. 2019;120(4):6502-6514
135. Ji S, Wang S, Zhao X. et al. Long noncoding RNA NEAT1 regulates the development of osteosarcoma through sponging miR-34a-5p to mediate HOXA13 expression as a competitive endogenous RNA. Mol Genet Genomic Med. 2019;7(6):e673
136. Patel VG, Oh WK, Galsky MD. Treatment of muscle-invasive and advanced bladder cancer in 2020. CA Cancer J Clin. 2020;70(5):404-423
137. Shan G, Tang T, Xia Y. et al. Long non-coding RNA NEAT1 promotes bladder progression through regulating miR-410 mediated HMGB1. Biomed Pharmacother. 2020;121:109248
138. Rapoport BL, Steel HC, Theron AJ. et al. High Mobility Group Box 1 in Human Cancer. Cells. 2020;9(7):1664
139. Liu F, Chen N, Gong Y. et al. The long non-coding RNA NEAT1 enhances epithelial-to-mesenchymal transition and chemoresistance via the miR-34a/c-Met axis in renal cell carcinoma. Oncotarget. 2017;8(38):62927-62938
140. Pothula SP, Xu Z, Goldstein D. et al. Targeting HGF/c-MET Axis in Pancreatic Cancer. Int J Mol Sci. 2020;21(23):9170
141. Kuczynski EA, Sargent DJ, Grothey A. et al. Drug rechallenge and treatment beyond progression-implications for drug resistance. Nat Rev Clin Oncol. 2013;10(10):571-587
142. Gottesman MM, Lavi O, Hall MD. et al. Toward a Better Understanding of the Complexity of Cancer Drug Resistance. Annu Rev Pharmacol Toxicol. 2016;56:85-102
143. Rebucci M, Michiels C.Molecular aspects of cancer cell resistance to chemotherapy. Biochem Pharmacol, 2013; 85(9): 1219-1226
144. Li X, Wang S, Li Z. et al. The lncRNA NEAT1 facilitates cell growth and invasion via the miR-211/HMGA2 axis in breast cancer. Int J Biol Macromol. 2017;105(Pt 1):346-353
145. Wang X, Jiang G, Ren W. et al. LncRNA NEAT1 Regulates 5-Fu Sensitivity, Apoptosis and Invasion in Colorectal Cancer Through the MiR-150-5p/CPSF4 Axis. Onco Targets Ther. 2020;13:6373-6383
146. Zhu M, Yang L, Wang X. NEAT1 Knockdown Suppresses the Cisplatin Resistance in Ovarian Cancer by Regulating miR-770-5p/PARP1 Axis. Cancer Manag Res. 2020;12:7277-7289
147. Yan P, Su Z, Zhang Z. et al. LncRNA NEAT1 enhances the resistance of anaplastic thyroid carcinoma cells to cisplatin by sponging miR-9-5p and regulating SPAG9 expression. Int J Oncol. 2019;55(5):988-1002
148. Hu Y, Yang Q, Wang L. et al. Knockdown of the oncogene lncRNA NEAT1 restores the availability of miR-34c and improves the sensitivity to cisplatin in osteosarcoma. Biosci Rep. 2018;38(3):BSR20180375
149. Jiang X, Guo S, Zhang Y. et al. LncRNA NEAT1 promotes docetaxel resistance in prostate cancer by regulating ACSL4 via sponging miR-34a-5p and miR-204-5p. Cell Signal. 2020;65:109422
150. Guo Y, Zhang H, Xie D. et al. Non-coding RNA NEAT1/miR-214-3p contribute to doxorubicin resistance of urothelial bladder cancer preliminary through the Wnt/β-catenin pathway. Cancer Manag Res. 2018;10:4371-4380
151. An J, Lv W, Zhang Y. LncRNA NEAT1 contributes to paclitaxel resistance of ovarian cancer cells by regulating ZEB1 expression via miR-194. Onco Targets Ther. 2017;10:5377-5390
152. Dong P, Xiong Y, Yue J. et al. Long noncoding RNA NEAT1 drives aggressive endometrial cancer progression via miR-361-regulated networks involving STAT3 and tumor microenvironment-related genes. J Exp Clin Cancer Res. 2019;38(1):295
153. Li X, Zhou Y, Yang L. et al. LncRNA NEAT1 promotes autophagy via regulating miR-204/ATG3 and enhanced cell resistance to sorafenib in hepatocellular carcinoma. J Cell Physiol. 2020;235(4):3402-3413
154. Chen S, Xia X. Long noncoding RNA NEAT1 suppresses sorafenib sensitivity of hepatocellular carcinoma cells via regulating miR-335-c-Met. J Cell Physiol. 2019 doi: 10.1002/jcp.27567
155. Xue F, Cheng Y, Xu L. et al. LncRNA NEAT1/miR-129/Bcl-2 signaling axis contributes to HDAC inhibitor tolerance in nasopharyngeal cancer. Aging (Albany NY). 2020;12(14):14174-14188
156. Lu Y, Li T, Wei G. et al. The long non-coding RNA NEAT1 regulates epithelial to mesenchymal transition and radioresistance in through miR-204/ZEB1 axis in nasopharyngeal carcinoma. Tumour Biol. 2016;37(9):11733-11741
157. Chen X, Zhang N. Downregulation of lncRNA NEAT1_2 radiosensitizes hepatocellular carcinoma cells through regulation of miR-101-3p/WEE1 axis. Cell Biol Int. 2019;43(1):44-55
158. Han D, Wang J, Cheng G. LncRNA NEAT1 enhances the radio-resistance of cervical cancer via miR-193b-3p/CCND1 axis. Oncotarget. 2017;9(2):2395-2409
Author contact
![]() Corresponding authors: Ziqiang Wang. E-mail: wangziqiangedu.cn. Wen Li. E-mail: 0686com.cn.
Corresponding authors: Ziqiang Wang. E-mail: wangziqiangedu.cn. Wen Li. E-mail: 0686com.cn.

 Global reach, higher impact
Global reach, higher impact